Abstract
Tamoxifen resistance has been largely attributed to genetic alterations in the epithelial tumor cells themselves, such as overexpression of HER-2/Neu. However, in the clinic, only about 15–20% of cases of HER-2/ Neu amplification has actually been correlated to the acquisition of endocrine resistance, suggesting that other mechanisms must be involved as well. Using the epithelial LM05-E and the fibroblastic LM05-F cell lines, derived from the estrogen dependent spontaneous M05 mouse mammary tumor, as well as MCF-7 cells, we analyzed whether soluble stromal factors or extracellular matrix components protected against tamoxifen induced cell death. Involvement of signaling pathways was determined by using specific inhibitors and western blot, and phosphorylation of the estrogen receptor alpha by western blot and immunofluorescence. Soluble factors produced by the fibroblastic cells protect the epithelial tumor cells from tamoxifen-induced cell death through a mechanism that involves EGFR and matrix metalloproteinases upstream of PI3K/AKT. Exogenous fibronectin by itself confers endocrine resistance through interaction with β1 integrin and activation of PI3K/AKT and MAPK/ERK 1/2 pathways. The conferred resistance is reversed by blocking β1 integrin. We show also that treatment with both conditioned medium and fibronectin leads to the phosphorylation of the estrogen receptor at serine-118, suggesting stromal factors as modulators of ER activity. Our results show that the tumor microenvironment can modulate tamoxifen resistance, providing an alternative explanation for why patients become refractory to hormone-therapy.
Keywords: Breast cancer, Tamoxifen resistance, Estrogen receptor, Tumor microenvironment, Fibronectin, Soluble stromal factors, β1 integrin
Introduction
Tamoxifen, a selective estrogen receptor modulator, has been the main adjuvant treatment for estrogen receptor (ER) positive breast cancer patients for approximately 30 years [1]. However, the development of both de novo and acquired resistance are still important clinical problems that need to be fully understood. Research focused on understanding the mechanisms of acquired tamoxifen resistance has been mostly carried out using the human MCF-7 cell line and as such current hypothesis are based on autocrine, or paracrine mechanisms between epithelial cells. Deregulation of the HER-2/Neu pathway has been shown to contribute to tumor progression and tamoxifen resistance, leading to the development of drugs such as Trastuzamab [2, 3]. However, clinical data show that only a relatively small group (15–20%) of ER positive patients who acquire tamoxifen resistance actually show changes in HER-2 status [4–6], implicating the involvement of other mechanisms.
Tumors are complex organs composed not only of neoplastic cells, but also contain fibroblasts, blood vessels, immune cells, and extracellular matrix [7, 8]. Evidence suggests that both tumor progression and response to therapy are modulated by the tumor microenvironment [9, 10]. Indeed, several papers have implicated stromal signatures as predictors of response to therapy in breast cancer [11, 12]. Moreover, response to tamoxifen is associated with the overexpression of an extracellular matrix gene cluster [13, 14]. However, probably due to the lack of adequate experimental models, the molecular link between stromal factors and tamoxifen resistance remains to be explored. We recently characterized the spontaneous M05 mouse mammary tumor that arose in a BALB/c mouse in our animal facility and showed that it is estrogen dependent and tamoxifen sensitive in early passages and progresses to endocrine resistance [15]. From it, we generated a bicellular cell line, LM05-Mix, composed of both epithelial and fibroblastic cells that were subsequently separated to generate the epithelial LM05-E and fibroblastic LM05-F cell lines, respectively [16]. Our previous work showed that the fibroblastic LM05-F cells conferred tamoxifen resistance to the otherwise tamoxifen-sensitive LM05-E cells [16]. In this paper, we unravel the mediators involved in this protective effect and show that fibroblast-derived soluble factors, and fibronectin through its interaction with β1 integrin, modulate resistance to tamoxifen in epithelial cells. Our results support the above-mentioned clinical data and sustain the notion that changes in the stroma may play a key role in the development of tamoxifen resistance in breast cancer.
Materials and methods
Cell culture
The MCF-7 and LM05-Mix, -E, -F cell lines were routinely maintained in growth medium, consisting of DMEM/F12 medium (Sigma-Aldrich, St. Louis, MO), supplemented with 10% fetal calf serum (FCS, GenSA, Buenos Aires, Argentina) and gentamicin, in a humidified 5% CO2/air atmosphere. Serial passages were carried out by treatment of 80% confluent monolayers with 0.25% trypsin (Invitrogen, Carlsbad, CA) and 0.02% EDTA in Ca2+-free and Mg2+-free PBS.
Cell treatments
For cell death experiments, 35,000 cells were plated in eight well LabTek chamber slides (Nunc, Thermo Fisher Scientific, Roskilde, Denmark) in growth medium. The next day, they were washed twice with phenol red free DMEM/F12, and treatments were carried out in this culture medium supplemented with 1% charcoal stripped FCS. 17-β-estradiol (Sigma-Aldrich) was used at a final concentration of 10 nM, and 4-OH-tamoxifen (Sigma-Aldrich) at 1 µM, as previously described [16]. Both were prepared as 1000× stock solutions in absolute ethanol, and the corresponding dilutions of ethanol were used for the control cells. To test the role of fibronectin (Millipore, Billerica, MA), chamber slides were previously coated using 200 µl per well of a solution of fibronectin (2 µg/100 µl in water) or the equivalent amount of fatty acid-free BSA (Sigma-Aldrich) as a control. When used in a soluble fashion, fibronectin was added to the media at a concentration of 30 µg/ml. For treatments with LM05-F-conditioned media (FCM), the day after seeding, cells were washed and treated with a mixture of 70% FCM + 30% fresh medium, plus the corresponding treatments. To prepare FCM, 1 × 106 LM05-F cells were plated in growth medium in 100-mm dishes. The next day, they were washed three times with PBS and incubated for 18 h in 5 ml of serum free, phenol red free DMEM/F12. This medium was collected, centrifuged to remove any floating cells and cell debris, and finally aliquoted and stored at −20°C until used. Regarding specific inhibitors, the following were used: MAPK pathway inhibitor PD98059 (10 µM; Calbiochem, Darmstadt, Germany), PI3K/AKT pathway inhibitor LY294002 (10 µM; Calbiochem), the matrix metalloproteinase inhibitor GM6001 (10 µM; Santa Cruz Biotechnology, Santa Cruz, CA) and the EGFR inhibitor AG1478 (6.4 µM; Calbiochem). We did not detect estrogenic activity on MCF-7 cells by ERE-Luc reporter assays or proliferation assays in the PD98059 (10 µM) we used (our unpublished observations). At least 10009 stock solutions of the inhibitors were prepared in DMSO, and the equivalent dilutions of DMSO were used as controls. To block β1 integrin, cells were incubated for 15 min with the AIIB2 monoclonal antibody (0.15 µg/ml; Aragen Bioscience, Morgan Hill, CA) or with control pre-immune IgG before being plated in the chamber slides, as previously described by others [17].
Cell death assays
We previously showed that treatment of LM05-E cells with 4-OH-tamoxifen leads to cell death as measured by the TUNEL assay (In Situ Cell Death Detection Kit, Flourescein, Roche Applied Science, Bromma, Sweden) or propidium iodide (PI) exclusion [16]. Thus, for these experiments, cells were seeded in LabTek chamber slides as described above and after the 48 h of treatment concluded, slides were washed with PBS, and cells were incubated for 1 min in a PI solution (Sigma-Aldrich, 50 µg/ml in PBS), washed in PBS, and fixed with 4% formalin in PBS for 10 min. Nuclei were counterstained with 4′,6-diamino-2-phenylindole (DAPI, Research Organics Inc., Cleveland, OH), and slides were mounted with Vectashield (Vector Laboratories, Burlingame, CA). Experiments were carried out in triplicates, and 10 random fields with an average of 200 cells/field were counted per well. The percentage of PI-positive cells relative to the total number of cells (stained with DAPI) was calculated as a measure of cell death and is expressed as % dead cells in the graphs. Images were taken using a Nikon TE2000S inverted fluorescent microscope and counted using the Image Pro Plus software.
Western blot
To evaluate the activation of the different signaling pathways by fibronectin and FCM, 600.000 LM05-E cells were plated on 60-mm culture dishes in growth medium. The next day, cells were washed twice, and media was replaced by phenol red free medium. Cells were starved for 48 h and were then subjected to treatments with FCM or fibronectin (30 µg/ml) for the indicated periods of time. Protein extracts were prepared by homogenizing cells on ice in RIPA buffer (50 mM Tris, pH 8.0 containing 150 mM NaCl, 0.1% SDS, 0.5% deoxycholate and 1% NP40) containing protease inhibitors (40 µm phenylmethylsulfonyl fluoride, 5 µg/ml leupeptin, 50 µg/ml aprotinin and 200 µM orthovanadate). Protein concentrations were measured using the Bradford method. Samples were mixed with 4× sample buffer containing β-mercaptoethanol and boiled for 2 min. Fifty micrograms of each sample was then separated in SDS-PAGE mini gels and transferred to PVDF membranes (Amersham Biosciences, Uppsala, Sweden). The membranes were blocked overnight in 5% fat free milk, 0.1% Tween-20 in PBS at 4°C. Primary antibodies were used at a 1/500–1/2000 dilution in PBS containing 0.1% Tween-20 (PBST) and 2.5% fat free milk and were incubated at 4°C overnight. After washing with PBST, membranes were incubated with secondary antibodies at a 1/1000 dilution for 1 h at room temperature. Signals were detected with an enhanced chemiluminescence kit (ECL, Amersham Biosciences). The following primary antibodies were used: mouse anti-phospho ERK1/2, mouse anti-ERK 1/2, rabbit anti-phospho AKT, rabbit anti-AKT, rabbit anti-β1 integrin, rabbit anti-E-cadherin (all from Santa Cruz Biotechnology), rabbit anti-phospho-serine 188 ER (Cell Signaling, Danvers, MA), mouse anti-phosphor-EGFR (Tyr1 173) and rabbit anti-EGFR (Millipore). The following secondary antibodies were used: donkey anti-rabbit HRP and goat anti-mouse HRP (Santa Cruz Biotechnology). E-cad-herin was used as loading control.
Zymography
Conditioned media prepared from LM05-F and LM05-E cells as indicated above was analyzed by gelatin substrate gels as described previously [18]. In brief, 10 µl of conditioned medium was mixed with Laemmli sample buffer without reducing agents, incubated for 15 min at 37°C, and separated on 8.8% sodium dodecyl sulfate (SDS)-poly-acrylamide slab gels containing 1 mg/ml of gelatin (Sigma-Aldrich). After electrophoresis, gels were incubated for 30 min with 2.5% Triton X-100 and subsequently overnight at 37°C in 100 mM Tris–HCl, pH 7.4, containing 15 mM CaCl2. Gels were stained with Coomassie Blue R-250 (Sigma-Aldrich) and destained with water. Clear zones emerged against a blue background, indicating proteolytic activity. The same procedure was used to analyze proteolytic activity in LM05-E-conditioned media.
Immunofluorescence
Cells grown on LabTek assemblies were fixed in 4% formalin in PBS for 20 min at room temperature and permeabilized with 0.1% Triton X-100 in PBS at 37°C for 20 additional minutes. Non-specific sites were blocked with 2% FCS in PBS for 1 h at room temperature. The primary antibody against phosphor-serine 118 ER (Cell Signaling) and the primary antibody against fibronectin (a kind gift from Dr. Alberto Kornblihtt) were prepared in blocking buffer at a 1/100 dilution and applied overnight at 4°C in a humidified chamber. Samples were washed in PBS and incubated with the secondary goat anti-rabbit antibody (FITC, Invitrogen) or the rabbit anti-mouse (FITC, Invitrogen) prepared in blocking buffer for 1 h at room temperature. The slides were then washed and counterstained with DAPI or PI and mounted with Vectashield. Samples were analyzed under a Nikon Laser Confocal Microscope (Nikon, Tokyo, Japan) or a Nikon TE2000S inverted fluorescent microscope. For each experiment, the same gain was used to acquire all images by confocal microscopy. Staining intensity was calculated using Image Pro Plus software. For each time point, the staining value was normalized to propidium iodide.
Statistical analysis
The statistical significance of differences between groups was calculated by applying one-way ANOVA, followed by Bonferroni multiple comparisons test. A value of P < 0.05 was considered significant.
Results
Fibroblast-derived soluble factors confer tamoxifen resistance to otherwise tamoxifen-sensitive epithelial cells: involvement of EGFR, MMPs and PI3K/AKT
From the estrogen-dependent M05 mouse mammary tumor [15], we established a continuous cell line referred to as LM05-Mix [16]. Two distinct cell populations were identified in the LM05-Mix cell line that were subsequently cloned to generate the epithelial LM05-E and the fibroblastic LM05-F cell lines, respectively [16]. We previously showed that the proliferation of the LM05-E cells was stimulated by 10 nM estradiol and inhibited by 1 µM 4-OH-tamoxifen [16]. On the other hand, the LM05-F cells did not respond to either estradiol or 4-OH-tamoxifen. Moreover, we demonstrated that 4-OH-tamoxifen-induced LM05-E inhibition of proliferation involved cell death as determined by the TUNEL assay and propidium iodide exclusion. However, when both cell types were cultivated together, the induction of 4-OH-tamoxifen cell death was inhibited [16]. Thus, our results suggested that the presence of fibroblastic cells was allowing the epithelial cells to escape from 4-OH-tamoxifen-induced cell death.
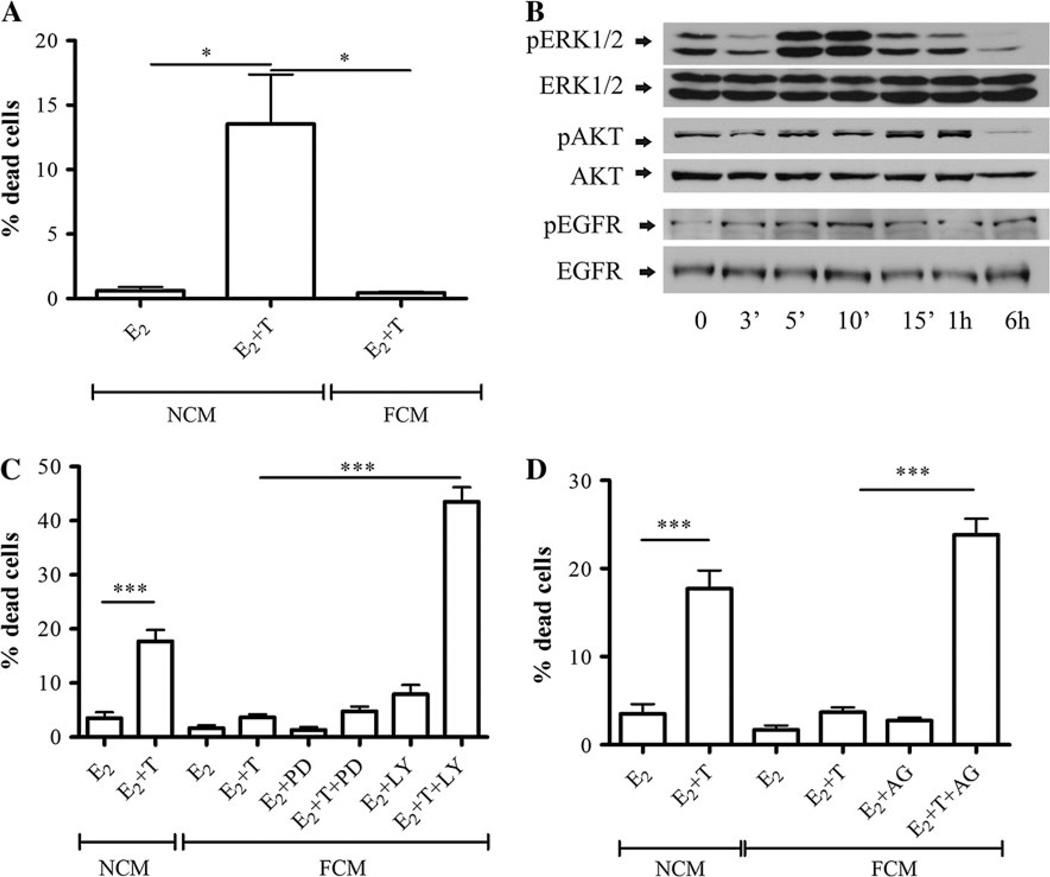
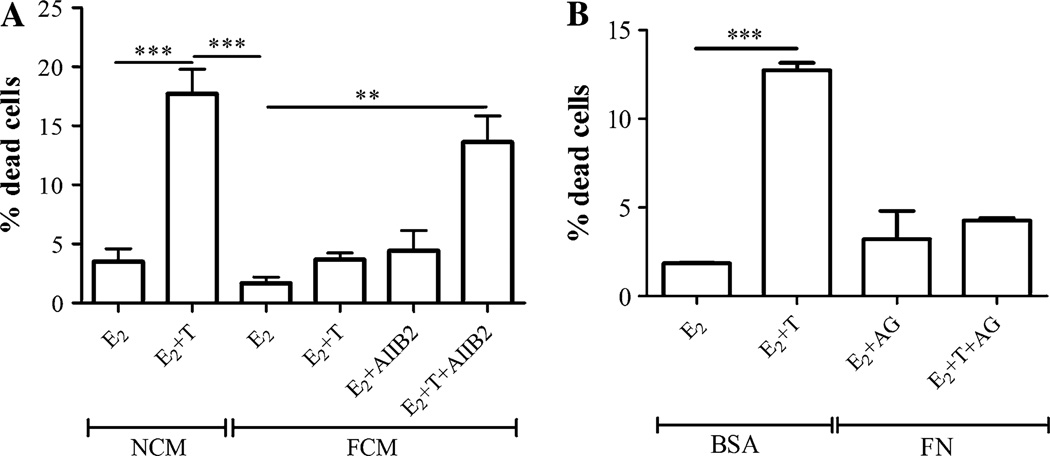
To understand the molecular mechanisms by which stromal–epithelial interactions conferred tamoxifen resistance and determine whether physical interaction between the fibroblasts and epithelial cells was necessary for the protective effect, LM05-E cells were treated with 4-OH-tamoxifen for 48 h in the presence of LM05-F-conditioned media (FCM). FCM was sufficient to protect the epithelial cells from the tamoxifen-induced cell death, compared to non-conditioned media (NCM) (Fig. 1a). These results indicate that stromal-derived soluble factors, but not the physical interaction between the two cell types, are required for the protective effect. Aberrant activation of PI3K/AKT and MAPK/ERK1/2 pathways has been associated with the development of tamoxifen resistance in breast cancer [19]. Western blot analysis showed that FCM strongly activated the MAPK/ERK1/2 pathway, and to a lesser degree the PI3K/AKT pathway (Fig. 1b). To determine whether both pathways are implicated in mediating the protective effect, LM05-E cells were treated with tamoxifen in the presence of FCM with the addition of the signaling inhibitors PD98059 or LY294002. Only the PI3K/AKT inhibitor LY294002 reversed the protective effect of FCM (Fig. 2c). The effectiveness of the inhibitory effects of PD98059 and LY294002 can be seen in Supplementary Fig. 1a and b.
Fig. 1.
LM05-F-conditioned media induces tamoxifen resistance through a mechanism that involves EGFR and PI3K/AKT. a LM05-E cells were cultured in non-conditioned media (NCM) or LM05-F-conditioned media (FCM) for 48 h in the presence of 10 nM estradiol (E2) or 10 nM estradiol plus 1 µM 4-OH-tamoxifen (E2 + T). Tamoxifen induced cell death in the presence of NCM; however, this effect was suppressed in the presence of FCM (*P<0.05). To establish the % of dead cells, once the treatments were over, cells were washed, incubated with PI, fixed, counterstained with DAPI, and mounted as explained in materials and methods. Cell death is expressed as the % of PI-positive cells/total number of cells. b Activation of signaling pathways by FCM in LM05-E cells. LM05-E cells starved for 48 h were treated for the indicated periods of time with FCM. Western blot analysis shows phosphorylation of ERK1/2, AKT and EGFR. E-cadherin was used as loading control. c LM05-E cells were treated as described in (a) with the addition of the MAPK/ERK inhibitor PD98059 (PD) or the PI3K/AKT inhibitor LY294002 (LY) (both at 10 µM) to the FCM-treated cells. Only LY294002 reversed the protective effect of FCM, (***P < 0.001). d LM05-E cells were treated as described in (a) with the addition of the EGFR inhibitor AG1478 (AG; 6.4 µM) to the FCM-treated cells. AG1478 reversed the FCM protective effect, but did not on its own affect the viability of the cells (***P<0.001). One of three experiments is shown
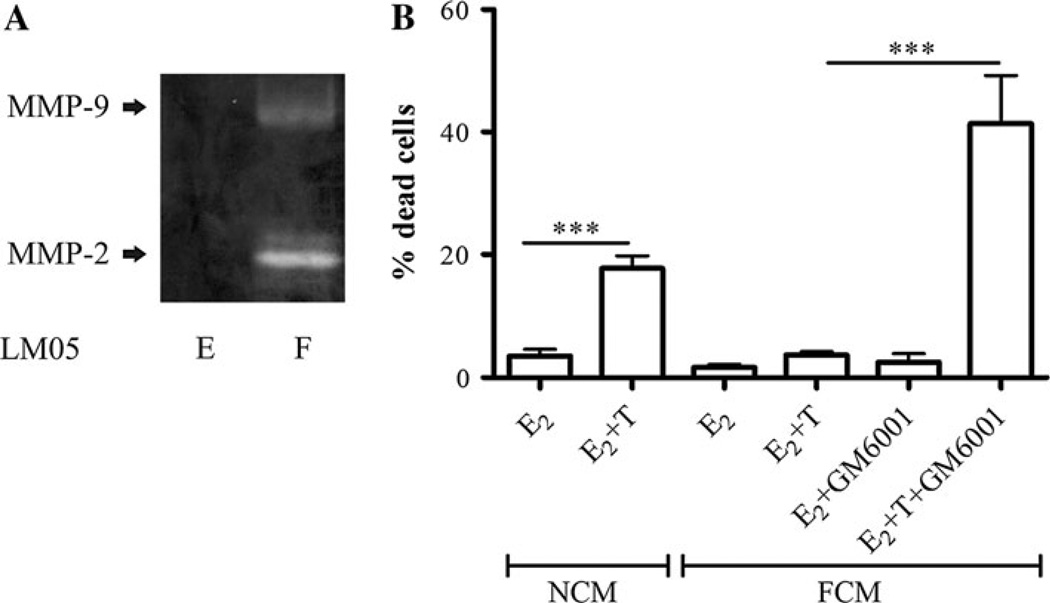
Fig. 2.
MMP activity in FCM is required for paracrine-induced tamoxifen resistance. a LM05-E- and LM05-F-conditioned media were analyzed by gelatin zymography; activities of MMP-9 and MMP-2 were only detected in conditioned media derived from LM05-F cells. b LM05-E cells were cultured in NCM or FCM and treated for 48 h with 10 nM estradiol (E2) or 10 nM E2 + 1 µM 4-OH-tamoxifen (E + T). Addition of the broad spectrum MMP inhibitor GM6001 (10 µM) reversed the FCM protective effect, but did not have any effects on its own (***P< 0.001). One of two experiments is shown
The EGFR receptor family has been implicated in the induction of tamoxifen resistance and is known to activate the MAPK/ERK and PI3K/AKT pathways in breast cancer cells [19]. Most previous studies used MCF-7 cells that were modified to over express EGFR or Her-2 to show the involvement of this receptor family in the induction of tamoxifen resistance [20, 21]. LM05-E cells are EGFR positive, and we have not modified them to over express or over-activate this receptor. Treatment with FCM led to modest phosphorylation of EGFR (Fig. 1b). To investigate whether the paracrine activation of the EGFR pathway was involved in the protective effect conferred by FCM, LM05-E cells were treated with tamoxifen in the presence of FCM and the specific EGFR inhibitor, AG1478. The protective effect of the conditioned media was reversed by AG1478 treatment (Fig. 1d), implying that the paracrine activation of EGFR is necessary for the induction of tamoxifen resistance in LM05-E cells. As an indicator of the effectiveness of AG1478, we measured MAPK/ERK1/2 activation, which is downstream of EGFR, in the presence of the inhibitor (Supplementary Fig. 1c)
Matrix metalloproteinases (MMPs) not only play a role in breaking down the extracellular matrix (ECM), but are involved also in the regulation of cell behavior by affecting, for example, the release of growth factors such as heparin-bound EGF [22]. We wondered whether proteases could be involved in the FCM protective effect. Analysis of MMP activity in conditioned media of LM05-F cells revealed the presence of both MMP-9 and MMP-2 activities (Fig. 2a). On the other hand, MMP activity was not detected by zymography in the conditioned media of LM05-E cells under the same experimental conditions (Fig. 2a). To test whether stromal-derived MMPs were involved in the FCM protective effect, LM05-E cells were treated with 4-OH-tamoxifen in the presence of FCM and GM6001, a broad spectrum MMP inhibitor. In the presence of tamoxifen, GM6001 reversed the FCM protective effect but had no effect on cells that did not receive 4-OH-tamoxifen (Fig. 2b). Our results suggest that soluble factors produced by fibroblasts, including growth factors and MMPs, are involved in the paracrine induction of tamoxifen resistance through EGFR and the PI3K/AKT pathway.
Fibronectin induced tamoxifen resistance through ligation of β1 integrins
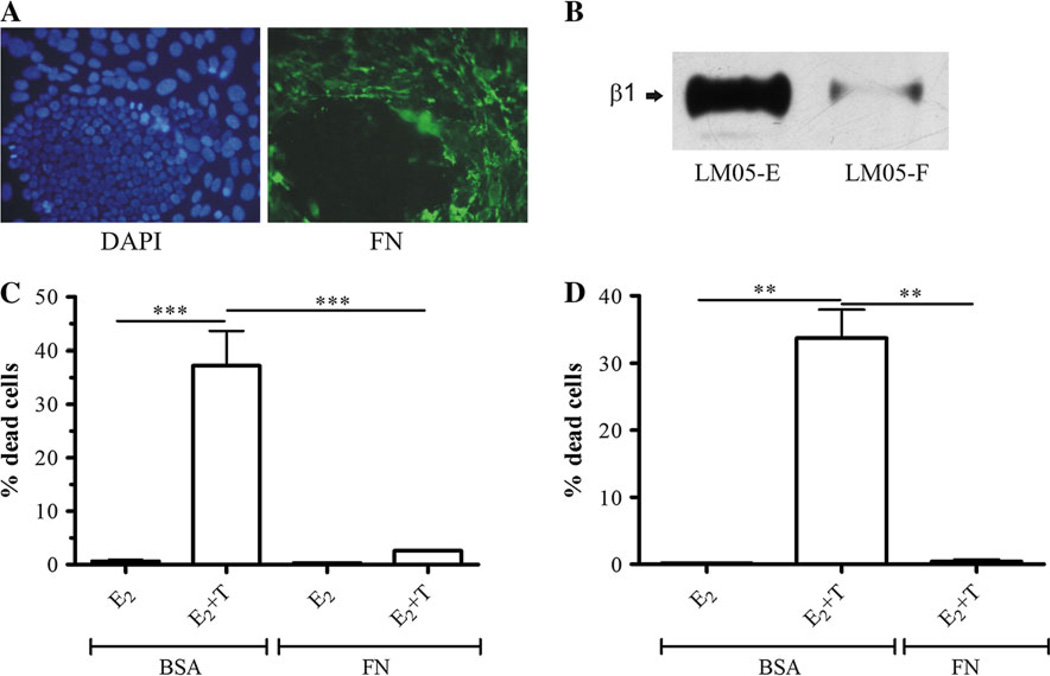
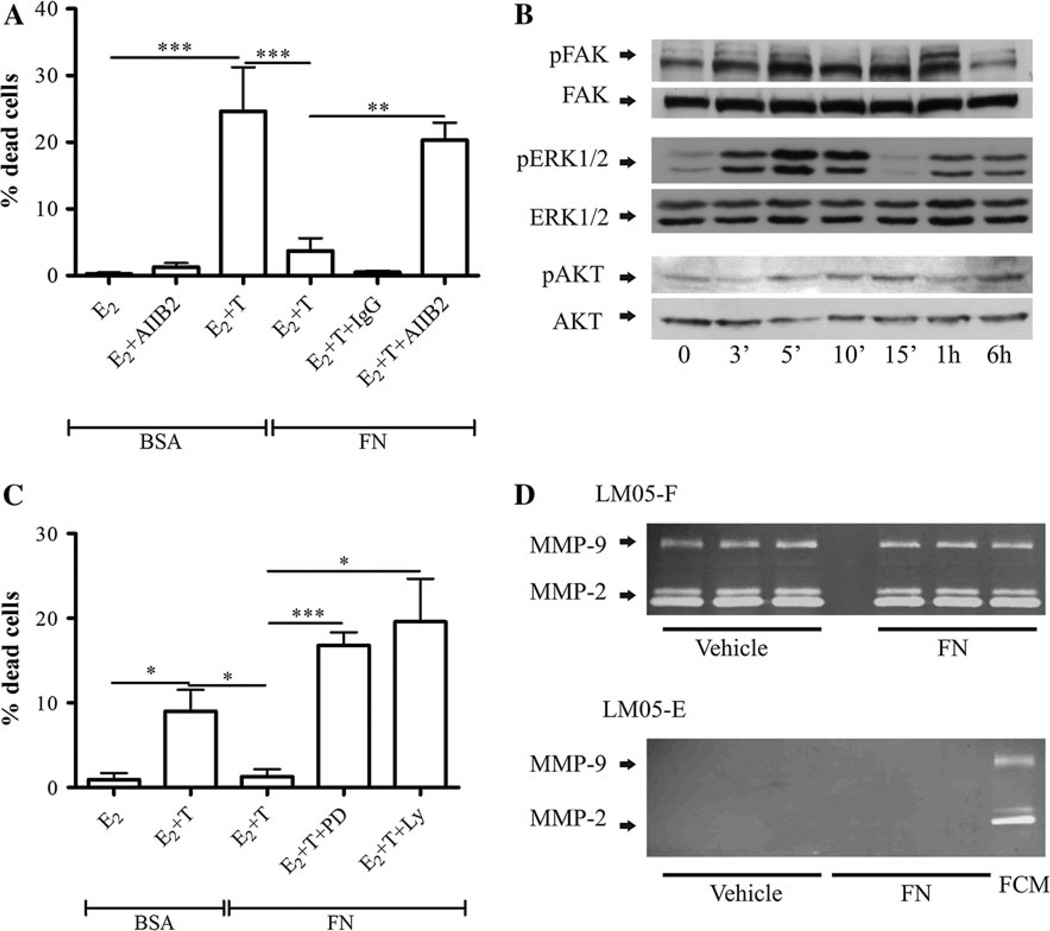
The interaction of tumor cells with the ECM has been shown to induce protection against cell death [7]. Moreover, the overexpression of fibronectin correlates with tamoxifen resistance in human breast cancer samples [13, 14]. However, to our knowledge, this has not been experimentally tested at the bench. Immunofluorescence analysis showed that fibronectin expression was specifically associated with the fibroblastic compartment in the LM05-Mix cell cultures (Fig. 3a). Interestingly, levels of β1 integrin, the main fibronectin receptor, were much higher in the epithelial LM05-E cells compared to the fibroblastic LM05-F cells (Fig. 3b). To test whether matrix components are involved in conferring tamoxifen resistance, LM05-E cells were cultured on fibronectin-coated plastic. We did not detect a significant increase in cell death when cells adhering to fibronectin were treated with tamoxifen (Fig. 3c). This same protective effect was observed when MCF-7 cells plated on fibronectin-coated plastic were treated with tamoxifen (Fig. 3d). To determine whether the protective effect of fibronectin was mediated by β1 integrin, LM05-E cells were pre-incubated with the AIIB2 β1 integrin blocking antibody and then seeded on fibronectin. Treatment with AIIB2 dramatically reduced the protective effect of fibronectin on tamoxifen-induced cell death (Fig. 4a). We were unsuccessful in carrying out these experiments with MCF-7 cells given that blocking β1 integrin impeded cell adhesion to fibronectin and caused cell death (not shown), confirming previous data showing an increase in cell death by AIIB2 in this cell line [17]. Analysis of signaling pathways downstream of β1 integrin in LM05-E cells showed phosphorylation of FAK and activation of MAPK/ERK 1/2 and to a lower degree of PI3K/AKT (Fig. 4b). To test whether these pathways were directly involved in the protective effect, LM05-E cells seeded on fibronectin were treated with MAPK/ERK1/2 and PI3K/AKT inhibitors in the presence of 4-OH-tamoxifen. In both cases, the protective effect of fibronectin was reversed (Fig. 4c). None of the inhibitors affected cell viability in the absence of 4-OH-tamoxifen (Supplementary Fig. 1d). These results indicate that both pathways were directly involved in fibronectin conferred tamoxifen resistance. Finally, we tested whether fibronectin could be involved in regulating MMP activity in LM05-E or -F cells. We found no association with MMP-2 and MMP-9 activity as determined by exposure to fibronectin followed by zymography of conditioned media (Fig. 4e).
Fig. 3.
Fibronectin (FN) induces tamoxifen resistance in LM05-E and MCF-7 cells. a Immunofluorescence for FN (green, right panel) in LM05-Mix cell cultures (nuclei were counterstained with DAPI, left panel). FN was especially associated with the stromal compartment in the Mix cultures. b Western blot analyzing β1 integrin expression in LM05-E and LM05-F cells plated on plastic. High levels of β1 were especially detected for the epithelial LM05-E cells. c LM05-E cells were cultured on BSA (control) or FN and treated for 48 h with 10 nM estradiol (E2) or 10 nM E2 + 1 µM 4-OH-tamoxifen (E + T). The induction of cell death produced by tamoxifen was counteracted only in the group of cells cultured on FN (***P<0.001). d MCF-7 cells were treated as described for the LM05-E cells in (c) and FN, again, induced tamoxifen resistance confirming that the observed result is not specific to LM05-E cells (**P<0.01). One of three experiments is shown
Fig. 4.
The protective effect of FN is mediated by the PI3K/AKT and MAPK/ERK pathways and requires β1 integrin. a LM05-E cells were plated on BSA or FN and treated with 10 nM estradiol (E2) or estradiol plus 1 µM 4-OH-tamoxifen (E2 + T) in the presence of the β1 integrin blocking antibody AIIB2, or control IgG. AIIB2, but not the control IgG, reversed the protective effect (**P < 0.01; ***P < 0.001). b LM05-E cells were treated with FN (30 µg/ml) for the indicated periods of time followed by western blots analyzing the phosphorylation of FAK, ERK1/2, and AKT. E-cadherin was used as loading control. c LM05-E cells were cultured on FN and treated with 10 nM estradiol (E2) or estradiol plus 1 µM 4-OH-tamoxifen (E2 + T) in the presence of the MAPK/ERK inhibitor PD98059 or the PI3K/AKT inhibitor LY294002 (both at 10 µM). Both inhibitors reversed the protective effect of FN (*P<0.05; ***P<0.001). d To determine whether FN was affecting the levels of secreted MMPs by LM05-E and LM05-F cells, conditioned media was prepared as explained in materials and methods from cells treated ON with vehicle or FN (30 µg/ml). The conditioned media was analyzed by zymography. No increase in MMP activity was detected in the conditioned media derived from FN treated wells as compared to controls. FCM was used as a positive control in the zymogram that analyzed the activity of the conditioned media prepared from LM05-E cells. Experiments were carried out in triplicates, each lane represents one well
We initially showed that the treatment of LM05-E cells with conditioned media led to tamoxifen resistance through a mechanism involving EGFR. EGFR has been shown to cross talk with β1 integrin in breast cancer cells [23]. On the other hand, it was possible that soluble fibronectin fragments could be also involved in the FCM protective effect, inducing activation of EGFR. To determine whether β1 integrin was involved in the conditioned media protective effect, cells were treated with tamoxifen and AIIB2 in the presence of FCM. As shown in Fig. 5a, AIIB2 blocked the conditioned media protective effect. However, when we treated LM05-E cells seeded on fibronectin with the EGFR inhibitor AG1478, we did not reverse the protective effect of fibronectin (Fig. 5b). Our results thus show that both soluble factors and ECM components such as fibronectin confer tamoxifen resistance, with β1 integrin playing a key role in mediating the protective effect.
Fig. 5.
β1 integrin mediates the protective effect of LM05-F-conditioned media, but EGFR is not involved in FN’s protective effect. a To establish whether β1 integrin played a role in LM05-F-conditioned media (FCM) induced tamoxifen resistance, LM05-E cells were plated in the presence of non-conditioned media (NCM) or FCM and treated with 10 nM estradiol (E2) or estradiol plus 1 µM 4-OH-tamoxifen (E2 + T). The addition of AIIB2 reversed the protective effect of FCM on E2 + T treated cells, but did not significantly affect the E2 treated cells; (**P<0.01; ***P<0.001). b To test the involvement of EGFR on FN’s protective effect, LM05-E cells were cultured on BSA (control) or FN and treated for 48 h with 10 nM estradiol (E2) or 10 nM E2 + 1 µM 4-OH-tamoxifen (E2 + T) in the presence of the EGFR inhibitor AG1478 (AG; 6.4 µM). AG1478 did not reverse the protective effect of FN (***P<0.001). One of at least two experiments is shown
Soluble factors and fibronectin induce the phosphorylation of the estrogen receptor
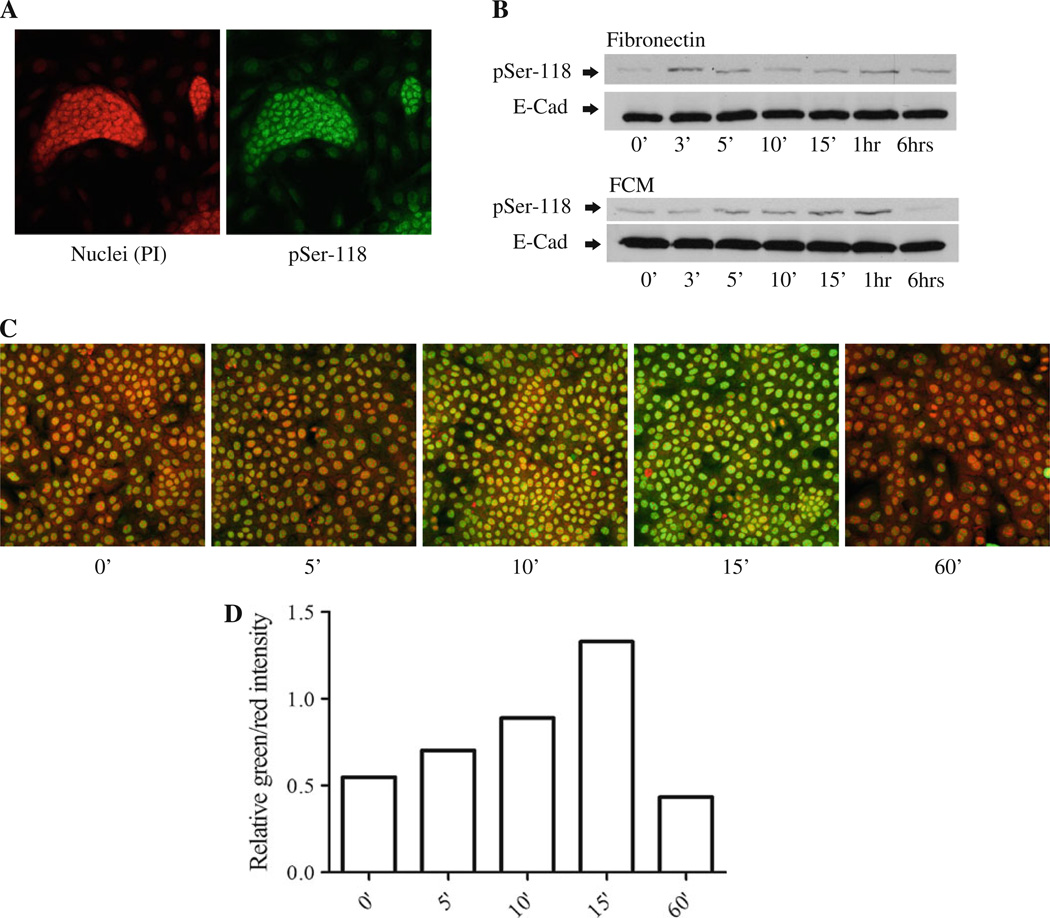
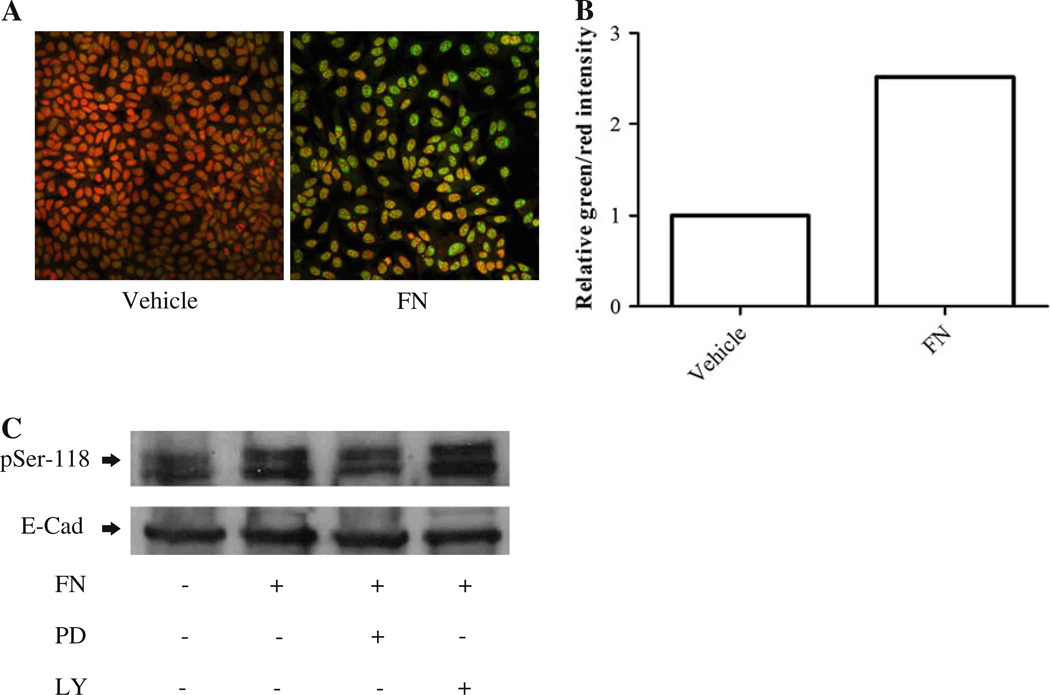
Phosphorylation of specific serines on ER-α is associated with tamoxifen resistance in breast cancer [24]. To determine whether phosphorylation of serine-118 was regulated by stromal-derived factors, we preformed immunofluorescence staining to check the localization of phosphor-serine-118 ER (pSer-118 ER) in starved LM05-Mix cells. The staining revealed that pSer-118 ER was very strong in the epithelial islets (Fig. 6a). Next, to establish whether soluble factors within FCM and/or fibronectin were involved in the induction of ER phosphorylation, LM05-E cells were starved for 48 h and treated with conditioned media, or fibronectin, for different periods of time. Western blot analysis revealed that both treatments increased the levels of pSer-118 ER (Fig. 6b). To further confirm the effect of fibronectin, immunofluorescence and confocal microscopy were carried out on LM05-E cells treated with fibronectin for 15 min. As can be appreciated in Fig. 6c and d, staining for pSer-118 ER-α increased in the fibronectin treated cells, as compared to the controls. Similar results were obtained when cells were treated with FCM (not shown). Moreover, we did not observe high levels of pSer-118 ER in starved epithelial LM05-E cells as shown for the epithelial compartment of the LM05-Mix cells where stromal–epithelial interactions are present (Fig. 6a), further supporting a role for stromal factors as modulators of ER-α phosphorylation. Finally, we also found that fibronectin affected the phosphorylation of ER-α in MCF-7 cells as determined by immunofluorescence and western blot (Fig. 7a–c). Moreover, phosphorylation of ER-α was inhibited by pre-treating the cells with PD98059 but not with LY294002, implying the involvement of MAPK/ERK1/2 in fibronectin induced ER-α phosphorylation (Fig. 7c). Our results clearly show that stromal-derived soluble factors and fibronectin induce ER-α phosphorylation in the epithelial cells and that this finding correlates with induction of tamoxifen resistance.
Fig. 6.
Fibronectin- and LM05-F-conditioned media induce phosphorylation of ER-α at serine-118 in LM05-E cells. a Immunofluorescence analyzing the levels of phospho-serine 118 ER (pSer-118). LM05-Mix cells were grown to 80% confluency in growth medium and were then starved for 48 h in phenol red free DMEM/F12. Staining to pSer-118 was carried out (green), and nuclei were counterstained with propidium iodide (red). Confocal images showed high levels of pSer-118 especially associated with the epithelial islets. b LM05-E cells were starved for 48 h and then treated for the indicated periods of time with FN or FCM. The levels of pSer-118 ER were analyzed by western blot. An increase in the levels of pSer-118 ER was detected in both the FN- and FCM-treated cells. E-cadherin was used as a loading control. c Immunofluorescence analyzing pSer-118 in control and FN treated LM05-E cells. LM05-E cells were seeded in chamber slides at 70% confluency and starved for 48 h. They were then treated with vehicle (water) or FN as indicated. Phospho-ser-118 staining was carried out using a FITC-labeled secondary antibody (green), and nuclei were counterstained with propidium iodide (red). FN induced an increase in the staining for pSer-118, in accordance with the results obtained from the western blots. d Graph showing the quantification of the pSer-118 staining corresponding to images shown in (c). Values for pSer-118 were normalized to propidium iodide for each time point. One of at least two experiments is shown
Fig. 7.
Fibronectin induces phosphorylation of ER at serine-118 in MCF-7 cells through MAPK/ERK1/2. a MCF-7 cells were grown to 80% confluency and starved for 48 h in phenol red free DMEM/F12. They were then treated with vehicle (water) or FN (30 µg/ml) for 15 min. Phospho-serine-118 ER was detected by immunofluorescence as described for LM05-E cells in Fig. 6. We found an increase in the levels of pSer-118 by treatment with FN. b Quantification of the staining intensity for pSer-118 corresponding to images shown in (a). Values for pSer-118 were normalized to propidium iodide for each treatment. c Western blot showing phosphorylation of ER at serine- 118 in MCF-7 cells. MCF-7 cells were grown to 80% confluency and then starved for 48 h. To determine the involvement of the MAPK/ ERK1/2 and PI3K/AKT pathways on the phosphorylation of ER-α, starved cells were pre-treated for 1 h with either vehicle (DMSO), PD98059 (10 µM), or LY294002 (10 µM). They were then subjected to a 15-min pulse of FN (30 µg/ml) or water as a control. Western blot analysis shows that FN leads to the phosphorylation of ER-α, and that it is inhibited by PD98059. No effect was detected with LY294002. One of at least two experiments is shown
Discussion
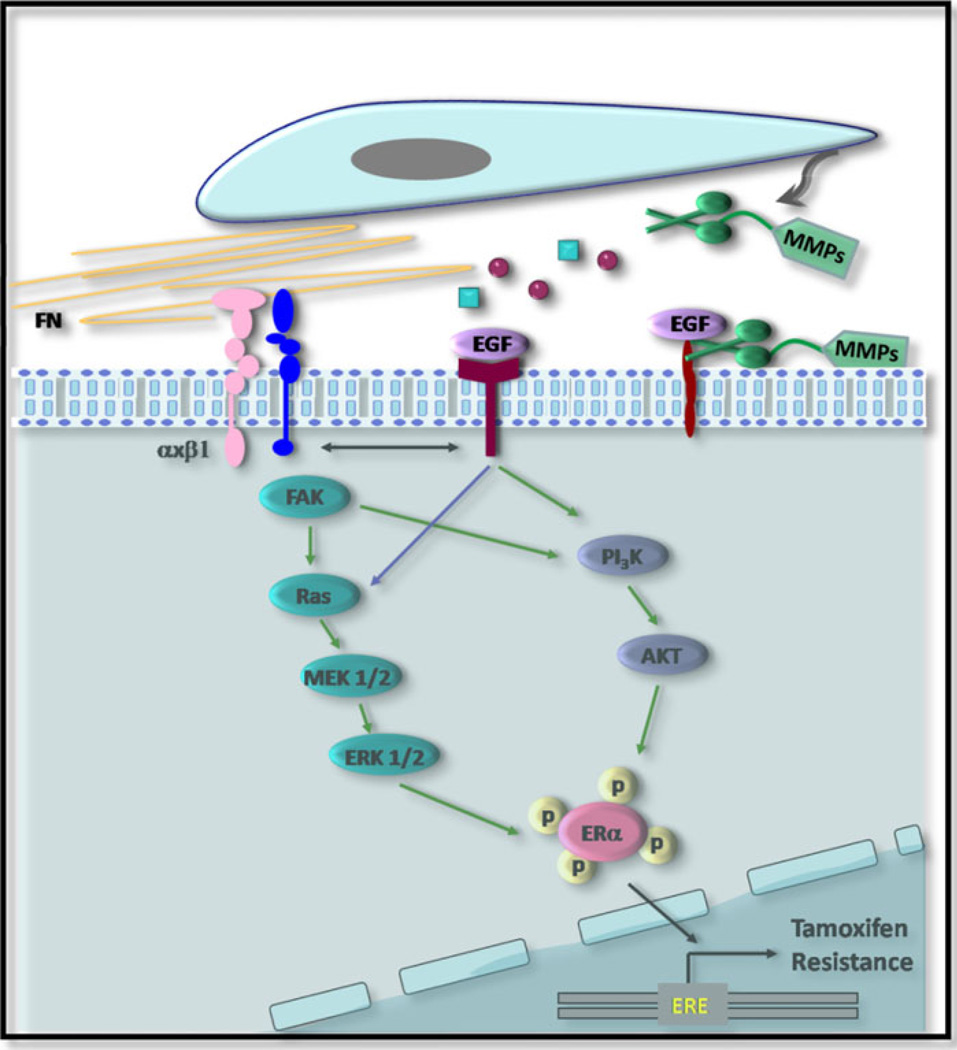
Resistance to endocrine therapy is today a major clinical problem. Understanding the molecular events involved in resistance will most certainly contribute to the development of agents that may enhance clinical success. Although research carried out in the last decade has convincingly shown that the tumor stroma co-evolves with the neoplastic cells determining not only progression, but response to therapy, very few attempts have been carried out to investigate whether stromal–epithelial interactions play a role in the development of endocrine resistance in breast cancer. We show here that factors produced by fibroblasts derived from the M05 mouse mammary tumor confer tamoxifen resistance to otherwise sensitive epithelial cells. We demonstrate that unidentified soluble factors present in the conditioned media induce resistance through activation of EGFR and PI3K/AKT, with the involvement of β1 integrin. Moreover, MMP activity is required to induce the protective effect. On the other hand, fibronectin makes the epithelial cells refractory to tamoxifen through binding to β1 integrin and the activation of the MAPK/ERK1/2 and PI3K/AKT pathways. Both types of microenvironmental factors lead to the phosphorylation of ER-α at serine-118. Our results question the current experimental models that only consider autocrine or paracrine mechanisms between identical cells as responsible for endocrine resistance. The illustration shown in Fig. 8 portrays the model of tamoxifen resistance we are proposing in this paper.
Fig. 8.
Illustration portraying the proposed mechanism of tamoxifen resistance. Soluble factors produced by carcinoma fibroblasts induce resistance through activation of EGFR and PI3K/AKT, with the involvement of β1 integrin; MMP activity is required to induce the protective effect. On the other hand, stromal fibronectin makes the epithelial cells refractory to tamoxifen through binding to β1 integrin and the activation of the MAPK/ ERK1/2 and PI3K/AKT pathways. Both types of microenvironmental factors lead to the phosphorylation of ER-α at serine-118. Green arrows indicate signaling pathways that are activated and are involved in the protective effect; blue arrows indicate pathways that are activated but that do not play a key role downstream. Black arrows indicates cross talk between different types of receptors
We showed previously that tumor-derived LM05-F fibroblasts protected epithelial LM05-E cells from tamoxifen-induced cell death [16]. Here, we demonstrate that treatment with FCM induces tamoxifen resistance, suggesting that physical interaction of the fibroblasts and the epithelial cells is not necessary to convey the protective effect. Cancer-associated fibroblasts secrete a variety of growth factors that acting as paracrine factors induce the PI3K/AKT and MAPK/ERK1/2 pathways in cancer cells [25]. Indeed, we find that the protective effect of FCM is blocked by PI3K/AKT inhibitors. However, inhibition of MAPK/ERK1/2 does not restore sensitivity to tamoxifen in FCM-treated epithelial cells, suggesting that this pathway does not play a key role in the protective effect induced by the stromal-derived soluble factors. In addition, cancer-associated fibroblasts produce large amounts of MMPs that remodel the tumor microenvironment [25]. We show that inhibiting MMP activity abolished the protective effect of FCM. MMPs have been shown to play a role in growth factor release, such as heparin-bound EGF [22], production of bioactive ECM fragments [26], and shedding of growth factor receptors [27] and surface molecules such as E-cadherin [28]. It remains to be established which of these events may intervene in the induction of tamoxifen resistance.
It has been well documented that the EGFR/HER-2 receptors are crucial for the development of tamoxifen resistance in breast cancer. However, the amplification of the genes that code for these receptors was detected in only 15–20% of patients who acquired tamoxifen resistance [6, 29]. We show that stromal-derived soluble factors activate EGFR and that blocking the activation prevents induction of tamoxifen resistance. Recently, Shekhar et al. showed that fibroblasts derived from ER−/PR− human breast tumors conferred tamoxifen resistance to otherwise sensitive premalignant EIII8 and tumorigenic MCF-7 cells, when co-cultured [30]. They suggest that EGFR is not involved in fibroblast-induced tamoxifen resistance because an association between tamoxifen resistance and levels of EGFR or phospho-EGFR and endocrine sensitivity was not found. However, it remains to be tested whether an EGFR inhibitor, such as AG1478, reverses the protective effect.
Deposition of ECM components such as fibronectin is associated with the breast tumor stroma. Previously, an extracellular matrix gene cluster, which includes fibronectin, was associated with tamoxifen resistance in breast tumor samples [13, 14]. However, the precise function of fibronectin in the acquisition of tamoxifen resistance has not been addressed before. We show that fibronectin is exclusively associated with the stromal compartment in the LM05-Mix cultures. Importantly, breast cancer cells cultured on fibronectin are resistant to tamoxifen-induced cell death. The β1 integrin antibody reversed the protective effect in LM05-E, indicating fibronectin–integrin binding as necessary. We further show that the effect of fibronectin led to the activation of FAK and was mediated by the PI3K/AKT as well as the MAPK/ERK1/2 pathway. Others have previously shown that laminin inhibits estrogen action in MCF-7 and T47D cells [31]. On the other hand, Hiscox et al. recently showed that in endocrine-resistant variants of MCF-7 cells, FAK activation is increased compared to the endocrine-sensitive parental cell line [32]. We did not find an association between fibronectin–integrin interaction and release of MMPs. Others have previously shown that α5β1-fibronectin binding leads to MMP-1-dependent invasion by breast cancer and mammary epithelial cells [33]. However, we did not detect MMP-1 in our zymogram assays either in the presence of absence of fibronectin (data not shown). Deregulation of integrins has been previously associated with resistance to chemotherapy in breast cancer [34], drug-induced apoptosis in myeloma [35], glioma [36] and non-small lung cancer cells [37]. A study carried out on 149 patients showed that high β1 integrin expression was associated with decreased overall and disease-free survival among patients with invasive breast cancer. Furthermore, extracellular fibronectin expression was also associated with decreased overall and disease-free survival, and β1 integrin expression was significantly correlated with fibronectin, implicating that integrin–ligand interactions were correlated with a more aggressive disease [38].
Although there is still controversy over this matter, tamoxifen resistance is mechanistically associated with the phosphorylation of the ER-α at serines located in the AF-1 region of the receptor [39]. We found that LM05-Mix cells cultured for 48 h in serum free media showed high levels of staining for pSer-118 ER-α in the epithelial cell compartment, implicating stromal–epithelial interactions in the modulation of ER-α phosphorylation. Accordingly, we established that both FCM and fibronectin induced the phosphorylation of ER-α at this location. Interestingly, the effect of fibronectin was reproducible in MCF-7 cells. These results have important implications given the fact that as mentioned above, there is still controversy over the correlation between levels of phosphorylation of ER-α in the AF-1 region and tamoxifen resistance [39].
In summary, our work shows that stromal-derived factors confer tamoxifen resistance to otherwise sensitive mammary epithelial tumor cells. The diversity of pathways involved suggests that the tumor microenvironment confers endocrine resistance through an array of overlapping mechanisms that are redundant. This implies that strategies that target only one signaling pathway may not re-sensitize endocrine-resistant tumors to tamoxifen and that the possibility of modulating the tumor microenvironment itself, or its interaction with the tumor cells, may be a more effective approach. Our results are the first, to our knowledge, to show an involvement of fibronectin in the induction of resistance to tamoxifen. Previous results from microarray analysis of human tumors had suggested a link between the stromal factors and tamoxifen resistance [13, 14]. Here, we confirm, in an experimental mouse setting, that the tumor stroma contributes to tamoxifen resistance and support the notion that endocrine therapy in combination with therapies that target the tumor microenvironment could be a future alternative to overcome endocrine resistance in breast cancer.
Supplementary Material
Acknowledgments
Financial support: MS work is supported by a Grant from the Susan G. Komen for the Cure Foundation (BCTR0600341) and ANPCyT (PICT2008-0325/Préstamo BID); EBKJ by ANPCyT (PICT 00417/Préstamo BID) and UBACyT (M003). MJB’s laboratory is supported by grants from the U.S. Department of Energy, OBER Office of Biological and Environmental Research (DE-AC02-05CH1123), a Distinguished Fellow Award and Low Dose Radiation Program and the Office of Health and Environmental Research, Health Effects Division, (03-76SF00098); by National Cancer Institute awards 5 R01CA064786, R01CA057621, U54CA126552 and U54CA112970; by U.S. Department of Defense (W81XWH0810736).
Abbreviations
- MMP
Matrix metalloproteinase
- ER
Estrogen receptor
- FN
Fibronectin
- FCM
LM05-F conditioned medium
- NMC
Non conditioned medium
- PI
Propidium iodide
- DAPI
4′,6-Diamino-2-phenylindole
- EGFR
Epidermal growth factor receptor
- pSer-118 ER
Phosphor-serine-118 ER
- ECM
Extracellular matrix
- E2
Estradiol
- PD
PD98059
- LY
LY294002
- T
4-OH-tamoxifen;
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-011-1766-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare they have no competing interests.
Contributor Information
Osvaldo Pontiggia, Área de Investigaciones, Instituto de Oncología, “Ángel H. Roffo”, Av. San Martín 5481, C1417DTB Buenos Aires, Argentina.
Rocio Sampayo, Área de Investigaciones, Instituto de Oncología, “Ángel H. Roffo”, Av. San Martín 5481, C1417DTB Buenos Aires, Argentina.
Diego Raffo, Área de Investigaciones, Instituto de Oncología, “Ángel H. Roffo”, Av. San Martín 5481, C1417DTB Buenos Aires, Argentina.
Andrea Motter, Área de Investigaciones, Instituto de Oncología, “Ángel H. Roffo”, Av. San Martín 5481, C1417DTB Buenos Aires, Argentina.
Ren Xu, Life Sciences Division, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, CA 94720, USA.
Mina J. Bissell, Life Sciences Division, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, CA 94720, USA
Elisa Bal de Kier Joffé, Área de Investigaciones, Instituto de Oncología, “Ángel H. Roffo”, Av. San Martín 5481, C1417DTB Buenos Aires, Argentina.
Marina Simian, Email: marina.simian@galuzzi.com, Área de Investigaciones, Instituto de Oncología, “Ángel H. Roffo”, Av. San Martín 5481, C1417DTB Buenos Aires, Argentina.
References
- 1.Jordan C. Historical perspective on hormonal therapy of advanced breast cancer. Clin Ther. 2002;24(Suppl A):A3–A16. doi: 10.1016/s0149-2918(02)85031-7. [DOI] [PubMed] [Google Scholar]
- 2.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232:123–138. doi: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/Neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 4.Dowsett M, Johnston S, Martin LA, Salter J, Hills M, Detre S, Gutierrez MC, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Smith I. Growth factor signalling and response to endocrine therapy: the Royal Marsden Experience. Endocr Relat Cancer. 2005;12(Suppl 1):S113–S117. doi: 10.1677/erc.1.01044. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Dowsett M. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 6.Newby JC, Johnston SR, Smith IE, Dowsett M. Expression of epidermal growth factor receptor and c-erbB2 during the development of tamoxifen resistance in human breast cancer. Clin Cancer Res. 1997;3:1643–1651. [PubMed] [Google Scholar]
- 7.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Littlepage LE, Egeblad M, Werb Z. Coevolution of cancer and stromal cellular responses. Cancer Cell. 2005;7:499–500. doi: 10.1016/j.ccr.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Tlsty T. Cancer: whispering sweet somethings. Nature. 2008;453:604–605. doi: 10.1038/453604a. [DOI] [PubMed] [Google Scholar]
- 10.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 11.Bergamaschi A, Tagliabue E, Sorlie T, Naume B, Triulzi T, Orlandi R, Russnes HG, Nesland JM, Tammi R, Auvinen P, Kosma VM, Menard S, Borresen-Dale AL. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol. 2008;214:357–367. doi: 10.1002/path.2278. [DOI] [PubMed] [Google Scholar]
- 12.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 13.Helleman J, Jansen MPHM, Ruigrok-Ritstier K, van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn JGM, Sleijfer S, Foekens JA, Berns EMJJ. Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response. Clin Cancer Res. 2008;14:5555–5564. doi: 10.1158/1078-0432.CCR-08-0555. [DOI] [PubMed] [Google Scholar]
- 14.Jansen MP, Foekens JA, van Staveren IL, Dirkzwager-Kiel MM, Ritstier K, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Portengen H, Dorssers LC, Klijn JG, Berns EM. Molecular classification of tamoxifen-resistant breast carcinomas by gene expression profiling. J Clin Oncol. 2005;23:732–740. doi: 10.1200/JCO.2005.05.145. [DOI] [PubMed] [Google Scholar]
- 15.Simian M, Manzur T, Rodriguez V, de Kier Joffe EB, Klein S. A spontaneous estrogen dependent, tamoxifen sensitive mouse mammary tumor: a new model system to study hormone-responsiveness in immune competent mice. Breast Cancer Res Treat. 2009;113:1–8. doi: 10.1007/s10549-007-9888-x. [DOI] [PubMed] [Google Scholar]
- 16.Pontiggia O, Rodriguez V, Fabris V, Raffo D, Bumaschny V, Fiszman G, de Kier Joffe EB, Simian M. Establishment of an in vitro estrogen-dependent mouse mammary tumor model: a new tool to understand estrogen responsiveness and development of tamoxifen resistance in the context of stromal-epithelial interactions. Breast Cancer Res Treat. 2009;116:247–255. doi: 10.1007/s10549-008-0113-3. [DOI] [PubMed] [Google Scholar]
- 17.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117–3131. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sengupta S, Jordan VC. Selective estrogen modulators as an anticancer tool: mechanisms of efficiency and resistance. Adv Exp Med Biol. 2008;630:206–219. doi: 10.1007/978-0-387-78818-0_13. [DOI] [PubMed] [Google Scholar]
- 20.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurokawa H, Arteaga CL. ErbB (HER) receptors can abrogate antiestrogen action in human breast cancer by multiple signaling mechanisms. Clin Cancer Res. 2003;9:511S–515S. [PubMed] [Google Scholar]
- 22.Yu WH, Woessner JF, Jr., McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 2002;16:307–323. doi: 10.1101/gad.925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massarweh S, Schiff R. Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer. 2006;13(Suppl 1):S15–S24. doi: 10.1677/erc.1.01273. [DOI] [PubMed] [Google Scholar]
- 25.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 26.Koshikawa N, Schenk S, Moeckel G, Sharabi A, Miyazaki K, Gardner H, Zent R, Quaranta V. Proteolytic processing of laminin-5 by MT1-MMP in tissues and its effects on epithelial cell morphology. FASEB J. 2004;18:364–366. doi: 10.1096/fj.03-0584fje. [DOI] [PubMed] [Google Scholar]
- 27.Chabottaux V, Noel A. Breast cancer progression: insights into multifaceted matrix metalloproteinases. Clin Exp Metastasis. 2007;24:647–656. doi: 10.1007/s10585-007-9113-7. [DOI] [PubMed] [Google Scholar]
- 28.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arpino G, Green SJ, Allred DC, Lew D, Martino S, Osborne CK, Elledge RM. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin Cancer Res. 2004;10:5670–5676. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 30.Shekhar MP, Santner S, Carolin KA, Tait L. Direct involvement of breast tumor fibroblasts in the modulation of tamoxifen sensitivity. Am J Pathol. 2007;170:1546–1560. doi: 10.2353/ajpath.2007.061004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodward TL, Lu H, Haslam SZ. Laminin inhibits estrogen action in human breast cancer cells. Endocrinology. 2000;141:2814–2821. doi: 10.1210/endo.141.8.7607. [DOI] [PubMed] [Google Scholar]
- 32.Hiscox S, Barnfather P, Hayes E, Bramble P, Christensen J, Nicholson RI, Barrett-Lee P. Inhibition of focal adhesion kinase suppresses the adverse phenotype of endocrine-resistant breast cancer cells and improves endocrine response in endocrine-sensitive cells. Breast Cancer Res Treat. 2011;125:659–669. doi: 10.1007/s10549-010-0857-4. [DOI] [PubMed] [Google Scholar]
- 33.Jia Y, Zeng ZZ, Markwart SM, Rockwood KF, Woods Ignatoski KM, Ethier SP, Livant DL. Integrin fibronectin receptors in matrix metalloproteinase-1-dependent invasion by breast cancer and mammary epithelial cells. Cancer Res. 2004;64:8674–8681. doi: 10.1158/0008-5472.CAN-04-0069. [DOI] [PubMed] [Google Scholar]
- 34.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 35.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 36.Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res. 1999;5:1587–1594. [PubMed] [Google Scholar]
- 37.Hodkinson PS, MacKinnon AC, Sethi T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Int J Radiat Biol. 2007;83:733–741. doi: 10.1080/09553000701570204. [DOI] [PubMed] [Google Scholar]
- 38.Yao ES, Zhang H, Chen YY, Lee B, Chew K, Moore D, Park C. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67:659–664. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- 39.Murphy LC, Weitsman GE, Skliris GP, Teh EM, Li L, Peng B, Davie JR, Ung K, Niu YL, Troup S, Tomes L, Watson PH. Potential role of estrogen receptor alpha (ERalpha) phosphorylated at Serine118 in human breast cancer in vivo. J Steroid Biochem Mol Biol. 2006;102:139–146. doi: 10.1016/j.jsbmb.2006.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.