Summary
Subarachnoid hemorrhage (SAH), predominantly caused by a ruptured aneurysm, is a devastating neurological disease that has a morbidity and mortality rate higher than 50%. Most of the traditional in vivo research has focused on the pathophysiological or morphological changes of large-arteries after intracisternal blood injection. This was due to a widely held assumption that delayed vasospasm following SAH was the major cause of delayed cerebral ischemia and poor outcome. However, the results of the CONSCIOUS-1 trial implicated some other pathophysiological factors, independent of angiographic vasospasm, in contributing to the poor clinical outcome. The term early brain injury (EBI) has been coined and describes the immediate injury to the brain after SAH, before onset of delayed vasospasm. During the EBI period, a ruptured aneurysm brings on many physiological derangements such as increasing intracranial pressure (ICP), decreased cerebral blood flow (CBF), and global cerebral ischemia. These events initiate secondary injuries such as blood-brain barrier disruption, inflammation, and oxidative cascades that all ultimately lead to cell death. Given the fact that the reversal of vasospasm does not appear to improve patient outcome, it could be argued that the treatment of EBI may successfully attenuate some of the devastating secondary injuries and improve the outcome of patients with SAH. In this review, we provide an overview of the major advances in EBI after SAH research.
Keywords: Subarachnoid hemorrhage, Early brain injury, Cerebral vasospasm, Animal model
Introduction
Subarachnoid hemorrhage (SAH) is a common and frequently devastating condition, accounting for 5% of all stroke types [118]. Each year, approximately 1 in 10,000 North Americans suffer from an aneurysmal SAH, and this carries with it a greater than 50% combined morbidity and mortality rate [60]. Despite advances in diagnosis and surgical treatment of SAH, effective therapeutic interventions are still limited and clinical outcomes remain disappointing. Traditionally, delayed cerebral vasospasm (CVS) has been considered the single and most important cause of delayed cerebral ischemia and poor outcomes [57]. Although animal studies have found many agents which inactivate spasmogenic substances or block arterial smooth muscle contraction, no agent has brought tremendous improvement in the human patient outcome after SAH. Early brain injury (EBI) was reported as a primary cause of mortality in SAH patients [12], and many important pathological mechanisms have been recognized to be initiated within minutes after aneurysmal SAH [81]. Recently, intensive research efforts have aimed to reveal the mechanisms of EBI. In this review, we provide an overview of the major advances in EBI after SAH research.
The SAH Experiments before Early Brain Injury
Experimental Focus on Delayed Cerebral Vasospasm after SAH
Since the first demonstration of CVS, about 60 years ago [29], many experimental and clinical studies have tried to disclose mechanisms responsible for this persistent vasoconstriction and to find proper treatment for its prevention and/or reversal. In humans, CVS usually occurs on day 3 after SAH, peaks at day 6-8, and lasts for 2-3 weeks [125]. Delayed cerebral ischemia has been considered to be induced by CVS because several studies found a strong association between radiologically confirmed vasospasm and clinical signs of delayed cerebral ischemia [35, 37, 92]. Therefore, there was a widely held assumption that CVS was the major cause of the high mortality and poor outcome after an otherwise successful treatment of a ruptured intracranial aneurysm [25]. Thus the majority of research performed worldwide has focused on strategies to limit arterial narrowing and delayed cerebral ischemia following SAH [57]. Restoration of narrowed large-arteries, using pharmacological agents, was believed to improve vasospasm as a whole. This conclusion was arrived at by using, the most common model of SAH and vasospasm, the canine “two-hemorrhage” model, in which two injections of blood, into the dog’s basal cistern, are performed 48 hours apart in order to observe the large artery pathophysiological or morphological changes [76].
Translational Trials for Cerebral Vasospasm: from Animals to Humans
Many pathophysiological mediators have been demonstrated in CVS such as i) the dysfunction of nitric oxide (NO) - nitric oxide synthase (NOS) pathway, ii) endothelin-1, iii) ferrous hemoglobin released from the subarachnoid clot and subsequent oxidative stress, iv) inflammatory pathways, v) blood-brain barrier (BBB) breakdown by endothelial apoptosis or thrombin, vi) excitotoxicity and membrane pathology of Ca2+ channels [90, 137]. Numerous interventions are currently being investigated for CVS treatment [61]. Several promising pharmacological treatments, previously demonstrated in pre-clinical animal experiments, have translated to human randomized and blinded clinical trials such as: calcium channel antagonists (nimodipine and nicardipine) [48, 79, 87, 88], endothelin antagonists [73, 119, 121], erythropoietin [107], fasudil [102], magnesium sulfate [126], statins [23, 117], tirilazad [47, 56], and tissue plasminogen activator (tPA) [36]. However, most of them failed in clinical trials for prevention and treatment of CVS [85], except fasudil which is used clinically in Japan and China [69]. Nimodipine had a beneficial effect on the reduction of in morbidity and improvement in functional outcome but not CVS [9].
Therefore, even now patients suffering from CVS receive complex treatments with calcium antagonists (oral nimodipine treatment), hypertensive drugs, hemodilution and hypervolemia (triple H therapy), risky and often only temporarily effective intra-arterial administration of vasodilator drugs, or balloon angioplasty [26].
The Next Targets for SAH Research after the CONSCIOUS-1 Trial
Clazosentan, a selective endothelin receptor type A antagonist, has been proven effective to decrease CVS after experimental SAH [93]. The patients after SAH treated with clazosentan demonstrated a 65% relative risk reduction in angiographic vasospasm. However, mortality or clinical outcome was not improved in the CONSCIOUS-1 trail (clazosentan to overcome neurological ischemia and infarction occurring after SAH) [73]. These observations indicate that the pathophysiology underlying delayed cerebral ischemia is multifactorial and that other pathophysiological factors, which are independent of angiographic vasospasm, can contribute to the outcome [74]. Additionally, it may be that the pathological mechanisms, activating within minutes after SAH and leading to EBI, play an important role in the pathogenesis of delayed ischemic injury and poor outcome [14].
Experiments on Early Brain Injury after SAH
Experimental transition from Cerebral Vasospasm to Early Brian Injury
The term EBI has been coined as the period which spans from the moment of initial bleeding to the onset of CVS. It describes the immediate injury to the brain after aneurysmal SAH as a whole, reported by Kusaka et al in 2004 [62]. It can be suggested that the EBI precipitates the occurrence of CVS in many ways, including vascular injury from acute ischemia, inflammation, and blood products, which may result in damage of NO-releasing neurons [89].
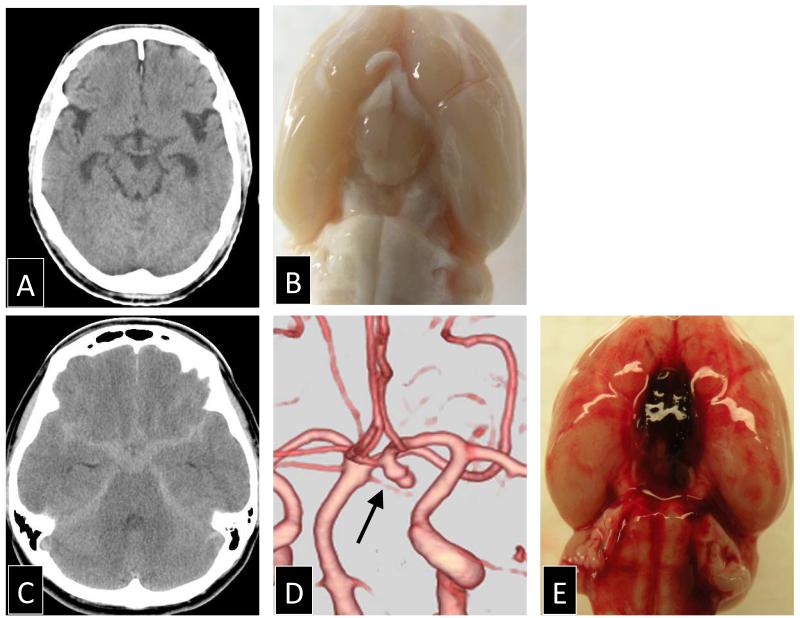
Since the main pathophysiological stage has changed from the large-arteries to the brain parenchyma, the experimental modeling of EBI began to simulate the intracranial artery rupture, and the common experimental model changed to the rodent “endovascular puncture” model. This model was independently described by Bederson et al and Veelken et al [10, 120], and the surgical procedure aims to perforate the internal carotid bifurcation, without craniotomy, by means of a sharp ended suture inserted through the external carotid artery (Figure 1).
Figure 1.
Comparison of subarachnoid hemorrhage in human and experimental endovascular perforation model of rat: (A) normal brain computed tomography (CT) scan in human around the circle of Willis, (B) a photograph of a sham-operated rat after cardiac perfusion, (C) high density area in the basal cistern on the CT scan after subarachnoid hemorrhage in human, (D) the cause of subarachnoid hemorrhage was an ruptured aneurysm in human (arrow), and (E) subarachnoid hemorrhage at the ventral surface induced by the endovascular perforation of the internal carotid artery in rat.
Vascular injury highly correlates with brain edema generally evaluated by brain water content in rat experimental SAH, showing increased intracranial pressure (ICP), and decreased microvascular flow, as well as injury to neuronal tissues [28, 59, 80]. Since 48 hours after SAH is the time point at which maximal cerebral vasospasm is observed in rats, the 24-hour time point seems to be correct for the analysis of EBI after SAH [130]. Furthermore, understanding EBI has become more and more important than that of CVS itself. Many recent studies using interventions such as: pharmacological agents, transgenic and knockout animals, or hyperbaric oxygen have been used to elucidate the numerous intracellular second messenger cascades and to find a promising treatment for EBI (Table 1).
Table 1.
Experimental in vivo studies of pathomechanisms in EBI after SAH, using any drugs and interventions
| Experimental paradigm | Intervention | Pathogenic factor | Contributing pathway and/or mechanism | Key effect | Outcome after treatment | Reference |
|---|---|---|---|---|---|---|
| EVP, rat | MK-801 (NMDA receptor antagonist) | Activation of c-fos and c-jun | Glutamate pathway | Spreading depression, cell death | Not tested | [49] |
| EVP, rat | NOS inhibitor | Blood components released during SAH | Scavenging NO by blood, impaired NO vasodilation | Acute vasoconstriction and ischemia | Not tested | [99] |
| SHI, mice | Mutant mice deficient in Mn-superoxide dismutase | Subarachnoid hemolysate | Superoxide production and cytochrome c release | DNA fragmentation and cell death | Not tested | [75] |
| SIN, rat | Isamoltane hemifumarate(5-HT1B receptor antagonist) or HET0016 (an inhibitor of the synthesis of 20-HETE) | Activation of 5-HT1B receptors | Synthesis of 20-HETE and rise in intracellular Ca2+ | Acute fall in rCBF | Not tested | [15] |
| SIN, rat | Recombinant adenovirus encoding human Cu/Zn SOD-1 | Superoxide anion mesured as a vascular NADPH oxidase activation | Sounperoxide production | Impairment of autoregulatory CBF and oxydative stress | Not tested | [103] |
| EVP, rat | z-VAD-FMK (a pan-caspase inhibitor) | Acute ischemia | Caspase-3 activity | Apoptosis, BBB disruption, and vasogenic brain edema | NS↑, MT↓ | [86] |
| EVP, rat | Hypertonic fluid (NaCl 7.5% plus 6% dextran 70) | Global ischemia | Osmotic mobilization of parenchymal water and improvement of microcirculation | Increasing ICP and decreased CBF | NS↑ | [133] |
| EVP, rat | PP1 (an Src-family kinase inhibitor) | VEGF | Src tyrosine kinase and ERK1/2, p38, and JNK pathways | BBB disruption, brain edema, and incresed ICP | MT↓ | [62] |
| EVP, rat | Hyperbaric oxygen | Acute ischemia | HIF-1alpha dependent Bcl-2/adenovirus E1B 19kDa-interacting protein 3 (BNIP3) activation | Decreasing CBF and CPP, increasing ICP, brain edema, and neuronal damage | NS↑, MT↓ | [80] |
| EVP, rat | Hypertonic fluid (NaCl 7.5% plus 6% dextran 70) | Global ischemia | Small volume resuscitation | Increasing ICP and neuronal damage | MT↓ | [11] |
| EVP, mice | ApoE-mimetic peptide | Inflammation | APOE4 genotype expression | Increasing brain edema | NS↑, MT↓ | [40] |
| SHI, rat | ZnPPIX (zinc protoporphyrin IX ; heme oxygenase(HO)) | Inhibited the production of endogenous carbon monoxide (CO) | Heme oxygenase/CO pathway | Brain damage (LDH activitivation in serum) | Not tested | [109] |
| EVP, rat | Hyperbaric oxygen | Expression and activation of NADPH oxidase | Superoxide anion production, increased neuronal immunoreactivity of gp91phox and regulation of gp91phox mRNA | Neuronal injury | NS↑, MT→ | [83] |
| EVP, rat | Hyperbaric oxygen | Up-regulated NADPH oxidase | Superoxide anion production and enhancing gp91 (phox) | Decrease CBF and production of lipid peroxidation | NA | [82] |
| EVP, rat | LY294002 (Phosphoinositide 3-kinase(PI3K) inhibitor | Neuronal injury by ischemia | PI3K/Akt/Glycogen synthase kinase-3beta (GSK3β) pathway | Apoptotic cell death | Not tested | [30] |
| EVP, rat | p-toluenesulfonate (iNOS inhibitor) | Transient global ischemia | Not formating BBB disruption and brain edema by iNOS | Increasing iNOS expression and NO metabolites concentration | Non improvement in NS or MT | [130] |
| EVP, rat | Cu/Zn SOD-1 transgenic (Tg) rats | Oxidative stress | Decrease of SOD-Akt-Glycogen synthase kinase-3beta activation | Apoptotic cell death | MT↓ | [31] |
| EVP, rat | Pifithrin-alpha (a selective inhibitor of p53-mediated transcription) | Up-regulation of p53 | Activation of the caspase -dependent and -independent pathways and the mitochondrial cascades | Increasing neuronal apoptosis and brain edema | NS↑, MT↓ | [13] |
| EVP, rat | SP600125 (JNK inhibitor) | Phosphorylation of JNK | c-Jun phosphorylation, aquaporin-1 expression, MMP-9 activity, increased VEGF tissue level, and cleaved caspase-3 expression | BBB disruption, brain swelling, and apoptosis | NS↑, MT→ | [131] |
| EVP, rat | S-nitrosoglutathione (NO donor) | depletion of NO | Collagen IV decrease, and collagenase activity increase | BBB disruption in the microvessels | Not tested | [97] |
| SIN, rat | Felbamate (a NMDA receptor antagonist) | Ischemia induced glutamate and aspartate | Stimulation of NMDA receptor | BBB disruption and brain edema | NS↑ | [42] |
| EVP,mice | gp91phox knockout mice | NADPH oxidase | Superoxide production | Not reducing the intensity of the oxidative stress | MT→ | [70] |
| EVP, rat | Melatonin | Oxidative stress | Melatonin may reduce down regulation of VEGF and astrocytic aquaporin 4 protein expression. | Brain edema | MT↓ | [6] |
| EVP, rat | 3% Hypertonic saline | Ischemic brain injury | Osmotic and rheologic properties | Not decrease brain edema | NS→ | [66] |
| DIN, rat | Magnesium | Vasoconstriction effect through Ca2+ influx | Anti-vasodilation effect | CBF reduction | Not tested | [77] |
| EVP, rat | Melatonin | Oxidative stress | No effect on the lipid peroxidation | Increasing brain edema | NS→, MT↓ | [5] |
| EVP, rat | Tetramethylpyrazine (an oxygen free radical scavenger) | Oxidative stress | Increasing cleaved caspase-3, no expression change of bax or bcl-2 | BBB disruption, brain edema, and apoptotic cell death | NS↑, MT→ | [39] |
| EVP, rat | Pifithrin-alpha (a selective inhibitor of p53-mediated transcription) | p53 gene | NF-kappaB/MMP-9 expression pathway activation and decreasing occludin and collagen IV | BBB disruption and brain edema | Not tested | [128] |
| SIN, rat | Clazosentan (an endothelin A receptor antagonist) | Immediate increase in ICP | Acute decrease in CPP and loss of autoregulation | CPP-dependent and -independent hypoperfusion in first hours | Not tested | [95] |
| SIN, rabbit | Dexmedetomidine (an α2-adrenoceptor agonist) | Catecholamine release and excessive free radicals production | Activated lipid peroxidation and Xanthine oxidase, and decreasing of antioxidant mechanisms | Neuronal degenerative change | Not tested | [24] |
| EVP, rat | Glibenclamide (selective sulfonylurea receptor 1 (SUR1) inhibitor) | Inflammation | TNFα/NF-kappaB/Abcc8 mRNA/SUR1 receptor upregulation and disruption of ZO-1 expression | BBB disruption and caspase-3 activation | Not tested | [104] |
| EVP, rat | Atorvastatin (HMG-CoA reductase inhibitor) | Global ischemia | Caspase-dependent apoptosis without p53 expression pathway | BBB disruption, brain edema, apoptotic cell death, and mRNA expression of caspase-3 and caspase-8 | NS↑, MT↓ | [21] |
| SIN, rat | Melatonin (antioxidant) | Oxidative stress | Increasing myeloperoxidase and malondialdehyde levels, and decreasing glutathione levels and Na+-K+-ATPase activity | BBB disruption and brain edema | NS↑, MT→ | [33] |
| EVP, rat | Argatroban (a direct thrombin inhibitor) | Thrombin | decreasing ZO-1 level, and increasing IL-1β level | BBB disruption, brain edema, apoptotic cell death, and inflammatory response | NS↑, MT→ | [108] |
| SIN, rat | N-acetylcysteine (a sulfhydryl-containing antioxidant) | Oxidative stress | Decreasing Cu/Zn SOD and glutathione peroxidase activity, and increasing lipid peroxidation product | Brain edema | NS↑ | [71] |
| EVP, mice | Ac-YVAD-CMK (a selective inhibitor of IL-1beta converting enzyme) | IL-1β | JNK and MMP-9 activation, and ZO-1 degradation | BBB disruption and brain edema | NS↑, MT↓ | [106] |
| SIN, rat | Extract of Ginkgo biloba (including flavonol glycosides and other common compound | Oyhemoglobin from extravasated blood | Enhanced VEGF mRNA and VEGF protein but not enough | VEGF mRNA and VEGF protein expression | Not tested | [110] |
| EVP, rat | Anatibant (a bradykinin B2 receptor antagonist) | Impairment of cerebral autoregulation | Increasing expression of bradykinin B2 receptors and kininogen (Kng1) mRNA | Brain edema | NS↑, MT→ | [116] |
| EVP, rat | Edaravone (=MCI-186, a potent free radical scavenger) | Oxidative stress | Increasing malondialdehyde | Reduced SOD, and apoptotic neuronal cell death | NS↑, MT↓ | [41] |
| PCI, rat | SB-3CT (a selective MMP-9 inhibitor) | Inflammation | MMP-9 activation and laminin degradation | Apoptotic neuronal cell death | NS↑ | [44] |
| SIN, rat | Alpha lipoic acid (a dithiol antioxidant) | Free radical generation and neutrophil accumulation | Increasing ROS formation, DNA fragmentation ratio, malondialdehyde, and myeloperoxidase activity ; and decreasing glutathione content and Na+-K+-ATPase activity |
BBB disruption and brain edema | NS↑ | [32] |
| EVP, rat | Osteopontin (an extracellular matrix glycoprotein) | Inflammation | Activation of NF-kappaB improving the balance between up-regulated MMP-9 and down- regulated TIMP-1 expression, and degradation of substrates of MMP-9 (laminin and ZO-1) pathway |
BBB disruption and brain edema | NS↑, MT→ | [111] |
| EVP, mice | Adenosine A(2A) receptor knockout mice | increased ICP and decreased CPP | Increasing collagen type IV by early Adenosine A(2A) receptor response | CBF reduction by decreasing of internal diameter of major cerebral vessels | Not tested | [96] |
| SIN, rabbit | Simvastatin | Global ischemia | PI3K/Akt/Glycogen synthase kinase-3beta (GSK3β) pathway | BBB disruption, brain edema and apoptotic cell death | NS→, MT→ | [20] |
| EVP, rat | Octonal and carbenoxolone (gap junction inhibitors) | Global ischemia | Connexin 43 phosphorylation | Not reducing apoptotic cell death | NS→, MT→(octanol), MT↑ (carbenoxolone) |
[3] |
| PCI, rat | U46619 and GR3219b (a thromboxane A2 receptor agonist and antagonist) | Cerebral ischemia | Up-regulation of Thromboxane A2 receptors and their mRNA levels | Global and rCBF redution | Not tested | [2] |
| SIN, mice | Aquaporin-4 null mice | Impairment of glial water channel protein | Reduction in glia limitans osmotic permeability and increasing ICP | BBB disruption and brain edema | NS↑, MT→ | [115] |
| SIN, rat | Neutalized IL-1β by anti-rat IL-1beta antibodies | Extravasated blood and inflammation | IL-1β inducing S-100β protein production | Brain injury and BBB disruption | Not tested | [54] |
| SIN, rat | Ghrelin (an endogenous ligand for growth hormone secretagogue receptor) | Oxidative stress | Increasing plasma levels of TNF-α and IL-1β, ROS generation, lipid peroxidation, and accumulation of neutrophils, reducing antioxidant status and Na+-K+-ATPase activity |
BBB disruption, brain edema, and cell death | NS↑ | [34] |
| DIN, rat | Ginsenoside Rb1 (an active component of Chinese medicine Panax Ginseng) | Global ischemia | P53 and Bax dependent proapoptosis pathway | BBB disruption, brain edema, and apoptotic cell death | NS↑, MT↓ | [68] |
| EVP, rat | Osteopontin (OPN) siRNA | Inflammation | Reduction of angiopoietin-1 and MAPK phosphatase-1,and activation of MAPKs and its both upstream and downstream VEGF-A |
BBB disruption | NS↓, MT→ | [114] |
| EVP, rat | Deferoxamine (an iron chelator) | Blood breakdown products and oxidative stress | increasing nonheme iron levels, heme-oxygenase-1 (HO-1) expression, and iron-handling proteins (transferrin and its receptor) |
Apoptotic cell death and oxidative DNA damage with ferritin | MT↓ | [63] |
| PCI, rat | Recombinant human erythropoietin | Oxidative stress | Erythroid 2-related factor 2 and antioxidant responsive element (Nrf2-ARE) pathway | BBB disruption, brain edema, and cortical apoptosis | Not tested | [135] |
| EVP, rat | CL-IB-MECA (a selective Adenosine A3 receptor agonist) | Inflammation | Increasing TNF-α and IL-1β, and microglial activation | Brain edema | NS↑, MT↓ | [72] |
| EVP, rat | Sodium orthovanadate(a tyrosine phosphatase inhibitor) | Global ischemia | Tyrosine phosphatase activation | Brain edema and apoptosis cell death | NS↑, MT→ | [51] |
| PCI, rat | Minocycline | Global ischemia | MMP-9 expression | Clinical assessments | NS↑ | [45] |
| EVP, rat | Osteopontin (OPN) siRNA | Inflammation | Activation of NF-kappaB, inhibition of MMP-9 induction and TIMP-1 reduction, and the consequent preservation of laminin and ZO-1 pathway |
BBB disruption and brain edema | NS↓ | [112] |
| PCI, rat | SB-3CT (a selective MMP-9 inhibitor) | Global ischemia | MMP-9 expression and laminin decrease | Apoptotic cell death | NA | [46] |
| DIN, rat | Ginsenoside Rb1 (an active component of Chinese medicine Panax Ginseng) | Ischemic brain injury | Vasculature thickening | Brain edema | NS↑ | [67] |
| EVP, rat | Sodium orthovanadate(a tyrosine phosphatase inhibitor) | Global ischemia | Mature brain-derived neurotrophic factor/phosphorylated TrkB/Akt pathway | Brain edema and apoptotic cell death | NS↑, MT→ | [50] |
| EVP, mice | S-nitrosylated hemoglobin enhanced by ethyl nitrite inhalation | Arteriopathy due to disruption of NO bioactivity | Decreasing of cortical tissue PO2 and parenchymal RBC flow velocity without blood pressure change | Brain edema and cerebral vessel diameters | NS↑, MT→ | [100] |
| PCI, rat | Sulforaphane (a specific Nrf2 activator) | Oxidative stress | Erythroid 2-related factor 2 and antioxidant responsive element (Nrf2-ARE) pathway | BBB disruption, brain edema, and cortical apoptosis | NS↑ | [18] |
| PCI or EVP, rat | NAT (n-acetyl-l-tryptophan, a neuropeptide substance P blocker) | Global ischemia | Albumin immunoreactivity and secondary ICP elevation | No change of brain edema and ICP elevation | NS→ | [8] |
| DIN, rat | Z-ligustilide (a primary lipophilic component of the radix Angelica sinensis) | Global ischemia | Increasing expression of p53 and cleaved caspase-3, and decreasing Bcl-2 expression on day7 | BBB disruption and brain edema | NS↑, MT→ | [16] |
| PCI, rat | Progesterone | Inflammation | Increasing toll-like receptor 4/NF-κB pathway, and up-regulation of pro-inflammatory cytokines, MCP-1, and ICAM-1 |
BBB disruption and brain edema | NS↑ | [122] |
| PCI, mice | Clazosentan (an endothelin A receptor antagonist) | Oxidative stress | Clazosentan treatment did not affect superoxide anion radical, peroxynitrite, microthromboemboli in the brain, or reduction of endothelial NOS uncoupling and neuronal injury after SAH |
Decreasing CBF and NO levels, and increasing uncoupled and phosphorylated eNOS and superoxide level |
MT→ | [94] |
| PCI, rat | Clazosentan (an endothelin A receptor antagonist) | Secondary complication other than large-artery vasospasm | Clazosentan treatment did not affect microthromboemboli, neuronal degeneration, apoptosis, or loss of long-term potensiation after SAH |
Increasing microthromboemboli, neuronal degeneration, and apoptotic cell death, and decreasing long-term potentiation |
MT→ | [19] |
| EVP, rat | PUMA (p53 upregulated modulator of apoptosis) siRNA | Global ischemia | PUMA, BAX, BAK, GRP78, and DRP1 expression | BBB disruption, brain edema, and apoptotic endothelial cell death | NS↑, MT↓ | [129] |
| EVP, rat | Osteopontin (OPN) siRNA | Inflammation | Activation of NF-kappaB and JNK pathways, activation of MMP-9 induction, and VEGF expresison | BBB disruption and brain edema | NS→, MT→ | [113] |
| EVP, mice | NS398 (a specific COX-2 inhibitor) | Inflammation | Increasing BBB disruption | Neurological funtion | NS↑, MT→ | [4] |
| EVP, rat | Deferoxamine (an iron chelator) | Blood breakdown products and oxidative stress | Increasing non-heme iron and ferritin levels, and heme-oxygenase-1 (HO-1) up-regulation | Apoptotic cell death | NA | [64] |
| EVP, rat | PNU-282987 (an α7 nicotinic acetylcholine receptor agonist) | Global ischemia | PI3K/Akt/caspase-3 pathway | BBB disruption and apoptotic cell death | NS↑, MT→ | [27] |
| EVP, rat | Minocycline | Inflammation | MMP activation | BBB disruption and neuronal loss | NS↑, MT→ | [101] |
| EVP, rat | Hydroxyfasudil (Rho kinase inhibitor) | BBB disruption | Increasing occludin and ZO-1 disruption | BBB disruption and brain edema | NS↑, MT→ | [38] |
| EVP, rat | small interfering RNAs for CHOP | Global ischemia | Bim-Caspase-3 pathway | BBB disruption and apoptotic cell death | NS↑, MT→ | [53] |
| EVP, rat | Hydrogen gas inhalation | Oxidative stress | Oxidative injury of lipid, protein, and DNA | BBB disruption, brain edema, and apoptotic cell death | NS↑ | [134] |
| NA, rabbit | Hydrogen-rich saline (a cytotoxic oxygen radical scavenger) | Oxidative stress | upregulated MDA, caspase-12/3, and brain edema | BBB disruption and apoptotic cell death | NA | [138] |
| EVP, mice | Isoflurane inhalation | Global ischemia | Sphingosine kinase 1/Akt/caspase-3 pathway | BBB disruption, brain edema, and apoptotic cell death | NS↑ | [1] |
| PCI, rat | Rapamycin (autophagy inducer) or 3-methyladenine (autophagy inhibitor) | Autophagy and apoptosis | Rapamycin ameliorated NS and brain edema via increasing MP1 LC3-II to LC3-I ratio and reducing caspase-3 activity |
BBB disruption, brain edema, and apoptotic cell death | NS↑ | [55] |
| SIN, rat | Heparin | Inflammation | Neutrophils invasion, activated phagocytic microglia, increasing NF-kappa B and IL-1β | Neuroinflammation, demyelination, and transsynaptic apoptosis | Not tested | [105] |
| NA, rat | SP600125 (JNK inhibitor) | Global ischemia | Increasing claudin-5 and ZO-1, up-regulated JNK1 and JNK3 | BBB disruption, apoptotic cell death | NA | [17] |
| PCI, rat | Melatonin | Oxidative stress | Nrf2-ARE pathway | BBB disruption, brain edema, and apoptotic cell death | NS↑ | [123] |
| PCI, rat | Anti-aquaporin-4 antibody, minocycline (an inhibitor of MMP-9) , or 2-methoxyestradiol (an inhibitor of HIF-1α) |
BBB disruption | Inhibition of HIF-1α significantly suppressed the level of aquaporin-4 and MMP-9 | BBB disruption | NA | [124] |
| PCI, rat | Cyclosporin A | Mitochondrial permeability transition pore opening | Increasing cytochrome C, apoptosis-inducing factor, and cleaved caspase-3 | BBB disruption, brain edema, and apoptotic cell death | NS↑ | [127] |
| EVP, rat | Rapamycin (autophagy inducer), simvastatin | Autophagy and apoptosis | Autophagy flux by microtubule-associated protein light chain-3 (LC3 II/I) and beclin-1 expression | Autophagy activation ameliorated BBB disruption and neuronal apoptosis | NS↑ | [136] |
BBB blood-brain barrier, CBF cerebral blood flow, CHOP cyclophosphamide, doxorubicin, vincristine, and prednisone, CL-IB-MECA 2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N- methyluronamide, CPP cerebral perfusion pressure, CSF cerebrospinal fluid, Cu/Zn SOD copper/zinc superoxide dismutase, DIN Double blood injection model, ERK extracellular signal-related kinase protein, EVP Endovascular perforation model, 20-HETE 20-hydroxyeiscosatetraenoic acid, HIF-1 Hypoxia-inducible factor-1, HMG-CoA 3-hydroxyl-3-methyl-glutaryl-coenzyme A, 5-HT1B 5-hydroxytryptamine1B, ICAM-1 intercellular adhesion molecule-1, ICP intracranial pressure, IL interleukin, iNOS inducible nitric oxide synthase, JNK c-Jun N-terminal kinase, LC3 light chain 3, LDH lactate dehydrogenase, MAPKs mitogen-activated protein kinases, MCP-1 monocyte chemotactic protein-1, MMP matrix metalloproteinase, MP1 microtubule-associated protein 1, MT mortality, NA not applicable, NADPH nicotinamide adenine dinucleotide phosphate, NF-kappaB nuclear factor kappa B, NO nitric oxide, NOS Nitric oxide synthase, Nrf2-ARE nuclear factor erythroid 2-related factor 2 and antioxidant responsive element, NS neurological score, PCI pre-chiamatic blood injection model, RBC red blood cell, rCBF regional cerebral blood flow, ROS reactive oxygen species, SHI Subarachnoid hemolysate injection model, SIN Single blood injection model, siRNA small interfering RNA, SOD superoxide dismutase, TIMP tissue inhibitor of MMP, TNF tumor necrosis factor, VEGF vascular endothelial growth factor, ZO-1 zona occludens 1.
The Mechanisms of Early Brain Injury after SAH
Perhaps the most immediate event following the rupture of an intracranial aneurysm is an arrest in intracranial circulation, caused by a peak of ICP (rising as high as mean arterial blood pressure within 1 minute of ictus). The ICP then falls over several minutes to reach a much lower baseline, but remains higher than normal [43]. The temporary intracranial circulatory arrest promotes hemostasis and contributes to severe global ischemic injury, all leading to loss of autoregulation, the reduction in cerebral perfusion pressure (CPP), secondary raised ICP and decreased cerebral blood flow (CBF) [14, 81]. This hypoxic state also culminates in energy failure in neurons and glia, and initiates the cascade of events leading to cytotoxic edema [81]. Ischemia also results in apoptosis of cells that constitute the BBB [58]. Death of endothelial cells and perivascular astrocytes cause increased diffusion of serum from the vascular lumen into cerebral tissues (vasogenic edema). SAH also impacts brain parenchyma by activating astrocytes and microglia, and triggering up-regulation of the pro-inflammatory cytokines [78, 91].
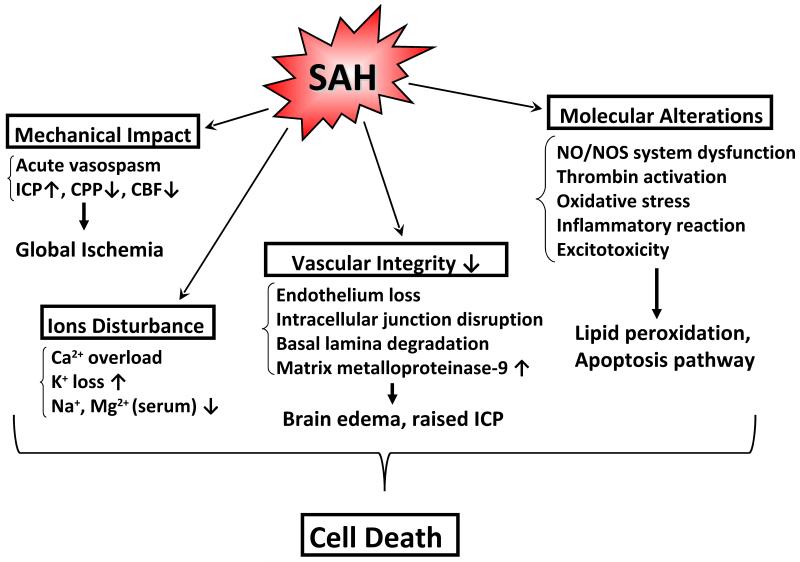
Therefore, factors stemming from the initial bleeding in SAH include: raised ICP, decreases in CBF and CPP, BBB disruption, brain swelling, brain edema, acute vasospasm and dysfunction of autoregulation, all of which constitute pathophysiological variables occurring during the EBI period (within the first 72 hours after SAH) [81]. Acute global ischemia, altered ionic homeostasis, degradation of vascular integrity, excitotoxicity, thrombin activation, oxidative stress, inflammation, elevated matrix metalloproteinase (MMP) 9, and activation of the NO-NOS pathway are all clinically relevant through their involvement in cell death and ultimate dysfunction that follows SAH (Figure 2) [7, 22, 98].
Figure 2.
Mechanism of early brain injury after SAH: SAH causes acute global ischemia, altered ionic homeostasis, degradation of vascular integrity, and molecular alterations, all leading to cell death.
Cell Death and Anti-Apoptotic Therapy in Early Brain Injury after SAH
Even a brief ischemic insult to the brain may trigger complex cellular events which lead to progressive apoptotic and necrotic cell death [132]. In general, apoptosis can be regarded as an energy-dependent process whereas necrosis is not. In SAH, if the initial bleed were severe enough to prevent blood flow to the brain as in a global stroke, it is unlikely that the brain tissue would survive. As a result, necrosis is not a major factor in SAH [14], and apoptosis may play an important role in EBI after SAH. Akt (protein kinase B), a serine/threonine kinase, is one of the key antiapoptotic signaling molecules downstream of phosphoinositide 3-kinase (PI3K) in EBI after SAH [20, 27, 30]. Mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38, have all been studied in EBI. JNK and p38 are activated in response to inflammatory cytokines and cellular stress, up-regulating apoptotic cascades [52]. The tumor suppressor gene, p53, also regulates apoptosis. In EBI after SAH, anti-apoptotic therapies have reported to ameliorate outcomes by targeting the MAPK pathway [62, 106, 114, 131], activating p53 [13, 16, 68, 76], and hypoxia inducible factor-1 (HIF-1) target genes by hyperbaric oxygen [84].
Future Directions of SAH Research
Now, EBI is considered to have a great potential for the implementation of treatment modalities in patients with SAH, attenuating some of the devastating secondary injuries that can be seen in the long term [14]. Mortality should be examined, and neurological functioning ought to be thoroughly evaluated because this information is very important in terms of translation from animals to humans. The mismatch between treatment of angiographical CVS and poor clinical outcome could result from mechanisms other than vasospasm, such as EBI, but also from the use of inadequate animal models of vasospasm. Since both EBI and CVS may contribute to the pathogenesis of delayed neurological deficits, experimental CVS should also be made by mimicking human SAH, in terms of having an injured artery and direct hemorrhagic brain lesion under arterial blood pressure [90]. The endovascular perforation model seems suitable to employ in acute SAH research, as it can produce more severe pathophysiological changes and a comparable insult to a ruptured aneurysm, as opposed to the double blood injection model [65].
Research regarding EBI after SAH is limited, and further studies are needed to clarify the exact mechanisms involved. Furthermore, it is postulated that cell death mechanisms such as apoptosis, autophagy, necroptosis and endoplasmic reticulum stress, as well as microcirculatory dysfunction, cortical spreading ischemia, and delayed neuronal injury may also be contributing to the outcomes.
Conclusion
Given the fact that the reversal of CVS does not appear to improve the outcome, it could be argued that the treatment of EBI may successfully attenuate some of the devastating secondary injuries following SAH. Further studies targeting EBI may lead to the development of new therapies and the improvement of outcomes for patients suffering from SAH.
Acknowledgments
This study was partially supports by grants from National Institutes of Health NS 053407 to JHZ
Footnotes
Conflict of interest statement
We declare that we have no conflicts of interest.
References
- 1.Altay O, Hasegawa Y, Sherchan P, Suzuki H, Khatibi NH, Tang J, Zhang JH. Isoflurane delays the development of early brain injury after subarachnoid hemorrhage through sphingosine-related pathway activation in mice. Crit Care Med. 2012;40:1908–13. doi: 10.1097/CCM.0b013e3182474bc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansar S, Larsen C, Maddahi A, Edvinsson L. Subarachnoid hemorrhage induces enhanced expression of thromboxane A2 receptors in rat cerebral arteries. Brain Res. 2010;1316:163–72. doi: 10.1016/j.brainres.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Ayer R, Chen W, Sugawara T, Suzuki H, Zhang JH. Role of gap junctions in early brain injury following subarachnoid hemorrhage. Brain Res. 2010;1315:150–8. doi: 10.1016/j.brainres.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayer R, Jadhav V, Sugawara T, Zhang JH. The neuroprotective effects of cyclooxygenase-2 inhibition in a mouse model of aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;111:145–9. doi: 10.1007/978-3-7091-0693-8_24. [DOI] [PubMed] [Google Scholar]
- 5.Ayer RE, Sugawara T, Chen W, Tong W, Zhang JH. Melatonin decreases mortality following severe subarachnoid hemorrhage. J Pineal Res. 2008;44:197–204. doi: 10.1111/j.1600-079X.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 6.Ayer RE, Sugawara T, Zhang JH. Effects of melatonin in early brain injury following subarachnoid hemorrhage. Acta Neurochir Suppl. 2008;102:327–30. doi: 10.1007/978-3-211-85578-2_62. [DOI] [PubMed] [Google Scholar]
- 7.Ayer R, Zhang J. Connecting the early brain injury of aneurysmal subarachnoid hemorrhage to clinical practice. Turk Neurosurg. 2010;20:159–66. doi: 10.5137/1019-5149.JTN.2714-09.0. [DOI] [PubMed] [Google Scholar]
- 8.Barry CM, Helps SC, den Heuvel C, Vink R. Characterizing the role of the neuropeptide substance P in experimental subarachnoid hemorrhage. Brain Res. 2011;1389:143–51. doi: 10.1016/j.brainres.2011.02.082. [DOI] [PubMed] [Google Scholar]
- 9.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr, Harbaugh RE, Patel AB, Rosenwasser RH, American Heart Association Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 10.Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–92. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- 11.Bermueller C, Thal SC, Plesnila N, Schmid-Elsaesser R, Kreimeier U, Zausinger S. Hypertonic fluid resuscitation from subarachnoid hemorrhage in rats: a comparison between small volume resuscitation and mannitol. J Neurol Sci. 2006;241:73–82. doi: 10.1016/j.jns.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–7. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 13.Cahill J, Calvert JW, Marcantonio S, Zhang JH. p53 may play an orchestrating role in apoptotic cell death after experimental subarachnoid hemorrhage. Neurosurgery. 2007;60:531–45. doi: 10.1227/01.NEU.0000249287.99878.9B. [DOI] [PubMed] [Google Scholar]
- 14.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–53. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 15.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34:1269–75. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Tang J, Khatibi NH, Zhu M, Li Y, Wang C, Jiang R, Tu L, Wang S. Treatment with Z-ligustilide, a component of Angelica sinensis, reduces brain injury after a subarachnoid hemorrhage in rats. J Pharmacol Exp Ther. 2011;337:663–72. doi: 10.1124/jpet.110.177055. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, Wei XT, Guan JH, Yuan JW, Peng YT, Song L, Liu YH. Inhibition of c-Jun N-terminal kinase prevents blood-brain barrier disruption and normalizes the expression of tight junction proteins clautin-5 and ZO-1 in a rat model of subarachnoid hemorrhage. Acta Neurochir (Wien) 2012;154:1469–76. doi: 10.1007/s00701-012-1328-y. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Fang Q, Zhang J, Zhou D, Wang Z. Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J Neurosci Res. 2011;89:515–23. doi: 10.1002/jnr.22577. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Tariq A, Ai J, Sabri M, Jeon HJ, Tang EJ, Lakovic K, Wan H, Macdonald RL. Different effects of clazosentan on consequences of subarachnoid hemorrhage in rats. Brain Res. 2011;1392:132–9. doi: 10.1016/j.brainres.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 20.Cheng G, Chunlei W, Pei W, Zhen L, Xiangzhen L. Simvastatin activates Akt/glycogen synthase kinase-3beta signal and inhibits caspase-3 activation after experimental subarachnoid hemorrhage. Vascul Pharmacol. 2010;52:77–83. doi: 10.1016/j.vph.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Cheng G, Wei L, Zhi-Dan S, Shi-Guang Z, Xiang-Zhen L. Atorvastatin ameliorates cerebral vasospasm and early brain injury after subarachnoid hemorrhage and inhibits caspase-dependent apoptosis pathway. BMC Neurosci. 2009;10:7. doi: 10.1186/1471-2202-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou SH, Feske SK, Simmons SL, Konigsberg RG, Orzell SC, Marckmann A, Bourget G, Bauer DJ, De Jager PL, Du R, Arai K, Lo EH, Ning MM. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res. 2011;2:600–7. doi: 10.1007/s12975-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou SH, Smith EE, Badjatia N, Nogueira RG, Sims JR, 2nd, Ogilvy CS, Rordorf GA, Ayata C. A randomized, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:2891–3. doi: 10.1161/STROKEAHA.107.505875. [DOI] [PubMed] [Google Scholar]
- 24.Cosar M, Eser O, Fidan H, Sahin O, Buyukbas S, Ela Y, Yagmurca M, Ozen OA. The neuroprotective effect of dexmedetomidine in the hippocampus of rabbits after subarachnoid hemorrhage. Surg Neurol. 2009;71:54–9. doi: 10.1016/j.surneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Dorsch NW. Cerebral arterial spasm--a clinical review. Br J Neurosurg. 1995;9:403–12. doi: 10.1080/02688699550041403. [DOI] [PubMed] [Google Scholar]
- 26.Dorsch NW. Therapeutic approaches to vasospasm in subarachnoid hemorrhage. Curr Opin Crit Care. 2002;8:128–33. doi: 10.1097/00075198-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH. α7 Nicotinic Acetylcholine Receptor Agonist PNU-282987 Attenuates Early Brain Injury in a Perforation Model of Subarachnoid Hemorrhage in Rats. Stroke. 2011;42:3530–6. doi: 10.1161/STROKEAHA.111.619965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duris K, Manaenko A, Suzuki H, Rolland W, Tang J, Zhang JH. Sampling of CSF via the cisterna magna and blood collection via the heart affects brain water content in a rat SAH model. Transl Stroke Res. 2011;2:232–7. doi: 10.1007/s12975-010-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ecker A, Riemenschneider PA. Arteriographic demonstration of spasm of the intracranial arteries, with special reference to saccular arterial aneurysms. J Neurosurg. 1951;8:660–7. doi: 10.3171/jns.1951.8.6.0660. [DOI] [PubMed] [Google Scholar]
- 30.Endo H, Nito C, Kamada H, Yu F, Chan PH. Akt/GSK3beta survival signaling is involved in acute brain injury after subarachnoid hemorrhage in rats. Stroke. 2006;37:2140–6. doi: 10.1161/01.STR.0000229888.55078.72. [DOI] [PubMed] [Google Scholar]
- 31.Endo H, Nito C, Kamada H, Yu F, Chan PH. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3beta survival signaling. J Cereb Blood Flow Metab. 2007;27:975–82. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ErQahin M, Toklu HZ, Cetinel S, Yüksel M, Erzik C, Berkman MZ, Yeğen BC, Sener G. Alpha lipoic acid alleviates oxidative stress and preserves blood brain permeability in rats with subarachnoid hemorrhage. Neurochem Res. 2010;35:418–28. doi: 10.1007/s11064-009-0072-z. [DOI] [PubMed] [Google Scholar]
- 33.Ersahin M, Toklu HZ, Cetinel S, Yüksel M, Yeğen BC, Sener G. Melatonin reduces experimental subarachnoid hemorrhage-induced oxidative brain damage and neurological symptoms. J Pineal Res. 2009;46:324–32. doi: 10.1111/j.1600-079X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 34.ErQahin M, Toklu HZ, Erzik C, Cetinel S, Akakin D, Velioğlu-Oğünç A, Tetik S, Ozdemir ZN, Sener G, Yeğen BC. The anti-inflammatory and neuroprotective effects of ghrelin in subarachnoid hemorrhage-induced oxidative brain damage in rats. J Neurotrauma. 2010;27:1143–55. doi: 10.1089/neu.2009.1210. [DOI] [PubMed] [Google Scholar]
- 35.Fergusen S, Macdonald RL. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007 Apr;60(4):658–67. doi: 10.1227/01.NEU.0000255396.23280.31. [DOI] [PubMed] [Google Scholar]
- 36.Findlay JM, Kassell NF, Weir BK, Haley EC, Jr, Kongable G, Germanson T, Truskowski L, Alves WM, Holness RO, Knuckey NW, Knuckey NW, Yonas H, Steinberg GK, West M, Winn HR, Ferguson G. A randomized trial of intraoperative, intracisternal tissue plasminogen activator for the prevention of vasospasm. Neurosurgery. 1995;37:168–78. [PubMed] [Google Scholar]
- 37.Fisher CM, Roberson GH, Ojemann RG. Cerebral vasospasm with ruptured saccular aneurysm--the clinical manifestations. Neurosurgery. 1977;1:245–8. doi: 10.1227/00006123-197711000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Fujii M, Duris K, Altay O, Soejima Y, Sherchan P, Zhang JH. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem Int. 2012;60:327–33. doi: 10.1016/j.neuint.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao C, Liu X, Liu W, Shi H, Zhao Z, Chen H, Zhao S. Anti-apoptotic and neuroprotective effects of Tetramethylpyrazine following subarachnoid hemorrhage in rats. Auton Neurosci. 2008;141:22–30. doi: 10.1016/j.autneu.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Gao J, Wang H, Sheng H, Lynch JR, Warner DS, Durham L, Vitek MP, Laskowitz DT. A novel apoE-derived therapeutic reduces vasospasm and improves outcome in a murine model of subarachnoid hemorrhage. Neurocrit Care. 2006;4:25–31. doi: 10.1385/NCC:4:1:025. [DOI] [PubMed] [Google Scholar]
- 41.Gao Y, Ding XS, Xu S, Wang W, Zuo QL, Kuai F. Neuroprotective effects of edaravone on early brain injury in rats after subarachnoid hemorrhage. Chin Med J (Engl) 2009;122:1935–40. [PubMed] [Google Scholar]
- 42.Germanò A, Caffo M, Angileri FF, Arcadi F, Newcomb-Fernandez J, Caruso G, Meli F, Pineda JA, Lewis SB, Wang KK, Bramanti P, Costa C, Hayes RL. NMDA receptor antagonist felbamate reduces behavioral deficits and blood-brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 2007;24:732–44. doi: 10.1089/neu.2006.0181. [DOI] [PubMed] [Google Scholar]
- 43.Grote E, Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988;22:654–61. doi: 10.1227/00006123-198804000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Guo Z, Sun X, He Z, Jiang Y, Zhang X, Zhang JH. Matrix metalloproteinase-9 potentiates early brain injury after subarachnoid hemorrhage. Neurol Res. 2010;32:715–20. doi: 10.1179/016164109X12478302362491. [DOI] [PubMed] [Google Scholar]
- 45.Guo ZD, Wu HT, Sun XC, Zhang XD, Zhang JH. Protection of minocycline on early brain injury after subarachnoid hemorrhage in rats. Acta Neurochir Suppl. 2011;110:71–4. doi: 10.1007/978-3-7091-0353-1_13. [DOI] [PubMed] [Google Scholar]
- 46.Guo ZD, Zhang XD, Wu HT, Lin B, Sun XC, Zhang JH. Matrix metalloproteinase 9 inhibition reduces early brain injury in cortex after subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110:81–4. doi: 10.1007/978-3-7091-0353-1_15. [DOI] [PubMed] [Google Scholar]
- 47.Haley EC, Jr, Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. J Neurosurg. 1997;86:467–74. doi: 10.3171/jns.1997.86.3.0467. [DOI] [PubMed] [Google Scholar]
- 48.Haley EC, Jr, Kassell NF, Torner JC. A randomized trial of nicardipine in subarachnoid hemorrhage: angiographic and transcranial Doppler ultrasound results. A report of the Cooperative Aneurysm Study. J Neurosurg. 1993;78:548–53. doi: 10.3171/jns.1993.78.4.0548. [DOI] [PubMed] [Google Scholar]
- 49.Harada S, Kamiya K, Masago A, Iwata A, Yamada K. Subarachnoid hemorrhage induces c-fos, c-jun and hsp70 mRNA expression in rat brain. Neuroreport. 1997;8:3399–404. doi: 10.1097/00001756-199710200-00041. [DOI] [PubMed] [Google Scholar]
- 50.Hasegawa Y, Suzuki H, Altay O, Zhang JH. Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011;42:477–83. doi: 10.1161/STROKEAHA.110.597344. [DOI] [PubMed] [Google Scholar]
- 51.Hasegawa Y, Suzuki H, Sherchan P, Zhan Y, Duris K, Zhang JH. Tyrosine phosphatase inhibition attenuates early brain injury after subarachnoid hemorrhage in rats. Acta Neurochir Suppl. 2011;110:67–70. doi: 10.1007/978-3-7091-0353-1_12. [DOI] [PubMed] [Google Scholar]
- 52.Hasegawa Y, Suzuki H, Sozen T, Altay O, Zhang JH. Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110:43–8. doi: 10.1007/978-3-7091-0353-1_8. [DOI] [PubMed] [Google Scholar]
- 53.He Z, Ostrowski RP, Sun X, Ma Q, Huang B, Zhan Y, Zhang JH. CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke. 2012;43:484–90. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jedrzejowska-Szypulka H, Straszak G, Larysz-Brysz M, Karpe J, Marcol W, Olakowska E, Woszczycka-Korczyńska I, Lewin-Kowalik J. Interleukin-1beta plays a role in the activation of peripheral leukocytes after blood-brain barrier rupture in the course of subarachnoid hemorrhage. Curr Neurovasc Res. 2010;7:39–48. doi: 10.2174/156720210790820226. [DOI] [PubMed] [Google Scholar]
- 55.Jing CH, Wang L, Liu PP, Wu C, Ruan D, Chen G. Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience. 2012;213:144–53. doi: 10.1016/j.neuroscience.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 56.Kassell NF, Haley EC, Jr, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84:221–8. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- 57.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–72. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 58.Keep RF, Andjelkovic AV, Stamatovic SM, Shakui P, Ennis SR. Ischemia-induced endothelial cell dysfunction. Acta Neurochir Suppl. 2005;95:399–402. doi: 10.1007/3-211-32318-x_81. [DOI] [PubMed] [Google Scholar]
- 59.Keep RF, Hua Y, Xi G. Brain water content. A misunderstood measurement? Transl Stroke Res. 2012;3:2–365. doi: 10.1007/s12975-012-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King JT., Jr Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am. 1997;7:659–68. [PubMed] [Google Scholar]
- 61.Koźniewska E, Michalik R, Rafalowska J, Gadamski R, Walski M, Frontczak-Baniewicz M, Piotrowski P, Czernicki Z. Mechanisms of vascular dysfunction after subarachnoid hemorrhage. J Physiol Pharmacol. 2006;57(Suppl 11):145–60. [PubMed] [Google Scholar]
- 62.Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–25. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 63.Lee JY, Keep RF, He Y, Sagher O, Hua Y, Xi G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab. 2010;30:1793–803. doi: 10.1038/jcbfm.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JY, Keep RF, Hua Y, Ernestus RI, Xi G. Deferoxamine reduces early brain injury following subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;112:101–6. doi: 10.1007/978-3-7091-0661-7_18. [DOI] [PubMed] [Google Scholar]
- 65.Lee JY, Sagher O, Keep R, Hua Y, Xi G. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery. 2009;65:331–43. doi: 10.1227/01.NEU.0000345649.78556.26. [DOI] [PubMed] [Google Scholar]
- 66.Lee S, Stier G, Marcantonio S, Lekic T, Allard M, Martin R, Zhang J. 3% hypertonic saline following subarachnoid hemorrhage in rats. Acta Neurochir Suppl. 2008;102:405–8. doi: 10.1007/978-3-211-85578-2_79. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Tang J, Khatibi NH, Zhu M, Chen D, Tu L, Chen L, Wang S. Treatment with ginsenoside rb1, a component of panax ginseng, provides neuroprotection in rats subjected to subarachnoid hemorrhage-induced brain injury. Acta Neurochir Suppl. 2011;110:75–9. doi: 10.1007/978-3-7091-0356-2_14. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Tang J, Khatibi NH, Zhu M, Chen D, Zheng W, Wang S. Ginsenoside Rbeta1 reduces neurologic damage, is anti-apoptotic, and down-regulates p53 and BAX in subarachnoid hemorrhage. Curr Neurovasc Res. 2010;7:85–94. doi: 10.2174/156720210791184952. [DOI] [PubMed] [Google Scholar]
- 69.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu S, Tang J, Ostrowski RP, Titova E, Monroe C, Chen W, Lo W, Martin R, Zhang JH. Oxidative stress after subarachnoid hemorrhage in gp91phox knockout mice. Can J Neurol Sci. 2007;34:356–61. doi: 10.1017/s031716710000682x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu H, Zhang DM, Chen HL, Lin YX, Hang CH, Yin HX, Shi JX. N-acetylcysteine suppresses oxidative stress in experimental rats with subarachnoid hemorrhage. J Clin Neurosci. 2009;16:684–8. doi: 10.1016/j.jocn.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 72.Luo C, Yi B, Tao G, Li M, Chen Z, Tang W, Zhang JH, Feng H. Adenosine A3 receptor agonist reduces early brain injury in subarachnoid haemorrhage. Neuroreport. 2010;21:892–6. doi: 10.1097/WNR.0b013e32833dbd13. [DOI] [PubMed] [Google Scholar]
- 73.Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, Pasqualin A. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–21. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 74.Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–63. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- 75.Matz PG, Fujimura M, Lewen A, Morita-Fujimura Y, Chan PH. Increased cytochrome c-mediated DNA fragmentation and cell death in manganese-superoxide dismutase-deficient mice after exposure to subarachnoid hemolysate. Stroke. 2001;32:506–15. doi: 10.1161/01.str.32.2.506. [DOI] [PubMed] [Google Scholar]
- 76.Megyesi JF, Vollrath B, Cook DA, Findlay JM. In vivo animal models of cerebral vasospasm: a review. Neurosurgery. 2000;46:448–61. [PubMed] [Google Scholar]
- 77.Mori K, Miyazaki M, Iwata J, Yamamoto T, Nakao Y. Intracisternal infusion of magnesium sulfate solution improved reduced cerebral blood flow induced by experimental subarachnoid hemorrhage in the rat. Neurosurg Rev. 2008;31:197–203. doi: 10.1007/s10143-008-0122-z. [DOI] [PubMed] [Google Scholar]
- 78.Murakami K, Koide M, Dumont TM, Russell SR, Tranmer BI, Wellman GC. Subarachnoid hemorrhage induces gliosis and increased expression of the pro-inflammatory cytokine high mobility group box 1 protein. Transl Stroke Res. 2011;2:72–9. doi: 10.1007/s12975-010-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neil-Dwyer G, Mee E, Dorrance D, Lowe D. Early intervention with nimodipine in subarachnoid haemorrhage. Eur Heart J. 1987;8(Suppl K):41–7. doi: 10.1093/eurheartj/8.suppl_k.41. [DOI] [PubMed] [Google Scholar]
- 80.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–71. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 81.Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- 82.Ostrowski RP, Colohan AR, Zhang JH. Neuroprotective effect of hyperbaric oxygen in a rat model of subarachnoid hemorrhage. Acta Neurochir Suppl. 2006;96:188–93. doi: 10.1007/3-211-30714-1_41. [DOI] [PubMed] [Google Scholar]
- 83.Ostrowski RP, Tang J, Zhang JH. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke. 2006;37:1314–8. doi: 10.1161/01.STR.0000217310.88450.c3. [DOI] [PubMed] [Google Scholar]
- 84.Ostrowski RP, Zhang JH. Hyperbaric oxygen for cerebral vasospasm and brain injury following subarachnoid hemorrhage. Transl Stroke Res. 2011;2:316–27. doi: 10.1007/s12975-011-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Otten ML, Mocco J, Connolly ES, Jr, Solomon RA. A review of medical treatments of cerebral vasospasm. Neurol Res. 2008;30:444–9. doi: 10.1179/174313208X284089. [DOI] [PubMed] [Google Scholar]
- 86.Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–7. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- 87.Petruk KC, West M, Mohr G, Weir BK, Benoit BG, Gentili F, Disney LB, Khan MI, Grace M, Holness RO, Karwon MS, Ford RM, G. Cameron S, Tucker WS, Purves GB, Miller JDR, Hunter KM, Richard MT, Durity FA, Chan R, Clein LJ, Maroun FB, Godon A. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J Neurosurg. 1988;68:505–17. doi: 10.3171/jns.1988.68.4.0505. [DOI] [PubMed] [Google Scholar]
- 88.Philippon J, Grob R, Dagreou F, Guggiari M, Rivierez M, Viars P. Prevention of vasospasm in subarachnoid haemorrhage. A controlled study with nimodipine. Acta Neurochir (Wien) 1986;82:110–4. doi: 10.1007/BF01456369. [DOI] [PubMed] [Google Scholar]
- 89.Pluta RM. Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacol Ther. 2005;105:23–56. doi: 10.1016/j.pharmthera.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Pluta RM, Hansen-Schwartz J, Dreier J, Vajkoczy P, Macdonald RL, Nishizawa S, Kasuya H, Wellman G, Keller E, Zauner A, Dorsch N, Clark J, Ono S, Kiris T, Leroux P, Zhang JH. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31:151–8. doi: 10.1179/174313209X393564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Inflammation in the brain after experimental subarachnoid hemorrhage. Neurosurgery. 2005;56:1082–92. [PubMed] [Google Scholar]
- 92.Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, Atkinson JL, Wijdicks EF. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:1862–6. doi: 10.1161/01.STR.0000133132.76983.8e. [DOI] [PubMed] [Google Scholar]
- 93.Roux S, Breu V, Giller T, Neidhart W, Ramuz H, Coassolo P, Clozel JP, Clozel M. Ro 61-1790, a new hydrosoluble endothelin antagonist: general pharmacology and effects on experimental cerebral vasospasm. J Pharmacol Exp Ther. 1997;28:1110–8. [PubMed] [Google Scholar]
- 94.Sabri M, Ai J, Macdonald RL. Dissociation of vasospasm and secondary effects of experimental subarachnoid hemorrhage by clazosentan. Stroke. 2011;42:1454–60. doi: 10.1161/STROKEAHA.110.604728. [DOI] [PubMed] [Google Scholar]
- 95.Schubert GA, Schilling L, Thomé C. Clazosentan, an endothelin receptor antagonist, prevents early hypoperfusion during the acute phase of massive experimental subarachnoid hemorrhage: a laser Doppler flowmetry study in rats. J Neurosurg. 2008;109:1134–40. doi: 10.3171/JNS.2008.109.12.1134. [DOI] [PubMed] [Google Scholar]
- 96.Sehba FA, Flores R, Muller A, Friedrich V, Chen JF, Britz GW, Winn HR, Bederson JB. Adenosine A(2A) receptors in early ischemic vascular injury after subarachnoid hemorrhage. Laboratory investigation. J Neurosurg. 2010;113:826–34. doi: 10.3171/2009.9.JNS09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sehba FA, Friedrich V, Jr, Makonnen G, Bederson JB. Acute cerebral vascular injury after subarachnoid hemorrhage and its prevention by administration of a nitric oxide donor. J Neurosurg. 2007;106:321–9. doi: 10.3171/jns.2007.106.2.321. [DOI] [PubMed] [Google Scholar]
- 98.Sehba FA, Pluta RM, Zhang JH. Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol. 2011;43:27–40. doi: 10.1007/s12035-010-8155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sehba FA, Schwartz AY, Chereshnev I, Bederson JB. Acute decrease in cerebral nitric oxide levels after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2000;20:604–11. doi: 10.1097/00004647-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 100.Sheng H, Reynolds JD, Auten RL, Demchenko IT, Piantadosi CA, Stamler JS, Warner DS. Pharmacologically augmented S-nitrosylated hemoglobin improves recovery from murine subarachnoid hemorrhage. Stroke. 2011;42:471–6. doi: 10.1161/STROKEAHA.110.600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sherchan P, Lekic T, Suzuki H, Hasegawa Y, Rolland W, Duris K, Zhan Y, Tang J, Zhang JH. Minocycline Improves Functional Outcomes, Memory Deficits, and Histopathology after Endovascular Perforation-Induced Subarachnoid Hemorrhage in Rats. J Neurotrauma. 2011;28:2503–12. doi: 10.1089/neu.2011.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, Nagata I, Kikuchi H, Takemae T, Hidaka H, Nakashima M. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76:571–7. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- 103.Shin HK, Lee JH, Kim CD, Kim YK, Hong JY, Hong KW. Prevention of impairment of cerebral blood flow autoregulation during acute stage of subarachnoid hemorrhage by gene transfer of Cu/Zn SOD-1 to cerebral vessels. J Cereb Blood Flow Metab. 2003;23:111–20. doi: 10.1097/01.WCB.0000036561.60552.63. [DOI] [PubMed] [Google Scholar]
- 104.Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29:317–30. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simard JM, Tosun C, Ivanova S, Kurland DB, Hong C, Radecki L, Gisriel C, Mehta R, Schreibman D, Gerzanich V. Heparin reduces neuroinflammation and transsynaptic neuronal apoptosis in a model of subarachnoid hemorrhage. Transl Stroke Res. 2012;3:155–165. doi: 10.1007/s12975-012-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sozen T, Tsuchiyama R, Hasegawa Y, Suzuki H, Jadhav V, Nishizawa S, Zhang JH. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–25. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Springborg JB, Møller C, Gideon P, Jørgensen OS, Juhler M, Olsen NV. Erythropoietin in patients with aneurysmal subarachnoid haemorrhage: a double blind randomised clinical trial. Acta Neurochir (Wien) 2007;149:1089–101. doi: 10.1007/s00701-007-1284-z. [DOI] [PubMed] [Google Scholar]
- 108.Sugawara T, Jadhav V, Ayer R, Chen W, Suzuki H, Zhang JH. Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats. Stroke. 2009;40:1530–2. doi: 10.1161/STROKEAHA.108.531699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun BL, An W, Xia ZL, Zheng CB, Li WX, Yang MF, Zhao T, Ye WJ. Zinc protoporphyrin aggravates cerebral ischemic injury following experimental subarachnoid hemorrhage. Clin Hemorheol Microcirc. 2006;34:241–6. [PubMed] [Google Scholar]
- 110.Sun BL, Hu DM, Yuan H, Ye WJ, Wang XC, Xia ZL, Zhang SM, Wang LX. Extract of Ginkgo biloba promotes the expression of VEGF following subarachnoid hemorrhage in rats. Int J Neurosci. 2009;119:995–1005. doi: 10.1080/00207450902815842. [DOI] [PubMed] [Google Scholar]
- 111.Suzuki H, Ayer R, Sugawara T, Chen W, Sozen T, Hasegawa Y, Kanamaru K, Zhang JH. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2010;38:612–8. doi: 10.1097/CCM.0b013e3181c027ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suzuki H, Ayer R, Sugawara T, Chen W, Sozen T, Hasegawa Y, Kanamaru K, Zhang JH. Role of osteopontin in early brain injury after subarachnoid hemorrhage in rats. Acta Neurochir Suppl. 2011;110:75–9. doi: 10.1007/978-3-7091-0353-1_14. [DOI] [PubMed] [Google Scholar]
- 113.Suzuki H, Hasegawa Y, Ayer R, Sugawara T, Chen W, Sozen T, Kanamaru K, Taki W, Zhang JH. Effects of recombinant osteopontin on blood-brain barrier disruption after subarachnoid hemorrhage in rats. Acta Neurochir Suppl. 2011;111:231–6. doi: 10.1007/978-3-7091-0693-8_39. [DOI] [PubMed] [Google Scholar]
- 114.Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41:1783–90. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tait MJ, Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Increased brain edema in aqp4-null mice in an experimental model of subarachnoid hemorrhage. Neuroscience. 2010;167:60–7. doi: 10.1016/j.neuroscience.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thal SC, Sporer S, Schmid6Elsaesser R, Plesnila N, Zausinger S. Inhibition of bradykinin B2 receptors before, not after onset of experimental subarachnoid hemorrhage prevents brain edema formation and improves functional outcome. Crit Care Med. 2009;37:2228–34. doi: 10.1097/CCM.0b013e3181a068fc. [DOI] [PubMed] [Google Scholar]
- 117.Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–32. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- 118.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–18. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 119.Vajkoczy P, Meyer B, Weidauer S, Raabe A, Thome C, Ringel F, Breu V, Schmiedek P. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg. 2005;103:9–17. doi: 10.3171/jns.2005.103.1.0009. [DOI] [PubMed] [Google Scholar]
- 120.Veelken JA, Laing RJ, Jakubowski J. The Sheffield model of subarachnoid hemorrhage in rats. Stroke. 1995;26:1279–84. doi: 10.1161/01.str.26.7.1279. [DOI] [PubMed] [Google Scholar]
- 121.Vergouwen MD. Effect of endothelin-receptor antagonists on delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage remains unclear. Stroke. 200(40):e714–6. doi: 10.1161/STROKEAHA.109.565887. [DOI] [PubMed] [Google Scholar]
- 122.Wang Z, Zuo G, Shi XY, Zhang J, Fang Q, Chen G. Progesterone administration modulates cortical TLR4/NF-κB signaling pathway after subarachnoid hemorrhage in male rats. Mediators Inflamm. 2011;2011:848309. doi: 10.1155/2011/848309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Z, Ma C, Meng CJ, Zhu GQ, Sun XB, Huo L, Zhang J, Liu HX, He WC, Shen XM, Shu Z, Chen G. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J Pineal Res. 2012;53:129–37. doi: 10.1111/j.1600-079X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 124.Wang Z, Meng CJ, Shen XM, Shu Z, Ma C, Zhu GQ, Liu HX, He WC, Sun XB, Huo L, Zhang J, Chen G. Potential contribution of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 to blood-brain barrier disruption and brain edema after experimental subarachnoid hemorrhage. J Mol Neurosci. 2012;48:273–80. doi: 10.1007/s12031-012-9769-6. [DOI] [PubMed] [Google Scholar]
- 125.Wilkins RH. Cerebral vasospasm. Crit Rev Neurobiol. 1990;6:51–77. [PubMed] [Google Scholar]
- 126.Wong GK, Chan MT, Boet R, Poon WS, Gin T. Intravenous magnesium sulfate after aneurysmal subarachnoid hemorrhage: a prospective randomized pilot study. J Neurosurg Anesthesiol. 2006;18:142–8. doi: 10.1097/00008506-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 127.Xie Z, Lei B, Huang Q, Deng J, Wu M, Shen W, Cheng Y. Neuroprotective effect of Cyclosporin A on the development of early brain injury in a subarachnoid hemorrhage model: a pilot study. Brain Res. 2012;1472:113–23. doi: 10.1016/j.brainres.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 128.Yan J, Chen C, Hu Q, Yang X, Lei J, Yang L, Wang K, Qin L, Huang H, Zhou C. The role of p53 in brain edema after 24 h of experimental subarachnoid hemorrhage in a rat model. Exp Neurol. 2008;214:37–46. doi: 10.1016/j.expneurol.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 129.Yan J, Li L, Khatibi NH, Yang L, Wang K, Zhang W, Martin RD, Han J, Zhang J, Zhou C. Blood-brain barrier disruption following subarchnoid hemorrhage may be faciliated through PUMA induction of endothelial cell apoptosis from the endoplasmic reticulum. Exp Neurol. 2011;230:240–7. doi: 10.1016/j.expneurol.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 130.Yatsushige H, Calvert JW, Cahill J, Zhang JH. Limited role of inducible nitric oxide synthase in blood-brain barrier function after experimental subarachnoid hemorrhage. J Neurotrauma. 2006;23:1874–82. doi: 10.1089/neu.2006.23.1874. [DOI] [PubMed] [Google Scholar]
- 131.Yatsushige H, Ostrowski RP, Tsubokawa T, Colohan A, Zhang JH. Role of c-Jun N-terminal kinase in early brain injury after subarachnoid hemorrhage. J Neurosci Res. 2007;85:1436–48. doi: 10.1002/jnr.21281. [DOI] [PubMed] [Google Scholar]
- 132.Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14:469–77. doi: 10.1007/s10495-008-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zausinger S, Thal SC, Kreimeier U, Messmer K, Schmid-Elsaesser R. Hypertonic fluid resuscitation from subarachnoid hemorrhage in rats. Neurosurgery. 2004;55:679–87. doi: 10.1227/01.neu.0000134558.28977.ee. [DOI] [PubMed] [Google Scholar]
- 134.Zhan Y, Chen C, Suzuki H, Hu Q, Zhi X, Zhang JH. Hydrogen gas ameliorates oxidative stress in early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2012;40:1291–6. doi: 10.1097/CCM.0b013e31823da96d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang J, Zhu Y, Zhou D, Wang Z, Chen G. Recombinant human erythropoietin (rhEPO) alleviates early brain injury following subarachnoid hemorrhage in rats: possible involvement of Nrf2-ARE pathway. Cytokine. 2010;52:252–7. doi: 10.1016/j.cyto.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 136.Zhao H, Ji Z, Tang D, Yan C, Zhao W, Gao C. Role of autophagy in early brain injury after subarachnoid hemorrhage in rats. Mol Biol Rep. 2012 doi: 10.1007/s11033-012-2120-z. in press. [DOI] [PubMed] [Google Scholar]
- 137.Zhou Y, Martin RD, Zhang JH. Advances in experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110:15–21. doi: 10.1007/978-3-7091-0353-1_3. [DOI] [PubMed] [Google Scholar]
- 138.Zhuang Z, Zhou ML, You WC, Zhu L, Ma CY, Sun XJ, Shi JX. Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci. 2012 May 15;13:47. doi: 10.1186/1471-2202-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]