Abstract
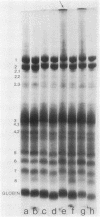
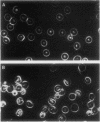
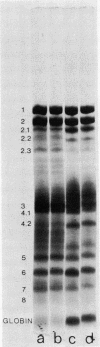
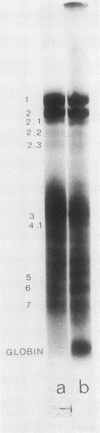
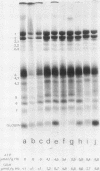
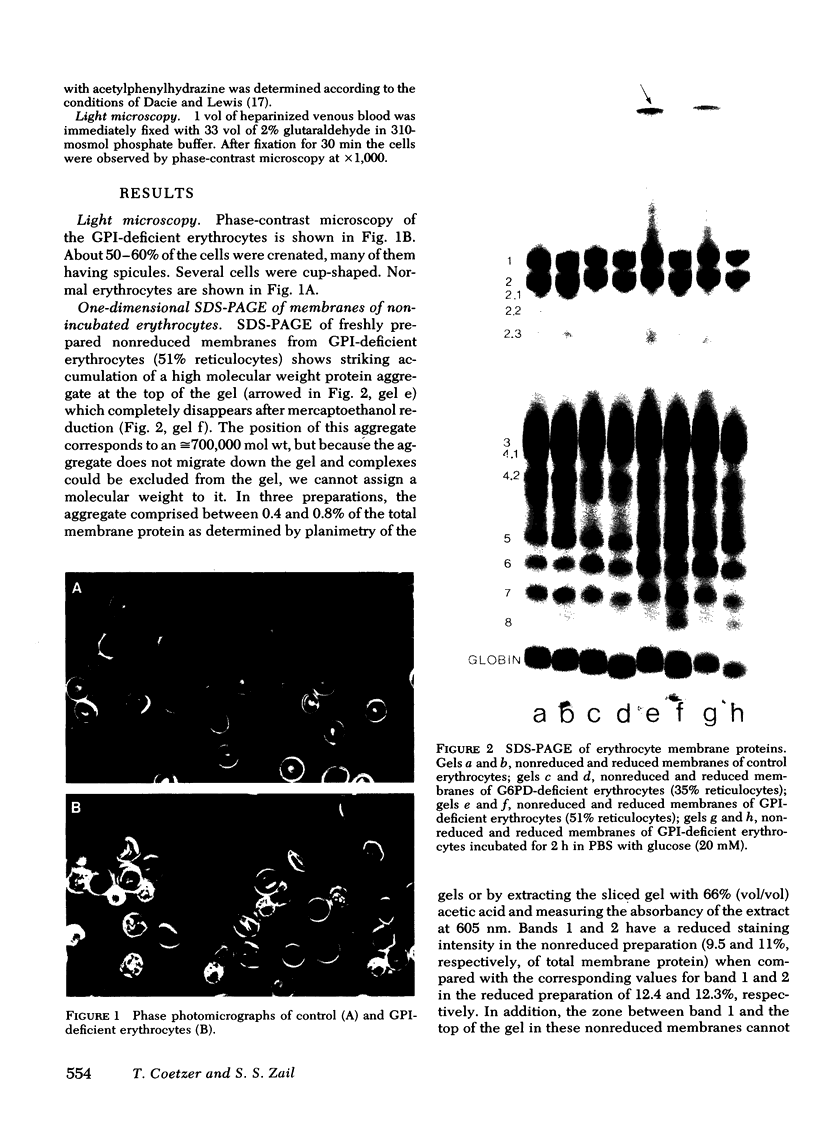
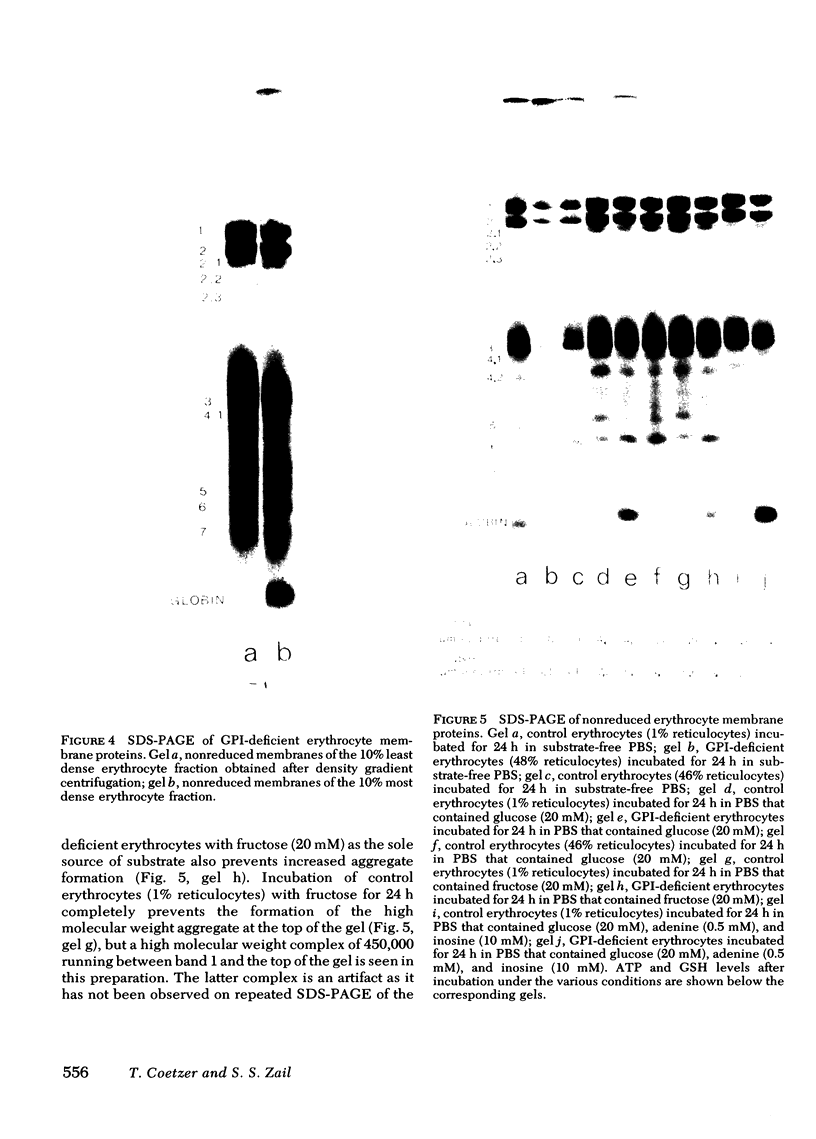
Erythrocytes (approximately equal to 50% reticulocytes) obtained from a splenectomized patient with a thermolabile variant of glucosephosphate isomerase (GPI) deficiency showed a striking degree of crenation and decreased filterability through 3-micrometer Nuclepore filters (Nuclepore Corp., Pleasanton, Calif.). Membranes prepared by hypotonic lysis of such erythrocytes were found to contain a high molecular weight aggregate which was probably disulphide-bonded. The 10% most dense erythrocyte fraction showed an accentuation of aggregate formation while aggregates could not be detected in the 10% least dense erythrocyte fraction. The aggregate consisted mainly of spectrin (band 1) and a protein with the mobility of 4.2. "Extractability" of spectrin from these membranes was also markedly diminished. Incubation of the erythrocytes for 24 h in substrate-free medium caused more pronounced spectrin aggregation than in low or high reticulocyte controls. Incubation of low or high reticulocyte controls for 24 h in medium that contained glucose completely prevented the formation of the high molecular weight aggregate. GPI-deficient erythrocytes incubated with glucose in the medium showed an accentuation of membrane protein aggregate formation; however, this was almost completely reversed by the addition of adenine and inosine to the incubation medium or by the use of fructose, the intermediate just distal to the "block" in glycolysis, as the sole substrate. ATP and reduced glutathione levels in the GPI-deficient erythrocytes incubated with glucose were similar to that found in the low and high reticulocyte controls. Our findings suggest that only a proportion of erythrocytes (the older, more dense population of cells) are susceptible to the formation of disulphide-bonded aggregates, and that this is directly related to an impairment of substrate flow through the glycolytic sequence. The exact mechanism of aggregate formation in these erythrocytes remains to be elucidated.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. W., Johnson G. J., Cadman S., Kaplan M. E. Membrane polypeptide aggregates in glucose 6-phosphate dehydrogenase-deficient and in vitro aged red blood cells. J Lab Clin Med. 1978 Feb;91(2):321–327. [PubMed] [Google Scholar]
- Cayanis E., Penfold G. K., Freiman I., MacDougall L. G. Haemolytic anaemia associated with glucosephosphate isomerase (GPI) deficiency in a Black South African child. Br J Haematol. 1977 Nov;37(3):363–371. doi: 10.1111/j.1365-2141.1977.tb01007.x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons J. F., Koler R. D., Jones R. T. Red cell age-related changes of hemoglobins AIa+b and AIc in normal and diabetic subjects. J Clin Invest. 1976 Oct;58(4):820–824. doi: 10.1172/JCI108534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu S. C., Fairbanks G., Palek J. Spontaneous, reversible protein cross-linking in the human erythrocyte membrane. Temperature and pH dependence. Biochemistry. 1977 Sep 6;16(18):4066–4074. doi: 10.1021/bi00637a020. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M. Isolation and partial characterization of a high molecular weight red cell membrane protein complex normally removed by the spleen. Blood. 1977 Oct;50(4):625–641. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Ukena T. E. Diminished spectrin extraction from ATP-depleted human erythrocytes. Evidence relating spectrin to changes in erythrocyte shape and deformability. J Clin Invest. 1978 Mar;61(3):815–827. doi: 10.1172/JCI108996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSKI F. A., NAIMAN J. L. RED CELL METABOLISM IN THE PREMATURE INFANT. I. ADENOSINE TRIPHOSPHATE LEVELS, ADENOSINE TRIPHOSPHATE STABILITY, AND GLUCOSE CONSUMPTION. Pediatrics. 1965 Jul;36:104–112. [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Hereditary glucosephosphate isomerase deficiency. A review. Am J Clin Pathol. 1974 Dec;62(6):740–751. doi: 10.1093/ajcp/62.6.740. [DOI] [PubMed] [Google Scholar]
- Palek J., Liu A., Liu D., Snyder L. M., Fortier N. L., Njoku G., Kiernan F., Funk D., Crusberg T. Effect of procaine HCLl on ATP: calcium-dependent alterations in red cell shape and deformability. Blood. 1977 Jul;50(1):155–164. [PubMed] [Google Scholar]
- Palek J., Liu S. C., Snyder L. M. Metabolic dependence of protein arrangement in human erythrocyte membranes. I. Analysis of spectrin-rich complexes in ATP-depleted red cells. Blood. 1978 Mar;51(3):385–395. [PubMed] [Google Scholar]
- Schröter W., Tillmann W. Congenital nonspherocytic hemolytic anemia associated with glucosephosphate isomerase deficiency: variant Paderborn. Klin Wochenschr. 1977 Apr 15;55(8):393–396. doi: 10.1007/BF01488625. [DOI] [PubMed] [Google Scholar]
- Schröter W., Tillmann W. Decreased deformability of erythrocytes in haemolytic anaemia associated with glucosephosphate isomerase deficiency. Br J Haematol. 1977 Aug;36(4):475–484. doi: 10.1111/j.1365-2141.1977.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]