Abstract
Context
Levels of high-sensitivity cardiac troponin T (hs-cTnT) predict secondary cardiovascular events in patients with stable coronary heart disease (CHD).
Objectives
To determine the association of hs-cTnT with structural and functional measures of heart disease and the extent to which these measures explain the relationship between hs-cTnT and secondary events.
Design, Setting, and Participants
We measured serum concentrations of hs-cTnT and performed exercise treadmill testing with stress echocardiography in a prospective cohort study of 984 outpatients with CHD who were enrolled between September 2000 and December 2002 and followed for a median of 8.2 years.
Main Outcomes Measures
Cardiovascular events (myocardial infarction, heart failure, or cardiovascular death), determined by review of medical records and death certificates.
Results
Of 984 participants, 794 (80.7%) had detectable hs-cTnT levels. At baseline, higher hs-cTnT was associated with greater inducible ischemia and with worse left ventricular ejection fraction, left atrial function, diastolic function, left ventricular mass, and treadmill exercise capacity. During follow-up, 317 participants (32.2%) experienced a cardiovascular event. After adjustment for clinical risk factors, baseline cardiac structure and function, and other biomarkers (NT-proBNP and C-reactive protein), each doubling in hs-cTnT remained associated with a 37% higher rate of cardiovascular events (HR 1.37, 95%CI 1.14, 1.65; P=0.001).
Conclusions
In outpatients with stable CHD, higher hs-cTnT levels were associated with multiple abnormalities of cardiac structure and function but remained independently predictive of secondary events. These findings suggest that hs-cTnT may detect an element of risk that is not captured by existing measures of cardiac disease severity.
Introduction
Standard serum cardiac troponin assays can detect measurable levels of troponin in the setting of myocardial injury,1 but most healthy individuals and ambulatory patients with coronary heart disease (CHD) have undetectable levels.2 Recently, high-sensitivity assays have been developed that can detect cardiac troponin in most healthy individuals.3
In the general population, levels of troponin within the range detected by high-sensitivity assays are associated with structural heart disease.4 In patients with stable coronary heart disease, elevated high-sensitivity troponin cardiac troponin T (hs-cTnT) levels predict greater risk of secondary events.5 However, it is unclear whether the increased risk of secondary events associated with hs-cTnT is independent of existing measures of cardiac structural and functional abnormalities.
Therefore, we sought to evaluate the association of hs-cTnT with baseline measures of cardiac structure and function and to determine whether the association of hs-cTnT with cardiovascular events was explained by baseline cardiac structure and function, in a prospective cohort study of 984 outpatients with CHD.
Methods
Participants
We evaluated 984 patients from the Heart and Soul Study, a prospective cohort study that was originally designed to investigate the effects of psychosocial factors on health outcomes in outpatients with stable CHD. Methods have been previously described.6 Patients were eligible if they had at least 1 of the following: history of myocardial infarction, angiographic evidence of ≥50% stenosis in ≥1 coronary vessels, evidence of exercise-induced ischemia by treadmill ECG or stress nuclear perfusion imaging, or a history of coronary revascularization. Patients were excluded if they were unable to walk one block, had an acute coronary syndrome within the previous six months, or were likely to move out of the area within three years.
Between September 11, 2000 and December 20, 2002, 1024 subjects were enrolled from 12 outpatient clinics in the San Francisco Bay Area, including 549 (54%) with a history of myocardial infarction, 237 (23%) with a history of revascularization but not myocardial infarction, and 238 (23%) with a diagnosis of coronary disease that was documented by their physician, based on a positive angiogram or treadmill test in over 98% of cases. All study participants completed a full-day study including medical history, extensive questionnaires, and an exercise treadmill test with baseline and stress echocardiograms. Twelve hour fasting serum samples were obtained on the morning prior to stress test. They were centrifuged, aliquoted and frozen at −80°C within 60 minutes of the blood draw. Samples underwent two freeze-thaw cycles prior to the hs-cTnT assays in October 2012. Frozen samples were not available for 40 participants, leaving 984 subjects for this analysis.
High sensitivity cardiac troponin T assay
Serum samples were available in 984 participants, who formed the analytic cohort for this study. Cardiac troponin T concentrations were measured with highly sensitive cardiac troponin T reagents on an Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, IN). The analytical measurement range of the assay was 3 to 10,000 pg/mL. The limit of blank was 3 pg/mL and limit of detection was 5 pg/mL. Values below the limit of detection were analyzed as 4.99 pg/mL. In validation studies of hs-cTnT, the 99th percentile for a healthy reference population has been reported to be 13.5 pg/mL (10.0 pg/mL in women and 14.5 pg/mL in men)7 and 14.5 pg/mL,8 with a coefficient of variation at or near 10% at 99th percentile levels.7,8
Outcomes ascertainment
Annual telephone interviews were conducted with participants or their proxy to inquire about interval emergency department visit, hospitalization, or death. For any reported event, medical records, electrocardiograms, death certificates, autopsy, and coroner's reports were obtained. Each event was adjudicated by 2 independent and blinded reviewers. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator, if needed.
The primary outcome was time to secondary cardiovascular event (myocardial infarction, heart failure, or cardiovascular death). Myocardial infarction was defined using standard diagnostic criteria.9 Heart failure was defined as hospitalization for signs and symptoms of heart failure. Death and cause of death were verified by death certificates and review of medical records.
Other Patient Characteristics
Demographic characteristics, medical history, and smoking status were collected by self-report questionnaire. Depressive symptoms were assessed using the 9-item Patient Health Questionnaire, a self-report instrument that measures the frequency of depressive symptoms, with a score of 10 or higher being classified as having depressive symptoms.10 We measured weight and height and calculated the body mass index (kg/m2). Resting blood pressure was measured in the supine position with a standard sphygmomanometer. Participants were asked to bring their medication bottles to the study appointment, and research personnel recorded all current medications. Medications were categorized using Epocrates Rx (Epocrates, Inc., San Mateo, CA).
Total cholesterol, high-density lipoprotein cholesterol, amino-terminal portion of the prohormone of brain-type natriuretic peptide (NT-proBNP) (Elecsys proBNP, Roche Diagnostics), high sensitivity C-reactive protein, and standard cardiac troponin T (Elecsys TnT third-generation assay, Roche Diagnostics) were determined from 12-h fasting serum samples. Estimated glomerular filtration rate was calculated from levels of creatinine and cystatin C.11
Measures of cardiac structure and function
Participants underwent symptom-limited exercise stress testing according to a standard Bruce protocol (those unable to complete the standard protocol were converted to a manual protocol) with continuous 12-lead electrocardiogram monitoring. Exercise capacity was estimated as the total metabolic equivalents (METs) achieved at peak exercise.12 Prior to exercise, participants underwent complete resting two-dimensional echocardiograms with all standard views using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, CA) with a 3.5-MHz transducer and Doppler ultrasound examination. Standard two-dimensional parasternal short-axis and apical two- and four-chamber views were obtained during held inspiration and were used to calculate chamber sizes and left ventricular ejection fraction.13 Chamber size measurements were indexed to body surface area. Using pulse wave Doppler, we measured the velocity time integral of the left ventricular outflow tract from the apical five-chamber view. Left atrial function index was calculated as ([left atrial emptying fraction × left ventricular outflow tract-velocity time integral] / [left atrial end-systolic volume index)], where left atrial emptying fraction was defined as: (left atrial end systolic volume - left atrial end-diastolic volume) / left atrial end-systolic volume.14 Diastolic dysfunction was defined as pseudonormal or restrictive filling on mitral inflow.6 At peak exercise, precordial long- and short-axis and apical two- and four-chamber views were obtained to ascertain wall motion abnormalities. We defined exercise-induced ischemia as the presence of one or more new wall motion abnormalities at peak exercise that were not present at rest. A single experienced cardiologist (NBS), who was blinded to the results of hs-cTnT and clinical histories, interpreted all echocardiograms.
Statistical Analysis
For descriptive purposes, we divided participants into tertiles of hs-cTnT. To compare the differences amongst tertiles, we used the X2 test for categorical variables and oneway analysis of variance for continuous variables.
For the primary combined outcome of myocardial infarction, heart failure, or cardiovascular death, we constructed Kaplan-Meier curves for event-free survival by tertile of hs-cTnT and compared groups using the log-rank test. We calculated event rates per 100 person-years by category of hs-cTnT for myocardial infarction, heart failure, cardiovascular death, and the primary combined outcome. Event rates by category were compared with the Mantel-Haenszel test for trend and Cox proportional hazards models.
To rule out the possibility that the association between cTnT and cardiovascular events was due to our definition of categories, we analyzed hs-cTnT as a continuous variable. We used Cox proportional hazards models to evaluate the association of each doubling in hs-cTnT (base-2 logarithm transformed hs-cTnT) with secondary cardiovascular events. We adjusted for clinical risk factors, stress echocardiogram measures, and cardiac biomarkers found to be associated with hs-cTnT with p<0.10. We tested for interactions of hs-cTnT, age, sex, smoking, diabetes, eGFR, left ventricular ejection fraction, and left ventricular mass in predicting cardiovascular events. Finally, we evaluated the association between sex-specific tertiles of hs-cTnT and cardiovascular events.
Using a logistic regression model for cardiovascular events, we estimated the area under the receiver operating curve (C-statistic) for natural log-transformed hs-cTnT. Natural log-transformed hs-cTnT was added to baseline models of clinical risk factors (age, sex, current smoking, myocardial infarction, heart failure, revascularization, diabetes mellitus, systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, body mass index, estimated glomerular filtration rate) and clinical risk factors plus natural log-transformed NT-proBNP and natural log-transformed C-reactive protein. We calculated C-statistics, category-free net reclassification improvement, and integrated discrimination improvement for these models.15-17 We evaluated model calibration with Hosmer-Lemeshow goodness of fit with 10 groups.
All analyses were performed using Stata (Version 12, StataCorp LP).
Results
Among 984 participants, 794 (80.7%) had detectable levels of hs-cTnT (>5 pg/mL). With the standard troponin T assay, only 58 participants (6% of the entire cohort) had detectable troponin levels.2 Age, sex, hypertension, history of heart failure, diabetes mellitus, revascularization, physical inactivity, blood pressure, eGFR, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use, diuretic use, NT-proBNP, and C-reactive protein varied across levels of hs-cTnT (Table 1).
Table 1.
Baseline characteristics of 988 participants by tertile of high-sensitivity troponin T.
| Tertile of high-sensitivity troponin T |
||||
|---|---|---|---|---|
| Low | Intermediate | High | P-value | |
| Number in Tertile | 330 | 326 | 328 | |
| Range (pg/mL) | <5 - 7.09 | 7.12 - 13.67 | 13.78 - 540.2 | |
| Demographics | ||||
| Age (y) | 60.6 ± 9.8 | 68.1 ± 9.9 | 71.5 ± 10.3 | <.001 |
| Male Sex | 225 (68.2) | 277 (83.9) | 300 (90.9) | <.001 |
| Caucasian | 188 (57.0) | 200 (60.6) | 205 (62.1) | 0.34 |
| Current smoking | 99 (30.0) | 55 (16.7) | 42 (12.7) | <.001 |
| History | ||||
| Hypertension | 217 (65.8) | 231 (70.0) | 245 (74.2) | 0.05 |
| Myocardial Infarction | 163 (49.4) | 174 (52.7) | 189 (57.3) | 0.14 |
| Heart Failure | 36 (10.9) | 48 (14.5) | 89 (27.0) | <.001 |
| Diabetes | 55 (16.7) | 79 (23.9) | 124 (37.6) | <.001 |
| Revascularization | 168 (50.9) | 205 (62.1) | 202 (61.2) | 0.004 |
| Depressive Symptoms | 67 (20.3) | 59 (17.9) | 64 (19.4) | 0.77 |
| Physical Inactivity | 117 (35.5) | 101 (30.6) | 142 (43.0) | 0.004 |
| Measurements | ||||
| Body Mass Index (kg/m2) | 28.5 ± 5.7 | 28.5 ± 5.2 | 28.3 ± 5.2 | 0.9 |
| Systolic Blood Pressure (mmHg) | 130.6 ± 19.0 | 133.3 ± 21.1 | 135.1 ± 22.7 | 0.02 |
| Diastolic Blood Pressure (mmHg) | 75.9 ± 10.4 | 73.9 ± 11.1 | 74.0 ± 12.4 | 0.04 |
| eGFR (mL/min) | 84.1 ± 17.6 | 71.8 ± 17.0 | 55.8 ± 21.7 | <.001 |
| Total Cholesterol (mg/dL) | 181.5 ± 41.4 | 176.0 ± 40.9 | 175.5 ± 45.9 | 0.15 |
| HDL Cholesterol (mg/dL) | 46.7 ± 14.2 | 45.9 ± 13.6 | 44.4 ± 14.3 | 0.11 |
| Medications | ||||
| Beta-blocker | 181 (54.8) | 189 (57.3) | 194 (58.8) | 0.68 |
| ACE-inhibitor or ARB | 129 (39.1) | 175 (53.0) | 200 (60.6) | <.001 |
| Statin | 200 (60.6) | 225 (68.2) | 205 (62.1) | 0.09 |
| Aspirin | 233 (70.6) | 244 (73.9) | 231 (70.0) | 0.39 |
| Diuretics | 62 (18.8) | 84 (25.5) | 145 (43.9) | <.001 |
| Biomarkers | ||||
| NT-proBNP (pg/mL) | 86 (45, 182) | 174 (87, 358) | 473 (175, 1246) | <.001 |
| C-reactive Protein (mg/L) | 2.0 (0.8, 4.3) | 2.0 (0.8, 4.4) | 2.7 (1.2, 6.3) | 0.02 |
Values expressed as N (%), mean ± SD, or median (interquartile range)
Abbreviations: eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; NT-proBNP: amino-terminal portion of the prohormone of brain natriuretic peptide.
Elevated hs-cTnT was associated with greater severity of cardiac structural and functional abnormalities, measured by left ventricular mass index, left ventricular ejection fraction, left atrial function index, diastolic dysfunction, inducible ischemia, and treadmill exercise capacity (Table 2).
Table 2.
Structural and functional measures of cardiac disease severity by tertile of hs-troponin T.
| Tertile of high-sensitivity troponin T |
|||||
|---|---|---|---|---|---|
| Low | Intermediate | High | P-value | ||
| Range (pg/mL) | <5 - 7.09 | 7.12 - 13.67 | 13.78 - 540.2 | ||
| Echocardiogram Measure | No. | ||||
| Left ventricular mass index (g/m2) | 975 | 89.5 ± 20.9 | 94.8 ± 22.2 | 110.4 ± 30.5 | <.001 |
| Left ventricular ejection fraction (%) | 958 | 64.1 ± 7.1 | 62.5 ± 9.2 | 58.2 ± 11.4 | <.001 |
| Left atrial function index (Units) | 944 | 45.5 ± 17.3 | 40.6 ± 17.3 | 34.2 ± 19.7 | <.001 |
| Diastolic Dysfunction | 872 | 21/294 (7.1) | 28/294 (9.5) | 59/284 (20.8) | <.001 |
| Inducible Ischemia | 898 | 47/307 (15.3) | 74/307 (24.1) | 97/284 (34.2) | <.001 |
| Treadmill Exercise Capacity (METs) | 904 | 8.6 ± 3.6 | 7.4 ± 3.0 | 5.9 ± 2.9 | <.001 |
Values expressed as N (%) or mean ± SD
Abbreviations: METs: metabolic equivalents
During a median follow-up of 8.2 years (interquartile range 3.7 - 9.0), 133 participants experienced myocardial infarction, 180 had hospital visits for heart failure, 146 died from cardiovascular causes, and 317 experienced the primary outcome of cardiovascular events (myocardial infarction, heart failure, or cardiovascular death).
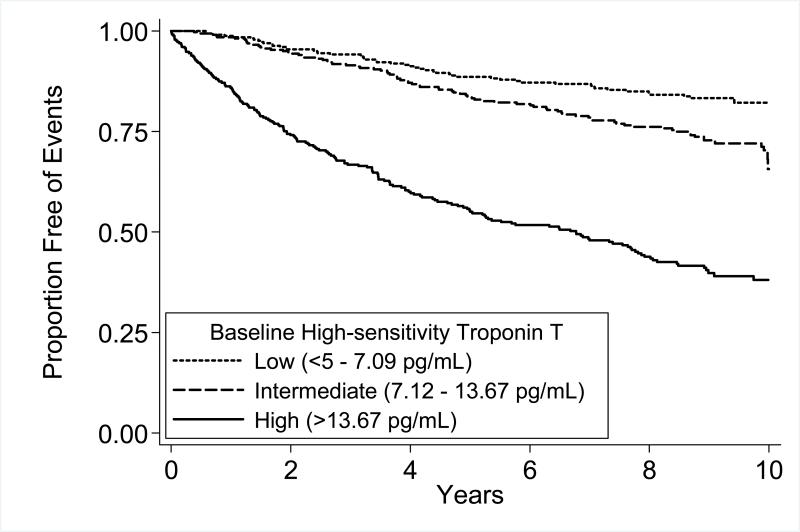
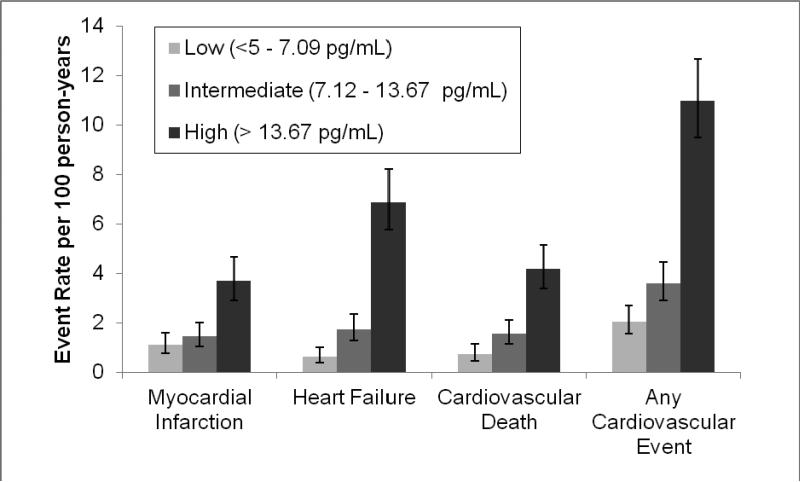
Participants with intermediate or high hs-cTnT were more likely to experience secondary cardiovascular events than those with undetectable hs-cTnT (Figure 1). For the individual components of the primary outcome (myocardial infarction, heart failure, and cardiovascular death), we found that higher hs-cTnT was associated with higher rates of cardiovascular events (Figure 2). Participants in the intermediate tertile of hs-cTnT experienced cardiovascular events at 1.75 times the rate of participants in the lowest tertile of hs-cTnT (HR 1.75, 95%CI 1.23, 2.47; P=0.002). Participants in the highest tertile of hs-cTnT experienced cardiovascular events at 5 times the rate of participants in the lowest tertile of hs-cTnT (HR 5.20, 95%CI 3.80, 7.09; P<.001).
Figure 1.
Cardiovascular events (myocardial infarction, heart failure, or cardiovascular death) by tertile of high-sensitivity troponin T (hs-cTnT). P<.001 by log-rank test.
Figure 2.
Rates of cardiovascular events (myocardial infarction, heart failure, or cardiovascular death) by tertile of high-sensitivity troponin T. Error bars indicate 95% confidence intervals for event rates. Test for trend P<.001 for all events.
After adjustment for clinical risk factors (age, male sex, smoking, hypertension, heart failure, diabetes mellitus, revascularization, physical inactivity, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use, statin use, diuretic use) and stress echocardiogram variables (left ventricular mass index, left ventricular ejection fraction, left atrial function index, diastolic dysfunction, inducible ischemia, treadmill exercise capacity), high hs-cTnT remained associated with a 76% higher rate of cardiovascular events (adjusted HR 1.76, 95%CI 1.07, 2.88; p=0.03). When participants were categorized by sex-specific tertiles of hs-cTnT, this association was similar (adjusted HR for highest tertile vs. lowest tertile: 2.04, 95% CI 1.28, 3.25; p=0.003) (Supplemental Table 1).
Evaluating continuous values of hs-cTnT with Cox proportional hazards models, we found that each doubling in hs-cTnT was associated with almost twice the rate of cardiovascular events (HR 1.98, 95%CI 1.80, 2.17; P<.001)(Table 3). With adjustment for clinical risk factors (age, male sex, smoking, hypertension, heart failure, diabetes mellitus, revascularization, physical inactivity, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use, statin use, diuretic use), echocardiogram measurements (left ventricular mass index, left ventricular ejection fraction, left atrial function index, diastolic dysfunction, inducible ischemia, treadmill exercise capacity), and cardiovascular biomarkers (NT-proBNP, C-reactive protein), each doubling in hs-cTnT remained independently associated with a 37% greater risk of cardiovascular events (HR 1.37, 95%CI 1.14, 1.65; P=0.001).
Table 3.
Association of high-sensitivity troponin T (per doubling) with cardiovascular events after adjusting for clinical risk factors and measures of disease severity.
|
Myocardial Infarction |
Heart Failure |
Cardiovascular Death |
Any Event |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI)a | P-value | HR (95%CI)a | P-value | HR (95%CI)a | P-value | HR (95%CI)a | P-value | |
| Unadjusted | 1.74 (1.50, 2.03) | <.001 | 2.30 (2.04, 2.59) | <.001 | 1.95 (1.70, 2.23) | <.001 | 1.98 (1.80, 2.17) | <.001 |
| Model 1b | 1.55 (1.26, 1.91) | <.001 | 1.92 (1.62, 2.28) | <.001 | 1.78 (1.46, 2.16) | <.001 | 1.68 (1.47, 1.92) | <.001 |
| Model 2c | 1.32 (1.02, 1.71) | 0.03 | 1.55 (1.19, 2.01) | 0.001 | 1.39 (1.06, 1.83) | 0.02 | 1.42 (1.19, 1.70) | <.001 |
| Model 3d | 1.28 (0.99, 1.67) | 0.06 | 1.44 (1.10, 1.89) | 0.007 | 1.36 (1.03, 1.81) | 0.03 | 1.37 (1.14, 1.65) | 0.001 |
Hazard Ratio (HR) for cardiovascular events per doubling in high-sensitivity troponin T.
Model 1 - Adjusted for age, male sex, smoking, history of hypertension, history of heart failure, history of diabetes mellitus, revascularization, physical inactivity, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate, ace-inhibitor or angiotensin receptor blocker use, statin use, diuretic use.
Model 2 - Adjusted for Model 1 plus left ventricular mass index, left ventricular ejection fraction, left atrial function index, diastolic dysfunction, inducible ischemia, treadmill exercise capacity.
Model 3 - Adjusted for Model 2 plus natural logarithm of NT-proBNP, natural logarithm of C-reactive protein.
In unadjusted analysis, the association between hs-cTnT and cardiovascular events was modified by sex (P=0.008 for interaction). For each doubling in hs-cTnT, women had greater increases in rates of cardiovascular events (HR 2.64, 95%CI 2.09, 3.34; P<.001) than men (HR 1.89, 95%CI 1.70, 2.10; P<.001). However, after adjustment for echocardiogram measures, sex no longer significantly modified the association between hs-cTnT and cardiovascular events (P=0.54 for interaction) (Supplemental Table 2). We found no evidence for an interaction of hs-cTnT with age, smoking, diabetes, eGFR, left ventricular ejection fraction, or left ventricular mass as predictors of cardiovascular events (P>0.05 for all).
We evaluated discrimination and reclassification with the addition of hs-cTnT. With hs-cTnT alone, the c-statistic was 0.73 (95%CI 0.69, 0.76). Compared to a baseline model of clinical risk factors (age, sex, current smoking, myocardial infarction, heart failure, revascularization, diabetes mellitus, systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, body mass index, estimated glomerular filtration rate), the addition of hs-cTnT increased the c-statistic from 0.72 (95%CI 0.66, 0.74) to 0.75 (95%CI 0.70, 0.77) (P=0.002), resulted in category-free net reclassification improvement of 33% (95%CI 19%, 49%), and resulted in integrated discrimination improvement of 3.8% (95%CI 1.7%, 6.7%) (Table 4). Compared to a baseline model of clinical risk factors plus NT-proBNP and C-reactive protein, the addition of hs-cTnT increased the c-statistic from 0.76 (95%CI 0.72, 0.79) to 0.77 (95%CI 0.74, 0.80) (P=0.08), resulted in category-free net reclassification improvement of 13% (95%CI 0.2%, 32%), and resulted in integrated discrimination improvement of 1.3% (95%CI 0.2%, 3.1%). There was no evidence of poor calibration in any model (all goodness of fit P-values > 0.10).
Table 4.
Discrimination and risk reclassification for cardiovascular events with the addition of biomarkers to a clinical risk factor model.
| c-statistic (95%CI) | Integrated Discrimination Improvement (IDI) % (95%CI) | Net Reclassification Improvement (Category-free) % (95%CI) | |
|---|---|---|---|
| Clinical Risk Factorsa | 0.72 (0.66, 0.74) | 0 (Reference) | 0 (Reference) |
| +NT-proBNP | 0.76 (0.71, 0.78)b | 6.4 (3.4, 10.3) | 48 (30, 63) |
| +CRP | 0.72 (0.66, 0.75)c | 0.4 (−0.1, 1.7) | 12 (−6, 28) |
| +hs-cTnT | 0.75 (0.70, 0.77)d | 3.8 (1.7, 6.7) | 33 (19, 49) |
| +NT-proBNP + CRP | 0.76 (0.72, 0.79)e | 6.4 (3.4, 9.8) | 49 (28, 59) |
| +NT-proBNP + CRP + hs-cTnT | 0.78 (0.74, 0.80)f | 1.3 (0.2, 3.1)g | 13 (0.2, 32)g |
Clinical risk factors of age, sex, current smoking, myocardial infarction, heart failure, revascularization, diabetes mellitus, systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, body mass index, estimated glomerular filtration rate.
P<.001 for comparison with clinical risk factor model.
P=0.57 for comparison with clinical risk factor model.
P=0.002 for comparison with clinical risk factor model.
P<.001 for comparison with clinical risk factor model.
P=0.08 for comparison with clinical risk factor, plus NT-proBNP and CRP model.
Improvement in reference to clinical risk factor, plus NT-proBNP and CRP model.
Abbreviations: CRP: C-reactive protein; NT-proBNP: NT-proBNP: amino-terminal portion of the prohormone of brain natriuretic peptide; hs-cTnT: high-sensitivity cardiac troponin T.
Conclusions
In a sample of 984 outpatients with stable CHD, we found that elevated levels of hs-cTnT were associated with structural and functional measures of heart disease, including increased left ventricular mass index, lower left ventricular ejection fraction, reduced left atrial function index, diastolic dysfunction, inducible ischemia, and poor treadmill exercise capacity. After adjustment for these and other measures, hs-cTnT remained independently predictive of secondary cardiovascular events. These findings suggest that hs-cTnT quantifies an element of risk that is not captured by existing measures of cardiovascular disease severity.
Two previous studies have evaluated the association between hs-cTnT and secondary cardiovascular events in patients with stable CHD. In a relatively low-risk clinical trial population of patients with stable CHD,5 Omland et al found that levels of troponin detected with a high-sensitivity assay for cardiac troponin T (hs-cTnT) were associated with subsequent cardiovascular events and improved risk prediction. A study by Koenig et al of patients upon discharge from inpatient cardiac rehabilitation confirmed these findings.18 Another study found that elevated levels of a different assay (high-sensitivity troponin I) were associated with secondary events in a clinical trial population of patients with stable CHD.19 Our study confirms and extends these findings in 3 important ways. First, we have found that hs-cTnT is detectable in over three-fourths of patients in a general population of outpatients with CHD. Second, we have shown that hs-cTnT is associated with cardiac structural and functional abnormalities. Third, we have demonstrated that, even after adjustment for available measures of cardiac disease severity, hs-cTnT is associated with secondary cardiovascular events in a contemporary cohort of patients with stable CHD. It is possible that hs-cTnT may be capturing more elusive disease such as microvascular ischemia, inflammation, or wall stress. Indeed, in a study of patients with stable CHD undergoing cardiac computed tomography, hs-cTnT levels were not associated with stenosis severity, but were associated with non-calcified plaque, implicating clinically silent plaque rupture and subsequent microembolism as a potential mechanism for myocardial injury resulting in hs-cTnT release.20
Additionally, our findings align with studies of hs-cTnT in both higher- and lower-risk populations. The finding that hs-cTnT was associated with heart failure and death complements previous research in higher-risk outpatients with chronic heart failure, most of whom had ischemic heart disease.21 The finding that levels of hs-cTnT below the 99th percentile for a normal population were associated with cardiovascular events also is consistent with data from prospective cohort studies of lower-risk, mostly healthy individuals.4,22,23
Since participants in this study underwent exercise treadmill testing with stress echocardiography, we were able to evaluate the associations between measures of cardiac structure and function and levels of hs-cTnT. Previous studies have shown that left ventricular mass, left ventricular ejection fraction, and left atrial size were associated with hs-cTnT levels in the general population.4,22 In addition to finding that cardiac structure was associated with hs-cTnT levels, we revealed that functional measures of cardiac disease, including left ventricular ejection fraction, left atrial function index, diastolic dysfunction, inducible ischemia, and treadmill exercise capacity, were associated with hs-cTnT levels. This suggests that hs-cTnT levels are related to both structural and functional cardiac abnormalities.
We found that the association between hs-cTnT and cardiovascular events was modified by sex in the unadjusted analysis. It is known that hs-cTnT levels vary between women and men and suspected that the variation by gender is related to heart size.24 An analysis of hs-cTnT in a prospective cohort study of participants without cardiovascular disease at baseline also noted an interaction between gender and hs-cTnT levels.23 In our study, higher hs-cTnT was associated with a higher rate of cardiovascular events in women than in men, but the interaction between sex and hs-cTnT was no longer statistically significant after adjustment for measures of cardiac structure and function. This may suggest that sex differences in the relationship between hs-cTnT and cardiovascular events could be related to sex differences in cardiac structure and function.
Our study has several limitations. First, our population included mostly urban men, thus the results may not be generalizable to other populations. The finding that sex differences in hs-cTnT modified the association with cardiovascular events, but may be related to cardiac structure and function, should be validated in populations including more women. Second, we analyzed data from the full range of hs-cTnT levels, including those levels between 5 pg/mL and 14 pg/mL where the coefficient of variation for the assay is > 10%. This limits the precision of our results within this range of values. However, the finding that participants with intermediate levels of troponin had higher event rates than those participants in the lowest tertile of hs-cTnT suggests that measurement of hs-cTnT in this range can provide prognostic information. Finally, we did not report values above the limit of blank of the assay but below the limit of detection (3-5 pg/mL), which differs from previous reports of hs-cTnT, but is more reflective of the range of values in which hs-cTnT can be detected.
In conclusion, we found that levels of hs-cTnT were associated with measures of cardiac structure and function, and that hs-cTnT was associated with cardiovascular events independent of clinical risk factors, echocardiographic measures of heart disease, exercise capacity, and biomarkers. These findings support the potential of hs-cTnT as a marker of risk in stable outpatients with CHD.
Supplementary Material
Acknowledgments
Dr. Beatty and Dr. Whooley had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Sources
Alexis Beatty is supported by Award Number F32 HL110518 from the National Heart, Lung, and Blood Institute. The Heart and Soul Study was supported by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program), the National Heart, Lung and Blood Institute (R01 HL079235), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Ischemia Research and Education Foundation, Nancy Kirwan Heart Research Fund, and Roche Diagnostics. None of these funding sources had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Financial Disclosures
This research is supported by an investigator-initiated grant from Roche Diagnostics.
References
- 1.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction: Kristian Thygesen, Joseph S. Alpert and Harvey D. White on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. European Heart Journal. 2007;28(20):2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh BPC, Rogers AM, Na B, Wu AHB, Schiller NB, Whooley MA. Prevalence and prognostic significance of incidental cardiac troponin T elevation in ambulatory patients with stable coronary artery disease: Data from The Heart and Soul Study. American Heart Journal. 2009;158(4):673–679. doi: 10.1016/j.ahj.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apple FS, Collinson PO. Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays. Clin Chem. 2012 Sep 30;58(1):54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 4.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010 Dec 8;304(22):2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009 Dec 24;361(26):2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. Jama. 2008 Nov 26;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical Validation of a High-Sensitivity Cardiac Troponin T Assay. Clinical Chemistry. 2010;56(2):254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 8.Saenger AK, Beyrau R, Braun S, et al. Multicenter analytical evaluation of a high- sensitivity troponin T assay. Clin Chim Acta. 2011 Apr 11;412(9-10):748–754. doi: 10.1016/j.cca.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Luepker RV. Case Definitions for Acute Coronary Heart Disease in Epidemiology and Clinical Research Studies: A Statement From the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 10.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012 Jul 5;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Sports Medicine Guidelines for Exercise Testing and Prescription. 6th ed. Lippincott Williams & Wilkins; Baltimore, MD: 2000. [Google Scholar]
- 13.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989 Sep-Oct;2(5):358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 14.Thomas L, Hoy M, Byth K, Schiller NB. The left atrial function index: a rhythm independent marker of atrial function. Eur J Echocardiogr. 2008 May;9(3):356–362. doi: 10.1016/j.euje.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D'Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 16.Pepe MS. Problems with risk reclassification methods for evaluating prediction models. Am J Epidemiol. 2011 Jun 1;173(11):1327–1335. doi: 10.1093/aje/kwr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepe MS, Kerr KF, Longton GM, Wang Z. Testing for improvement in prediction model performance. [6/19/2012];UW Biostatistics Working Paper Series. 2012 http://www.bepress.com/uwbiostat/paper379.
- 18.Koenig W, Breitling LP, Hahmann H, Wusten B, Brenner H, Rothenbacher D. Cardiac troponin T measured by a high-sensitivity assay predicts recurrent cardiovascular events in stable coronary heart disease patients with 8-year follow-up. Clinical Chemistry. 2012 Aug;58(8):1215–1224. doi: 10.1373/clinchem.2012.183319. [DOI] [PubMed] [Google Scholar]
- 19.Kavsak PA, Xu L, Yusuf S, McQueen MJ. High-sensitivity cardiac troponin I measurement for risk stratification in a stable high-risk population. Clin Chem. 2011 Aug;57(8):1146–1153. doi: 10.1373/clinchem.2011.164574. [DOI] [PubMed] [Google Scholar]
- 20.Korosoglou G, Lehrke S, Mueller D, et al. Determinants of troponin release in patients with stable coronary artery disease: insights from CT angiography characteristics of atherosclerotic plaque. Heart. 2011 May;97(10):823–831. doi: 10.1136/hrt.2010.193201. [DOI] [PubMed] [Google Scholar]
- 21.Latini R, Masson S, Anand IS, et al. Prognostic Value of Very Low Plasma Concentrations of Troponin T in Patients With Stable Chronic Heart Failure. Circulation. 2007;116(11):1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 22.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010 Dec 8;304(22):2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011 Apr 5;123(13):1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collinson PO, Heung YM, Gaze D, et al. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clinical Chemistry. 2012 Jan;58(1):219–225. doi: 10.1373/clinchem.2011.171082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.