Abstract
The antibacterial activity of gas discharge plasma has been studied for quiet some time. However, high biofilm inactivation activity of plasma was only recently reported. Studies indicate that the etching effect associated with plasmas generated represent an undesired effect, which may cause live bacteria relocation and thus contamination spreading. Meanwhile, the strong etching effects from these high power plasmas may also alter the surface chemistry and affect the biocompatibility of biomaterials. In this study, we examined the efficiency and effectiveness of low power gas discharge plasma for biofilm inactivation and removal. Among the three tested gases, oxygen, nitrogen, and argon, discharge oxygen demonstrated the best anti-biofilm activity because of its excellent ability in killing bacteria in biofilms and mild etching effects. Low power discharge oxygen completely killed and then removed the dead bacteria from attached surface but had negligible effects on the biocompatibility of materials. DNA left on the regenerated surface after removal of biofilms did not have any negative impact on tissue cell growth. On the contrary, dramatically increased growth was found for these cells seeded on regenerated surfaces. These results demonstrate the potential applications of low power discharge oxygen in biofilm treatments of biomaterials and indwelling device decontaminations.
Keywords: Gas discharge plasma, biofilms, biomaterials, contamination, sterilization, infections
1. INTRODUCTION
Bacteria prefer to attach to solid surfaces and grow into biofilms [1]. Biofilms are an assemblage of bacteria that are irreversibly associated with a surface and are enclosed in a matrix of primarily polysaccharides. It is estimated that over 95 % of bacteria existing in nature are found in biofilms [1]. The solid-liquid interfaces between the surfaces of various implants and body fluids/blood provide ideal environments for biofilm growth. Bacterial biofilms are found on a wide variety of indwelling medical devices. One of the most important features of biofilms is their resistant nature towards both the host’s immune system and antimicrobial treatment [2–4]. Existing antimicrobial agents at typical dosages only lead to incomplete killing of bacteria. Although a very small fraction of phenotypic variants survive antimicrobial treatment, these resistant bacteria are able to start biofilm development once the treatment is discontinued. Therefore, compared to other types of infections, medical device-associated biofilm infections are extremely difficult to treat. Systemic antibiotic treatment of biofilms can hardly be accepted, and in most cases, replacement of the prosthesis is the only remedy [3, 4]. The formation of biofilms on biomedical devices leads to increased suffering, prolonged hospital visits, rejection of implants, recurrent operations, and even death.
Plasma mediated sterilization technology has been studied for many years [5, 6]. Over the past two decades, discharge gas generated from plasma has been widely used and investigated in many industrial and medical applications for the sterilization and decontamination of medical devices from the formation of biofilms [7]. This method results in shorter treatment times along with no change in the chemical properties of the medical device when generated at lower powers in comparison to conventional experimental treatment parameters [8–10]. The excellent ability of discharge gases to cause the complete inactivation of biofilms was confirmed in recent publications [11–13, 23] and makes gas discharge plasma a more attractive approach for biomaterial sterilization. More specifically, these studies have gone into great detail regarding the bacterial inactivation ability of plasmas [14–16]. However, the antibacterial mechanism of plasma has not been fully revealed yet. In most studies, plasma mediated sterilizations have focused on discharge gases such as air and oxygen, both generated at extremely high powers (~20K watts) [14,16–19]. Although these studies prove the efficiency of antibacterial activity of plasmas generated at high powers, the severe etching effect associated with discharge gases, as we discovered in our recent study [13], represents an undesired effect in sterilization practices.
We have proven in our previous study that plasma is a very powerful and effective technique in the treatment of biofilms. Bacteria in mature biofilms are completely killed by gas discharge plasma within several minutes [13]. Further studies revealed that this plasma-mediated materials sterilization approach involved two mechanisms: 1) killing adhered bacteria and bacteria embedded in biofilms, and 2) removing bacteria (both live and dead) from the contaminated substrate through surface etching. The surface etching and bacterial killing activities of high electrical power plasmas proved to be coupled events that happened simultaneously during plasma sterilization. Since bacteria can be released from attached surfaces before they are completely killed, the etching activity of plasma represents an undesired and negative effect. Etching caused live bacteria relocation to uncontaminated areas, which may lead to contamination spreading and biomaterial device failure. In addition, strong etching effects of plasma may change the chemistry of surfaces and compromise the biocompatibility of biomaterials.
In this paper, we tested the efficiency and effectiveness of low power gas discharge plasmas for the inactivation and removal of biofilms from various material surfaces.
2. MATERIALS AND METHODS
2.1. Bacterial strains and medium
Staphylococcus aureus (penicillin resistant, ATCC 29213), a good biofilm forming S. aureus strain, and Escherichia coli (ATCC 25404) were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Staphylococcus epidermidis (NJ 9709) was from Dr. Kaplan at University of Medicine and Dentistry of New Jersey (UMDNJ). We grew staphylococcus bacteria in tryptic soy broth (TSB) supplemented with 0.2% glucose (TSBG) and E. coli in Luria Broth (LB). For each experiment, an isolated single bacterial colony was picked from an agar plate, transferred to 10–15 ml of medium, and then incubated under orbital agitation (100–150 rpm) at 37 °C for 18–24 h.
2.2. Reagents and solutions
A LIVE/DEAD staining kit was purchased from Invitrogen Life Technologies (Carlsbad, CA) for the staining of bacteria within biofilms. Also, 5% methylthiazolyldiphenyl-tetrazolium bromide, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), in phosphate buffered saline (PBS), crystal violet (CV), ethidium bromide (EB), and other reagents were all purchased from the Sigma Chemical Laboratory (St Louis, MO).
2.3. Biofilm Growth
For each experiment, an isolated single bacterial colony was picked from an agar plate, transferred to 10–15 ml of TSBG or LB medium, and then incubated under orbital agitation (100–150 rpm) at 37 °C for 18–24 h. An overnight culture of bacteria were diluted in TSBG to 2×106 cells ml−1, and then inoculated on surfaces of polyethylene terephthalate (PET) films, on silicon wafers, and on LabTek 8-well cover-glass chambers. Biofilms of 15~20 μm in thickness were formed on all tested materials within 24 hrs. At the end of incubation, the formed biofilms (about 12–18 μm in thickness) were washed with PBS to remove planktonic and loosely attached bacteria.
2.4. Biofilm assays
Widely used CV staining method in combination with the MTT based viability assay was used to assess biofilm susceptibility to discharge gases. Unlike CV staining, which is used for staining bacterial cells (both live and dead) and other macromolecules such as polysaccharides, DNA, and proteins in biofilm extracellular matrix, the MTT assay was designed for live bacteria by measuring the overall metabolic activity of bacterial cells in biofilms. There is an excellent correlation between formazan concentrations (absorbance at OD 570 nm) and CFU counting [13, 20]. Thus, CV staining was used for the quantification of biofilms (total biomass of biofilm) while MTT assay and EB staining were utilized to evaluate viability of bacteria in biofilms and DNA/polysaccharides in bioiflm extracellular matrix.
In CV staining, biofilms were stained with 0.1% (w/v) CV for 10 min. The excess dye was removed by thoroughly rinsing the plate with water. CV dye associated with biofilms was then extracted by 33% glacial acetic acid and quantified using a microplate reader by measuring solution absorbance values at 570 nm.
In the MTT assay, biofilms were incubated with MTT at 37 °C for 10 min. After washing, the purple formazan formed inside the bacterial cells was dissolved by SDS and then measured using a microplate reader by setting the detecting and reference wavelengths at 570 nm and 630 nm, respectively.
In the EB staining, EB (conc) was added into the TSBG culture medium used for biofilm growth. Distribution of EB stained DNAs in formed biofilms were visualized under confocal fluorescence microscopy by setting the excitation and emission wavelengths at 488 nm and 530 nm, respectively
2.5. Generation of gas discharge plasma
Discharge gases were generated using Plasma Prep III device (SPI Supplies) as described previously [9]. Bottled gases of oxygen, nitrogen and argon were purchased from Praxair (Keasbey, NJ) and were prepared by Cryogenic Air separation which led to a purity of >99.9%. Discharge powers were controlled in the range between 0 and 100 W with fixed gas flow rate at 2.4 ft3 h−1. Preformed biofilm samples were placed at in 6-well plates which is 6 cm away from the gas outlet.
2.6. Staining of live and dead bacteria in biofilms
Live and dead bacterial distributions in biofilms were studied by confocal laser scanning microscopy using a LIVE/DEAD staining kit as described previously [13]. Biofilms grown on LabTek 8-well cover-glass chambers were washed with PBS to remove planktonic bacteria and TSBG medium. After that LIVE/DEAD dyes in water were added and incubated for 15 minutes at room temperature. Stained live (green) and dead (red) bacteria in biofilms were visualized confocal fluorescence microscopy according to the protocol provided by the manufacture.
2.7. Scanning electron microscopy (SEM) Analysis
Preformed biofilm were treated with discharge oxygen at 60 W for a period of time. These treated samples were then coated with gold, and SEM images were taken at Auriga Modular CrossBeam workstation (Carl Zeiss, Inc.) using accelerating voltage of 2kV [21].
2.8. Biocompatibility assay performed on cultured tissue cells
Biocompatibility studies on plasma treated contaminated surfaces were conducted on MC-3T3 cells (2 × 104 cells/well), which were cultured in alpha MEM medium supplemented with 10% fetal bovine serum and incubated at 37°C in a humidified atmosphere of 5% CO2 for 48 hours. Before the assay, cells were washed with PBS three times and stained with the LIVE/DEAD staining kit for 15 min. After the 48 hours, cell images were recorded using a Zeiss LSM510 Confocal Microscope. The excitation wavelength was fixed at 488 nm and the emission wavelengths were set at 505–530 nm (for the live cells) and 560 nm (for the dead cells) [22].
3. RESULTS AND DISCUSSION
3.1. The anti-biofilm ability of low power gas discharge plasmas
It is known that discharge powers can greatly affect the antibacterial activity of plasmas by altering the types of species generated. Plasmas generated at high radio frequencies are always associated with better antibacterial activity [23]. We found in the previous study that discharge gases would have greatly decreased anti-biofilm activity when the discharge powers were set below 60 watts [13]. Sixty watts seems to be the minimal discharge power required to maintain the excellent antibacterial activity of gas discharge plasmas to bacteria in preformed biofilms. Therefore, we choose to compare the effectiveness of gas discharge plasmas in terms of biofilm inactivation and removal ability by setting the discharge power to 60 watts.
In this study, three gases with distinct chemical and physical properties, oxygen, nitrogen, and argon, were tested on mature one-day old biofilms from S. aureus. These S. aureus biofilms had typical biofilm structures (Fig. 1) and with proven resistance to antibiotics treatment [20]. Individual bacteria were protected and attached to one another by the extracellular polymeric substances secreted by the bacteria.
Fig. 1.
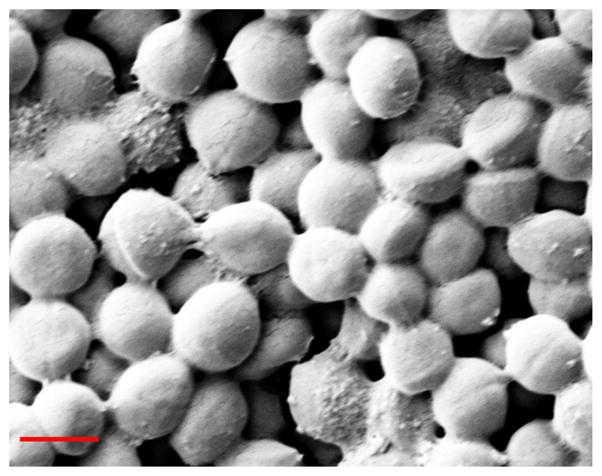
SEM image of one-day old S. aureus biofilms. Scale bar =1.0 μm
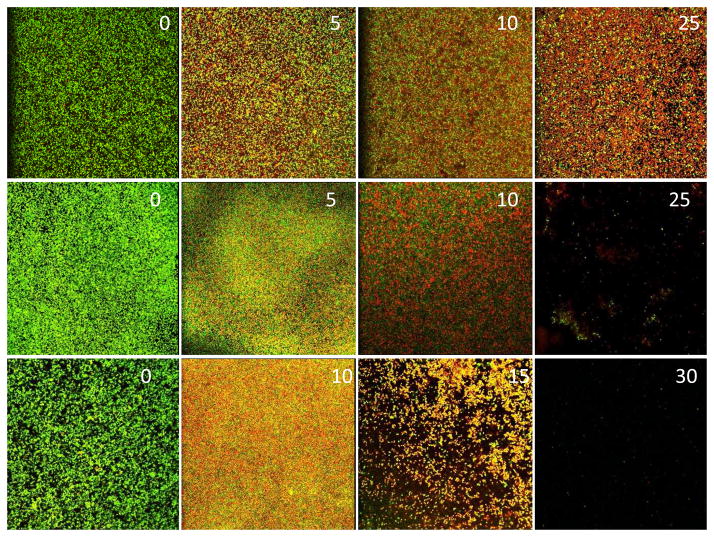
Although all three discharge gases showed good antibacterial activity, they each demonstrated different biofilm inactivation mechanisms (Fig. 2). Discharge argon and oxygen were more potent than discharge nitrogen and the majority of bacteria in biofilms were killed by discharge argon and oxygen within the first 5 minutes of treatment. However, extended exposure time (> 10 minutes) was needed for discharge nitrogen in order to achieve the same biofilm inactivation as discharge argon and oxygen. Unlike plasmas generated at high discharge powers, the etching ability of low power gas discharge plasmas was dramatically decreased and occurred only after prolonged time (>25 minutes) exposure. Discharge argon behaved differently from discharge oxygen and nitrogen. Low power discharge argon did not show any notable biofilm etching activity even after extended treatment times of 60 minutes. Although both discharge oxygen and nitrogen caused the removal of biofilms from attached surfaces, the etching effect of discharge oxygen was more profound and began at an earlier phase during plasma treatment after all the bacteria in biofilms were killed. Nearly all bacteria in S. aureus biofilms were removed from the surfaces of PET films by discharge after 25–30 minutes exposure.
Fig. 2.
Plasma mediated biofilm inactivation and removal. S. aureus biofilms on PET films treated with discharge argon (top panel), nitrogen (middle panel), and oxygen (bottom panel) generated at 60 watts for the indicated time (minutes) given in each image. Fluorescence images of biofilms were acquired using confocal microscopy. Live and dead bacteria were stained green and red, respectively, by Live/Dead staining kit.
3.2. Decontamination efficacy of low power oxygen discharge plasma
It should be noted that since bacteria in biofilms were quickly inactivated by discharge oxygen and biofilm removal occurred at the late phase of discharge oxygen treatment (Fig. 2), therefore, low power oxygen discharge plasma would have dramatically reduced risk in causing contamination spreading in comparison with high power discharge in which bacteria killing and removal happened simultaneously [9]. Therefore low power discharge oxygen is more suitable for the treatment of biofilm associated biomaterials contaminations.
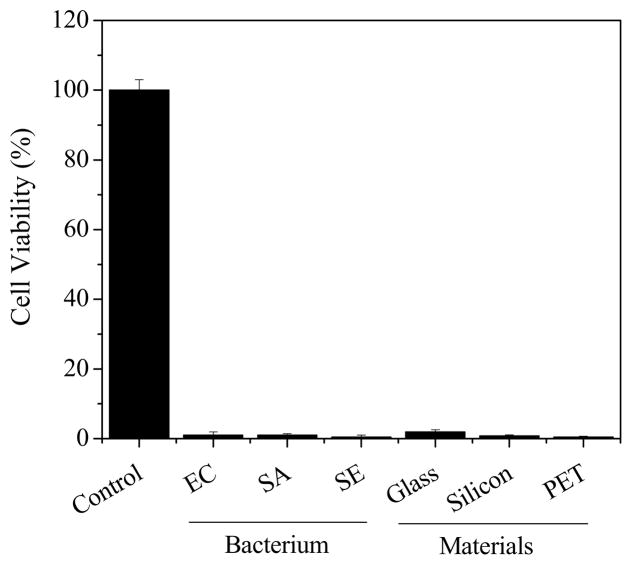
It is known that different bacteria may form biofilm with completely different extracellular matrix. Meanwhile, the chemical and physical properties of materials can also dramatically affect the bacterial adhesion and formation biofilms [15]. We found that unlike other decontamination methods, low power plasma had low selectivity and was active to biofilms from different bacteria and formed on materials with distinct chemical/physical properties (Fig. 3). This was consistent with results from previous studies [12] and confirmed the ability of plasma in biofilm inactivation/decontamination.
Fig. 3.
The ability of low power discharge oxygen in the inactivation of biofilms from different bacterial strains (SA=S. aureus; EC=E. Coli; SE=S. epidermidis) and formed on various materials (glass, silicon wafer, and PET). One-day old biofilms treated by low power (60 watts) discharge argon for 30 minutes. Live bacteria in biofilms before and after plasma treatments were estimated using MTT assay.
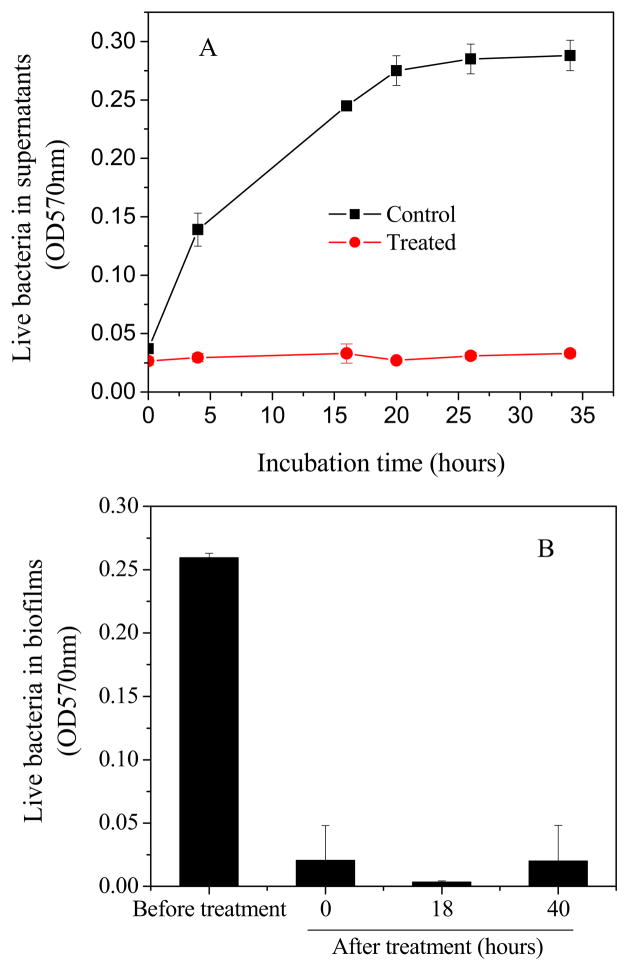
In order to further evaluate the biofilm sterilization efficiency of low power discharge oxygen, a well designed re-growth assay was conducted on the regenerated surfaces (Fig. 4). Although untreated biofilms experienced a rapid growth in the first 24h after the medium change, no bacteria or biofilm re-growth was observed in low power discharge oxygen treated biofilms even after 40 h of incubation. This is consistent with the results from Live/Dead staining assays (Fig. 2) and confirms the effectiveness of low power discharge oxygen in biofilms inactivation/sterilization.
Fig. 4.
Bacterial re-growth from 1-day old S. aureus biofilms treated by low power (60 watts) discharge argon for 30 minutes. A) Bacteria in culture supernatants measured as absorbance at 570 nm. B) live bacteria in biofilms before and after plasma treatments as measured using MTT assay.
Surprisingly, despite the efficiency of discharge oxygen in inactivating and removing bacteria from attached surface (Fig. 2), discharge oxygen treatment did not lead to a completely clean surface. Although no bacteria were found, polymer like residues from the previously removed biofilm was left behind on regenerated silicone wafer surfaces (Fig. 5). Improvement was found when the exposure times were extended, but these polymer-like materials left on the surfaces could not be completely removed even after 60 minute treatments.
Fig. 5.
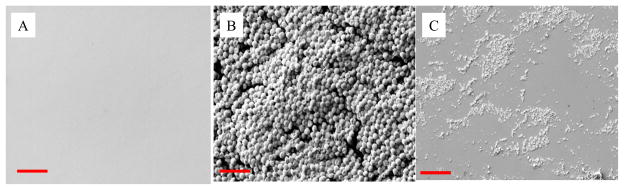
SEM images of silicone wafer surface (A), one-day old S. aureus biofilms on silicone wafer (B), and silicone wafer surface after S. aureus biofilms were removed by discharge oxygen (60 watts, 30 minutes treatment) (C). Scale bar =5.0 μm.
3.3. Biocompatibility of Regenerated Surfaces from Plasma Treatment
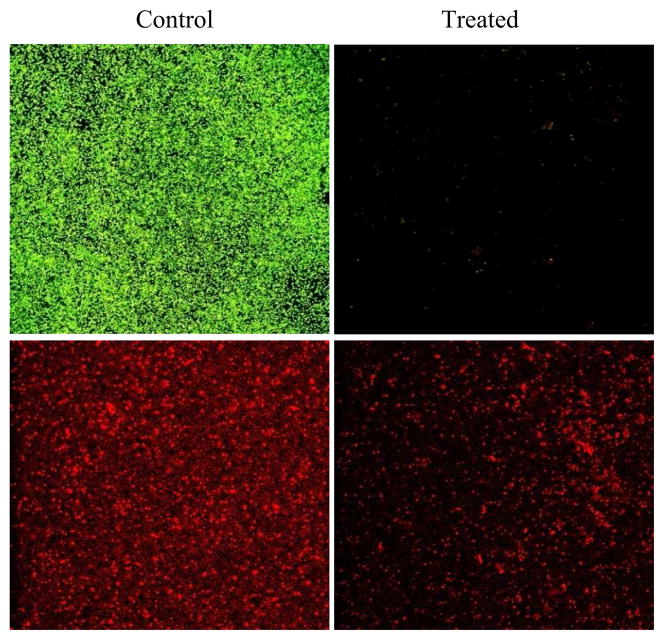
Depending on the types of bacteria, biofilms may have different extracellular polymeric substances. However, the main components of biofilm extracellular matrix are always polysaccharides [24,25]. A surface staining assay using EB revealed that the majority of the substances left on the regenerated surfaces were DNA (Fig. 6). We know that due to their fragile lipid structure, cells are more sensitive to plasma treatment than macromolecules such as DNA and proteins. Therefore, bacteria can be easily damaged, leading to cell lysis, which explains why bacteria in biofilms were effectively removed but extracellular matrix, DNA in the case of S. sureus biofilms, were left behind on the discharge oxygen treated surfaces.
Fig. 6.
Surface assays using Live/Dead (top panel) and EB staining (bottom panel). One-day old S. aureus biofilms on PET films were treated by discharge oxygen for 30 minutes at 60 watts.
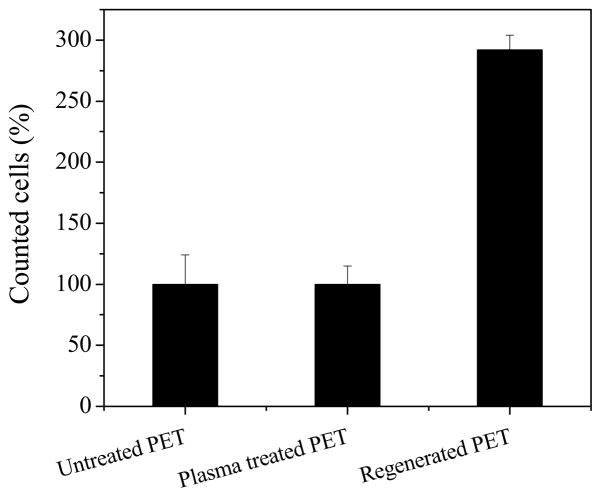
It should be pointed out that the detection of DNA on biofilm removed surfaces indicate that low power discharge oxygen treatment has limited or minimal effects on the chemistry of biomaterials. Although DNAs are biomolecules, they are different from proteins, bioactive peptides, and lipopolysaccharides (LPS). DNA has proven to be a very poor antigen and thus can hardly elicit immune responses [26]. In addition, because of its exogentic nature to mammalian cells, bacterial DNA by itself should not have any biological implications regardless they exist outside cells or is uptaken by the tissue cells. In this regard, bacterial DNA left on the regenerated surfaces will have very limited impact on the biocompatibility of biomaterials. To prove this, we tested tissue cell growth on the PET films regenerated from biofilm contaminated surfaces using low power discharge plasma. As shown in Fig. 7, tissue cells grew normally on low power discharge oxygen treated surfaces as well as non-treated surfaces. Interestingly, tissue cells on regenerated surfaces from S. aureus biofilm contaminated PET films were more healthy and grew much more rapidly (nearly three times) than the same cells on the other (control and plasma treated) PET films (Fig. 8). These results demonstrate the potentials of applying low power discharge oxygen in biofilm treatments and biomaterials and indwelling device decontaminations.
Fig. 7.
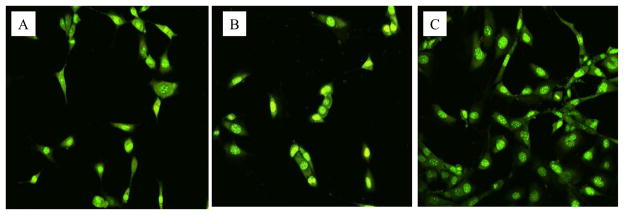
Confocal microscopy images of tissue cells (MC-3T3) grew on PET film (A), PET film treated using low power discharge oxygen (60 watts, 30 minutes) (B), and regenerated PET film from biofilm contaminated films using low power discharge oxygen (60 watts, 30 minutes) (C). Cells were cultured on PET films for two days and then stained using Live/Dead kit.
Fig. 8.
Counting of tissue cells on PET film, PET film treated using low power discharge oxygen (60 watts, 30 minutes), and regenerated PET film from biofilm contaminated surfaces using low power discharge oxygen (60 watts, 30 minutes). Cells on PET films were taken as 100%.
4. CONCLUSION
This study has confirmed the anti-biofilm ability of low power discharge oxygen on S. aureus biofilms in which antibiotic resistance has developed. Low power discharge oxygen completely killed and then removed the dead bacteria from the attached surface. This low power generated plasma also had a negligible effect on the biocompatibility of materials. DNA left behind on the regenerated surfaces did not have any negative impact on tissue cell growth. On the contrary, dramatically increased growth was found for the tissue cells seeded on these surfaces. These results demonstrate the potential application of low power discharge oxygen in biofilm treatments and biomaterials and indwelling device decontaminations.
Highlights.
We tested the anti-biofilm ability of plasma generated at different powers
Low power discharge plasma was effective in inactivating biofilms
Low power discharge oxygen showed reduced etching effect and good biocompatibility
Tissue cells grow rapidly on the regenerated surfaces from plasma treatment
Acknowledgments
This work was supported by NIH grant AI078176 and AI079608. Mr. Traba is a recipient of the Stanly Fellowship. Mr. Chen is a recipient of the Innovation and Entrepreneurship Doctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Overman PR. Biofilm: A new view of plaque. J Contempt Dent Pract. 2006;1:1–11. [PubMed] [Google Scholar]
- 2.Patel R. Biofilms and antimicrobial resistance. Clin Orthop Relat Res. 2005;437:41–47. doi: 10.1097/01.blo.0000175714.68624.74. [DOI] [PubMed] [Google Scholar]
- 3.NNIS System. National Nosocomial Infections Surveillance vative and multidisciplinary approaches for prophy- (NNIS) System Report, data summary from January 1992 through June 2003. Am J Infect Control. 2003;8:481–98. doi: 10.1016/j.ajic.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein RA. Biofilm Elimination on Intravascular Catheters: Important Considerations for the Infectious Disease Practitioner. Clin Infect Dis. 2011;8:1038–1045. doi: 10.1093/cid/cir077. [DOI] [PubMed] [Google Scholar]
- 5.Morris AD, McCombs GB, Akan T, Hynes W, Laroussi M, Tolle SL. Cold plasma technology: bactericidal effects on Geobacillus stearothermophilus and Bacillus cereus microorganisms. J Dent Hyg. 2009;83:55–61. [PubMed] [Google Scholar]
- 6.Sureshkumar A, Sankar R, Mandal M, Neogi S. Effective bacterial inactivation using low temperature radio frequency plasma. Int J Pharm. 2010;396:17–22. doi: 10.1016/j.ijpharm.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 7.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 8.Moisan M, Barbeau J, Crevier MC, Pelletier J, Philip N, Saoudi B. Plasma sterilization. Methods and mechanisms. Pure Appl Chem. 2002;74:349–358. [Google Scholar]
- 9.Lademann O, Kramer A, Richter H, Patzelt A, Meinke MC, Czaika V, et al. Skin disinfection by plasma-tissue interaction: comparison of the effectivity of tissue-tolerable plasma and a standard antiseptic. Skin Pharmacol Physiol. 2011;24(5):284–288. doi: 10.1159/000329913. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrievska S, Petit A, Doillon CJ, Epure L, Ajji A, Yahia L, et al. Effect of sterilization on non-woven polyethylene terephthalate fiber structures for vascular grafts. Macromol Biosci. 2011;11(1):13–21. doi: 10.1002/mabi.201000268. [DOI] [PubMed] [Google Scholar]
- 11.Joshi SG, Paff M, Friedman G, Fridman G, Fridman A, Brooks AD. Control of methicillin resistant Staphylococcus aureus in planktonic form and biofilms: a biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am J Infect Control. 2010;38:293–301. doi: 10.1016/j.ajic.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Joaquin J, Kwan C, Abramzon N, Vandervoort K, Brelles-Marino G. Is gas Discharge plasma a new solution to the old problem of biofilm inactivation? Microbiology. 2009;155:724–732. doi: 10.1099/mic.0.021501-0. [DOI] [PubMed] [Google Scholar]
- 13.Traba C, Liang JF. Susceptibility of Staphylococcus aureus biofilms to reactive discharge gases. Biofouling. 2011;27:763–772. doi: 10.1080/08927014.2011.602188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia LH. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm. 2001;226:1–21. doi: 10.1016/s0378-5173(01)00752-9. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Olivares-Navarrete R, Baier RE, Meyer AE, Tannenbaum R, Boyan BD, et al. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 2012;5:1966–75. doi: 10.1016/j.actbio.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laroussi M, Leipold F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom. 2004;233:81–86. [Google Scholar]
- 17.Min J, Lee CW, Gu MB. Gamma-radiation dose-rate effects on DNA damage and toxicity in bacterial cells. Radiat Environ Biophys. 2003;3:189–192. doi: 10.1007/s00411-003-0205-8. [DOI] [PubMed] [Google Scholar]
- 18.Moreau S, Moisan M, Barbeau J, Pelletier J, Ricard A. Using the flowing afterglow of a plasma to inactivate bacillus subtilis spores: Influence of the operating conditions. J Appl Phys. 2000;88(2):1166–1174. [Google Scholar]
- 19.Walsh JL, Iza F, Kong MG. Atmospheric glow discharges from the high-frequency to very high-frequency bands. Appl Phys Lett. 2008;93:251502. [Google Scholar]
- 20.Kharidia R, Liang JF. The activity of a small lytic peptide PTP-7 on Staphylococcus aureus biofilms. J Microbiol. 2011;49(4):663–668. doi: 10.1007/s12275-011-1013-5. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Patrone N, Liang JF. Biomacromolecules. 2012 doi: 10.1021/bm301106p. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Tu Z, Voloshchuk N, Liang JF. J Pharm Sci. 2012;101:1508–1517. doi: 10.1002/jps.23043. [DOI] [PubMed] [Google Scholar]
- 23.Traba C, Chen L, Azzam R, Liang JF. Sterilization of sophisticated biomaterials and biomedical devices using discharge argon. Biomaterials. (Forthcoming) [Google Scholar]
- 24.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay D, von Holy A. Bacterial biofilms within the clinical setting: what healthcare professionals should know. J Hosp Infect. 2006;64(4):313–25. doi: 10.1016/j.jhin.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Habib S, Moinuddin, Ali R. Acquired antigenicity of DNA after modification with peroxynitrite. Int J Biol Macromol. 2005;35(3–4):221–5. doi: 10.1016/j.ijbiomac.2005.02.005. [DOI] [PubMed] [Google Scholar]