In human immunodeficiency virus (HIV)/tuberculosis–coinfected patients, coadministration of efavirenz (EFV) and rifampin-based tuberculosis therapy was associated with a trend toward higher, not lower, EFV trough concentrations compared to EFV alone. Neither weight ≥50 kg nor ≥60 kg was associated with decreased HIV virologic suppression.
Keywords: HIV/AIDS, tuberculosis, efavirenz, rifampin, pharmacokinetics
Abstract
Background. Rifampin (RIF) upregulates CYP 450 isoenzymes, potentially lowering efavirenz (EFV) exposure. The US EFV package insert recommends an EFV dose increase for patients on RIF weighing ≥50 kg. We conducted a pharmacokinetic study to evaluate EFV trough concentrations (Cmin) and human immunodeficiency virus (HIV) virologic suppression in patients on EFV (600 mg) and RIF-based tuberculosis treatment in the multicenter randomized trial (ACTG A5221).
Methods. EFV Cmin was measured 20–28 hours post–EFV dose at weeks 4, 8, 16, 24 on-RIF and weeks 4, 8 off-RIF. Results were evaluated with 2-sided Wilcoxon rank-sum, χ2, Fisher exact tests and logistic regression (5% type I error rate).
Results. Seven hundred eighty patients received EFV; 543 provided ≥1 EFV Cmin. Median weight was 52.8 kg (interquartile range [IQR], 48.0–59.5), body mass index 19.4 kg/m2 (IQR, 17.5–21.6), and age 34 years (IQR, 29–41); 63% were male, 74% black. Median Cmin was 1.96 µg/mL on-RIF versus 1.80 off-RIF (P = .067). Cmin were significantly higher on-RIF versus off-RIF in blacks (2.08 vs 1.75, P = .005). Weight ≥60 kg on-RIF, compared to <60 kg, was associated with lower EFV Cmin (1.68 vs 2.02, P = .021). However, weight ≥60 kg was associated with more frequent HIV RNA < 400 copies/mL at week 48, compared to weight <60 kg (81.9% vs 73.8%, P = .023).
Conclusions. EFV and RIF-based tuberculosis therapy coadministration was associated with a trend toward higher, not lower, EFV Cmin compared to EFV alone. Patients weighing ≥60 kg had lower median EFV Cmin versus those <60 kg, but there was no association of higher weight with reduced virologic suppression. These data do not support weight-based dosing of EFV with RIF.
Tuberculosis is the leading cause of death in human immunodeficiency virus (HIV)–coinfected individuals worldwide. Concomitant treatment of HIV and tuberculosis is required to reduce the risk of death and HIV progression [1–3]. However, antiretroviral therapy (ART) can be complicated by drug–drug interactions with tuberculosis medications, particularly rifampin (RIF), which induces cytochrome (CYP) P450 enzymes. Efavirenz (EFV) is recommended as a component of first-line ART in HIV/tuberculosis coinfection [4] and is metabolized primarily through hepatic cytochrome P450 CYP2B6. EFV pharmacokinetic exposure is significantly increased by several genetic polymorphisms in CYP2B6 [5–9]. Slow-metabolizing CYP2B6 alleles are present in all populations at varying frequencies, with 516G→T (rs3745274) most frequent with African or Asian ancestry, 983T→C (rs28399499) most frequent with African ancestry, and 15582C→T (rs4803419) most frequent with Asian or European ancestry [6, 10–12]. In addition, patients taking multidrug therapy for tuberculosis also receive isoniazid, an inhibitor of CYP2A6 and other isoenzymes [13], potentially impacting EFV concentrations as well as rifampin. CYP2A6 is an alternative pathway for EFV elimination that may be of particular importance in patients with slow EFV metabolizer phenotypes [14, 15].
The appropriate EFV dose for HIV-infected patients receiving concomitant RIF continues to be debated because available data are conflicting. Traditional pharmacokinetic (PK) studies enrolling healthy volunteers in the United States combined with limited data from patients coinfected with HIV and tuberculosis have demonstrated a 30% decrease in plasma EFV area under the concentration time curve (AUC) with RIF coadministration [16–18]. In contrast, several larger, population-based studies in patients from primarily resource-limited settings indicate either that there is no effect of RIF on EFV concentrations [7, 19] or that RIF coadminstration increases EFV concentrations in African patients [20, 21]. Focusing on intensive PK data gathered primarily in developed settings, the US Food and Drug Administration (FDA) recently approved a revised EFV package insert to recommend that EFV be increased from a standard daily dose of 600 mg to 800 mg for patients taking concomitant RIF who weigh >50 kilograms [22], whereas the British HIV/tuberculosis treatment guidelines recommend EFV dose increase for those weighing >60 kg [23]. In contrast, based on clinical trial and observational data, the World Health Organization does not recommend increased EFV dosing based on weight in tuberculosis patients [4, 24]. Determining the appropriate dosing of EFV during tuberculosis treatment is essential because very high EFV concentrations may increase drug-related toxicity, while very low EFV concentrations may result in treatment failure with emergence of drug-resistant HIV [25, 26].
To evaluate the relationship between weight, EFV concentrations, and HIV RNA suppression, we conducted a population-based pharmacokinetic analysis in the STRIDE (A Strategy Study of Immediate Versus Deferred Initiation of Antiretroviral Therapy for AIDS Disease-Free Survival in HIV-Infected Persons Treated for Tuberculosis with CD4 < 250 Cells/mm3) study participants. The STRIDE study (A5221) was an open-label, randomized study comparing ART started earlier (within 2 weeks of tuberculosis treatment initiation) versus later (8–12 weeks after tuberculosis treatment initiation) in HIV-infected participants receiving RIF-based tuberculosis treatment [2]. The impact of weight ≥50 kg and ≥60 kg on EFV concentrations was evaluated, given the differing weight cutoffs for recommended EFV dose [22, 23, 27].
METHODS
Study Population
The STRIDE study enrolled HIV-infected ART-naive participants with CD4+ cell counts <250 cells/mm3 who had confirmed or probable tuberculosis and randomized them to early ART initiation (within 2 weeks of tuberculosis treatment start) versus delayed ART initiation (between 8 and 12 weeks of tuberculosis treatment start). All eligibility criteria for the STRIDE study are described in detail elsewhere [2]. The current PK study population consisted of STRIDE participants with 1 or more EFV Cmin values available for analysis. Participants received 600 mg of EFV daily (Stocrin, donated by Merck), with no dose adjustment for weight and a fixed-dose combination of emtricitabine 200 mg daily and tenofovir disoproxil fumarate 300 mg daily (Truvada, donated by Gilead Sciences). The study protocol was approved by institutional review board or ethics committee at each participating site. The National Institutes of Health funded this study and provided study oversight.
Study Evaluations
Single-trough EFV concentrations (Cmin) were measured at ART treatment weeks 4, 8, 16, and 24, and at weeks 4 and 8 after the discontinuation of RIF. Cmin was obtained 20–28 hours after EFV administration in participants with self-report of no missed EFV or RIF doses (when on RIF) for the prior 3 days. Fasting was not required; however, EFV dosing was recommended on an empty stomach. EFV was measured using a validated high-performance liquid chromatography methodology with a lower limit of quantitation of 0.1 µg/mL [28]. Therapeutic EFV levels were prespecified as ≥1 µg/mL and supratherapeutic levels as >4 µg/mL. Plasma HIV-1 RNA (Roche Amplicor assay) had a lower limit of detection of 400 copies/mL.
Statistical Analysis
HIV virologic suppression was defined as participants with plasma HIV RNA <400 copies/mL at study week 48, with those missing week 48 RNA, lost to follow-up, dead, or with RNA >400 were classified as not virologically suppressed. On-RIF and/or off-RIF EFV Cmin values were available from participants at multiple timepoints. Primary PK endpoints were within-participant averages of EFV Cmin values at all available on-RIF and off-RIF collections; however, some comparisons are presented as week-specific Cmin values. Continuous variables were summarized using the median and first and third quartiles (IQR). Between-group comparisons of continuous endpoints used Wilcoxon rank-sum tests (signed-rank for paired data), and percents used Pearson χ2 test (Fisher exact test for small sample). Logistic regression was used to evaluate predictors of binary outcomes. All tests and confidence intervals (CIs) were 2-sided with a 5% type 1 error rate.
RESULTS
From July 2007 through June 2010, 543 STRIDE participants took part in the PK study by contributing 1 or more EFV Cmin concentrations; 505 with on-RIF values, and 362 with off-RIF values. Of PK study participants, 63% were male, median age 34 (IQR, 29–41), 74% black, 20% Hispanic, 5% non-Hispanic white, and 1% Asian (see Table 1). Seventy percent of participants were enrolled from sub-Saharan Africa, 25% from South America (Brazil and Peru), 3% from Haiti, and 1% each from Thailand and the United States, with a total of 11 countries represented. Median weight was 52.8 kg (IQR, 48.0–59.5), and median body mass index (BMI) was 19.4 kg/m2 (IQR, 17.5–21.6). Median weight gain at week 48 was 8.5 kg (IQR, 4.5–12.7), among 494 participants with PK data and weight at both day 0 and week 48.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Total (n = 543) |
|---|---|
| Sex | |
| Female | 203 (37%) |
| Male | 340 (63%) |
| Age at study entry, median (IQR) | 34 (29–41) |
| Race/ethnicity | |
| White non-Hispanic | 26 (5%) |
| Black non-Hispanic | 403 (74%) |
| Hispanic, regardless of race | 108 (20%) |
| Asian/Pacific Islander | 5 (1%) |
| Unknown/missing | 1 (0%) |
| Country | |
| Botswana | 24 (4%) |
| Brazil | 95 (17%) |
| Haiti | 18 (3%) |
| Kenya | 31 (6%) |
| Malawi | 120 (22%) |
| Peru | 39 (7%) |
| South Africa | 175 (32%) |
| Thailand | 4 (1%) |
| United States | 7 (1%) |
| Uganda | 27 (5%) |
| Zimbabwe | 3 (1%) |
| Baseline weight, kg, median (IQR) | 52.80 (48.00–59.50) |
| Baseline body mass index, median (IQR) | 19.37 (17.51–21.61) |
| Entry CD4+ cells/mm3, median (IQR) | 80 (36–140) |
| Entry HIV RNA copies, log10/mL, median (IQR) | 5.41 (4.93–5.79) |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
EFV Exposure During and After RIF Administration
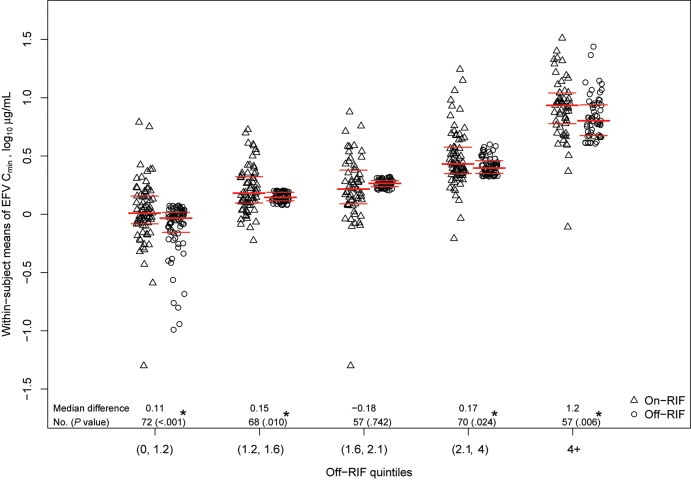
Median EFV Cmin concentration (ie, within-participant average) on-RIF was 1.96 µg/mL (IQR, 1.24–3.79 µg/mL), which was not significantly different from 1.80 µg/mL (IQR, 1.26–2.63 µg/mL) off-RIF (P = .067). At least 1 on-RIF and 1 off-RIF concentration was available for 324 participants. For these participants, the median within-participant difference in EFV concentration, on-RIF minus off-RIF, was 0.12 µg/mL (IQR, −0.29 to 0.97 µg/mL; range, −8.89 to 24.33 µg/mL; Wilcoxon signed-rank P < .001). These participants were divided into quintiles based on their off-RIF EFV concentrations to evaluate the impact of rifampin coadministration across the spectrum of EFV exposure and metabolizer phenotypes (Figure 1). In all but the middle quartile, on versus off within-participant differences were significant, with on-RIF concentrations higher than off-RIF concentrations. When restricted to the 91 participants with both week 24 (on-RIF) and week 8 (off-RIF), medians of week-specific EFV Cmin values were 1.81 µg/mL (on-RIF) versus 1.70 µg/mL (off-RIF); in a paired analysis, the median within-participant difference was 0.16 µg/mL (IQR, –0.36 to 1.07 µg/mL; Wilcoxon signed-rank P = .012). EFV concentrations were similar at week 4 (1.79 µg/mL) compared to week 8 (1.80 µg/mL) after RIF discontinuation.
Figure 1.
Statistical summaries of within-participant mean efavirenz (EFV) trough concentrations (Cmin), log10 µg/mL. EFV Cmin from 324 participants with at least 1 on-rifampin (RIF; open triangles) and off-RIF (open circles) Cmin values. EFV Cmin from participants with EFV Cmin from multiple timepoints have values summarized as the within-participant mean EFV concentration on-RIF and off-RIF. Heavy horizontal lines indicate medians; light horizontal lines indicate Q1 and Q3. P values are obtained from a Wilcoxon signed-rank test of within-participant differences, on-RIF minus off-RIF EFV Cmin (within off-RIF quintile). *Statistically significant difference (P ≤ .05). Abbreviations: Cmin, trough concentrations; EFV, efavirenz; RIF, rifampin.
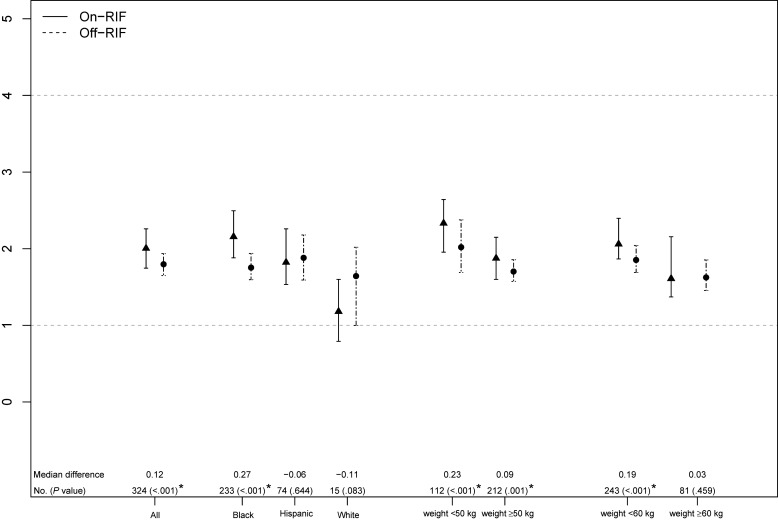
Figure 2.
Comparison of on- and off-rifampin (RIF) efavirenz (EFV) trough concentrations (Cmin) by race/ethnicity and by weight. On-RIF (closed triangle, solid line) and off-RIF (closed circle, dashed line) participant-specific mean EFV Cmin are presented as medians and 95% confidence intervals (CIs) around the medians. Data from 324 participants with both on- and off-RIF EFV Cmin values are plotted. The median within-participant on-RIF versus off-RIF EFV Cmin difference, number of participants contributing to each CI and a Wilcoxon signed-rank P value is shown. *Statistically significant difference (P ≤ 0.05). Two Asian male participants were omitted from the by-race/ethnicity comparison. Horizontal dashed lines at 1 and 4 µg/mL mark the commonly cited thresholds for sub- and supraoptimal EFV Cmin. Abbreviation: RIF, rifampin.
Black participants had significantly higher median EFV Cmin on-RIF compared to off-RIF (2.08 versus 1.75 µg/mL, P = .005). Conversely, there was a trend toward lower EFV concentrations on-RIF versus off-RIF in Hispanic and white participants (Figure 2). On RIF, EFV Cmin was lower in those with baseline weight ≥60 kg versus <60 kg (1.68 versus 2.02 µg/mL, P = .021), with no significant difference in Cmin in those with weight ≥50 kg versus <50 kg (1.86 versus 2.08 µg/mL, P = .087).
Evaluating participants with at least 1 on-RIF and off-RIF EFV Cmin, female participants had significantly higher the median on-RIF EFV Cmin versus off-RIF (2.37 versus 1.83 µg/mL, P < .001) while in men, the difference in on-RIF versus off-RIF Cmin was not statistically significant (1.87 versus 1.75 µg/mL, P = .018). When stratifying by weight ≥60 kg versus <60 kg, female participants had higher EFV levels on-RIF versus off-RIF in both weight categories. Male participants had higher EFV Cmin on-RIF in those <60 kg but slightly higher EFV concentrations off-RIF in those ≥60 kg.
EFV Levels Outside the Therapeutic Range
One or more time points with EFV Cmin <1 µg/mL occurred in 27.3% of participants during RIF coadministration versus 26.2% off-RIF (P = .723). Weight ≥50 kg was associated with a trend toward having all available EFV Cmin <1 µg/mL (13.6% versus 8.1%, P = .07), but not with having 1 or more EFV Cmin <1 µg/mL (29.5% vs 33%, P = .4). Weight ≥60 kg was significantly associated with having all available EFV trough concentrations <1 µg/mL (17.5% vs 9.9%, P = .023), but not with having 1 or more EFV Cmin <1 µg/mL (30.8% versus 26.2%, P = .324). A total of 19.6% (99/505) of participants had all available EFV levels above the therapeutic range (>4 µg/mL) during RIF coadminstration versus 18.8% (68/362) off-RIF (P = .763). However, a significantly higher proportion of black participants had all available EFV Cmin >4 µg/mL compared to whites or Hispanics, both during RIF coadminstration (22.9% versus 3.9% vs 12.3%, respectively, P = .004) and off-RIF (20.8% versus 0.0% vs 15.8%, P = .090).
EFV Exposure Association With Toxicity
Of the PK study participants, 6 of 543 (1.1%) discontinued EFV and replaced it with an alternate ART agent due to toxicity of any grade attributed to EFV; 1 of 6 toxicity changes was due to neuropsychiatric events, assessed by patient self-report and nursing evaluation. No formal neuropsychiatric scale or structured testing was administered. Neither any nor all EFV Cmin values >4 µg/mL were associated with EFV discontinuation. Forty-six of 780 (5.9%) STRIDE participants who initiated EFV experienced grade 3 or 4 neuropsychiatric adverse events. EFV Cmin >4 µg/mL was not significantly associated with occurrence of grade 3 or higher neurologic adverse events.
HIV Virologic Suppression
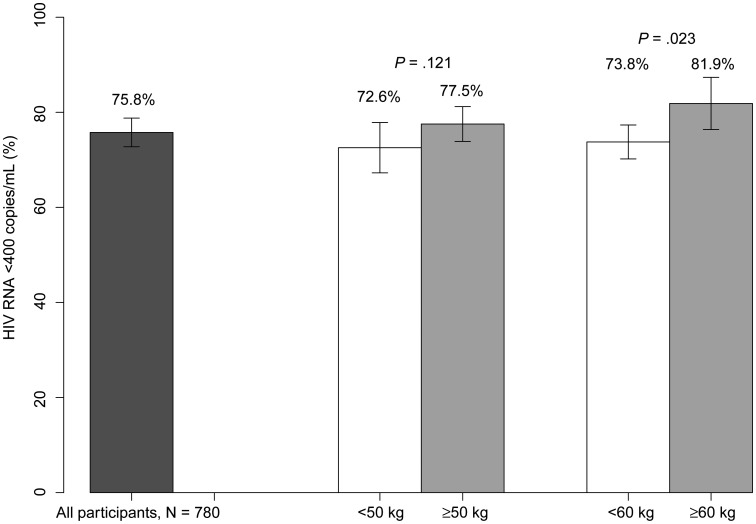
Of the 780 STRIDE participants who received EFV-based ART, 75.8% had HIV RNA <400 copies/mL at study week 48. A higher proportion of participants weighing ≥60 kg attained week 48 HIV suppression than those <60 kg (81.9% vs 73.8%, respectively; P = .023), with no significant difference in HIV suppression in those weighing ≥50 kg versus <50 kg (77.5% versus 72.6%, respectively; P = .121; Figure 3). When restricted to the 505 participants with EFV Cmin available on-RIF, the findings were similar, with 80.4% attaining RNA suppression at week 48, and a comparable proportion of those weighing ≥60 attaining suppression compared to <60 kg (85.0% vs 79.0%, P = .146). In univariate logistic regression, virologic suppression was associated with higher on-RIF EFV Cmin when expressed on the log scale, where differences at the lower end of the scale are emphasized (odds ratio [OR], 1.34; 95% CI, 1.09–1.65), higher weight (OR, 1.22 per 10 kg; 95% CI, 1.02–1.46 per 10 kg), as was higher BMI (OR, 1.08 per kg/m2; 95% CI, 1.03–1.14 per kg/m2). Using backward elimination in multivariate models, EFV Cmin and weight were jointly statistically significant; BMI was not significant when EFV Cmin was in the model. To take into account weight gain during study, weight ≥60 kg at study week 48 was associated with 90.1% suppression, compared weight <60 kg with 84.1% (P = .046). Having any or all on-RIF EFV Cmin <1 μg/mL was associated with lower likelihood of virologic suppression: OR, 0.57 (95% CI, .36–.91) and OR, 0.49 (95% CI, .26–.91), respectively.
Figure 3.
Human immunodeficiency virus RNA suppression at week 48, by weight. P values are obtained from a Pearson χ2 test. Missing RNA and participants lost to follow-up are considered not suppressed. Abbreviation: HIV, human immunodeficiency virus.
DISCUSSION
This study demonstrates that coadministration of EFV with RIF-based multidrug tuberculosis treatment in HIV/tuberculosis-coinfected patients did not lead to a reduction of EFV concentrations, compared to EFV levels in the absence of RIF, but rather was associated with a trend toward increased EFV exposures. Notably, in black participants, EFV Cmin was statistically significantly higher during RIF administration, compared to EFV Cmin after RIF discontinuation; a finding also found for patients with drug measurements available for all requested PK study visits. This paradoxical increase in EFV concentrations with concomitant RIF as part of multidrug tuberculosis therapy had previously been reported in black patients [18, 19]. RIF given with isoniazid and other antituberculosis agents appears to reduce EFV clearance [21], particularly in patients exhibiting CYP2B6 genetic polymorphisms associated with slow efavirenz metabolism (ie, CYP2B6 516 G → T) [20]. Interestingly, as demonstrated in Figure 1, the increase in EFV levels on-RIF was most pronounced in patients with the highest EFV concentrations when EFV was given alone (slow metabolizers). Patients characterized as slow metabolizers may be the most susceptible to paradoxical increases in EFV exposure with RIF coadministration due to possible metabolic inhibition of alternate metabolic pathways including CYP2A6 by isoniazid [14, 15, 20, 21]. CYP2B6 genotyping for the STRIDE pharmacokinetic substudy participants is planned for future analysis.
In our study, weight ≥60 kg at study entry was associated with lower on-RIF EFV concentrations with a higher proportion of patients (18%) exhibiting EFV troughs consistently <1 µg/mL. However, of participants weighing ≥60 kg, 69% still attained EFV concentrations ≥1 µg/mL for all measured time points. Importantly, weight ≥50 or ≥60 kg was not associated with decreased ART efficacy as measured by virologic suppression, suggesting that higher weight does not jeopardize EFV efficacy when coadministered with RIF, even in the context of lower EFV concentrations for some patients. To the contrary, baseline weight ≥60 kg was associated with significantly increased HIV RNA suppression compared to weight <60 kg; a finding potentially explained in that higher weight corresponds with less advanced HIV/tuberculosis disease and better nutritional status, 2 factors associated with better clinical outcomes with ART [29–31]
The current EFV package insert recommends weight-based EFV dosing, with an increase to 800 mg for patients weighing >50 kg and taking RIF. These recommendations largely stem from intensive PK evaluations carried out in healthy volunteers or small numbers of HIV/tuberculosis-coinfected patients from European settings. The STRIDE PK data, representing 543 coinfected patients from 4 continents, do not support this recommendation, nor does the growing body of literature demonstrating excellent clinical outcomes with standard 600 mg daily EFV dosing in RIF-treated tuberculosis patients [32–35], equivalent to outcomes attained in EFV-treated patients without tuberculosis [7, 36]. Furthermore, several studies comparing EFV 600 mg to 800 mg daily in the setting of RIF-based tuberculosis treatment have not demonstrated a virologic benefit in terms of improved HIV RNA suppression with EFV 800 mg [37, 38].
The recommended weight-based dose increase of EFV has 2 important potential downsides. First, although not seen in this study, EFV concentrations >4 µg/mL have been associated with increased central nervous system toxicity in several studies [6, 7, 39], as has EFV dosing of 800 mg daily [40]. Therefore, weight-based dosing may increase the risk of EFV toxicity without improving clinical outcomes. Second, weight-based dosing will increase the complexity of ART provision for tuberculosis patients as well as the cost, a critical consideration for resource-limited programs that are already burdened by the challenges to provide integrated care for HIV/tuberculosis-coinfected patients and for prompt initiation of ART in patients with tuberculosis.
This study was conducted in a population that was 74% black and 20% Hispanic, with only 5% white participants, limiting the ability to address the impact of RIF coadminstration with EFV in white populations. However, in the United States, in 2010, 85% of tuberculosis cases overall occurred in nonwhite populations [41], and globally HIV prevalence in new tuberculosis infection is highest in regions of sub-Saharan Africa, South America, and Southeast Asia, regions with substantial non-white populations [42]. Thus, it is important to evaluate EFV efficacy in RIF-treated tuberculosis patients from different racial and ethnic backgrounds.
This was a population PK analysis with sparse sampling, with not all STRIDE participants providing EFV Cmin at all timepoints. Participants were not required to be fasting for EFV Cmin evaluations; however, the median time from last meal was 3.45 hours (IQR = 2.25, 5.33; n = 1847 PK visits) indicating that EFV was taken on an empty stomach by the majority of participants. Adherence to EFV and RIF in the 3 days prior to PK collection was by self-report. However, a substantial number of participants (543) provided at least 1 EFV Cmin, and 91 provided samples at the last on- and off-RIF PK evaluations. Restricting the analysis to participants who provided all required PK analyses did not alter the study results. Although this PK analysis was not conducted in a strictly controlled inpatient environment with multiple samples per participant, the study provides important insight on the real-world impact of RIF coadministration on both EFV levels and HIV RNA suppression in a large number of HIV/tuberculosis-coinfected patients from diverse geographic settings. To date, the data used to inform the US package labeling on EFV dosing in tuberculosis coinfection comes from healthy volunteers and a very limited number of HIV-tuberculosis coinfected patients [43].
In conclusion, this study demonstrates that EFV-based ART, with standard EFV dosing, can be coadministered with rifampin with excellent HIV RNA outcomes, including in patients weighing >50 kg at baseline. EFV levels were paradoxically increased during rifampin coadminstration in this mostly nonwhite population. These data do not support weight-based dose increase of EFV during rifampin-based tuberculosis treatment.
Notes
Acknowledgments. We thank the study participants, the site principal investigators, and staff for their exceptional efforts to conduct the study, coordinate efforts with the in-country tuberculosis control programs, and help build the capacity of integrated HIV/tuberculosis services; the data managers, Carol Suckow, BSN, and Lynne Jones, BS; the DAIDS protocol pharmacist, Ana Martinez, RPh; the University of California, San Francisco AIDS Clinical Trials Group (ACTG) Pharmacology Specialty laboratory supervisor Patty Lizak; the field representative, Janet Nicotera, RN, BSN; the study's laboratory technologist, Patty Anthony, BS, CLS; the laboratory data coordinator, Travis Behm, BS; and the community representative, Martha Tholanah Mensah-King.
Author contributions. A. F. L., S. S., I. M. S., P. I., E. H., D. V. H., and F. A. are members of the protocol study team and were involved in study design and conduct. C. A. B. participated in study monitoring and provided ACTG leadership. F. M. conducted the PK assays. S. L. R. and D. L. were responsible for statistical analysis. All authors contributed to manuscript preparation.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (award number U01AI068636) and the Statistical and Data Management Center (UM1 AI068634) funded by the National Institute of Allergy and Infectious Diseases. Antiretroviral medications were donated by Gilead Sciences and Merck Pharmaceuticals.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Potential conflicts of interest. A. F. L., S. L. R., D. L., P. I., E. H., S. S., C. A. B., and D. V. H. have received research grant support to their institutions to support this work and to support travel to study-related meetings. A. F. L. has received research grant support to her institution from Cepheid. I. M. S. is on the advisory board for Mylan Pharmaceuticals. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. New Engl J Med. 2011;365:1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. Geneva, Switzerland: WHO: 2012. [PubMed] [Google Scholar]
- 5.Rotger M, Tegude H, Colombo S, et al. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther. 2007;81:557–66. doi: 10.1038/sj.clpt.6100072. [DOI] [PubMed] [Google Scholar]
- 6.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–400. [PubMed] [Google Scholar]
- 7.Cohen K, Grant A, Dandara C, et al. Effect of rifampicin-based antitubercular therapy and the cytochrome P450 2B6 516G>T polymorphism on efavirenz concentrations in adults in South Africa. Antivir Ther. 2009;14:687–95. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Sonnerborg A, Rane A, et al. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet Genomics. 2006;16:191–8. doi: 10.1097/01.fpc.0000189797.03845.90. [DOI] [PubMed] [Google Scholar]
- 9.Grady BJ, Ritchie MD, Acosta EP, et al. Genome wide association study (GWAS) of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group (ACTG) protocols. 2012 doi: 10.1097/FPC.0b013e32835a450b. 19th Conference on Retroviruses and Opportunistic Infections, Seattle, WA,. Abstract 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King J, Aberg JA. Clinical impact of patient population differences and genomic variation in efavirenz therapy. AIDS. 2008;22:1709–17. doi: 10.1097/QAD.0b013e32830163ad. [DOI] [PubMed] [Google Scholar]
- 11.Kwara A, Lartey M, Sagoe KW, Rzek NL, Court MH. CYP2B6 (c.516G–>T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br J Clin Pharmacol. 2009;67:427–36. doi: 10.1111/j.1365-2125.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran G, Hemanth Kumar AK, Rajasekaran S, et al. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob Agents Chemother. 2009;53:863–8. doi: 10.1128/AAC.00899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura Y, Kurata N, Sakurai E, Yasuhara H. Inhibitory effect of antituberculosis drugs on human cytochrome P450-mediated activities. J Pharmacol Sci. 2004;96:293–300. doi: 10.1254/jphs.fp0040296. [DOI] [PubMed] [Google Scholar]
- 14.Court MH, Almutain F, Greenblatt D, et al. Identification of isoniazid as a potent inhibitor of CYP2A6-mediated efavirenz 7-hydroxylation in CYP2B6*6 GENOTYPED HUMAN LIVER MICROSOMES. 2013 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA. Abstract 517. [Google Scholar]
- 15.Lee L, Soon GH, Chew N, Else N, Amara A, Khoo S. Differential induction of efavirenz metabolism by rifampin without and with isoniazid in healthy volunteers with CYP2B6 516GG and TT genotypes. 2013 20th Conference on Retroviruses and Opportunistic Infections Atlanta, GA,. Abstract 516. [Google Scholar]
- 16.Efavirenz package insert. Available at: http://packageinserts.bms.com/pi/pi_sustiva.pdf. Accessed 15 April 2013. [Google Scholar]
- 17.Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, et al. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–90. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- 18.Yenny N, Djoerban Z, Setiabudy R. Pharmacokinetic interaction between efavirenz and rifampicin in healthy volunteers. Int J Clin Pharmacol Ther. 2011;49:162–8. doi: 10.5414/cp201473. [DOI] [PubMed] [Google Scholar]
- 19.Ren Y, Nuttall JJ, Eley BS, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:439–43. doi: 10.1097/QAI.0b013e31819c33a3. [DOI] [PubMed] [Google Scholar]
- 20.Kwara A, Lartey M, Sagoe KW, Court MH. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS. 2010;25:388–90. doi: 10.1097/QAD.0b013e3283427e05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gengiah TN, Holford NH, Botha JH, Gray AL, Naidoo K, Abdool Karim SS. The influence of tuberculosis treatment on efavirenz clearance in patients co-infected with HIV and tuberculosis. Eur J Clin Pharmacol. 2011;68:689–95. doi: 10.1007/s00228-011-1166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Food and Drug Administration. Sustiva labeling update/dosing adjustment with rifampin. Available at: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm294476.htm. Accessed 12 August 2012. [Google Scholar]
- 23.Pozniak AL, Coyne KM, Miller RF, et al. British HIV Association guidelines for the treatment of TB/HIV coinfection 2011. HIV Med. 2011;12:517–24. doi: 10.1111/j.1468-1293.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Recommendations for a public health approach: 2010 revision. Geneva, Switzerland: WHO; 2010. Antiretroviral therapy for HIV infection in adults and adolescents. [PubMed] [Google Scholar]
- 25.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 26.Stahle L, Moberg L, Svensson JO, Sonnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004;26:267–70. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Morbidity and Mortality Weekly Report. 2009;58(RR-4):1–216. [PubMed] [Google Scholar]
- 28.Huang L, Parikh S, Rosenthal PJ, et al. Concomitant efavirenz reduces pharmacokinetic exposure to the antimalarial drug artemether-lumefantrine in healthy volunteers. J Acquir Immune Defic Syndr. 2012;61:310–6. doi: 10.1097/QAI.0b013e31826ebb5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu E, Spiegelman D, Semu H, et al. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis. 2011;204:282–90. doi: 10.1093/infdis/jir246. [DOI] [PubMed] [Google Scholar]
- 30.Thiebaut R, Malvy D, Marimoutou C, Davis F. Anthropometric indices as predictors of survival in AIDS adults. Aquitaine Cohort, France, 1985–1997. Groupe d'Epidemiologie Clinique du Sida en Aquitaine (GECSA) Eur J Epidemiol. 2000;16:633–9. doi: 10.1023/a:1007696530440. [DOI] [PubMed] [Google Scholar]
- 31.Van Lettow M, Kumwenda JJ, Harries AD, et al. Malnutrition and the severity of lung disease in adults with pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2004;8:211–7. [PubMed] [Google Scholar]
- 32.Pedral-Sampaio DB, Alves CR, Netto EM, Brites C, Oliveira AS, Badaro R. Efficacy and safety of efavirenz in HIV patients on rifampin for tuberculosis. Braz J Infect Dis. 2004;8:211–6. doi: 10.1590/s1413-86702004000300004. [DOI] [PubMed] [Google Scholar]
- 33.Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006;58:1299–302. doi: 10.1093/jac/dkl399. [DOI] [PubMed] [Google Scholar]
- 34.Swaminathan S, Padmapriyadarsini C, Venkatesan P, et al. Efficacy and safety of once-daily nevirapine- or efavirenz-based antiretroviral therapy in HIV-associated tuberculosis: a randomized clinical trial. Clin Infect Dis. 2011;53:716–24. doi: 10.1093/cid/cir447. [DOI] [PubMed] [Google Scholar]
- 35.Bonnet M, Bhatt N, Baudin E, et al. Nevirapine versus efavirenz for patients co-infected with HIV and tuberculosis: a randomised non-inferiority trial. Lancet Infect Dis. 2013;13:303–12. doi: 10.1016/S1473-3099(13)70007-0. [DOI] [PubMed] [Google Scholar]
- 36.Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–9. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 37.Manosuthi W, Kiertiburanakul S, Sungkanuparph S, et al. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. AIDS. 2006;20:131–2. doi: 10.1097/01.aids.0000196181.18916.9b. [DOI] [PubMed] [Google Scholar]
- 38.Orrell C, Cohen K, Conradie F, et al. Efavirenz and rifampicin in the South African context: is there a need to dose-increase efavirenz with concurrent rifampicin therapy? Antivir Ther. 2011;16:527–34. doi: 10.3851/IMP1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez F, Navarro A, Padilla S, et al. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis. 2005;41:1648–53. doi: 10.1086/497835. [DOI] [PubMed] [Google Scholar]
- 40.Brennan-Benson P, Lyus R, Harrison T, Pakianathan M, Macallan D. Pharmacokinetic interactions between efavirenz and rifampicin in the treatment of HIV and tuberculosis: one size does not fit all. AIDS. 2005;19:1541–3. doi: 10.1097/01.aids.0000183519.45137.a6. [DOI] [PubMed] [Google Scholar]
- 41.Trends in tuberculosis—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:333–7. [PubMed] [Google Scholar]
- 42.World Health Organization. WHO report 2011, global tuberculosis control. Available at: http://www.who.int/tb/publications/global_report/en/index.html. Accessed 15 April 2013. [Google Scholar]
- 43.Chan-Tack K, Liu J, Jadhav P, et al. Interaction- Why did the FDA approve EFV 800 mg When Co-adminstered with Rifampin? San Diego, CA: American College of Clinical Pharmacology Annual Meeting 2012. Abstract 1385545. [Google Scholar]