Abstract
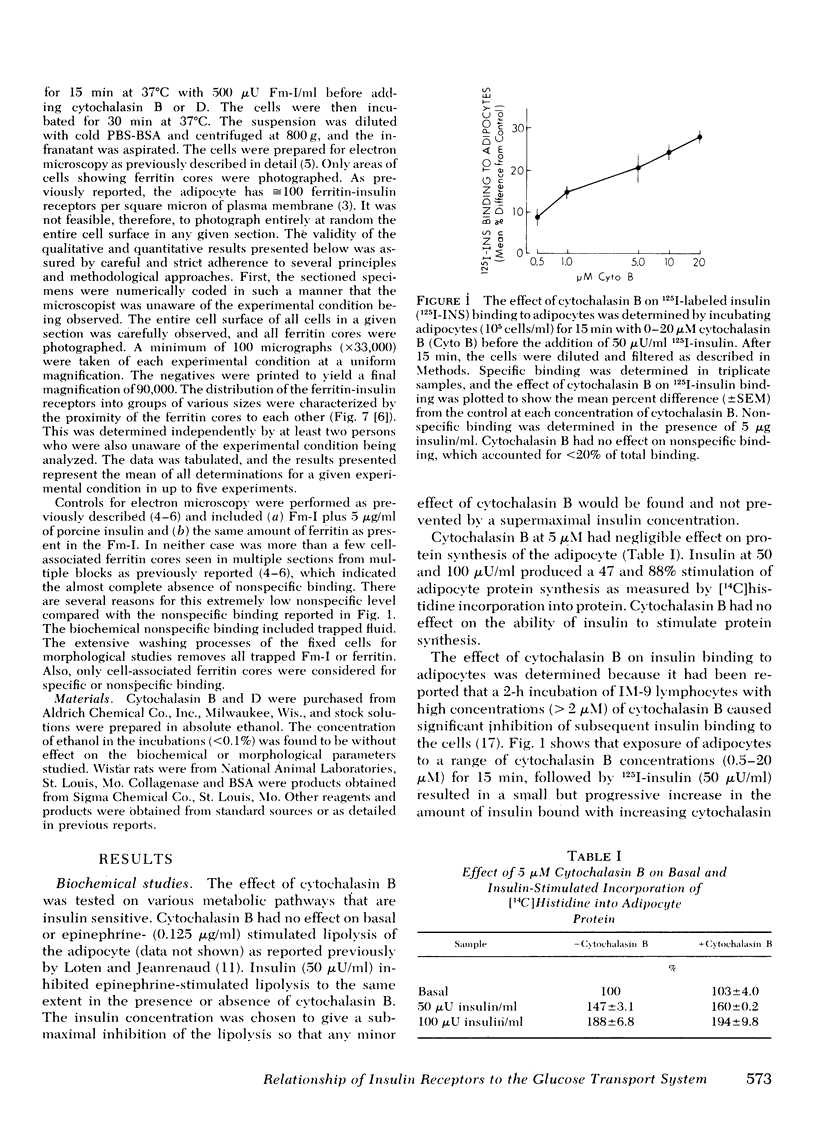
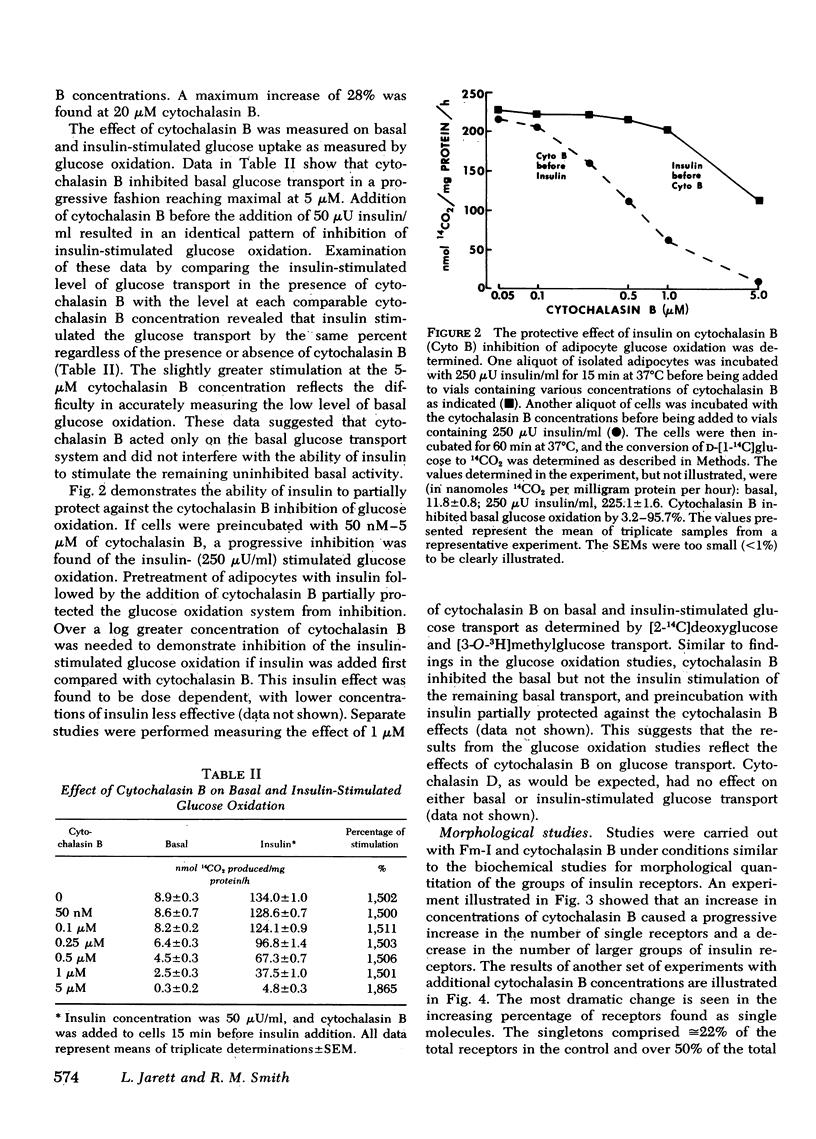
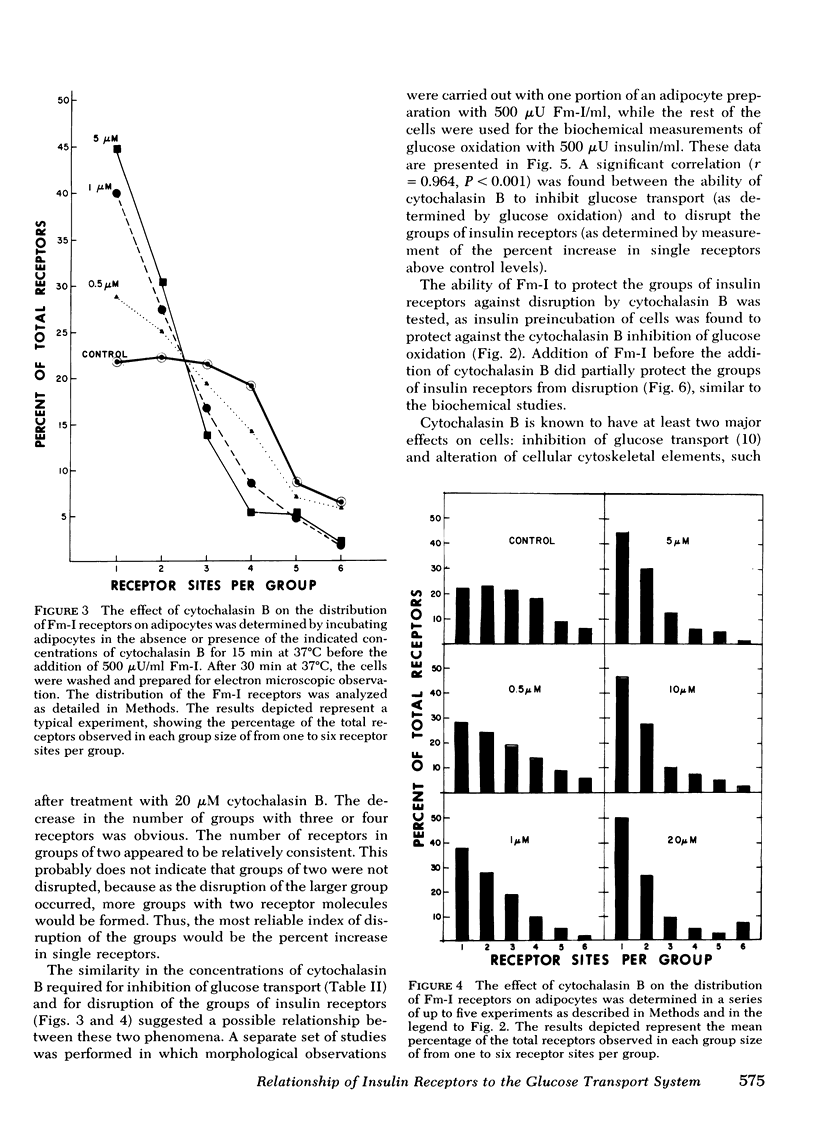
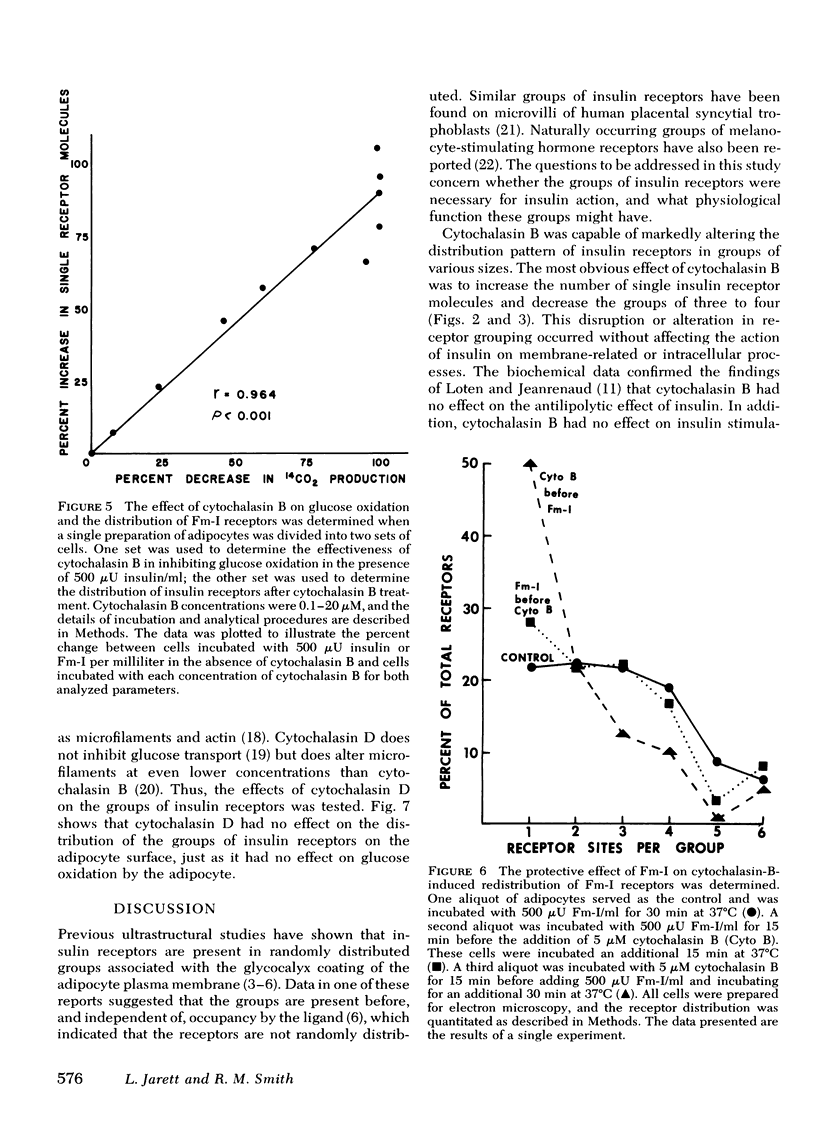
The possible physiological importance of the groups of insulin receptors on rat adipocytes and the relationship of these groups to insulin action were investigated. The effect of cytochalasin B and D on biological actions of insulin was measured and compared with the effect of these agents on the ultrastructural distribution of groups of insulin receptors. Cytochalasin B had no effect on epinephrine-stimulated lipolysis, insulin inhibition of epinephrine-stimulated lipolysis, or insulin stimulation of protein synthesis. Cytochalasin B, over a concentration range of 50 nM to 5 μM, progressively inhibited the basal glucose transport system, as measured by glucose oxidation, 2-deoxyglucose transport, and 3-O-methylglucose transport. Insulin was capable of fully stimulating remaining basal transport at submaximal concentrations of cytochalasin B. Insulin pretreatment of adipocytes partially protected the glucose transport system from inhibition by cytochalasin B. Cytochalasin B markedly altered the distribution pattern of insulin receptors, which caused an increase in the number of single receptor molecules by decreasing the number of larger groups. A significant correlation (r = 0.964; P < 0.001) was found between the percent increase in single receptors and the percent decrease in glucose transport. Ferritin-insulin pretreatment of adipocytes prevented disruption of the groups of insulin receptors by cytochalasin B. Cytochalasin D had no effect on the biological actions of insulin or on the groups of insulin receptors. These data suggest that the ability of insulin to affect adipocyte metabolism is independent of the hormone occupying adjacent, grouped receptor sites. The marked contrast in effects of cytochalasin B and D on groups of insulin receptors and glucose transport suggests that the microfilament system is not involved in insulin action or in holding the groups of insulin receptors together, as both agents are known disrupters of microfilaments and inhibitors of actin gelation. The correlation between the effects of cytochalasin B on insulin receptor distribution and glucose transport leads to the speculation that the glycoprotein molecules containing the insulin receptor are functionally linked with the glucose transport system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas S. J., Lin S. Dihydrocytochalasin B. Biological effects and binding to 3T3 cells. J Cell Biol. 1978 Feb;76(2):360–370. doi: 10.1083/jcb.76.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L. Peptide hormone receptors. Annu Rev Physiol. 1977;39:529–557. doi: 10.1146/annurev.ph.39.030177.002525. [DOI] [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of concanavalin A and wheat germ agglutinin with the insulin receptor of fat cells and liver. J Biol Chem. 1973 May 25;248(10):3528–3534. [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Lynn D. G., Lynn W. S. Cytochalasin B-sensitive 2-deoxy-D-glucose transport in adipose cell ghosts. J Biol Chem. 1973 May 25;248(10):3636–3641. [PubMed] [Google Scholar]
- Czech M. P. Regulation of the D-glucose transport system in isolated fat cells. Mol Cell Biochem. 1976 Mar 26;11(1):51–63. doi: 10.1007/BF01792833. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- Gliemann J. Assay of insulin-like activity by the isolated fat cell method. I. Factors influencing the response to crystalline insulin. Diabetologia. 1967 Aug;3(4):382–388. doi: 10.1007/BF02342631. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Gammeltoft S., Vinten J. Time course of insulin-receptor binding and insulin-induced lipogenesis in isolated rat fat cells. J Biol Chem. 1975 May 10;250(9):3368–3374. [PubMed] [Google Scholar]
- Guidotti G. The structure of intrinsic membrane proteins. J Supramol Struct. 1977;7(3-4):489–497. doi: 10.1002/jss.400070318. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Ho M. K., Guidotti G. A membrane protein from human erythrocytes involved in anion exchange. J Biol Chem. 1975 Jan 25;250(2):675–683. [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Electron microscopic demonstration of insulin receptors on adipocyte plasma membranes utilizing a ferritin-insulin conjugate. J Biol Chem. 1974 Nov 10;249(21):7024–7031. [PubMed] [Google Scholar]
- Jarett L., Smith R. M. The natural occurrence of insulin receptors in groups on adipocyte plasma membranes as demonstrated with monomeric ferritin-insulin. J Supramol Struct. 1977;6(1):45–59. doi: 10.1002/jss.400060104. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Ultrastructural localization of insulin receptors on adipocytes. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3526–3530. doi: 10.1073/pnas.72.9.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarett L., Steiner A. L., Smith R. M., Kipnis D. M. The involvement of cyclic AMP in the hormonal regulation of protein synthesis in rat adipocytes. Endocrinology. 1972 May;90(5):1277–1284. doi: 10.1210/endo-90-5-1277. [DOI] [PubMed] [Google Scholar]
- Karlin A. The acetylcholine receptor: progress report. Life Sci. 1974 Apr 16;14(8):1385–1415. doi: 10.1016/0024-3205(74)90150-7. [DOI] [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Gorden P., Flier J. S., Kahn C. R., Freychet P. Anti-insulin receptor antibodies inhibit insulin binding and stimulate glucose metabolism in skeletal muscle. Diabetologia. 1978 May;14(5):311–317. doi: 10.1007/BF01223022. [DOI] [PubMed] [Google Scholar]
- Loten E. G., Jeanrenaud B. Effects of cytochalasin B, colchicine and vincristine on the metabolism of isolated fat-cells. Biochem J. 1974 May;140(2):185–192. doi: 10.1042/bj1400185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANS R. J., NOVELLI G. D. A convenient, rapid and sensitive method for measuring the incorporation of radioactive amino acids into protein. Biochem Biophys Res Commun. 1960 Nov;3:540–543. doi: 10.1016/0006-291x(60)90171-6. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Holland P. C. Calcium transport in sarcoplasmic reticulum. Annu Rev Biophys Bioeng. 1975;4(00):377–404. doi: 10.1146/annurev.bb.04.060175.002113. [DOI] [PubMed] [Google Scholar]
- Mimura N., Asano A. Actin-related gelation of Ehrlich tumour cell extracts is reversibly inhibited by low concentrations of Ca2+. Nature. 1978 Mar 16;272(5650):273–276. doi: 10.1038/272273a0. [DOI] [PubMed] [Google Scholar]
- Mousa G. Y., Trevithick J. R. Differentiation of rat lens epithelial cells in tissue culture. II. Effects of cytochalasins B and D on actin organization and differentiation. Dev Biol. 1977 Oct 1;60(1):14–25. doi: 10.1016/0012-1606(77)90107-5. [DOI] [PubMed] [Google Scholar]
- Nelson D. M., Smith R. M., Jarett L. Nonuniform distribution and grouping of insulin receptors on the surface of human placental syncytial trophoblast. Diabetes. 1978 May;27(5):530–538. [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Pillion D. J., Czech M. P. Antibodies against intrinsic adipocyte plasma membrane proteins activate D-glucose transport independent of interaction with insulin binding sites. J Biol Chem. 1978 Jun 10;253(11):3761–3764. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Scott R. E., Maercklein P. B., Furcht L. T. Plasma membrane intramembranous particle topography in 3T3 and SV3T3 cells: the effect of cytochalasin B. J Cell Sci. 1977 Feb;23:173–192. doi: 10.1242/jcs.23.1.173. [DOI] [PubMed] [Google Scholar]
- Singer S. J. Thermodynamics, the structure of integral membrane proteins, and transport. J Supramol Struct. 1977;6(3):313–323. doi: 10.1002/jss.400060304. [DOI] [PubMed] [Google Scholar]
- Tannenbaum J., Tanenbaum S. W., Godman G. C. The binding sites of cytochalasin D. II. Their relationship to hexose transport and to cytochalasin B. J Cell Physiol. 1977 May;91(2):239–248. doi: 10.1002/jcp.1040910209. [DOI] [PubMed] [Google Scholar]
- Taylor N. F., Gagneja G. L. A model for the mode of action of cytochalasin B inhibition of D-glucose transport in the human erythrocyte. Can J Biochem. 1975 Oct;53(10):1078–1084. doi: 10.1139/o75-148. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., De Meyts P., Roth J. Cell surface receptors for insulin and human growth hormone. Effect of microtubule and microfilament modifiers. J Biol Chem. 1976 Nov 10;251(21):6844–6851. [PubMed] [Google Scholar]
- Varga J. M., Saper M. A., Lerner A. B., Fritsch P. Nonrandom distribution of receptors for melanocyte-stimulating hormone on the surface of mouse melanoma cells. J Supramol Struct. 1976;4(1):45–49. doi: 10.1002/jss.400040105. [DOI] [PubMed] [Google Scholar]
- Weihing R. R. Cytochalasin B inhibits actin-related gelation of HeLa cell extracts. J Cell Biol. 1976 Oct;71(1):303–307. doi: 10.1083/jcb.71.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]



