Abstract
Background. With prolonged replication, attenuated polioviruses used in oral polio vaccine (OPV) can mutate into vaccine-derived poliovirus (VDPV) and cause poliomyelitis outbreaks. Individuals with primary humoral immunodeficiencies can become chronically infected with vaccine poliovirus, allowing it to mutate into immunodeficiency-associated VDPV (iVDPV). It is unclear if children perinatally infected with the human immunodeficiency virus (HIV), who have humoral as well as cellular immunodeficiencies, might be sources of iVDPV.
Methods. We conducted a prospective study collecting stool and blood samples at multiple time points from Zimbabwean infants receiving OPV according to the national schedule. Nucleic acid extracted from stool was analyzed by real-time polymerase chain reaction for OPV serotypes.
Results. We analyzed 825 stool samples: 285 samples from 92 HIV-infected children and 540 from 251 HIV-uninfected children. Poliovirus shedding was similar after 0–2 OPV doses but significantly higher in the HIV-infected versus uninfected children after ≥3 OPV doses, particularly within 42 days of an OPV dose, independent of seroconversion status. HIV infection was not associated with prolonged or persistent poliovirus shedding. HIV infection was associated with significantly lower polio seroconversion rates.
Conclusions. HIV infection is associated with decreased mucosal and humoral immune responses to OPV but not the prolonged viral shedding required to form iVDPV.
Keywords: polio, OPV, shedding, immunity, HIV, infants, vaccine, VDPV
Wild poliovirus is nearing global eradication. Since 1988, when the World Health Organization proposed a plan to eradicate poliomyelitis, annual global cases have dropped from 350 000 to 650 in 2011 [1]. The last case of naturally acquired wild poliovirus type 2 was reported in 1999, and only 3 countries remain endemic with uninterrupted transmission [2]. The primary approach for global eradication has been administration of oral polio vaccine (OPV), an inexpensive live attenuated vaccine that is easy to administer and promotes community immunity through transmission from vaccinated children to community contacts. However, although OPV effectively prevents poliomyelitis, its continued use inevitably leads to emergence of vaccine-derived polioviruses (VDPVs), which may jeopardize the success of the eradication campaign.
VDPVs, whose sequences differ by 1%–15% from parent OPV strains (0.6%–15% for serotype 2), develop with prolonged viral replication of typically >1 year [3, 4]. In normal circumstances, children vaccinated with OPV and their infected close contacts shed virus for several weeks. However, people with primary humoral immunodeficiencies can shed virus for prolonged periods, in one case for >20 years, allowing the formation of immunodeficiency-associated VDPV (iVDPV) [5]. To date, 65 people with primary immunodeficiencies shedding iVDPV have been identified [4]. Furthermore, in communities with incomplete vaccine coverage, OPV-derived strains can spread person to person and circulate for years, allowing the virus to mutate into circulating VDPV (cVDPV). Twenty-one independent cVDPV outbreaks have been identified [6, 7], with an attack rate and disease severity similar to wild poliovirus infections [8].
One question yet to be fully explored is whether children perinatally infected with human immunodeficiency virus (HIV) might be sources of iVDPV. HIV infection during the perinatal period is associated with humoral as well as cellular immunodeficiencies [9]. Children perinatally infected with HIV often have a polyclonal hypergammaglobulinemia paradoxically associated with diminished antibody responses to specific antigens [10–12]. As approximately 90% of the 2 million children with HIV live in Africa [13], where OPV is predominantly used for polio vaccination regardless of HIV status, prolonged poliovirus shedding by HIV-infected children could impair the polio eradication effort.
Although there are several studies in the literature exploring whether HIV-infected children shed vaccine poliovirus for prolonged periods after OPV administration, they do not adequately answer the question of whether HIV-infected children might be sources of iVDPV. Two prospective studies did not show vaccine poliovirus shedding beyond 6 months, but they were small, and 1 had very low retention rates [14, 15]. Of 2 cross-sectional studies, 1 detected no poliovirus in stool samples from 94 HIV-infected Guatemalan children 6 months to ≥10 years after their last OPV dose, but the other did detect VDPV in the stools of hospitalized HIV-infected South African children [16–18]. However, as a cross-sectional study, it is unclear whether these isolates were cVDPV acquired in the community or iVDPV from chronic infection in the individual children.
To better examine whether children perinatally infected with HIV can be a source of iVDPV and to further evaluate the humoral and mucosal immune response to OPV in HIV-infected children, we conducted a prospective study evaluating stool shedding of vaccine strains and polio seroconversion in HIV-infected and -uninfected Zimbabwean children who received OPV according to the national immunization schedule.
METHODS
Study Design and Population
This was a prospective, longitudinal, observational study in Chitungwiza, the third-largest urban center in Zimbabwe, a southern African country with a population of approximately 11 million and HIV prevalence of 14.3% during the study period [19]. OPV is given to children regardless of HIV status at 3, 4, 5, and 18 months of age per the Zimbabwean vaccination schedule. During the study period, supplementary vaccination campaigns providing additional OPV doses were held 6–18 June 2009, 30 November–12 December 2009, and 24 May–1 June 2010. The last poliomyelitis case, clinically diagnosed but not virologically confirmed, occurred in Zimbabwe in 1999 [20]. The study protocol was approved by the Medical Research Council of Zimbabwe, the Research Council of Zimbabwe, and the institutional review board of Stanford University School of Medicine.
Enrollment occurred between September 2008 and December 2010 at 2 community clinics in Chitungwiza. Initial inclusion criteria included age between 2 and 4 months, a birth mother with known HIV status, and plans to receive OPV according to the national schedule. Due to lower-than-expected enrollment of HIV-infected children, enrollment was expanded (1) in October of 2009 to HIV-infected children aged ≤18 months (late enrollers) and (2) in April 2010 to the Epworth clinic in Harare (located 10 miles from Chitungwiza and serving the same ethnic group). Severely ill children were excluded.
After written informed consent was obtained from the parents, participation included 9 study visits at 3, 4, 5, 6, 9, 12, 18, 19, and 24 months of age. At each visit, the child's stool was collected, and a questionnaire detailing the child's health, vaccination history, and demographics was completed. At 5 of the study visits (enrollment and 5, 9, 18, and 24 months), blood was collected for HIV testing, T-cell subsets, and polio-neutralizing antibodies. Blood was drawn from late enrollers at enrollment, and further visits were conducted according to the initial protocol. Follow-up visits ended in June 2011. Mothers of children found to be infected with HIV were counseled and referred to an HIV clinic to start their child on antiretroviral therapy (ART).
Infants were included in the current study if (1) their HIV status was confirmed, (2) their OPV history could be verified, and (3) they had available stool. For the stool-shedding analysis, all of the stool samples from HIV-infected subjects were processed. Stool samples from HIV-uninfected control subjects, matched by age in months at sample collection, were randomly selected for processing at a ratio of approximately 2:1 control to HIV-infected samples.
Sample Processing
Blood was collected in a purple-top ethylenediaminetetraacetic acid–containing Vacutainer tube and a red-top Vacutainer tube and transported on ice to a laboratory in Harare. The red-top tubes were centrifuged, and the sera were transferred to cryovials (for polio-neutralizing antibody assays) and stored at −70°C until shipment on dry ice to the Maldonado laboratory at Stanford.
Complete Blood Count and T-Cell Subsets
Whole blood was analyzed within several hours of collection using a Sysmex KX-21N Automated Hematology Analyzer for the complete blood count and a Partec Cyflow Counter for lymphocyte subsets.
Determination of Child's HIV-1 Infection Status
HIV status was determined from whole blood by HIV polymerase chain reaction using an Amplicor HIV DNA Test Version 1.5 kit and a GeneAmp 9700 thermal cycler. At 18 months of age, HIV enzyme-linked immunosorbent assay tests were performed to confirm HIV status.
We defined confirmation of HIV status as ≥2 positive HIV tests with ≥1 done as part of the study, or ≥1 positive HIV test done as part of the study and clinical picture strongly suggestive of HIV infection.
Polio-Neutralizing Antibodies Assay
Three- and 9-month serum was shipped on dry ice to Dr Konstantin Chumakov's laboratory at the United States Food and Drug Administration for polio-neutralizing antibody assays according to the World Health Organization method [21]. A titer of ≥1:8 at the 9-month time point was considered positive for seroconversion.
For the analysis linking stool samples to seroconversion status), we assumed fixed seroconversion status from 1 week after administration of an OPV dose to 1 week after administration of the next dose, based on data that serum-neutralizing antibodies develop about 1 week after an OPV dose [22].
Stool Assays
At Stanford, stool underwent RNA extraction, reverse transcription, and real-time polymerase chain reaction to look for vaccine poliovirus serotypes 1, 2, and 3 and their revertant forms according to previously published methods with the following exceptions [23]. A Biorad CFX384 Real-Time System was used, and a single threshold, set at 400 RFU, was used for detecting fluorescence.
Statistical Analysis
Variables considered while estimating the proportions shedding vaccine poliovirus serotypes 1–3 included number of previous OPV doses administered, time from last OPV dose, HIV status, ART administration, poliovirus seroconversion, and CD4%.
HIV-infected children were considered to be on ART if they started ART at least 30 days before sample collection. One child who was on ART at enrollment without a start date listed was assumed to have been on ART for at least 1 month.
Severe immunosuppression was defined, per the World Health Organization, as CD4% <25 in children aged ≤11 months and CD4% <20 in children aged 12–35 months [24].
The average of the available CD4% for a child, with/without ART, was used in comparing pre-ART or post-ART HIV-infected children with HIV-uninfected children.
Categorical data were compared using a 2-tailed Fisher exact test. Continuous data were compared using a 2-tailed t test. Correlations were estimated with Pearson coefficients. Logistic regression with backwards elimination (stay criteria P < .05) was used for assessing the association of multiple explanatory variables with shedding. A P value ≤ .05 was considered statistically significant. The analysis was done with SAS 9.3.
RESULTS
Four hundred two of the 421 children originally enrolled were included in this study: 92 (23%) HIV-infected children and 310 (77%) HIV-uninfected children (excluded: 11 whose HIV status could not be confirmed, 5 missing OPV records, and 3 without stool samples). The demographics of the children in the study are shown in Table 1. The majority of HIV-infected children were severely immunosuppressed by World Health Organization criteria (75% [65 of 87] of HIV-infected children vs 42% [129 of 307] of uninfected children; P < .0001). HIV-infected children on and off ART had significantly lower mean CD4% than the uninfected children (20% for HIV-infected children not on ART vs 26% for HIV-infected children on ART vs 31% for uninfected children; P values < .01 for all comparisons).
Table 1.
Demographics of Study Subjects
| Demographics | HIV-infected | HIV-uninfected |
|---|---|---|
| Number of children | 92 | 310 |
| Age at enrollment, | ||
| median (IQR) months, | 7.1 (3.4–12.1) | 3.2 (3.0–3.4) |
| Normal enrollment, % (No.) | 29 (27) | 99 (308) |
| 4–9 mo at enrollment, % (No.) | 42 (39) | 1 (2) |
| ≥12 mo at enrollment, % (No.) | 28 (26) | 0 (0) |
| Date of birth, range | 23 July 2008–7 July 2010 | 27 June 2008–17 August 2010 |
| End of study | ||
| % (No.) who died during the study | 13 (12) | 2 (7) |
| % (No.) who came to last expected visit | 59 (54) | 54 (168) |
| Study visits | ||
| Total No. of expected visits | 389 | 2654 |
| % (No.) of recorded visits out of all expected visits | 78 (302) | 80 (2121) |
| ART | ||
| % (No.) on ART at end of study | 73 (67) | NA |
| Median (IQR) ART start age, months | 8 (5–11) | NA |
| Adherence with OPV schedule | ||
| % age ≥6 mo who received doses 1–3 on time | 82 (69/84) | 95 (269/282) |
| Demographics | ||
| % (No.) male | 55 (51) | 49 (153) |
| % (No.) mother is main caretaker | 95 (87) | 98 (304) |
| Median (IQR) mother's age, years | 28 (24–31) | 27 (23–31) |
| % (No.) breastfed ≥6 mo | 75 (69) | 80 (245) |
| % (No.) with siblings ≤5 y | 34 (31) | 42 (131) |
| Median (IQR) home density | 3 (2–4) | 3 (2–4) |
| % (No.) with working tap/borehole | 58 (54) | 91 (282) |
| % (No.) with working toilet | 36 (33) | 77 (238) |
Normal enrollment was defined as enrollment between 2 and 4 months of age.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable.
Altogether 825 stool samples were analyzed for poliovirus shedding: all 285 stool samples from 92 HIV-infected subjects and 540 age-matched samples from 251 HIV-uninfected subjects. Poliovirus was detected in 54 (19%) samples from HIV-infected subjects and 87 (16%) samples from uninfected subjects. Serotype 2 was detected in 87 samples (11%), serotype 1 in 56 samples (7%), and serotype 3 in 56 samples (7%). In samples containing poliovirus, 99 (70%) contained only 1 serotype, 26 (18%) contained 2 serotypes, and 16 (11%) contained 3 serotypes. Poliovirus shedding within 42 days after OPV vaccination was higher than shedding either >42 days after or before any OPV (31% vs 7% vs 9% respectively; P ≤ .0001). There was no evidence of correlations between shedding at one visit and shedding at another visit in individual subjects.
Although poliovirus shedding in samples collected after 0–2 OPV doses was not associated with HIV status, shedding was significantly higher in HIV-infected subjects after receipt of ≥3 OPV doses (16% [32 of 200 samples] vs 10% [42 of 414 samples]; P = .047). This difference was predominantly seen in samples collected within 42 days of an OPV dose (35% [19 of 54 samples] vs 18% [27 of 151 samples]; P = .01; Table 2, Figure 1). Shedding from >42 days after an OPV dose was higher in HIV-infected subjects, but the difference was not statistically significant. After controlling for HIV infection, CD4%, the enrolling clinic, and the demographic/social status of the children were not associated with poliovirus shedding after controlling for HIV infection. In addition, among HIV-infected children, being on ART was not associated with poliovirus shedding.
Table 2.
Vaccine Poliovirus Shedding Patterns by Prior Oral Polio Vaccine (OPV) Doses and Time from Last OPV Dose in Human Immunodeficiency Virus–Infected Versus -Uninfected Children
| Number of samples |
Mean age in months ± SE at sample collection |
Mean days ± SE from last OPV dose to sample collection |
No. (%) samples containing poliovirus |
No. (%) of samples containing poliovirus containing all 3 serotypes |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | |
| No prior OPV doses | 18 | 27 | 3.5 ± 0.2 | 3.4 ± 0.1 | NA | NA | 1 (6) | 3 (11) | 0 (0) | 0 (0) |
| <43 d from 1st OPV dose | 31 | 31 | 4.2 ± 0.2 | 3.8 ± 0.1 | 18 ± 3 | 15 ± 3 | 11 (35) | 15 (48) | 4 (36) | 3 (20) |
| <43 d from 2nd OPV dose | 24 | 57 | 5.0 ± 0.1 | 4.8 ± 0.1 | 18 ± 3 | 13 ± 2 | 9 (38) | 26 (46) | 0 (0) | 3 (12) |
| <43 days from ≥3rd OPV dose* | 54 | 151 | 10.3 ± 0.8 | 10.4 ± 0.5 | 22 ± 2 | 23 ± 1 | 19 (35)* | 27 (18)* | 6 (32)** | 0 (0)** |
| 43–92 d from ≥3d OPV dose | 20 | 23 | 12.2 ± 1.3 | 12.4 ± 1.0 | 62 ± 3 | 67 ± 4 | 4 (20) | 3 (13) | 0 (0) | 0 (0) |
| >92 d from ≥3rd OPV dose | 126 | 240 | 14.9 ± 0.4 | 14.0 ± 0.3 | 238 ± 15 | 207 ± 6 | 9 (8) | 12 (5) | 0 (0) | 0 (0) |
Except for those noted with asterisks, all P values comparing shedding patterns between HIV-infected and uninfected children were >0.3. Of note, the 11 samples from HIV-infected children and the 10 samples from HIV-uninfected children collected >42 days after the first or second OPV dose were not included in this table because the numbers were too low for a meaningful comparison. Forty-two days was used as the cutoff because it is normal for OPV recipients to shed poliovirus for up to 6 weeks [3].
Abbreviations: HIV, human immunodeficiency virus; OPV, oral polio vaccine; SE, standard error.
*P = .01.
**P = .003.
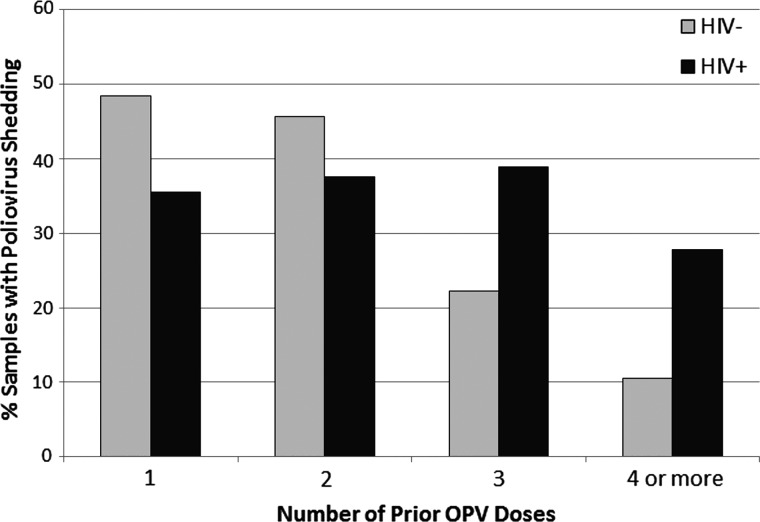
Figure 1.
Vaccine poliovirus shedding within 42 days of an oral polio vaccine (OPV) dose in human immunodeficiency virus (HIV)–infected versus -uninfected children. The number of samples, from HIV-infected and -uninfected subjects, respectively, were 31 and 31 after 1 OPV dose, 24 and 57 after 2 OPV doses, 36 and 94 after 3 OPV doses, and 18 and 57 after ≥4 OPV doses. Abbreviations: HIV, human immunodeficiency virus; OPV, oral polio vaccine.
There was no evidence from our data that HIV-infected children were more likely to have prolonged (≥6 months) poliovirus shedding. Overall shedding >180 days from the last OPV dose was 6% (14 of 230) with small and not statistically significant differences between HIV-infected and HIV-uninfected children (7% [6 of 90] HIV-infected; 6% [8 of 140] uninfected). Furthermore, the 4 (3 HIV-infected) of 14 late shedders who had another sample taken either before or after the positive sample without an intervening OPV dose did not have detectable poliovirus in the other sample.
Samples collected within 42 days of vaccination and containing detectable poliovirus were more likely to contain all 3 serotypes if they were from HIV-infected versus HIV-uninfected subjects (26% [10 of 39] vs 9% [6 of 68]; P = .03). This difference was most pronounced in subjects who had received ≥3 doses of OPV (30% [6 of 19] vs 0% [0/27]; P = .003; Table 2). Shedding >1 serotype decreased with successive OPV doses among HIV-uninfected subjects but not among HIV-infected subjects.
Among 35 HIV-infected subjects and 222 HIV-uninfected subjects who had received at least 3 prior doses of OPV, seroconversion was significantly lower in the HIV-infected subjects, as was geometric mean titer among the seroconverters (Table 3). Among the 61 analyzed stool samples collected within 42 days of OPV vaccination that could be linked to poliovirus seroconversion status, poliovirus shedding was significantly increased among HIV-infected children even after controlling for seroconversion, and seroconversion was not associated with a change in poliovirus shedding (Table 4).
Table 3.
Polio Seroconversion and Neutralizing Antibody Geometric Mean Titer in Children Who Received ≥3 Oral Polio Vaccine Doses, by Human Immunodeficiency Virus Status
| Serconversion |
GMT Among Seroconverters |
|||||
|---|---|---|---|---|---|---|
| HIV-Infected, No. (%) (n = 35) | HIV-Uninfected, No. (%) (n = 222) | P Value | HIV-Infected | HIV-Uninfected | P Value | |
| Serotype 1 | 24 (69) | 193 (87) | .01 | 126 | 371 | .01 |
| Serotype 2 | 26 (74) | 211 (95) | .0004 | 148 | 343 | .0003 |
| Serotype 3 | 13 (37) | 188 (85) | <.0001 | 60 | 116 | .03 |
| Any serotype | 30 (86) | 218 (98) | .003 | |||
| All serotypes | 12 (34) | 165 (74) | <.0001 | |||
Data were from the 9-month serum sample.
Abbreviations: GMT , geometric mean titer; HIV, human immunodeficiency virus.
Table 4.
Vaccine Poliovirus Shedding within 42 Days of Oral Polio Vaccine Receipt by Polio Seroconversion and Human Immunodeficiency Virus Status
| Serotype | HIV Status | Seroconverted | No Seroconversion |
|---|---|---|---|
| 1 | HIV+ | 33% (5/15) | 31% (5/16) |
| HIV− | 0% (0/19) | 9% (1/11) | |
| P Value | .01 | .35 | |
| 2 | HIV+ | 38% (6/16) | 33% (5/15) |
| HIV− | 4% (1/24) | 0% (0/6) | |
| P Value | .01 | .26 | |
| 3 | HIV+ | 33% (2/6) | 28% (7/25) |
| HIV− | 0% (0/14) | 0% (0/16) | |
| P Value | .08 | .03 |
Data are from 61 stool samples (31 from human immunodeficiency virus [HIV]–infected, 30 from HIV-uninfected children) that could be linked to seroconversion status and were collected within 42 days of an oral polio vaccine dose.
Abbreivation: HIV, human immunodeficiency virus.
DISCUSSION
This prospective study evaluated vaccine poliovirus shedding in stool and polio seroconversion in HIV-infected and -uninfected Zimbabwean children who received OPV according to the national immunization schedule. We found that although poliovirus shedding after 1 or 2 OPV doses was not associated with the children's HIV status, poliovirus shedding and shedding of multiple serotypes were significantly higher in the HIV-infected versus -uninfected children who had received ≥3 OPV doses. This reflects a decrease in shedding among uninfected children after 3 OPV doses that was not seen in the HIV-infected children (Figure 1). As decreased poliovirus shedding is considered a marker for the development of mucosal intestinal immunity [25], this suggests that although uninfected children developed intestinal mucosal immunity after 3 OPV doses, HIV-infected children often did not. The HIV-infected children also had significantly lower poliovirus seroconversion rates after receipt of equivalent doses of OPV, confirming our previous observations [12]. Poliovirus seroconversion among HIV-infected children was not associated with a decrease in poliovirus shedding, although the sample size for this particular analysis was small. This suggests that perinatal HIV infection is independently associated with both a defective humoral immune response and a defective intestinal mucosal immune response to vaccination with OPV.
Although poliovirus shedding rates were higher among the HIV-infected children after ≥3 OPV doses, we did not see significantly higher rates of prolonged shedding in the HIV-infected children. Poliovirus shedding >6 months after an OPV dose was often not preceded by earlier shedding, suggesting that the positive sample represented reinfection with vaccine poliovirus from the community rather than prolonged shedding. Based on the results of our study, it seems unlikely that children perinatally infected with HIV could be sources of iVDPV. However, these children's increased short-term shedding could increase the likelihood that cVDPV might develop in their communities.
There are 2 small prospective studies and 4 cross-sectional studies in the literature that have investigated whether HIV-infected individuals excrete vaccine poliovirus in their stools for prolonged periods of time. One study from Kenya examined 255 stool specimens from 15 HIV-infected and 9 HIV-uninfected children collected over 12 months and found no evidence of poliovirus shedding for ≥6 months in either group [15]. However, serotype 1 vaccine poliovirus was detected in 3 serial samples from 1 child with moderately symptomatic AIDS up to 87 days after OPV administration, and the VP1 region of the viral genome acquired 0.99% nucleotide substitution by the final positive sample. A prospective study in the Central African Republic enrolled 16 HIV-infected and 37 HIV-uninfected children who were receiving 3 doses of OPV during national immunization days, but low retention rates resulted in samples from only 12 HIV-infected and 32 HIV-uninfected children 1 month after the last OPV dose, down to 1 HIV-infected and 1 HIV-uninfected child 6 months after the last OPV dose [14]. Vaccine poliovirus was detected in 5.6% versus 2.1% of the samples from HIV-infected versus -uninfected children, but no poliovirus shedding was seen beyond 4 weeks. In 2 studies, the stools of 28 and 419 HIV-infected adults who were living with or nearby to children immunized with OPV during national immunization days in the Central African Republic and Cote d'Ivoire were found to contain no detectable poliovirus [26, 27]. A cross-sectional study in Guatemala examined the stools of 94 HIV-infected children (median age, 3.6 years) who had not received OPV for at least 6 months along with 101 HIV-infected adults and also found no detectable poliovirus [16]. In contrast, a cross-sectional study in South Africa examined 164 stool samples from hospitalized HIV-infected children and found 13 containing OPV-derived strains, 5 from children who had not received OPV for >6 months [17, 18]. However, when these polioviruses were sequenced, only 2 of the 5 viruses showed the degree of divergence expected had these children been excreting the virus continuously since their last vaccination. Additionally, 1 child who had last received OPV 16 months before shed a serotype 1 virus genetically identical to the serotype 1 virus shed by another HIV-infected child who had been hospitalized with chronic diarrhea 3 months before, suggesting that these children may have acquired these polioviruses from person-to-person contact, possibly while hospitalized.
Our study has several limitations. Thanks to successful prevention programs and increased ART accessibility, HIV prevalence in Zimbabwe decreased between study planning and execution from 20% to 14%, and mother-to-child transmission fell to 4%. Consequently, enrollment of sufficient HIV-infected children necessitated a protocol change that opened enrollment in another clinic and to older HIV-infected children who were not enrolled at the younger ages immediately after routine OPV immunization. However, the variations of home environment introduced by the added clinic were not associated with increased shedding, and late enrollment of HIV-infected children had no impact on their vaccination program, which followed the regular Zimbabwean schedule. The planned study period was shortened by a delay of the start time caused by a cholera outbreak at the study site, causing the study to close before some subjects completed their later follow-up visits. The high mobility of Zimbabwean families, caused by the unstable Zimbabwean economic situation during the study period, resulted in a high proportion of enrolled children who did not complete the study and/or missed intervening visits. As a result of these factors, only approximately 30% of subjects completed all 9 study visits. However, overall 80% of all expected study visits were completed. It is important to note that our conclusions are based on analyses done separately at each visit, therefore the missing study visits do not reduce the significance of our findings.
Despite these limitations, we demonstrate that perinatal HIV infection is associated with reduced mucosal and humoral immunity against polio in children with complete OPV vaccination. The decreased mucosal immunity could conceivably lead to more circulation of vaccine-derived strains and increase the likelihood of development of cVDPV in communities with a high HIV prevalence. However, in the largest study to date, we demonstrated no evidence that children perinatally infected with HIV shed poliovirus for the prolonged time periods required to form iVDPV.
In conclusion, although the decreased mucosal and humoral immune responses to OPV of HIV-infected children could contribute to increased circulation of polioviruses in their communities, these children are unlikely to be a source of iVDPV. This finding suggests that HIV infection would not be an impediment to the use of the Salk inactivated polio vaccine (IPV) in the final stage of the global effort to eradicate polio and that IPV use among HIV-infected children should be pursued to eradicate circulating wild and vaccine-derived polioviruses. Studies to determine the immunogenicity of IPV as the routine polio immunization regimen should be investigated among immunocompromised populations such as HIV-infected children living in developing settings. This approach could further support the feasibility of global use of IPV for several years after wild poliovirus eradication and global cessation of OPV to maintain high levels of population immunity until attenuated and vaccine-derived polioviruses cease to circulate.
Notes
Acknowledgements. The authors thank the study nurses, study counselors, ZAPP data manager (Lovemore Chinyere), Drs Nivedita Srinivas and Marisa Holubar for their assistance in obtaining and analyzing some of the samples, Dr LauraLe Dyner, Dr Micah Simoyi and the Chitungwiza Health Department, Professors Godfrey Woelk and Simbarashe Rusakaniko along with the ZAPP-UZ support staff, the UZ College of Health Sciences, the Zimbabwe National Poliomyelitis Reference Laboratory, and especially all the mothers and infants who participated in the study.
The authors wish to acknowledge the late Professor Isidore-Evans Pazvakavambwa, who helped design the study, and the late Professor Michael Chirara, who advised on the laboratory assays.
Financial support. This study was funded by the United States National Institutes of Health (NIH R01 AI068577 to Y. A. M.).
Potential conflicts of interest. Y. A. M. and A. K. S. have worked on the vaccine advisory board for Merck and Novartis. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission—worldwide, January 2010–March 2011. MMWR Morb Mortal Wkly Rep. 2011;60:582–6. [PubMed] [Google Scholar]
- 2.Global Polio Eradication Initiative. Polio this week. http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx. Accessed 20 February 2012. [Google Scholar]
- 3.Sutter R, Kew O, Cochi S. Poliovirus vaccine-live. In: Paul O, Orenstein W, Plotkin S, editors. Vaccines. 5th ed. Philadelphia: Saunders/Elsevier; 2008. pp. 631–85. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses—worldwide, April 2011–June 2012. MMWR Morb Mortal Wkly Rep. 2012;61:741–6. [PubMed] [Google Scholar]
- 5.Minor P. Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine. 2009;27:2649–52. doi: 10.1016/j.vaccine.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 6.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 7.Global Polio Eradication Initiative. Circulating vaccine-derived poliovirus (cVDPV) 2000–2011. http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Circulatingvaccinederivedpoliovirus.aspx. Accessed 9 January 2013. [Google Scholar]
- 8.Jenkins H, Aylward R, Gasasira A, et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N Engl J Med. 2010;362:2360–9. doi: 10.1056/NEJMoa0910074. [DOI] [PubMed] [Google Scholar]
- 9.Español T, Garcia X, Caragol I, Sauleda S, Muntane C. Immunological abnormalities in pediatric AIDS. Immunol Invest. 1991;20:215–21. doi: 10.3109/08820139109050790. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein L, Ochs H, Wedgwood R, Rubinstein A. Defective humoral immunity in pediatric acquired immune deficiency syndrome. J Pediatr. 1985;107:352–7. doi: 10.1016/s0022-3476(85)80505-9. [DOI] [PubMed] [Google Scholar]
- 11.Shearer W, Easley K, Goldfarb J, et al. Prospective 5-year study of peripheral blood CD4, CD8, and CD19/CD20 lymphocytes and serum Igs in children born to HIV-1 women. The P(2)C(2) HIV Study Group. J Allergy Clin Immunol. 2000;106:559–66. doi: 10.1067/mai.2000.109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnanashanmugam D, Troy SB, Musingwini G, et al. Immunologic response to oral polio vaccine in human immunodeficiency virus–infected and uninfected Zimbabwean children. Pediatr Infect Dis J. 2012;31:176–80. doi: 10.1097/INF.0b013e31823faa5f. [DOI] [PubMed] [Google Scholar]
- 13.WHO Technical Reference Group. WHO antiretroviral therapy for infants and children. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 14.Manirakiza A, Picard E, Ngbale R, Menard D, Gouandjika-Vasilache I. OPV strains circulation in HIV infected infants after National Immunisation Days in Bangui, Central African Republic. BMC Res Notes. 2010;3:136. doi: 10.1186/1756-0500-3-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khetsuriani N, Helfand R, Pallansch M, et al. Limited duration of vaccine poliovirus and other enterovirus excretion among human immunodeficiency virus infected children in Kenya. BMC Infect Dis. 2009;9:136. doi: 10.1186/1471-2334-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asturias E, Grazioso C, Luna-Fineman S, Torres O, Halsey N. Poliovirus excretion in Guatemalan adults and children with HIV infection and children with cancer. Biologicals. 2006;34:109–12. doi: 10.1016/j.biologicals.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Pavlov D, Van Zyl W, Kruger M, Blignaut L, Grabow W, Ehlers M. Poliovirus vaccine strains detected in stool specimens of immunodeficient children in South Africa. Diagn Microbiol Infect Dis. 2006;54:23–30. doi: 10.1016/j.diagmicrobio.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Pavlov D, Van Zyl W, Van Heerden J, et al. Prevalence of vaccine-derived polioviruses in stools of immunodeficient children in South Africa. J Appl Microbiol. 2006;101:1367–79. doi: 10.1111/j.1365-2672.2006.03020.x. [DOI] [PubMed] [Google Scholar]
- 19.United Nations. United Nations General Assembly special session report on HIV and AIDS: Zimbabwe country report. Reporting period: January 2008 to December 2009. 2010. http://data.unaids.org/pub/Report/2010/zimbabwe_2010_country_progress_report_en.pdf .
- 20.World Health Organization. Zimbabwe reported cases (vaccine-preventable diseases) http://apps.who.int/immunization_monitoring/en/globalsummary/timeseries/tsincidencebycountry.cfm?C=ZWE. Accessed 10 August 2011. [Google Scholar]
- 21.World Health Organization. Manual for the virological investigation of polio. Geneva, Switzerland: World Health Organization; 1997. Vol. WHO/EPI/GEN/97.01. [Google Scholar]
- 22.Troy S, Maldonado Y. Polioviruses. In: Long SS, Pickering LK, Prober CG, editors. Principles and practice of pediatric infectious diseases. 4th ed. China: Elsevier; 2012. http://www.amazon.com/dp/1437727026#reader_1437727026 . [Google Scholar]
- 23.Troy SB, Ferreyra-Reyes L, Huang C, et al. Use of a novel real-time PCR assay to detect oral polio vaccine shedding and reversion in stool and sewage samples after a Mexican national immunization day. J Clin Microbiol. 2011;49:1777–83. doi: 10.1128/JCM.02524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra S, Lo Y-R, Nyunt MZ, Macleod I. Management of HIV infection and antiretroviral therapy in infants and children: a clinical manual. India: World Health Organization; 2006. http://www.unicef.org/rosa/management_hiv.pdf . [Google Scholar]
- 25.Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8:e1002599. doi: 10.1371/journal.ppat.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouandjika-Vasilache I, Akoua-Koffi C, Begaud E, Dosseh A. No evidence of prolonged enterovirus excretion in HIV-seropositive patients. Trop Med Int Health. 2005;10:743–7. doi: 10.1111/j.1365-3156.2005.01454.x. [DOI] [PubMed] [Google Scholar]
- 27.Hennessey K, Lago H, Diomande F, et al. Poliovirus vaccine shedding among persons with HIV in Abidjan, Cote d'Ivoire. J Infect Dis. 2005;192:2124–8. doi: 10.1086/498166. [DOI] [PubMed] [Google Scholar]