To the Editor—Here, we discuss the residual risk of human immunodeficiency virus (HIV) transmission due to intermittent HIV shedding in semen during sustained long-term suppression of plasma viremia by antiretroviral therapy (ART) and the implications for serodiscordant heterosexual couples planning to conceive a child. There are several important issues to consider in these circumstances: (1) the residual risk of transmission, (2) the global impact of seminal HIV shedding during ART, and (3) the factors related to seminal HIV shedding that can be modified to further reduce the risk of HIV transmission.
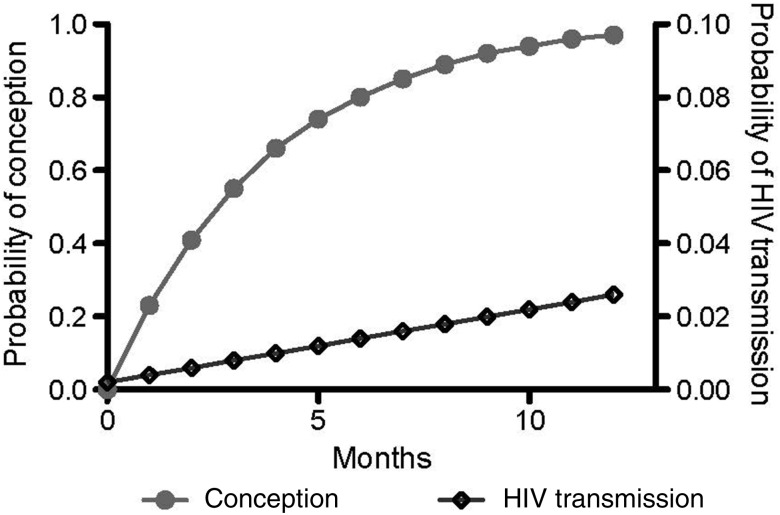
Among heterosexual discordant pairs, the HIV load in seminal plasma is the most predictive factor for HIV transmission [1]. Notably, HIV transmission can occur from individuals with low blood viral load (<1500 copies/mL) and those receiving successful ART [2–5]. In a recent study, only 1 of 103 genetically linked HIV transmissions was from an infected partner receiving ART, with a calculated rate of 0.37 transmissions per 100 person-years (95% confidence interval [CI], .09–2.04), compared with 2.24 transmissions per 100 person-years (95% CI, 1.84–2.72) for ART-naive partners, corresponding to 92% reduction [2]. This is similar to a pooled estimate of 0.46 transmissions per 100 person-years (95% CI, .19–1.09) for heterosexuals receiving ART [3]. Factoring a 92% reduction in HIV transmission during ART into the summary estimated risk of heterosexual transmission of 0.008 [6] results in a HIV transmission risk of 0.0007 per coital act involving a seropositive partner receiving ART. Although the risk of HIV transmission is likely a stochastic process (related to sporadic seminal HIV shedding), rather than a specific risk per episode of unprotected sex, this average estimate can be used to inform serodiscordant couples planning unprotected sexual activity for conception about the risk of HIV transmission. The probability of conception from a single coital act varies during the ovulatory cycle of the average woman, with estimates of 0.084 for day 12, 0.086 for day 13, and 0.081 for day 14 [7]. If couples had sex on all 3 days, the monthly cumulative probability of conception would be 0.23 [0.084 + (1–0.084)*0.086 + (1–0.079)*0.081], while the risk of transmitting HIV can be estimated similarly at 0.002. However, the probability of conception is highest during the first few months of trying, while the risk of HIV transmission likely accumulates steadily over time (Figure 1). This temporal probability trend should be taken into account when planning structured natural conception, to minimize the risk of HIV transmission.
Figure 1.
Theoretical cumulative monthly probability of conception and human immunodeficiency virus (HIV) acquisition over 1 year for a serodiscordant couple with average fertility and an HIV-infected male partner receiving antiretroviral therapy. Grey dots represent the cumulative probability of conception, whereas empty diamonds represents the cumulative probability of HIV transmission. Note the different scales in the y-axes for the risk of conception (0.0–1.0) and for HIV transmission (0.0–0.1).
In a broader view, the population effect of HIV shedding during ART in the United States will likely have the largest impact on men who have sex with men (MSM). We estimated the potential impact of transmission from MSM during ART by using a theoretical average of 2 unprotected anal sex acts per HIV-infected MSM per month with serodiscordant partners (California Collaborative Treatment Group, unpublished data), at a probability of transmission of 0.00136 per act (92% reduction from 0.017 [6]). On the assumptions that there are approximately 490 000 HIV-infected MSM living in the United States (Centers for Disease Control and Prevention, unpublished data, 2009) and that 59% are receiving ART, there are approximately 290 000 US MSM receiving ART. If sexually active men compose 85% of MSM [8], we estimate that approximately 8000 transmission events in the United States are related to MSM receiving ART every year, theoretically, that there are >1000 cases of transmitted drug resistance mutations (DRMs), assuming 15% rate of transmitted DRMs [9]. This suggests that a large pool of individuals receiving ART could still contribute substantially to the HIV epidemic.
Both in the case of serodiscordant couples trying to conceive children and in the overall HIV epidemic, additional measures for prevention of HIV transmission are needed. Our group focused on identifying factors associated with HIV seminal shedding in MSM [10, 11]. In ART-naive MSM, we identified that seminal shedding of cytomegalovirus (CMV), Epstein-Barr virus (EBV), and human herpes virus 8 were associated with increased HIV seminal shedding [10]; CMV and EBV were also associated with HIV transmission [11]. We recently demonstrated that high-level seminal CMV shedding is also associated with seminal shedding of HIV in MSM receiving ART [12]. Since we only included MSM in this study, it needs to be determined whether this remains true for heterosexual individuals trying to conceive. While the treatment of bacterial sexually transmitted infections is a recognized strategy to reduce HIV transmission [13], given our recent observations, an additional strategy might be the suppression of asymptomatic viral coinfections in the genital tract, especially those due to CMV. Other considerations for adjunctive prevention measures include preexposure prophylaxis with antiretroviral medication and alternative ART regimens. As suggested by Mounzer and DiNubile [14], there are still limitations of current methods to prevent HIV transmission among serodiscordant couples attempting to conceive a child, and this should lead to development of future strategies using combined approaches to minimize the risk.
Notes
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Department of Veterans Affairs, the James Pendleton Charitable Trust, the National Institutes of Health (awards AI69432, AI043638, MH62512, MH083552, AI077304, AI36214, AI047745, AI74621, GM093939 and AI080353, AI306214 [CFAR]), and the Swiss National Science Foundation (grant PASMP3_136983).
Potential conflicts of interest. D. M. S. has received grant support from ViiV Pharmaceuticals and consultant fees from Gen-Probe. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 4.Lu W, Zeng G, Luo J, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr. 2010;55:232–8. doi: 10.1097/QAI.0b013e3181e9b6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturmer M, Doerr HW, Berger A, Gute P. Is transmission of HIV-1 in non-viraemic serodiscordant couples possible? Antiviral Therapy. 2008;13:729–32. [PubMed] [Google Scholar]
- 6.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcox AJ, Dunson DB, Weinberg CR, Trussell J, Baird DD. Likelihood of conception with a single act of intercourse: providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001;63:211–5. doi: 10.1016/s0010-7824(01)00191-3. [DOI] [PubMed] [Google Scholar]
- 8.Schwarcz S, Scheer S, McFarland W, et al. Prevalence of HIV infection and predictors of high-transmission sexual risk behaviors among men who have sex with men. Am J Public Health. 2007;97:1067–75. doi: 10.2105/AJPH.2005.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheeler WH, Ziebell RA, Zabina H, et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS. 2010;24:1203–12. doi: 10.1097/QAD.0b013e3283388742. [DOI] [PubMed] [Google Scholar]
- 10.Gianella S, Morris SR, Anderson C, et al. Herpesviruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS. 2013;27:39–47. doi: 10.1097/QAD.0b013e3283573305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianella S, Morris SR, Vargas MV, et al. The role of seminal shedding of herpesviruses in HIV-1 transmission. J Infect Dis. 2012;207:257–61. doi: 10.1093/infdis/jis683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianella S, Morris S, Young JA, et al. Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1 infected men who have sex with men; 2013. [published online ahead of print April 17, 2013]. Clin Infect Dis. doi:10.1093/cid/cit252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez K, Bassett D, Schembri G, Lee V. STI screening in people living with HIV: are we getting the whole story? J Int AIDS Soc. 2012;15:18117. doi: 10.1177/0956462415575637. [DOI] [PubMed] [Google Scholar]
- 14.Mounzer KC, DiNubile MJ, et al. Seminal human immunodeficiency virus blips and structured natural conception in serodiscordant couples. J Infect Dis. 2013 doi: 10.1093/infdis/jit219. [DOI] [PubMed] [Google Scholar]