Abstract
Background. Increased intestinal permeability may be one of the mechanisms of transmission of human immunodeficiency virus (HIV) to infants through breast-feeding. Intestinal permeability correlates with microbial translocation, which can be measured through quantification of bacterial lipopolysaccharide (LPS).
Methods. We evaluated levels of plasma LPS (by the Limulus amebocyte lysate assay) and immune activation markers in serial specimens from infants exposed to but uninfected with HIV and infants infected with HIV from the Breastfeeding, Antiretrovirals, and Nutrition (BAN) study.
Results. Plasma LPS levels increased after infants in the BAN study were weaned from the breast, at 24 weeks of age. Cotrimoxazole prophylaxis was associated with higher plasma LPS levels (P = .004). Infants with HIV infection had higher LPS levels, compared with uninfected infants (P = .004). Higher preinfection plasma LPS levels were a significant predictor of infant HIV infection through breast-feeding (hazard ratio = 1.60 for every unit increase in plasma LPS level; P = .01) and of lower infant length-for-age z scores (P = .02).
Conclusions. These findings suggest that disruption in intestinal integrity is a mechanism of HIV transmission to infants through breast-feeding. Weaning from breast milk and use of antibiotic prophylaxis was associated with increased levels of microbial translocation, which could facilitate HIV entry through the intestine. Complementary approaches to enhance intestinal mucosal integrity in the infant may further reduce breast-feeding transmission of HIV.
Keywords: HIV, infant, breast-feeding, microbial translocation, immune activation, intestinal permeability
The majority of breast-fed infants of mothers infected with human immunodeficiency virus type 1 (HIV) remain uninfected despite ingesting liters of HIV-containing breast milk over several months [1]. The mechanisms of protection from infection and transmission are incompletely understood. In particular, much remains to be learned about the role of mucosal barriers and the innate and adaptive immune responses that defend infants against mucosal HIV transmission. Increased intestinal permeability may be one of the mechanisms facilitating HIV transmission through breast-feeding. In young infants, as the intestinal barrier matures, the tight junctions between enterocytes close and intestinal permeability decreases [2]. Gastrointestinal infections and malnutrition disrupt this maturational process and increase intestinal permeability, as does artificial (ie, formula) feeding [3]. This mechanism may account for the higher risk of HIV infection with mixed feeding, compared with exclusive breast-feeding [4]. A study showed that African infants displayed increasing intestinal permeability after 3 months of life, contrary to European infants, and by 12 months of life intestinal permeability was 5 times as high in African infants, compared with European infants [5]. Traditionally, assays to evaluate intestinal permeability have been based on differential absorption of carbohydrates (such as the lactulose-mannitol assay) [3, 5]. Such assays are cumbersome and time intensive, particularly for infants.
The intestinal epithelium acts as a barrier to luminal contents, including bacteria and endotoxins. Injuries to the intestinal barrier result in bacterial or endotoxin translocation from the intestinal lumen to the systemic circulation through the intestinal mucosa, in the absence of overt bacteremia [6]. This process is termed microbial translocation, and it can be quantified by measuring plasma levels of lipopolysaccharide (LPS), an endotoxin and component of gram-negative bacteria [6–8]. Intestinal permeability, measured by the lactulose-mannitol test, and systemic endotoxemia, measured by detection of LPS by the Limulus amebocyte lysate assay, showed a significant positive correlation in one study [6]. Elevated plasma LPS levels are indicative of disrupted intestinal mucosal integrity and can occur after invasive gastrointestinal surgery, in graft-versus-host disease, and in inflammatory bowel disease, as well as in HIV infection [6, 9–11]. LPS is a potent stimulator of macrophages, monocytes, and neutrophils and causes release of cytokines that are inflammatory mediators [11, 12].
We postulated that microbial translocation through the gut, a marker of disrupted intestinal mucosal integrity, may correlate with increased risk of acquisition of HIV infection through breast-feeding. We tested this hypothesis with samples from the Breastfeeding, Antiretrovirals, and Nutrition (BAN) study, a large prospectively followed cohort of breast-fed infants born to HIV-infected mothers. We studied plasma LPS levels and cellular and soluble markers of immune activation at various times during infants’ first year of life to determine the dynamics of microbial translocation through the gut and examine its relationship with feeding pattern (ie, breast-feeding vs weaned status), receipt of antibiotic prophylaxis, and infants' nutritional status; to explore the correlation of microbial translocation with cellular and soluble markers of immune activation; and to determine the role of microbial translocation in acquisition of HIV infection through breast-feeding.
METHODS
BAN Study Design and Subjects
All infants were enrolled in the BAN randomized, controlled trial, which has been described elsewhere [13]. Briefly, pregnant HIV-infected women with CD4+ T cell counts >250 cells/μL (≥200 cells/μL before 24 July 2006; the change accords with Malawi Ministry of Health guidelines) were enrolled and followed in Lilongwe, Malawi, from April 2004 to January 2010. All women provided written informed consent. The study protocol was approved by the Malawi National Health Science Research Committee and by institutional review boards at the University of North Carolina and the Centers for Disease Control and Prevention.
Mother-infant pairs were randomly assigned within 1 week of birth to a 2-group maternal nutritional intervention and to a 3-group antiretroviral intervention consisting of a triple-drug antiretroviral regimen for mothers (n = 849), daily nevirapine for infants (n = 852), or neither (control arm; n = 668). Irrespective of antiretroviral treatment assignment, all mothers in labor and their newborns were given a single dose of nevirapine and a 7-day course of zidovudine and lamivudine. The interventions began after delivery and were continued until breast-feeding cessation but for no longer than 28 weeks. Infants found to be HIV infected within the first 2 weeks of life were disenrolled from the study and referred for care. All mothers were counseled to breast-feed exclusively for the first 24 weeks of life, followed by a 4-week weaning period; a locally produced ready-to-use therapeutic food was provided as a weaning supplement [13]. Self-reported compliance with study feeding counseling was high—95% of mothers were breast-feeding at 24 weeks (88% were practicing exclusive breast-feeding)—but infants for >90% had been weaned by 28 weeks [14].
Infants were tested for HIV infection at birth and at 2, 12, 28, and 48 weeks of age with the Roche Amplicor 1.5 DNA polymerase chain reaction assay. HIV-infected infants were referred for care.
In accordance with the Malawi Ministry of Health and World Health Organization (WHO) guidelines, cotrimoxazole prophylaxis (240 mg once daily from 6 to 36 weeks of age or until weaning and HIV infection was ruled out) was initiated in infants in the BAN study in June 2006 [13]. Infant weight was measured at 0, 24, 28, 32, 36, 42, and 48 weeks of age, using Tanita digital infant scales (0.1-g increments), which were calibrated daily using a standard 1-kg weight. Recumbent length was measured using length boards made to UNICEF (United Nations Children's Fund) specifications (0.1-cm increments). Length-for-age and weight-for-age z scores were calculated using the WHO growth standards [15].
Infants were selected for the current analysis on the basis of the availability of serial samples of peripheral blood mononuclear cells (PBMCs) and plasma. The analysis included the 39 infants (19 males and 20 females) from the control arm who remained HIV uninfected and had available samples at 4 (n = 18) or 5 (n = 21) of the desired time points (birth, 12, 24, 36, and 48 weeks of age). The study also included a random sample of 30 infants (14 males and 16 females) who were determined to be infected perinatally (defined on the basis of a positive HIV DNA polymerase chain reaction test result within first 2 weeks of life); all 30 were from the group of 41 infants with available plasma and PBMCs at delivery. Twenty-six of the 30 perinatally infected infants received a diagnosis during the first 2 days of life and were thus considered to have acquired HIV in utero [16]. These infants provided samples only at the time of birth. In addition, 25 (17 males and 8 females) of the 93 BAN study infants who became HIV infected during breast-feeding (ie, after 2 weeks of life) and had plasma and PBMC specimens from at least 2 of the prespecified time points before infection and 1 time point after infection were included; 16 of these acquired HIV infection in the first 6 months of life, and 9 acquired infection in the second 6 months of life. These infants were from all BAN study arms. For time points before HIV was detected, these infants were considered HIV uninfected in the analyses. The median time of mother-reported weaning for infants who were HIV uninfected at 28 weeks of age was 24.6 weeks (interquartile range, 24.0–28.0 weeks). Almost all infants who were HIV infected by 28 weeks continued to breast-feed until the end of study follow-up, in accordance with current recommendations.
Cellular Markers
Lymphocyte immunophenotyping was performed as described elsewhere [17]. Briefly, cryopreserved PBMCs were quickly thawed, washed twice with complete medium (ie, Roswell Park Memorial Institute 1640 medium, 10% fetal bovine serum, penicillin/streptomycin, and l-glutamine), and resuspended at 5 × 106/mL in FACS buffer (phosphate-buffered saline containing 2% bovine serum albumin and 0.1% NaN3). An amount of 100 μL was stained at room temperature for 20 minutes and then washed with FACS buffer before fixing in 300 μL of 1% paraformaldehyde. The panel of markers used included the following: T-lymphocyte markers (CD3, CD4, and CD8), activation markers (CD38 and HLA-DR), and 7-AAD (for dead cell exclusion). All monoclonal antibodies used were from BD Biosciences (San Jose, CA). Acquisition of data and cell subset analysis were performed with FACSCalibur (BD Biosciences), using FlowJo software (Tree star).
Soluble Markers
Plasma LPS levels were quantified by the Limulus amebocyte lysate assay QCL-1000, according to the manufacturer's protocol (Cambrex Bioscience, Walkersville, MD). Briefly, samples were diluted 1:10 with endotoxin-free water, heat-inactivated at 65°C for 10 minutes, vigorously mixed, and tested in 96-well microplates according to the manufacturer's instructions. The optical densities (ODs) were converted into enzyme units (EU) per milliliter on the basis of a standard curve.
Plasma concentrations of soluble CD14 (sCD14; R&D Systems, Minneapolis, MN) and soluble L-selectin (sL-selectin; R&D Systems, Minneapolis, MN) were tested in duplicate by enzyme-linked immunosorbent assay per the manufacturer's specifications. The lower detection limits for LPS, sCD14, and sL-selectin were 0.1 EU/mL, 125 pg/mL, and 300 pg/mL, respectively.
Statistical Analysis
Results are reported as median values (interquartile ranges). Analysis of variance was determined using the Mann-Whitney U test. Spearman correlations (r and P values) were calculated to determine the relationships between plasma LPS levels, sCD14 levels, sL-selectin levels, and cellular activation markers. Linear mixed-effects models with an autoregressive covariance structure were used to evaluate the effect of infant age and HIV status on plasma levels of LPS and cellular and soluble markers and the effect of cotrimoxazole prophylaxis on plasma LPS levels, after adjustment for maternal CD4+ T-cell count at baseline and infant birth weight. Trends in plasma levels of LPS and cellular and soluble immune activation markers over the first year of life were assessed using nonparametric smoothing splines. Linear mixed models adjusted for study arm, maternal CD4+ T-cell count, time, infant birth weight, sex, and HIV status were used to evaluate whether plasma levels of immune markers were associated with z scores of infant weight-for-age and length-for-age. The Cox proportional hazards model was used to determine whether plasma levels were associated with time to infant HIV infection, after control for study arm, maternal CD4+ T-cell count, and birth weight.
RESULTS
Microbial Translocation and Immune Activation Increase During the First Year of Life in Infants Infected With HIV and Those Exposed to and Uninfected With HIV
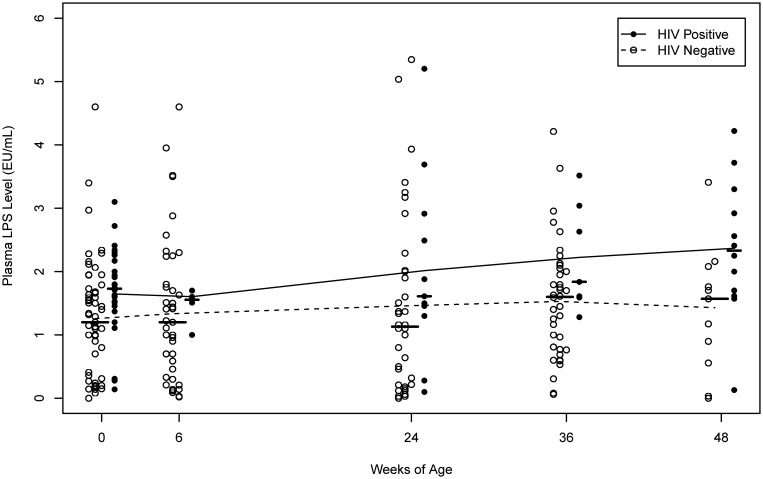
Plasma LPS, a marker of microbial translocation, was detectable in HIV-exposed but uninfected newborns, and levels remained stable until 24 weeks of age. Small increases in median plasma LPS levels were observed after the first 24 weeks of life in HIV-uninfected infants, but levels seemed to stabilize between 36 and 48 weeks (Table 1 and Figure 1). Infants with HIV infection displayed higher LPS levels at the time of birth and relatively stable levels until 36 weeks, with a subsequent increase at 48 weeks of age (Figure 1).
Table 1.
Plasma Levels of Lipopolysaccharide (LPS), Soluble CD14 (sCD14), Soluble L-Selectin (sL-Selectin), and Cellular Activation Markers in Infants, by Age and Human Immunodeficiency Virus (HIV) Status at Study Visit
| HIV Infected |
HIV Uninfected |
||||
|---|---|---|---|---|---|
| Marker, Infant Age | No. | Median (IQR) | No. | Median (IQR) | P |
| LPS level, EU/mL | |||||
| Delivery | 28 | 1.73 (1.41–2.17) | 58 | 1.20 (0.41–1.68) | .006 |
| 6 wk | 4 | 1.56 (1.26–1.65) | 43 | 1.20 (0.33–1.80) | .446 |
| 24 wk | 11 | 1.61 (1.30–2.92) | 36 | 1.13 (0.21–2.01) | .149 |
| 36 wk | 7 | 1.84 (1.59–3.04) | 38 | 1.60 (0.77–2.05) | .107 |
| 48 wk | 12 | 2.33 (1.66–3.11) | 11 | 1.57 (0.56–2.08) | .042 |
| sCD14 level, ng/mL | |||||
| Delivery | 28 | 793 (725–935) | 58 | 634 (501–813) | .004 |
| 6 wk | 4 | 1210 (1154–1447) | 43 | 1190 (966.8–1391) | .457 |
| 24 wk | 11 | 1841 (1288–2314) | 36 | 1393 (1123–1606) | .046 |
| 36 wk | 7 | 2323 (1862–2536) | 38 | 1477 (1245–1875) | .010 |
| 48 wk | 12 | 2107 (1910–2656) | 11 | 1410 (989.4–1859) | .004 |
| sL-selectin level, ng/mL | |||||
| Delivery | 28 | 882 (726–971) | 58 | 684 (635–762) | < .001 |
| 6 wk | 4 | 1273 (841.2–1508) | 43 | 1395 (1224–1719) | .294 |
| 24 wk | 11 | 2267 (1936–2591) | 36 | 1981 (1754–2235) | .081 |
| 36 wk | 7 | 2235 (1977–2850) | 38 | 2152 (1925–2426) | .266 |
| 48 wk | 12 | 2313 (2021–2736) | 11 | 2213 (2074–2563) | .735 |
| % CD4+CD38+HLA-DR+ | |||||
| Delivery | 28 | 0.70 (0.35–1.30) | 64 | 0.30 (0.20–0.50) | < .001 |
| 6 wk | 4 | 4.55 (3.15–7.75) | 44 | 0.95 (0.55–2.65) | .023 |
| 24 wk | 13 | 2.20 (1.00–3.30) | 37 | 1.00 (0.60–2.00) | .065 |
| 36 wk | 9 | 3.00 (1.90–3.20) | 36 | 2.00 (1.00–3.30) | .326 |
| 48 wk | 12 | 3.40 (2.15–5.15) | 11 | 3.00 (0.80–3.40) | .241 |
| % CD8+CD38+HLA-DR+ | |||||
| Delivery | 27 | 6.40 (2.20–16.0) | 55 | 0.50 (0.20–0.80) | <.001 |
| 6 wk | 4 | 23.0 (17.6–28.4) | 37 | 7.00 (2.00–12.8) | .012 |
| 24 wk | 13 | 14.1 (12.0–21.0) | 29 | 6.00 (2.00–13.0) | .018 |
| 36 wk | 7 | 16.0 (6.80–18.0) | 31 | 11.0 (4.00–15.0) | .187 |
| 48 wk | 12 | 15.2 (12.4–17.5) | 11 | 4.60 (3.20–12.7) | .029 |
Abbreviations: EU, enzyme units; IQR, interquartile range.
Figure 1.
Plasma bacterial lipopolysaccharide (LPS) levels, a marker of microbial translocation through the intestine, by infant age and human immunodeficiency virus (HIV) status at the particular BAN study visit. The median level at each study visit is indicated by a horizontal line, and the curves are estimated using nonparametric smoothing splines.
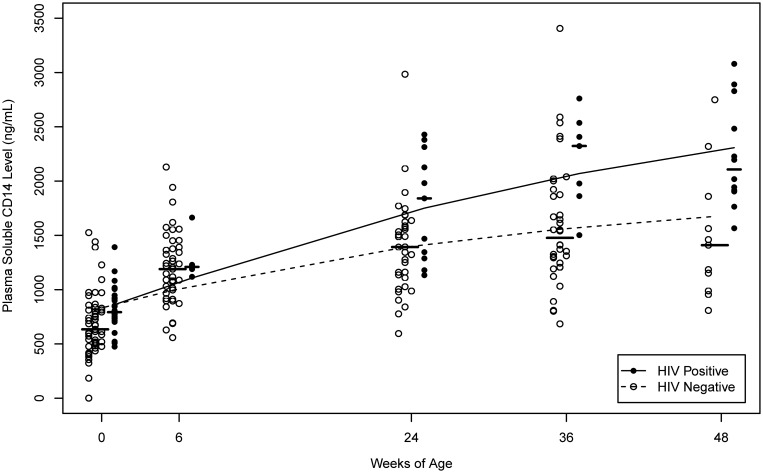
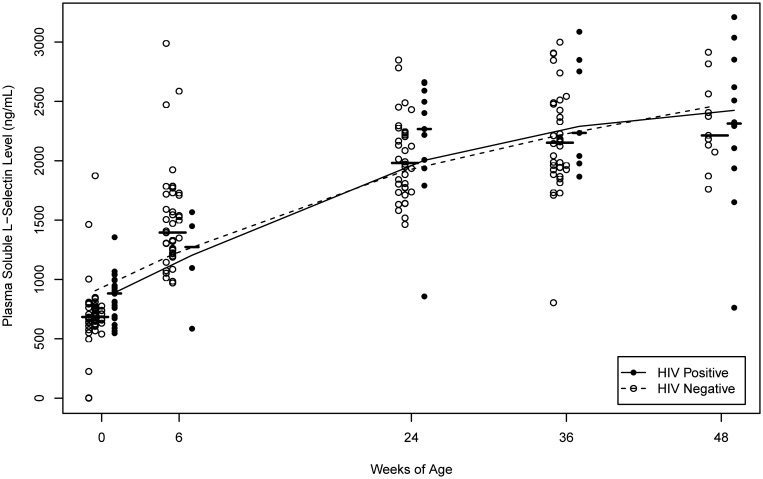
Plasma levels of sCD14, a marker of monocyte/macrophage activation, significantly increased with age for both groups of infants (P < .001; Figure 2). Increasing trends in sCD14 levels were noted earlier than for LPS levels in both HIV-infected infants and uninfected infants (Table 1 and Figures 1 and 2). Plasma levels of sL-selectin, a marker of lymphocyte activation, increased nonlinearly with age in both HIV-infected infants and uninfected infants (P < .001). The increase in sL-selectin level was most rapid very early in life, before weaning (Figure 3). Levels of CD4+ and CD8+ T-lymphocyte activation, measured by expression of the markers CD38 and HLA DR, also had a trend of increasing over the first year of life in both HIV-infected infants and uninfected infants (Table 1).
Figure 2.
Plasma soluble CD14 levels, a marker of monocyte/macrophage activity, by infant age and human immunodeficiency virus (HIV) status at the particular BAN study visit. The median level at each study visit is indicated by a horizontal line, and the curves are estimated using nonparametric smoothing splines.
Figure 3.
Plasma soluble L-selectin levels, a marker of lymphocyte activation, by infant age and human immunodeficiency virus (HIV) status at the particular BAN study visit. The median level at each study visit is indicated by a horizontal line, and the curves are estimated using nonparametric smoothing splines.
HIV-Infected Infants Have Higher Levels of Microbial Translocation and Immune Activation Than Uninfected Infants
Overall, median plasma LPS levels were higher in HIV-infected infants, compared with uninfected infants (P = .004 from a longitudinal mixed model; data not shown; Figure 1). The differences were statistically significant even at birth: infants with HIV infection detected in the first 2 days of life had a median LPS level of 1.73 EU/mL, compared with 1.20 EU/mL in uninfected infants (P = .006). At 48 weeks of age, HIV-infected infants also had higher median plasma LPS levels, compared with uninfected infants (P = .04) (Table 1).
Plasma levels of sCD14 were also elevated in HIV-infected infants, compared with uninfected infants (P = .002 from a longitudinal mixed model; data not shown; Figure 2). As with LPS, increased sCD14 levels in HIV-infected infants, compared with uninfected infants, were evident at birth (Table 1).
In contrast, there was no significant difference in sL-selectin levels between HIV-infected infants and uninfected infants except at the time of birth (Table 1 and Figure 3). At birth, HIV-infected infants (most of whom had in utero–acquired infections) had higher levels of lymphocyte activation than HIV-uninfected infants.
Levels of CD4+ and CD8+ T lymphocyte immune activation were generally higher in HIV-infected infants, compared with uninfected infants (Table 1). As expected, differences in activation levels between HIV-infected infants and uninfected infants were more pronounced for CD8+ T lymphocytes, rather than for CD4+ T lymphocytes, which are directly depleted by HIV (Table 1).
Immune Activation Markers Show Poor Correlation With Microbial Translocation
Levels of LPS and sCD14 from birth to 48 weeks of age were modestly (but statistically significantly) correlated in uninfected infants (r = 0.33, P < .001) but not in HIV-infected infants (r = 0.21, P = .107). Plasma LPS levels were not significantly correlated with sL-selectin levels in either HIV-infected infants (r = 0.21) or uninfected infants (r = 0.08).
LPS levels were modestly (but significantly) correlated with CD4+ T-lymphocyte immune activation in HIV-infected infants (r = 0.27, P = .04) but not in HIV-uninfected infants (r = 0.05, P = .47). There was no correlation between LPS and CD8+ T lymphocyte immune activation (r = 0.08 for HIV-uninfected infants, and r = 0.02 for HIV-infected infants).
Microbial Translocation Is Associated With HIV Transmission Through Breast-feeding, Infant Cotrimoxazole Prophylaxis, and Less Infant Growth
The preinfection plasma LPS level was a significant predictor of infant HIV infection through breast-feeding (hazard ratio = 1.60 for every 1 unit increase in plasma LPS level; P = .01, adjusted for study arm, maternal CD4+ T-cell count, and birth weight). Plasma levels of sCD14 or sL-selectin, on the other hand, were not significant predictors of infant HIV acquisition through breast-feeding. Receipt of cotrimoxazole prophylaxis by infants was a strong predictor of higher levels of plasma LPS (P = .004) and sCD14 (P < .001) in a linear regression model, after adjustment for maternal CD4+ T-cell count and infant birth weight. This association remained after removing data collected after 36 weeks of age from the model, when cotrimoxazole prophylaxis was stopped for HIV-uninfected infants. Plasma LPS levels were inversely associated with infant weight-for-age (P = .191) and length-for-age (P = .002) z scores, after adjustment for study arm, time, birth weight, maternal CD4+ T-cell count, and infant sex and HIV status. Each 1.0-EU/mL increase in LPS was significantly associated with a 0.05 decrease in weight-for-age z score and a 0.17 decrease in length-for-age z score. This equates to average decreases of 0.02 kg and 0.31 cm at birth and 0.06 kg and 0.41 cm by 1 year of age per 1.0-EU/mL increase in LPS level.
DISCUSSION
Our findings indicate that microbial translocation, as measured by plasma LPS levels, increased after the infants' first 6 months of life, which coincided with weaning from breast milk for HIV-uninfected infants in the study. Specifically, increases in plasma LPS levels were observed in the weeks that followed weaning (which occurred between 24 and 28 weeks of age), and levels seemed to stabilize by 48 weeks of life in uninfected infants. This pattern of postweaning increases in levels of microbial translocation temporally coincided with an increase in episodes of diarrhea that was previously reported in weaned infants in the BAN study [14]. Previous studies indicated that microbial translocation can be detected in healthy infants and young children but decreases after 2 years of age in healthy children [18]. To our knowledge, the impact of breast-feeding and weaning on LPS levels has not been reported. Our finding suggests that breast-feeding protects against disruption of the intestinal mucosal barrier or that artificial or formula feeding may stimulate gut inflammation. Breast milk contains a multitude of factors, such as lactoferrin or transforming growth factor β, with growth-promoting properties for the gut that help maintain the integrity of the epithelial barrier and may thus prevent microbial translocation [2, 19].
Plasma LPS levels in infants in this study were lower, compared with levels in those from a study of formula-fed infants [12]. Presence of LPS in infant formula or in the water used to dilute formula has been postulated as a reason to account for the early detection of LPS in formula-fed infants [12]. Plasma LPS levels in our study were comparable with those found in HIV-uninfected adults and children from Africa [18].
Compared with uninfected infants, HIV-infected infants had higher levels of microbial translocation at birth and at 48 weeks of life. Increased LPS levels have been observed in infants [12, 18], adults [20], and children [18, 21, 22] with HIV infection. This could be either the cause or the effect, or both, of HIV infection. It is now recognized that acute HIV infection causes depletion of mucosal lymphocytes, particularly subsets such as T-helper 17 cells in the gut, leading to disruption of the mucosal immune system and microbial translocation [23]. In young infants, the disruptions to the gut mucosa caused by HIV infection may be compounded by the physiologic immaturity of the gut [18, 24, 25]. However, the fact that most cases of HIV infection through breast-feeding in our study were predicted by prior increases in LPS, but not in immune activation markers, suggests a causal role of disrupted mucosal integrity in such transmission. A previous study did not find that increases in intestinal permeability measured by the lactulose-mannitol assay explained which infants became HIV-infected via breast-feeding [26]; however, assessments were made only in the first 14 weeks of life, and there were only 3 infants with confirmed HIV infection via breast-feeding by 14 weeks in that study.
Differences in gut microbiota may also affect levels of microbial translocation. This is supported by our finding that use of antibiotic prophylaxis was a predictor of increased plasma LPS levels, suggesting that disruption in the infants’ intestinal microflora, caused by the chronic administration of a broad-spectrum antibiotic, can lead to increased microbial translocation. Infant milk source and complementary foods are also known to affect the composition of gut flora [27]. Taken together, the observations of increases in microbial translocation with chronic antibiotic prophylaxis and after weaning from breast milk suggest a model in which disruption of the microbiota leads to increased levels of mucosal injury and immune activation. To our knowledge, this finding has not been previously reported. The use of cotrimoxazole prophylaxis in HIV-infected infants and HIV-exposed but uninfected infants has health benefits, as has been demonstrated in BAN and other studies [28–30]. Probiotics therapy or other approaches may need to be evaluated for their ability to reverse such adverse effects of antibiotics [31].
Microbial translocation was also correlated with slower infant linear growth in our study. This finding, to our knowledge, has not been previously reported. However, it is consistent with previous reports of association of increased intestinal permeability with infant growth retardation in resource-limited settings [5, 32, 33].
Microbial translocation is a force driving immune activation [9]. We found a modest correlation of plasma LPS levels with monocyte activity (measured by plasma sCD14 levels) in uninfected infants. However, LPS levels did not significantly correlate with lymphocyte activation (measured by expression of activation markers on CD4+ and CD8+ T lymphocytes and plasma levels of sL-selectin). Lack of correlation of LPS levels with lymphocyte activation markers has been reported in other studies, as well [12, 18, 22]. None of the immune activation markers was predictive of HIV transmission through breast-feeding, supporting the hypothesis that breeches in gut mucosa barrier are important for HIV transmission, rather than endotoxin-induced immune activation.
Our study has some limitations. HIV-uninfected infants were weaned at 6 months of age; thus, we were unable to compare the dynamics of LPS levels with those in corresponding infants who continued to breast-feed throughout the first year of life. Also, we did not have a control group of infants who received formula from birth. Varying degrees of mixed feeding likely occurred in our study cohort. The relatively small sample size studied precluded evaluation of a direct correlation of microbial translocation with gastrointestinal morbidity. All infants in our study were born to HIV-infected mothers in a resource-limited setting with a high background of endemic diarrhea, which could affect the generalizability of the results.
In conclusion, our findings indicate that levels of microbial translocation in infancy appear to increase with weaning from breast milk and with chronic receipt of antibiotic prophylaxis. Microbial translocation correlated modestly with monocyte and not with lymphocyte immune activation in the infants; microbial translocation was associated with poorer infant growth. Importantly, microbial translocation (but not immune activation) was a significant predictor of acquisition of HIV infection through breast-feeding after adjustment for covariates, suggesting that increased intestinal permeability may be one of the mechanisms of HIV infection through breast-feeding. These findings suggest that, in addition to antiretroviral prophylaxis, complementary pathways to further reduce HIV transmission through breast-feeding can be based on enhancing intestinal mucosal integrity. Such approaches may include promotion of more-prolonged breast-feeding and implementation of appropriate interventions, including vaccination (eg, rotavirus vaccine), hygiene-improvement activities (eg, water sanitation programs), and, perhaps, supplementation with probiotic or other preparations for infants receiving antibiotics, to minimize gastrointestinal infections.
Notes
Acknowledgments. We thank the BAN Study Team at the University of North Carolina–Chapel Hill, the Centers for Disease Control and Prevention, and UNC Project–Malawi, including Linda Adair, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Margaret Bentley, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, Charles Chasela, Charity Chavula, Joseph Chimerang'ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chiudzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, Denise Jamieson, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C. King, Rodney Knight, Athena P. Kourtis, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Charles van der Horst, Esther Waalberg, Elizabeth Widen, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, and Chifundo Zimba; G. Xia, S. Ramachandran, and Y. Khudyakov, for facilitating the HBV sequencing; and the women and infants who participated in the BAN study.
Disclaimer. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Prevention Research Centers Special Interest Project of the Centers for Disease Control and Prevention (grants SIP 13-01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944-01), the National Institute of Allergy and Infectious Diseases, the University of North Carolina Center for AIDS Research (grant P30-AI50410), the NIH Fogarty AIDS International Training and Research Program (grants DHHS/NIH/FIC 2-D43 Tw01039-06 and R24 Tw00798; the American Recovery and Reinvestment Act), and the Emory University Center For AIDS Research Immunology Core (grant P30A01050409). The antiretrovirals used in the BAN study were donated by Abbott Laboratories, GlaxoSmithKline, Boehringer Ingelheim, Roche Pharmaceuticals, and Bristol-Myers Squibb. The Call to Action PMTCT program was supported by the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children's Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson & Johnson, and the US Agency for International Development.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kourtis AP, Butera S, Ibegbu C, et al. Breast milk and HIV-1: vector of transmission or vehicle of protection? Lancet Infect Dis. 2003;3:786–93. doi: 10.1016/s1473-3099(03)00832-6. [DOI] [PubMed] [Google Scholar]
- 2.Sherman MP. New concepts of microbial translocation in the neonatal intestine: Mechanisms and Prevention. Clin Perinatol. 2010;37:565–79. doi: 10.1016/j.clp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catassi C, Bonucci A, Coppa G, et al. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21:383–6. doi: 10.1097/00005176-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant feeding patterns on early mother to child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African Vitamin A Study Group. Lancet. 1999;354:471–6. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 5.Lunn PG. The impact of infection and nutrition on gut function and growth in childhood. Proceed Nutr Society. 2000;59:147–54. doi: 10.1017/s0029665100000173. [DOI] [PubMed] [Google Scholar]
- 6.Schietroma M, Carlei F, Cappelli S, Amicucci G. Intestinal permeability and systemic endotoxemia after laparatomic or laparoscopic Cholecystectomy. Ann Surg. 2006;243:359–63. doi: 10.1097/01.sla.0000201455.89037.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suffredini AF, Hochstein HD, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O: 113 endotoxin. J Infect Dis. 1999;179:1278–82. doi: 10.1086/314717. [DOI] [PubMed] [Google Scholar]
- 8.Favre D, Ledere S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. Plos Pathog. 2009:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 10.Caradonna L, Amati L, Magrone T, et al. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–14. [PubMed] [Google Scholar]
- 11.Cooke KR, Olkiewicz K, Erickson N, Ferrara JL. The role of endotoxin and innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res. 2002;8:441–8. doi: 10.1179/096805102125001046. [DOI] [PubMed] [Google Scholar]
- 12.Papasavvas E, Azzoni L, Foulkes A, et al. Increased microbial translocation in less than 180 days old perinatally HIV-positive infants as compared with HIV-exposed uninfected infants of similar age. Pediatr Infect Dis J. 2011;30:877–82. doi: 10.1097/INF.0b013e31821d141e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Horst C, Chasela C, Ahmed Y, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33. doi: 10.1016/j.cct.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamieson DJ, Chasela CS, Hudgens MG, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48 week follow-up of the BAN randomized controlled trial. Lancet. 2012;379:2449–58. doi: 10.1016/S0140-6736(12)60321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 16.Bryson YJ, Luzuriaga K, Sullivan JL, et al. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327:1246–7. doi: 10.1056/NEJM199210223271718. [DOI] [PubMed] [Google Scholar]
- 17.Kourtis AP, Ibegbu CC, Theiler R, et al. Breast milk CD4+ T cells express high levels of C chemokine receptor 5 and CXC chemokine receptor 4 and are preserved in HIV-infected mothers receiving highly active antiretroviral therapy. J Infect Dis. 2007;195:965–72. doi: 10.1086/512082. [DOI] [PubMed] [Google Scholar]
- 18.Wallet MA, Rodriguez CA, Lin Y, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T cell activation following therapy. AIDS. 2010;24:1281–90. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planchon SM, Martins CA, Guerrant RL, et al. Regulation of intestinal epithelial barrier function. J Immunol. 1994;153:5370–9. [PubMed] [Google Scholar]
- 20.Cassol E, Malfeld S, Mahasha P, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–33. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 21.Anselmi A, Vendrame D, Rampon O, Giaquinto C, Zanchetta M, De Rossi A. Immune reconstitution in HIV type 1-infected children with different virological responses to antiretroviral therapy. Clin Exp Immunol. 2007;150:442–50. doi: 10.1111/j.1365-2249.2007.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilakka-Kanthikeel S, Huang S, Fenton T, Borkowsky W, Cunningham CK, Pahwa S. Increased gut microbial translocation in HIV-infected children persists in virologic responders and virologic failures after antiretroviral therapy. Pediatr Infect Dis J. 2012;31:583–91. doi: 10.1097/INF.0b013e31824da0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quing G, Howlett S, Bortolussi R. Lipopolysaccharide binding proteins on polymorphonuclear leucocytes: comparison of adult and neonatal cells. Infect Immun. 1996;64:4638–42. doi: 10.1128/iai.64.11.4638-4642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wold A, Adlerberth I. Breastfeeding and the intestinal microflora of the infant-implications for protection against infectious diseases. Adv Exp Med Biol. 2000;478:77–93. doi: 10.1007/0-306-46830-1_7. [DOI] [PubMed] [Google Scholar]
- 26.Rollins NC, Filteau SM, Coutsoudis A, Tomkin AM. Feeding mode, intestinal permeability, and neopterin excretion: a longitudinal study in infants of HIV-infected South African women. J Acquir Immune Defic Syndr. 2001;28:132–9. doi: 10.1097/00042560-200110010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen S, Nielsen DS, Lauritzen L, Jakobsen M, Michaelson K. Impact of diet on the intestinal microbiota in 10-month-old infants. J Pediatr Gastroenterol Nutr. 2007;44:613–8. doi: 10.1097/MPG.0b013e3180406a11. [DOI] [PubMed] [Google Scholar]
- 28.Kourtis AP, Wiener J, Kayira D, et al. Health outcomes of HIV-exposed uninfected African infants. AIDS. 2013;27:749–59. doi: 10.1097/QAD.0b013e32835ca29f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taha TE, Hoover DR, Chen S, et al. Effects of cessation of breastfeeding in HIV-1-exposed, uninfected children in Malawi. Clin Infect Dis. 2011;53:388–95. doi: 10.1093/cid/cir413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulenga V, Ford D, Walker AS, et al. Effect of cotrimoxazole on causes of death, hospital admissions and antibiotic use in HIV-infected children. AIDS. 2007;21:77–84. doi: 10.1097/QAD.0b013e3280114ed7. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham-Rundles S, Ahrne S, Johann-Liang R, et al. Effect of probiotic bacteria on microbial host defense, growth, and immune function in human immunodeficiency virus type-1 infection. Nutrients. 2011;3:1042–70. doi: 10.3390/nu3121042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133:1332–8. doi: 10.1093/jn/133.5.1332. [DOI] [PubMed] [Google Scholar]
- 33.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injurty, and growth faltering in Gambian infants. Lancet. 1991;338:907–10. doi: 10.1016/0140-6736(91)91772-m. [DOI] [PubMed] [Google Scholar]