Abstract
Background. Microbial translocation has been implicated in the pathogenesis of liver fibrosis and cirrhosis. We sought to determine whether markers of microbial translocation are associated with liver disease progression during coinfection with human immunodeficiency virus (HIV) and hepatitis C virus (HCV).
Methods. We measured serial plasma lipopolysaccharide (LPS), endotoxin core antibody, intestinal fatty acid–binding protein (I-FABP), soluble CD14 (sCD14), interleukin 6 (IL-6), interleukin 10, and tumor necrosis factor α (TNF-α) levels over a 5-year period in 44 HIV/HCV-coinfected women, 21 of whom experienced liver disease progression and 23 were nonprogressors.
Results. While LPS levels did not differ significantly over time between progressors and nonprogressors (P = .60), progressors had significantly higher plasma levels of sCD14, a marker of monocyte activation by LPS, at the first time point measured (P = .03) and throughout the study period (P = .001); progressors also had higher IL-6 and I-FABP levels over the 5-year study period (P = .02 and .03, respectively). The associations between progression and sCD14, I-FABP, and IL-6 levels were unchanged in models controlling for HIV RNA and CD4+ T-cell count.
Conclusions. Although LPS levels did not differ between liver disease progressors and nonprogressors, the association of sCD14, I-FABP, and IL-6 levels with liver disease progression suggests that impairment of gut epithelial integrity and consequent microbial translocation may play a role in the complex interaction of HIV and HCV pathogenesis.
Keywords: HIV, hepatitis C, microbial translocation, fibrosis, liver disease progression, soluble CD14
Liver disease, most often due to hepatitis C virus (HCV), is an increasingly common cause of morbidity and mortality among persons infected with human immunodeficiency virus (HIV) [1–3]. It is clear that HIV accelerates the course liver disease progression among coinfected persons, but the cause of this accelerated progression is not fully understood [1, 4].
Microbial translocation has been implicated in the pathogenesis of liver disease associated with alcoholism and graft versus host disease [5–7]. Kupffer cells, which are hepatic macrophages, can be activated by lipopolysaccharide (LPS). Free LPS binds to Kupffer cells via interaction with LPS binding protein and CD14 [8]. The LPS complex, via Toll-like receptor 4 (TLR4) and transcription factor nuclear factor κ-B, sensitizes hepatic stellate cells to transforming growth factor β and the activating effects of Kupffer cells. Activated stellate cells produce a matrix rich in type 1 collagen, leading to hepatic fibrosis [9]. How important these mechanisms are in HIV/HCV-associated liver disease is unclear.
Events early in HIV infection result in translocation of microbes and microbial products across the gut mucosa into the systemic circulation. The destruction of gut-associated lymphoid tissue occurs early in HIV infection through virus-induced apoptosis of CD4+ T lymphocytes of the intestinal submucosa [10–12] and increases the permeability of the gut. There are limited data on the influence of microbial translocation on HIV/HCV-associated liver disease progression during HIV infection. Balagopal et al, in a cross-sectional study of HIV/HCV-coinfected persons, demonstrated that low CD4+ T-cell counts and high LPS levels were independently associated with cirrhosis [13]. In a look-back study of 53 HIV-infected individuals with hepatitis C, elevated LPS levels were seen ≤1 year but not >1 year before cirrhosis diagnosis [13].
We sought to add to the understanding of the influence of microbial translocation on HIV/HCV-associated liver disease by comparing longitudinal plasma markers of microbial translocation, macrophage activation, and inflammation in HIV/HCV-coinfected women who experienced significant liver disease progression to markers in women who experienced minimal or no progression.
METHODS
This study involved women from the Chicago site of the Women's Interagency HIV Study (WIHS). The WIHS is a longitudinal study of HIV-infected and demographically similar uninfected women at 6 sites: Chicago, San Francisco Bay Area, Brooklyn and Bronx/Manhattan, New York, Washington, D. C., and Los Angeles. Women are seen semiannually for an interview, physical examination, and collection of blood and genital specimens. Informed consent was obtained from all participants in accordance with US Department of Health and Human Services guidelines and the institutional review boards of participating institutions. The cohort was designed to reflect the demographic characteristics of US women infected with HIV. Details of cohort recruitment, retention, and demographic characteristics are published elsewhere [14, 15].
Study subjects were HIV/HCV-coinfected women. HCV infection was defined as HCV antibody and RNA positivity at baseline measurement. Soluble markers of microbial translocation, intestinal mucosal integrity, and inflammation were measured from banked specimens, frozen at −80°C since collection. We compared markers from retrospectively defined intervals during which women manifested liver disease progression to intervals during which there was no clinical, pathological, or noninvasive-marker-based evidence of liver disease progression. Liver disease was ascertained by analysis of a liver biopsy specimen, confirmation of liver-associated death, or measurement of the following noninvasive markers: aspartate transaminase (AST) level to platelet count ratio index (APRI), calculated as ([AST level/upper limit of the normal AST level]/[platelet count]) × 100, where the AST level is expressed as units/liter and the platelet count is expressed as the number of platelets/liter multiplied by 109; and the fibrosis 4 (FIB-4) score, calculated as ([age × AST level]/[platelet count × ALT level])1/2, where the age is expressed in years, the AST and alanine transaminase (ALT) levels are expressed in units/liter, and the platelet count is expressed as the number of platelets/liter multiplied by 109 [16–19]. Liver-associated death was ascertained by review of death certificate. In the case of liver-associated death, the soluble markers were measured on a specimen obtained approximately 1 year before death to ensure that liver disease was present but to avoid measurement of markers that may reflect premortem infectious or inflammatory conditions.
Soluble markers were measured at 3 time points for each woman; time points were approximately 2.5 years apart. Time 1 (T1) for all women was a point when liver biopsy and both APRI (<0.5) and FIB-4 score (<1.45) indicated no or minimal fibrosis and self-report indicated no clinical evidence of end-stage liver disease. For liver disease progressors, T3 was a point 5 years later when there was biopsy-confirmed cirrhosis or bridging fibrosis, liver-associated death within approximately 1 year, or the APRI (>1.5) and FIB-4 score (>3.25) were consistent with cirrhosis. For nonprogressors, T3 was a point when there was no clinical evidence of end-stage liver disease and when liver biopsy or both APRI (<0.5) and FIB-4 score (<1.45) indicated no or minimal fibrosis. T2 was equidistant between T1 and T3, usually 2.5 years from each, with a range of 2 to 3 years.
APRI and FIB-4 score are the most commonly used noninvasive markers in the WIHS because the data necessary to calculate them are available at most WIHS visits. An APRI of >1.5 has been found to have area under the receiver operating curve of 0.76 for biopsy-proven significant fibrosis (METAVIR stages F2–4) and 0.82 for cirrhosis in a large meta-analysis including HCV-infected patients with and without HIV infection [20] and ranges of 0.71–0.82 for significant fibrosis and 0.81–0.92 and cirrhosis in large series [21]. The negative predictive values of an APRI of <0.5 for lack of significant fibrosis have been found to be 80%–91% in populations with a prevalence of fibrosis similar to that among WIHS participants [16, 20]. Several reviews have found that the accuracy of the APRI for cirrhosis was the same or better in studies of HIV/HCV-coinfected persons, compared with accuracy in studies of HCV-monoinfected persons [17, 20]. A FIB-4 score of >3.25 has been found to correlate with severe fibrosis (METAVIR stages F3–4), with an area under the receiver operating curve of 0.72–0.85, and a FIB-4 score of <1.45 has been found to have a negative predictive value of 95% for significant fibrosis in one large study of HIV/HCV-coinfected persons [20, 22].
Laboratory Methods
Plasma LPS levels were quantified in duplicate by dilution of plasma specimens to 10% with endotoxin-free water, using a Limulus amebocyte assay (Lonza Group, Switzerland). The background level was subtracted, and LPS levels were calculated by following the manufacturer's recommended protocol. Immunoglobulin M endotoxin core (EndoCAb) and intestinal fatty acid–binding protein (I-FABP) levels were quantified using an enzyme-linked immunosorbent assay (ELISA; Hycult Biotech, Uden, the Netherlands). Soluble CD14 (sCD14) levels were measured in plasma diluted to 1:200, and data were analyzed by ELISA according to the manufacturer's instructions (R & D Systems, Minneapolis, MN). Commercially available ELISAs were used to measure levels of tumor necrosis factor α (TNF-α; BD Biosciences, San Jose, CA), interleukin 6 (IL-6; R & D Systems), and interleukin 10 (IL-10; R & D Systems). Laboratory investigators were blinded to the liver disease progression status of participants.
Statistical Methods
Demographic and clinical characteristics were assessed at various periods (T1–3), which correlated with measures of the soluble plasma markers. At the initial study visit, age, mean CD4+ T-cell count, mean HIV RNA level, and injection drug or cocaine use in the previous 6 months (by self-report) was assessed. Additional characteristics evaluated included mean CD4+ T-cell count and mean HIV RNA level at each visit, use of highly active antiretroviral therapy during the study period, alcohol use (by self-report), and hepatitis C treatment. The crude association between progression and each categorical covariate was assessed using unadjusted odds ratios (ORs), 95% confidence intervals (CIs), and P values (calculated by the Fisher exact test); continuous variables were compared by the t test. Crude associations between progression and each plasma marker were also assessed at T1, T2, T3, and all visits, using t tests. Multiple generalized linear regression models (using Proc GLM) were fit to assess associations and slopes for each serum marker, stratifying by progression status and time and controlling for log HIV RNA level and CD4+ T-cell count. P-values from regression models are reported for demonstrating differences in slopes between progressors and nonprogressors for each plasma marker. All analyses were performed in SAS software, version 9.2 (SAS Institute, Cary, NC). Graphs were produced using STATA, version 10.0.
RESULTS
Forty-four participants in the Chicago WIHS site were studied, of whom 21 did and 23 did not experience progression of liver disease. Characteristics of the 44 women are in Table 1. The median interval between T1 and T3 was 4.9 years and did not differ between progressors (4.8 years) and nonprogressors (5.0 years). The majority of women in both groups were African American, and the mean age at T1 was 41.6 years. The CD4+ T-cell count was relatively preserved, with mean values at T1 of 421 cells/mm3 for progressors and 526 cells/mm3 for nonprogressors (P = .27); progressors had a significantly lower mean CD4+ T-cell count than nonprogressors on the basis of values from the entire study period (371 vs 492 cells/mm3; P = .02).
Table 1.
Demographic and Clinical Characteristics for 44 Liver Disease Progressor and Nonprogressor Study Subjects Coinfected With Human Immunodeficiency Virus (HIV) and Hepatitis C Virus in Whom Liver Disease Did or Did Not Progress
| Characteristic | Progressors (n = 21) | Nonprogressors (n = 23) | Univariate OR (95% CI) | Pa |
|---|---|---|---|---|
| Age at T1, y | 42.4 ± 7.4 | 40.8 ± 5.7 | … | .44 |
| Interval between T1 and T3, y | 4.8 ± 0.62 | 5.0 ± 0.33 | … | .17b |
| Race/ethnicity | ||||
| White, non-Hispanic | 8 (38.1) | 8 (34.8) | 1.04 (.31–3.52) | .62c |
| White, Hispanic | 0 (0) | 1 (4.3) | … | |
| African American, non-Hispanic | 13 (61.9) | 14 (60.9) | Reference | |
| CD4+ T-cell count, cells/mm3 | ||||
| T1 | 421.0 ± 267.8 | 526.0 ± 343.8 | … | .27b |
| All study visits (n = 131)d | 371.0 ± 271.4 | 491.7 ± 299.8 | … | .02b |
| CD4+ T-cell percentage | ||||
| T1 | 20.3 ± 8.5 | 27.0 ± 10.3 | … | .02b |
| All study visits (n = 131)d | 20.5 ± 10.4 | 27.4 ± 9.4 | … | .0001b |
| HIV RNA load, copies/mL | ||||
| T1 | 15 420.5 ± 30 190.4 | 12 156.1 ± 22 007.6 | … | .73b |
| All study visits (n = 131)d | 12 7894 ± 613 973 | 38 836.8 ± 185 216 | … | .28b |
| Visits during which HIV RNA load was undetectablee | 37/63 (58.7) | 36/68e (52.9) | 1.27 (.63–2.53) | .60 |
| HIV RNA load at T1 for those with a detectable HIV RNA load (n = 26) | 24 667.5 ± 36 585.8 | 19 079.3 ± 26 179.5 | … | .65b |
| HIV RNA load for those with a detectable HIV RNA load (n = 73)e | 213 830 ± 787 349 | 72 851.7 ± 251 275 | … | .31b |
| Visits during which HAART was usede | 36/63 (57.1) | 28/69 (40.6) | 1.95 (.98–3.90) | .08 |
| Maximum alcohol use, drinks/wk | ||||
| None | 7 (33.3) | 4 (17.4) | … | .16c |
| <8 | 5 (23.8) | 13 (56.5) | … | |
| 8–14 | 2 (9.5) | 2 (8.7) | … | |
| ≥15 | 7 (33.3) | 4 (17.4) | … | |
| Liver disease ascertainment | ||||
| By liver biopsy | 8 (38.1) | 8 (34.8) | … | |
| By confirmation of liver-associated death | 6 (28.6) | … | ||
| By serum marker analysis | 7 (33.3) | 15 (65.2) | ||
| Any hepatitis C treatment | 1 (4.8) | 1 (4.4) | 1.10 (.06–18.77) | >.999 |
| IDU since most recent visit before T1 | 2 (9.5) | 5 (21.7) | 0.38 (.07–2.21) | .42 |
| Cocaine use since most recent visit before T1 | 8 (38.1) | 6 (26.1) | 1.74 (.48–6.28) | .52 |
Data are mean ± SD or no. (%) of subjects, unless otherwise indicated. See Methods for definitions of T1 and T3.
Abbreviations: CI, confidence interval; HAART, highly active antiretroviral therapy; IDU, injection drug use; OR, odds ratio.
a By the Fisher exact test, unless otherwise indicated.
b By the t test.
c By global χ2 analysis.
d One subject did not have data for 1 visit.
e Data are for 3 visits per subject.
Relationship of Plasma Markers to Liver Disease Progression
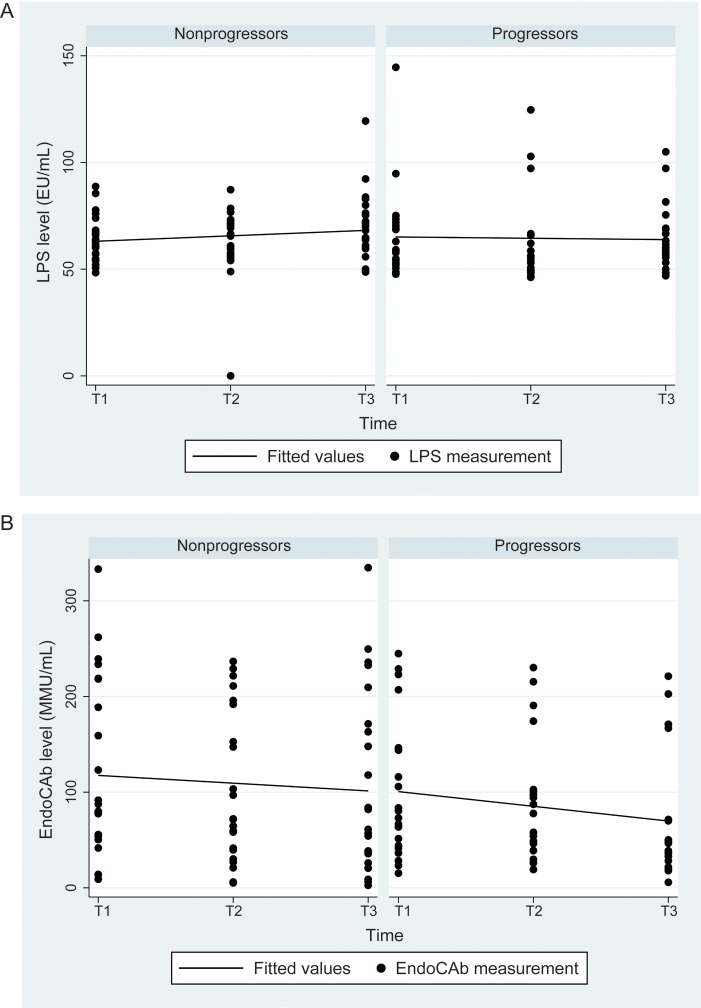
Table 2 summarizes the quantitative plasma marker data and the associations with liver disease progression, and Figures 1–4 depict the plasma markers values over time. Figure 1 shows LPS and EndoCAb levels over time in progressors and nonprogressors. There was no difference between progressors and nonprogressors in the LPS level or slope in unadjusted models or models with adjustment for HIV RNA load or CD4+ T-cell count. For EndoCAb, progressors had a nonsignificant trend toward a lower level overall (P = .07), which persisted in the model with HIV RNA load (P = .07) but was attenuated in the model with CD4+ T-cell count (P = .22). There was no change over time or difference in slope in crude models or those with adjustment for HIV RNA load and CD4+ T-cell count.
Table 2.
Associations Between Quantitative Plasma Marker Levels and Liver Disease Progression
|
Pa |
|||||
|---|---|---|---|---|---|
| Variable, by Time Point(s) | Overall | Progressors | Nonprogressors | Unadjusted | Adjustedb |
| T1 | n = 44 | n = 21 | n = 23 | ||
| LPS level, EU/mL (n = 43)c | 0.38 ± 0.33 | 0.35 ± 0.27 | 0.41 ± 0.38 | .53 | .59 |
| EndoCAb level, MMU/mL | 110.8 ± 82.5 | 99.5 ± 72.3 | 121.2 ± 91.3 | .39 | .42 |
| sCD14 level, pg/mL | 2359.9 ± 447.6 | 2510.7 ± 453.9 | 2222.2 ± 403.4 | .03 | .02 |
| IL-6 level, pg/mL | 3.1 ± 2.5 | 3.4 ± 2.0 | 2.9 ± 2.9 | .56 | .57 |
| IL-10 level, pg/mL | 10.6 ± 9.5 | 12.7 ± 12.5 | 8.8 ± 5.1 | .20 | .11 |
| TNF-α level, pg/mL | 2.2 ± 6.8 | 3.8 ± 8.8 | 0.6 ± 3.7 | .13 | .11 |
| I-FABP level, pg/mL | 476.1 ± 378.5 | 475.8 ± 356.6 | 476.4 ± 405.4 | .99 | .87 |
| T3 | n = 44 | n = 21 | n = 23 | ||
| LPS level, EU/mL | 0.32 ± 0.24 | 0.33 ± 0.18 | 0.32 ± 0.28 | .90 | .31 |
| EndoCAb level, MMU/mL | 87.7 ± 82.4 | 68.7 ± 63.6 | 105.0 ± 94.6 | .15 | .10 |
| sCD14 level, pg/mL | 2473.0 ± 607.6 | 2615.4 ± 603.7 | 2342.9 ± 594.3 | .14 | .33 |
| IL-6 level, pg/mL | 4.9 ± 3.8 | 5.5 ± 3.7 | 4.4 ± 3.9 | .33 | .69 |
| IL-10 level, pg/mL | 9.6 ± 4.7 | 9.9 ± 5.6 | 9.2 ± 3.7 | .63 | .74 |
| TNF-α level, pg/mL | 3.08 ± 8.1 | 3.2 ± 6.9 | 3.0 ± 9.2 | .94 | .97 |
| I-FABP level, pg/mL | 926.9 ± 799.3 | 1252.0 ± 1001.2 | 630.2 ± 380.2 | .01 | .001 |
| T1–3 | n = 132 | n = 63 | n = 69 | ||
| LPS level, EU/mL (n = 131)c | 0.37 ± 0.28 | 0.36 ± 0.25 | 0.37 ± 0.30 | .69 | .60 |
| EndoCAb level, MMU/mL | 97.9 ± 78.8 | 85.2 ± 66.7 | 109.4 ± 87.3 | .07 | .07 |
| sCD14 level, pg/mL | 2416.3 ± 531.0 | 2571.6 ± 483.6 | 2274.6 ± 536.0 | .001 | .003 |
| IL-6 level, pg/mL | 3.9 ± 3.3 | 4.6 ± 3.3 | 3.2 ± 3.2 | .02 | .04 |
| IL-10 level, pg/mL | 9.8 ± 6.9 | 11.0 ± 8.7 | 8.6 ± 4.6 | .05 | .04 |
| TNF-α level, pg/mL | 2.8 ± 7.7 | 3.8 ± 8.5 | 1.8 ± 6.9 | .13 | .14 |
| I-FABP level, pg/mL (n = 131)c | 673.2 ± 597.9 | 796.1 ± 734.5 | 559.4 ± 408.6 | .03 | .005 |
Data are mean ± SD. See Methods for definitions of T1–3.
Abbreviations: EndoCAb, antibody to endotoxin; EU, endotoxin units; I-FABP, intestinal fatty acid binding protein; IL-6, interleukin 6; IL-10, interleukin 10; LPS, lipopolysaccharide; MMU, immunoglobulin M median units; sCD14, soluble CD14; TNF-α, tumor necrosis factor α.
a By the t test.
b Adjusted for log human immunodeficiency virus RNA load.
c One subject did not have data for 1 visit.
Figure 1.
Plasma lipopolysaccharide (LPS) and endotoxin core antibody (EndoCAb) levels among liver disease progressors and nonprogressors over a 5-year period. A, There was no significant difference between progressors and nonprogressors in the LPS level in simple models (P = .70). There was also no significant difference in the slope, after adjustment for log human immunodeficiency virus (HIV) RNA level (P = .60) or CD4+ T-cell count (P = .63). B, There was a trend toward lower EndoCAb levels at all time points (P = .07). The statistical significance of the difference was unchanged in a model with log HIV RNA level (P = .07) but was attenuated in a model with CD4+ T-cell count (P = .22). There was no significant difference in slope. See Methods for definitions of T1–3. Abbreviations: EU, endotoxin units; MMU, immunoglobulin M median units.
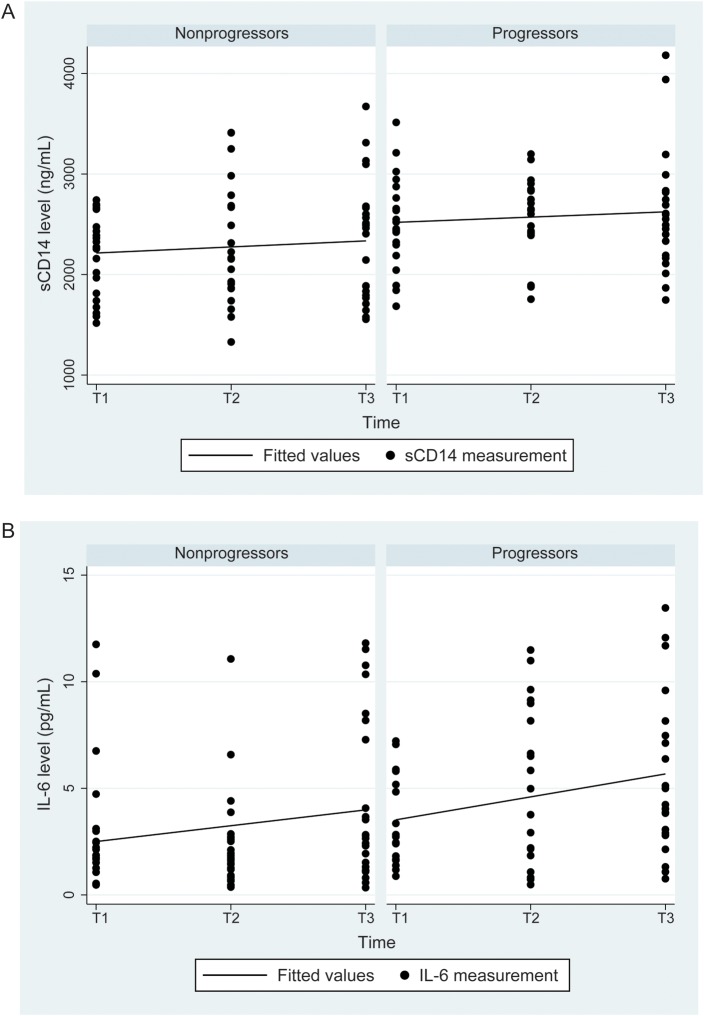
Figure 2.
Comparison of soluble CD14 (sCD14) and interleukin 6 (IL-6) levels between liver disease progressors and nonprogressors. A, For sCD14, levels were significantly higher in progressors overall (P = .001; P = .003 after adjustment for log human immunodeficiency virus [HIV] RNA load; P = .002 after adjustment for CD4+ T-cell count) and at T1 (P = .03). The association remained significant in a model that adjusted for log HIV RNA load and alcohol use (P = .01 over all time points). There was no difference in slope between progressors and nonprogressors after adjustment for log HIV RNA level (P = .75) or for log HIV RNA level and alcohol use (P = .94). B, For IL-6, levels were significantly higher in progressors for all visits (P = .02; P = .04 after adjustment for log HIV RNA load); adding alcohol use to the model attenuated the association to nonsignificance (P = .10). IL-6 levels increased significantly with time in progressors, with a slope of 1.1 (P = .03); the slope for nonprogressors was 0.74 (P = .12). However, when tested in the adjusted model with log HIV RNA load (P = .80) or in the adjusted model with log HIV RNA load and alcohol use (P = .62), the slopes did not differ. See Methods for definitions of T1–3.
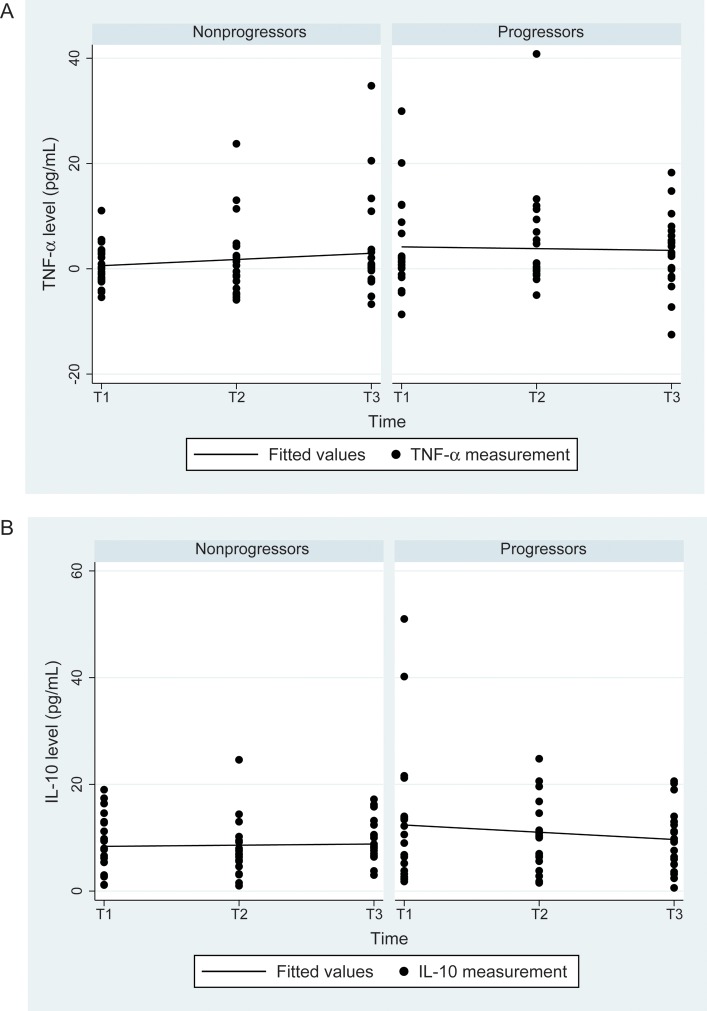
Figure 3.
Tumor necrosis factor α (TNF-α) and interleukin 10 (IL-10) levels over time in liver disease progressors and nonprogressors. A, For TNF-α, there were no differences in levels at all time points (P = .13) or in the slope between progressors and nonprogressors in simple or adjusted models. B, For IL-10, there was a moderately significant trend toward higher levels overall in progressors (11.0 vs 8.6 pg/mL; unadjusted P = .05; P = .04 after adjustment for log human immunodeficiency virus RNA load; P = .03 after adjustment for CD4+ T-cell count), but there was no difference in slope between progressors and nonprogressors in simple or adjusted models (P = .32). See Methods for definitions of T1–3.
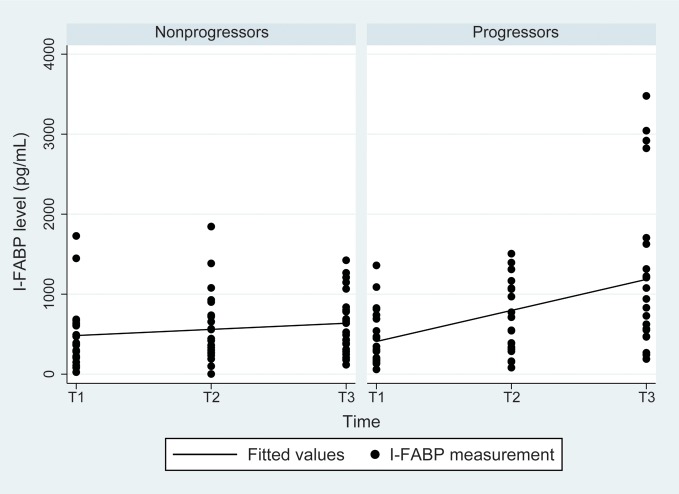
Figure 4.
Intestinal fatty acid–binding protein (I-FABP) levels over time in liver disease progressors and nonprogressors. The I-FABP level was significantly higher in progressors than nonprogressors at T3 (P = .01) and overall (P = .03). There was no significant difference at T1. Similar findings were found after adjustment for log human immunodeficiency virus (HIV) RNA level and CD4+ T-cell count. I-FABP levels increased significantly with time in progressors, with a slope of 388.1 (P = .0004); the slope for nonprogressors was 76.9 (P = .20). When tested in a model with adjustment for log HIV RNA level, the slopes were significantly different (P = .003). Similar findings were seen after adjustment for CD4+ T-cell count. See Methods for definitions of T1–3.
Figure 2 depicts levels of sCD14 and IL-6 in liver disease progressors and nonprogressors. The level of sCD14 was significantly higher in progressors at T1 (P = .03) and overall (P = .001), but the slope was not different (P = .75). When the HIV RNA load and CD4+ T-cell count data were added to the model testing the association between sCD14 level and liver disease progression, the relationship did not change (P = .003 and P = .002, respectively). Adding alcohol use to the model attenuated the association, but it remained significant (P = .01). Progressors had a higher level of IL-6 overall than nonprogressors (P = .02), and the level increased significantly over time for progressors (slope, 1.1; P = .03). In the models that adjusted for HIV RNA load and CD4+ T-cell count, the association between progression and IL-6 level remained significant (P = .04 and P = .04, respectively), but when alcohol was added to the model, the association attenuated to nonsignificance (P = .10).
Figure 3 shows results for TNF-α and IL-10 levels. TNF-α levels and slopes did not differ between progressors and nonprogressors. For IL-10, progressors had a higher level overall than nonprogressors (P = .05), with no change in relationship in adjusted models (HIV RNA load, P = .04; CD4+ T-cell count, P = .03) and no difference in slope. Figure 4 shows results for I-FABP. I-FABP levels were similar at visit 1 but significantly higher at visit 3 (P = .01) and overall (P = .03) in progressors. The I-FABP level increased significantly with time, with a slope of 388 for progressors (P < .001) and a slope of 76.9 for nonprogressors (P = .20). These findings were similar in models that included HIV RNA load and CD4+ T-cell count.
Relationship of Plasma Markers to HIV RNA Load
Two markers had significant relationships with HIV RNA level: sCD14 and IL-6 levels increased as the HIV RNA load increased (P = .0005 and P = .0007, respectively). Levels of LPS (P = .47), TNF-α (P = .86), IL-10 (P = .43), and EndoCAb (P = .47) showed no significant relationship with the HIV RNA level.
Relationship of Plasma Markers to CD4+ T-Cell Count
A higher IL-6 level was associated with a lower CD4+ T-cell count (P = .02), and a higher EndoCAb level were associated with a higher CD4+ T-cell count (P = .001). There was no significant relationship between CD4+ T-cell count and levels of LPS (P = .63), sCD14 (P = .34), IL-10 P = (.82), or TNF-α (P = .60).
DISCUSSION
In this study, we demonstrated that levels of markers of macrophage activation (sCD14), intestinal mucosal integrity (I-FABP), and inflammation (IL-6) were higher in HIV/HCV-coinfected women during intervals when significant liver disease progression occurred, compared with intervals when minimal or no progression occurred. While LPS levels did not differ between progressors and nonprogressors, there was a nonsignificant trend toward lower EndoCAb levels (P = .07), which may suggest increased clearance of LPS from the circulation in liver disease progressors, compared with nonprogressors [23].
Monocyte activation, as evidenced by elevated sCD14 levels, was associated with the most consistent and significant difference between progressors and nonprogressors, and the sCD14 level was significantly higher in progressors even at the first visit studied, 5 years before the liver disease end point. In the models with adjustment for HIV RNA level and CD4+ T-cell count, the relationship of the sCD14 level to liver disease progression remained highly significant (P = .003 and 0.002, respectively), although it was slightly attenuated when excess alcohol use was added to the model (P = .03). Although other stimuli may activate monocytes, an elevation in the sCD14 level is usually caused by LPS binding, and sCD14 is considered a marker of LPS-induced inflammation [24, 25]. The relationship between monocyte activation and liver disease progression is supported by what is known about the pathogenesis of liver fibrosis and from previous work involving HCV-monoinfected and HCV/HIV-coinfected persons. Kupffer cells, the hepatic macrophages, when stimulated by LPS, secrete inflammatory cytokines such as interleukin 1, IL-6, and TNF-α, which in turn lead to the activation of stellate cells. When activated, stellate cells produce a matrix rich in collagen, which leads to hepatic fibrosis [9]. In a cross-sectional study comparing patients with hepatitis B virus (HBV) or HCV monoinfection, with or without fibrosis, to uninfected controls, Sandler et al found that, while the LPS level was higher in patients with viral hepatitis, the level did not correlate with liver disease severity. However, the sCD14 level was associated with cirrhosis and markers of hepatic inflammation. As in our study, the downstream effects of microbial translocation, monocyte activation, and IL-6 secretion were associated with liver disease progression, but the LPS level itself was not. Of interest, Sandler et al found that a higher sCD14 level predicted nonresponse to hepatitis C therapy [25].
Our findings and those of Sandler et al contrast with results of a study by Balagopal et al, who found that a higher LPS level is associated with cirrhosis in HCV-infected persons with and those without HIV coinfection [13]. That the sCD14 level alone, rather than the LPS and sCD14 levels [25], is associated with liver disease progression in HIV/HCV-coinfected persons may reflect the rapid clearance of circulating LPS in vivo, the lack of bioactive LPS production by some translocated bacteria (eg, gram-positive organisms), or the limitations of the Limulus assay, which has been reported elsewhere [26]. Some investigators believe that sCD14 is a more relevant biomarker of microbial translocation because it reflects the host response to the stimulus rather than the stimulus itself [25]. Higher sCD14 levels may be a reflection of more cells responding to LPS or a genetic predisposition toward increased LPS responsiveness [27]. These findings also parallel findings by Douek et al, who observed that the sCD14 level, not the LPS level, predicts mortality in HIV-infected individuals, suggesting, again, that the host response to microbial translocation predicts outcome [28].
IL-6 levels increased with time in liver disease progressors, although when excess alcohol use was added to the model, the association was attenuated to nonsignificance. IL-6 is a marker of immune activation, hepatic inflammation, and regeneration and is secreted by T cells and macrophages (including Kupffer cells) in response to microbial products and other stimuli [29]. IL-6 production by monocytes can cause hepatic stellate cell proliferation [30]. IL-6 polymorphisms have been associated with more rapid disease progression in mild, untreated hepatitis C [31], and higher levels of IL-6 have been associated with nonresponse to HCV therapy in HIV/HCV-coinfected patients [32]. Elevated IL-6 levels may also be a consequence of liver disease progression. In our study, although IL-6 levels were approximately the same at T1, the slope of the increase in IL-6 levels over time was significantly higher among liver disease progressors than nonprogressors; a number of investigators have found that an elevated IL-6 level is associated with portal hypertension and decompensated hepatic cirrhosis in HIV-infected and HIV-uninfected patients [33]. Most likely, at the end stages of HIV/HCV-associated liver disease, microbial translocation is both a cause and effect of hepatic decompensation, leading to a vicious cycle of disease progression, as has been postulated by others [13]; the inflammatory effects of alcohol abuse further complicate the relationship.
I-FABP is a marker of enterocyte damage and has been found to correlate with severity of disease in patients with celiac disease [34] and intestinal ischemia [35]. Several investigators have found that higher I-FABP levels correlate with HIV disease itself [28] and with defective homing to the gut of CD4+ T-cell cells after therapy-associated immune recovery in patients with HIV infection [36]. In HBV- or HCV-monoinfected patients, Sandler et al found that I-FABP levels were positively correlated with other markers of microbial translocation and that levels decreased with successful treatment of viral hepatitis [25]. In our study, I-FABP levels were similar at T1 but increased significantly in liver disease progressors over the 5-year study period.
We found that progressors had higher IL-10 levels overall than nonprogressors and that levels remained higher over the period of observation. IL-10 is usually considered an antiinflammatory cytokine and has been found in WIHS and other studies to be associated positively with recovery in the CD4+ T-cell count. However, in patients with hepatitis C, the positive association of the IL-10 level and CD4+ T-cell count recovery was not seen [37]. In hepatitis C, elevated IL-10 levels due to monocyte activation have been described, and data from our cohort suggest that, in response to TLR3 and TLR4 in vitro, PBMCs from patients with IL-10 responses also secreted cytokines associated with inflammation, such as interleukin 1β, IL-6, and TNF-α [37], which may reflect activation of innate cells rather than an antiinflammatory response. IL-10 promoter polymorphisms have been associated with the ability to clear HCV infection [38]. Elevated levels of IL-10 due to monocytic activation have been previously described for patients with chronic HCV infection [39], and higher IL-10 (T-helper 2) responses in liver disease progressors could lead to suppression of the T-helper 1 cytokine response required for HCV clearance, augmenting hepatitis C progression [40].
Studying the effects of microbial translocation on hepatitis C progression is particularly relevant in HIV-coinfected patients because of the profound loss of gastrointestinal immune cells that characterizes early HIV infection. During acute and early infection, CD4+ T cells in the gastrointestinal tract, especially T-helper 17 cells, are lost, resulting in the loss of epithelial integrity and the translocation of microbes across the intestinal barrier, causing a generalized state of activation [41]. Brenchley et al found that a higher LPS level was associated with HIV disease stage, that effective HIV therapy was associated with a decreased LPS level, and that, in monkeys, antibiotic therapy to decrease intestinal bacterial concentration temporarily decreased the plasma LPS level [42]. However, subsequent investigations have not confirmed these observations [43].
Our findings should be considered in light of the limitations of our study. For many patients, we used noninvasive markers of fibrosis stage to define progressors and nonprogressors. While we used APRIs and FIB-4 scores at extremes that have been found to correlate highly with minimal or severe fibrosis, these measures are not the gold standard and may not accurately reflect liver histologic findings. We dichotomized women as either liver disease progressors or nonprogressors for the time points studied, but liver disease progression, in vivo, is a continuum. Women who were labeled nonprogressors may have experienced some liver disease progression during the 5-year study period. An obvious additional limitation is sample size: although the significant associations we found should be robust, we do not have power to assert a lack of association. Also, because of the sample size, we were unable to adjust for all variables that could potentially affect inflammation or liver disease progression.
Our findings of an association between markers of microbial translocation and liver disease progression suggest that further mechanistic and clinical study of this phenomenon is warranted. If further study confirms that microbial translocation contributes to the development of liver disease progression, interventions aimed at reducing translocation across the gut epithelium may have a salutary effect on the progression of liver disease in HIV/HCV-coinfected persons.
Notes
Financial support. This work was supported by the Chicago Consortium of the Women's Interagency HIV Study, which is funded by the National Institute of Allergy and Infectious Diseases (grants UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and the National Institute of Child Health and Human Development (grant UO1-HD-32632); the National Cancer Institute; the National Institute on Drug Abuse; the National Institute on Deafness and Other Communication Disorders; the National Center for Research Resources (grants MO1-RR-00071, MO1-RR-00079, and MO1-RR-00083); and the Chicago Developmental Center for AIDS Research (grant P30 AI-082151 to A. L. F.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weber R, Sabin CA, Friis-Moller N, et al. The Data Collection on Adverse Events of Anti-HIV Drugs Study Group. Liver-related deaths in persons infected with the human immunodeficiency virus. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 2.Lewden C, Salmon D, Morlat P, et al. Causes of death among HIV-infected adults in the era of potent antiretroviral therapy; emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–30. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 3.French AL, Gawel SH, Hershow RC, et al. Trends in mortality and causes of death for women with HIV in the US: a ten-year study. J Acquir Immune Defic Syndr. 2009;51:399–409. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–67. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Urbaschek R, McCuskey RS, Rudi V, et al. Endotoxin, endotoxin neutralizing capacity, sCD14, sICAM01 and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261–8. [PubMed] [Google Scholar]
- 6.Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease: role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:881–4. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 7.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft versus host disease. J Clin Invest. 1998;102:1882–91. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jerala R. Structural biology of LPS recognition. J Med Microbiol. 2007;297:353–63. doi: 10.1016/j.ijmm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Page EE, Nelson M, Kelleher P. HIV and hepatitis C coinfection: pathogenesis and microbial translocation. Curr Opin HIV AIDS. 2011;6:472–7. doi: 10.1097/COH.0b013e32834bbc71. [DOI] [PubMed] [Google Scholar]
- 10.Kotler DP. HIV infection and the gastrointestinal tract. AIDS. 2005;19:107–17. doi: 10.1097/00002030-200501280-00002. [DOI] [PubMed] [Google Scholar]
- 11.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veasey RS, Demaria MA, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell infection and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 13.Balogopal A, Philp FH, Asemborski J, et al. HIV-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–33. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 15.Hessol NA, Schneider M, Greenblatt RM, et al. Retention of women enrolled in a prospective study of HIV infection: impact of race, unstable housing and use of HIV therapy. Am J Epidemiol. 2001;154:563–73. doi: 10.1093/aje/154.6.563. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho-Filho RJ, Schiavon LL, Narciso-Schaivon JL, et al. Optimized cutoffs improve performance of aspartate aminotransferase to platelet ratio index for predicting significant liver fibrosis in HIV/HCV co-infection. Liver Int. 2008;1478:486–92. doi: 10.1111/j.1478-3231.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 17.Cacoub P, Carrat F, Bedossa P, et al. Comparison of non-invasive liver fibrosis biomarkers in HIV/HCV co-infected patients: the fibrovic study-ANRS HC02. J Hepatology. 2008;48:765–73. doi: 10.1016/j.jhep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Macias J, Giron-Gonzalez JA, Gonzalez-Serrano M, et al. Prediction of liver fibrosis in HIV/hepatitis C coinfected patients by simple non-invasive indexes. Gut. 2006;55:409–14. doi: 10.1136/gut.2005.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunes D, Fleming C, Offner G, et al. HIV infection does not effect the performance of noninvasive markers of fibrosis for the diagnosis of hepatitis C related liver disease. J Acquir Immune Defic Syndr. 2005;40:538–44. doi: 10.1097/01.qai.0000184856.31695.bf. [DOI] [PubMed] [Google Scholar]
- 20.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase to platelet ratio index for the prediction of hepatitis C related fibrosis: a systematic review. Hepatology. 2007;46:912–21. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 21.Stauber RE, Lackner C. Noninvasive diagnosis of hepatic fibrosis in chronic hepatitis C. World J Gastroenterol. 2007;13:4287–94. doi: 10.3748/wjg.v13.i32.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4; an inexpensive and accurate marker of fibrosis in HCV infection: comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 23.Windsor JA, Fearon KC, Ross JA, et al. Role of serum endotoxin and antiendotoxin core antibody levels in predicting the development of multiple organ failure in acute pancreatitis. Br J Surg. 1993;80:1042–6. doi: 10.1002/bjs.1800800840. [DOI] [PubMed] [Google Scholar]
- 24.Landmann R, Knopf HP, Link S, et al. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect immune. 1996;64:1762–69. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandler NG, Koh C, Roque A, et al. Host response to translocated microbial products predicts outcomes of patients with HBV and HCV infection. Gastroenterology. 2011;141:1220–30. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balagopal A, Gama L, Franco V, et al. Detection of microbial translocation in HIV and SIV infection using Limulus Amebocyte Lysate Assay is masked by serum and plasma. PLos One. 2012;7:e41258. doi: 10.1371/journal.pone.0041258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papasawas E, Pistilli M, Reynolds G, et al. Delayed loss of control of plasma lipopolysaccharide levels after therapy interruption in chronically HIV-1-infected patients. AIDS. 2009;23:369–75. doi: 10.1097/QAD.0b013e32831e9c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961–5. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toda K, Kumagai N, Tsuchimoto K, et al. Induction of hepatic stellate cell proliferation by LPS-stimulated mononuclear cells from patients with liver cirrhosis. J Gastroenterol. 2000;35:214–20. doi: 10.1007/s005350050333. [DOI] [PubMed] [Google Scholar]
- 31.Faleti E, Fabris C, Vandelli C, et al. Genetic polymorphisms of IL-6 modulate fibrosis progression in mild chronic hepatitis C. Hum Immunol. 2010;71:999–1004. doi: 10.1016/j.humimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Guzman-Fulgencio M, Jimenez JL, Berenguer J. Plasma IL-6 and IL-9 predict failure of interferon-α plus ribavirin therapy in HIV/HCV-coinfected patients. J Antimicrob Chemother. 2012;67:1238–45. doi: 10.1093/jac/dkr595. [DOI] [PubMed] [Google Scholar]
- 33.de-Oca MM, Marquez M, Soto MJ, et al. Bacterial translocation in HIV-infected patients with HCV cirrhosis: implications in hemodynamic alterations and mortality. J Acquir Immune Defic Syndr. 2011;56:42–7. doi: 10.1097/QAI.0b013e31820ef408. [DOI] [PubMed] [Google Scholar]
- 34.Adriaanse MP, Tack GJ, Passos VL, et al. Serum I-FABP as a marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharmacol Ther. 2013;37:482–90. doi: 10.1111/apt.12194. [DOI] [PubMed] [Google Scholar]
- 35.Pelsers MM, Hermens WT, Glatz JF. Fatty acid binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352:15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Mavigner M, Cazabat M, Dubois M, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest. 2012;122:62–9. doi: 10.1172/JCI59011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villacres M, Kono N, Mack W, et al. Interleukin-10 responses are associated with sustained CH3 T cell counts in treated HIV-monoinfection but not in HCV co-infection (abstract 780) 2012:374. doi: 10.1093/infdis/jis380. Program and abstracts of the 19th Conference on Retroviruses and Opportunistic Infections (Seattle, WA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paladino N, Fainbolm H, Theiler G, et al. Gender susceptibility to chronic hepatitis C infection associated with interleukin 10 promoter polymorphism. J Virol. 2006;80:9144–50. doi: 10.1128/JVI.00339-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woitas RP, Petersen U, Moshage D, et al. HCV-specific cytokine induction in monocytes of patients with different outcomes in hepatitis C. World J Gastroenterol. 2002;8:562–6. doi: 10.3748/wjg.v8.i3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reherman B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 41.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–54. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation as a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 43.Redd AD, Dabitao D, Breahm JH, et al. Microbial translocation, the innate cytokine response and HIV-1 disease progression in Africa. Proc Natl Acad Sci U S A. 2009;106:6718–23. doi: 10.1073/pnas.0901983106. [DOI] [PMC free article] [PubMed] [Google Scholar]