Abstract
Background. Nitric oxide (NO), a key macrophage antimycobacterial mediator that ameliorates immunopathology, is measurable in exhaled breath in individuals with pulmonary tuberculosis. We investigated relationships between fractional exhale NO (FENO) and initial pulmonary tuberculosis severity, change during treatment, and relationship with conversion of sputum culture to negative at 2 months.
Methods. In Papua, we measured FENO in patients with pulmonary tuberculosis at baseline and serially over 6 months and once in healthy controls. Treatment outcomes were conversion of sputum culture results at 2 months and time to conversion of sputum microscopy results.
Results. Among 200 patients with pulmonary tuberculosis and 88 controls, FENO was lower for patients with pulmonary tuberculosis at diagnosis (geometric mean FENO, 12.7 parts per billion [ppb]; 95% confidence interval [CI], 11.6–13.8) than for controls (geometric mean FENO, 16.6 ppb; 95% CI, 14.2–19.5; P = .002), fell further after treatment initiation (nadir at 1 week), and then recovered by 6 months (P = .03). Lower FENO was associated with more-severe tuberculosis disease, with FENO directly proportional to weight (P < .001) and forced vital-capacity (P = .001) and inversely proportional to radiological score (P = .03). People whose FENO increased or remained unchanged by 2 months were 2.7-fold more likely to achieve conversion of sputum culture than those whose FENO decreased (odds ratio, 2.72; 95% CI, 1.05–7.12; P = .04).
Conclusions. Among patients with pulmonary tuberculosis, impaired pulmonary NO bioavailability is associated with more-severe disease and delayed mycobacterial clearance. Measures to increase pulmonary NO warrant investigation as adjunctive tuberculosis treatments.
Keywords: tuberculosis, exhaled nitric oxide, L-arginine, M2 macrophages, biomarker
Investigation of tuberculosis pathophysiology provides the basis for research into tuberculosis adjunctive therapies and biomarkers. These fields are considered to be priority research areas as improved tuberculosis treatments are sought [1, 2]. Nitric oxide (NO) has a fundamental physiological role in innate immunity in tuberculosis. Derived chiefly from the amino acid L-arginine, its production in macrophages expressing the gene encoding nitric oxide synthase 2 (NOS2) provides a key component of the human immune response to Mycobacterium tuberculosis, both in mycobacterial killing and in ameliorating immunopathology (Table 1) [3–12]. M. tuberculosis, along with organisms in other bacterial genera, have specific immune-evasion strategies, including NO detoxification systems, to mitigate NO-mediated microorganism toxicity [13, 14], with variable susceptibility to NO [15]. Resistance of M. tuberculosis to reactive nitrogen species correlates with virulence in guinea pig models [16] and with resistance to isoniazid [17].
Table 1.
Evidence for the Role of Nitric Oxide (NO) in Tuberculosis Immunology
| Study Type, Results | Selected Reference(s) |
|---|---|
| M. tuberculosis in vitro studies | |
| Mycobacteria are susceptible to NO and other reactive nitrogen species in vitro | [16] |
| M. tuberculosis isolates differ in their susceptibility to reactive nitrogen intermediates: less susceptible isolates are more virulent in guinea pigs, and less susceptible isolates from human cases are less susceptible to antituberculosis drugs | [16, 17] |
| M. tuberculosis immune-evasion strategies include the induction of arginase expression (thereby decreasing NO availability) in macrophages | [18] |
| Mouse macrophage studies | |
| Arginine-derived reactive nitrogen intermediates in mouse macrophages effectively kill M. tuberculosis | [4] |
| M. tuberculosis lacking genetic resistance to reactive nitrogen intermediates cannot grow in mouse macrophages, in contrast with wild-type M. tuberculosis | [19] |
| Human macrophage studies | |
| Alveolar macrophages from healthy humans infected ex vivo with M. tuberculosis produce NO, and NO production correlates with intracellular growth inhibition of M. tuberculosis | [10] |
| Blood mononuclear cells from healthy donors infected ex vivo with M. tuberculosis and from people with preexisting tuberculosis produce NO | [7] |
| Pulmonary macrophages kill mycobacteria only if they express NOS2; killing is prevented with a NOS inhibitor | [9] |
| In vivo mouse studies | |
| NOS2 is expressed at sites of disease in immunocompetent mice but is deficient in immunocompromised mice with progressive tuberculosis | [20] |
| M. tuberculosis infection is poorly contained in mice treated with NOS inhibitors | [4] |
| Fulminant M. tuberculosis infection develops in NOS2 knockout mice (NOS2−/−) in contrast with controls | [20] |
| NO ameliorates inflammatory tissue damage in pulmonary tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β | [11] |
| In vivo human studies | |
| In lung resection studies, NOS2 and nitrotyrosine (a tissue marker of NO metabolism) are expressed in macrophages within granulomata and areas of tuberculosis pneumonitis | [6] |
| NOS2 expression is increased in peripheral blood monocytes from people with tuberculosis, compared with healthy controls | [8] |
| NOS2 is expressed in macrophages from lungs of patients with tuberculosis | [21] |
Abbreviations: IL-1β, interleukin 1β; M. tuberculosis, Mycobacterium tuberculosis; NOS2, nitric oxide synthase.
NO is measureable in exhaled breath, using validated chemiluminescence analyzers [21, 23]. Determinants of fractional exhaled NO (FENO) are shown in the Supplementary Panel. Analysis of breath components provides a noninvasive means of directly sampling the site of pathology in pulmonary tuberculosis. Differences in exhaled breath characteristics among people with tuberculosis, compared with controls, include significantly lower pH [24] and elevated levels of volatile metabolites [25]. Investigations of FENO in pulmonary tuberculosis to date have been limited by small sample sizes, post hoc analyses, and/or different methods for FENO measurement and have provided conflicting results, including reports of both increased [21] or decreased [26] FENO in patients with tuberculosis, compared with controls.
FENO has potential as a tuberculosis biomarker. Ideal biomarkers should be able to predict clinical and microbiological responses, identify active tuberculosis, and normalize with therapy [2]. Novel biomarkers capable of predicting early treatment response are sought for use as clinical trial outcome measures, because of the impracticality of relying on traditional, later tuberculosis treatment end points [2].
Given the immunological rationale and inconclusive findings to date, we aimed to investigate FENO in patients with pulmonary tuberculosis and healthy controls in a tropical setting where the tuberculosis burden is high. Our objectives were to determine the accuracy and precision of FENO measurement in the field, compare FENO in patients with pulmonary tuberculosis and healthy volunteers, investigate relationships between FENO and pulmonary tuberculosis severity, and determine the relationship between change in FENO and microbiological response (ie, sputum culture conversion at 2 months and time to sputum microscopy clearance).
METHODS
This study was performed as part of a clinical trial of adjunctive treatment with L-arginine and vitamin D for tuberculosis in Timika, Papua Province, Indonesia (clinicaltrials.gov identifier NCT00677339). In this study, neither intervention affected FENO [27]. The Timika population comprises people of Papuan (Melanesian) and non-Papuan (Asian) ethnicity. Eligible study participants had pulmonary tuberculosis, were enrolled at the tuberculosis clinic, were aged ≥15 years, were sputum smear–positive for acid fast bacilli, and provided written informed consent, with demographic details and human immunodeficiency virus (HIV) seroprevalence reported previously [28]. Standard tuberculosis treatment comprised rifampicin, isoniazid, pyrazinamide, and ethambutol for 2 months and then rifampicin and isoniazid for 4 months. Local healthy controls were aged ≥18 years, gave written informed consent, and had no comorbidities. Data (ie, age, sex, ethnicity, weight, and FENO only) from additional healthy controls from the same location whose results have been reported elsewhere [29] were also made available for this analysis. Enrollments of patients with tuberculosis and controls occurred across all months; environmental conditions in Timika, comprising tropical temperatures (25°C–30°C daily maxima) and high humidity (65%–90%), show minimal seasonality. The study was approved by the Human Research Ethics Committees of Menzies School of Health Research, Darwin, Australia, and the National Institute for Health Research and Development, Jakarta, Indonesia.
Procedures
FENO was measured using a portable NiOX MINO device (Aerocrine, Sweden). This device is well validated [22] and employs single-use, disposable, filtered mouthpieces without infection control risks. FENO measurements complied with 2005 American Thoracic Society guidelines [30]. FENO was measured on 1 occasion in healthy controls and serially, at baseline and 1 week, 2 weeks, 1 month, 2 months, and 6 months after baseline, in patients with tuberculosis. Quality control measures comparing results from biological controls (staff) or foil bags filled with varying NO concentrations were compared on a weekly basis throughout the 20-month study period between the NiOX MINO and a gold standard nonportable NiOX FLEX (not located at the tuberculosis clinic), calibrated fortnightly with 200 parts per billion (ppb) NO.
Chest radiographs, pulmonary function tests, a 6-minute walk test, and a locally adapted St George's Respiratory Questionnaire (SGRQ; in Indonesian) were performed at baseline and at 2 and 6 months. Chest Timika Tuberculosis X-ray were scored according to a previously reported method [31]. Pulmonary function (forced vital capacity [FVC] and forced expiratory volume in 1 minute [FEV1]) was measured outdoors (for infection control purposes), using a handheld spirometer (MicroLoop), with individual-use, filtered, 1-way mouthpieces (Sure-Gard) suitable for use in smear-positive pulmonary tuberculosis. Percentage of predicted FEV1 was calculated from local normal reference ranges [32]. A 6-minute walk test was assessed according to standard procedures [33].
Sputum microscopy was undertaken at the Timika laboratory weekly for 2 months and then at months 5 and 6. Baseline and month 2 sputum cultures were processed at the World Health Organization (WHO)–accredited laboratory at University of Indonesia, Jakarta, using the Bactec Mycobacterium Growth Indicator Tube 960 system. Conversion of sputum smear findings was defined as ≥2 consecutive M. tuberculosis–negative smears without a subsequent M. tuberculosis–positive smear. Conversion of sputum culture to negative at 2 months is a standard early measure of tuberculosis treatment response [2].
Statistical Methods
Analyses were undertaken using Stata, 12.1. Scatterplots and the Pearson correlation coefficient were used to evaluate differences in FENO within and between analyzers. FENO data were log normal; geometric means were compared between patients with tuberculosis and healthy controls, using the Student 2-sample t test, and between different time points using paired t tests. Associations between FENO and other variables were tested using univariate and multivariate regression models. Regression coefficients were exponentiated and interpreted as geometric mean ratios. Distribution of residuals was checked for normality. The relationship between FENO and weight was displayed graphically using values predicted from the regression model of FENO against weight. Incremental changes in FENO (ΔFENO) were calculated as [log FENO at week 8]−[log FENO at week 0]. The association between ΔFENO and culture or smear conversion at week 8 was tested using logistic regression, and receiver-operator characteristic (ROC) scores, given as areas under the curve (AUCs), were compared using χ2 tests.
RESULTS
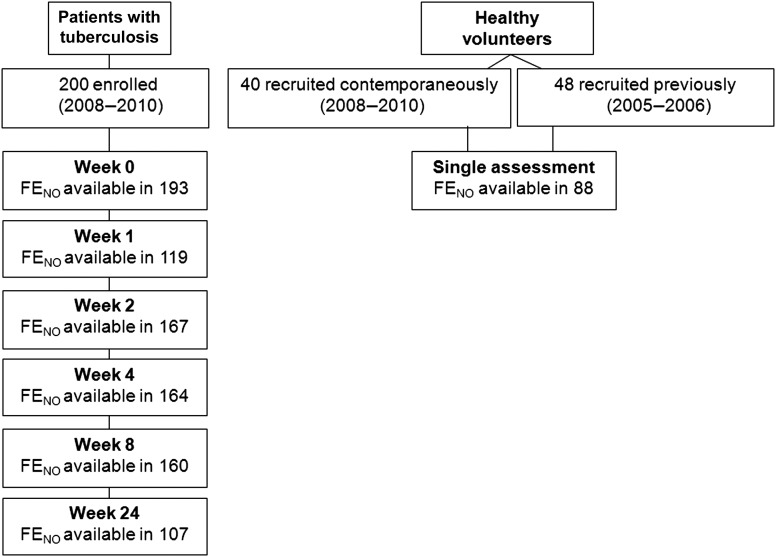
Two hundred participants with pulmonary tuberculosis and 40 healthy controls were enrolled during June 2008–February 2010, and 48 additional controls were enrolled in 2005 (Figure 1). Patients with pulmonary tuberculosis had lower weight, pulmonary function measures, 6-minute walk test results and worse SGQR scores than controls. Greater percentages of patients with pulmonary tuberculosis were non-Papuans and ex-smokers (Table 2).
Figure 1.
Number of patients with tuberculosis and healthy volunteers enrolled in the study and availability of fractional exhaled nitric oxide (FENO) data.
Table 2.
Baseline Characteristics of Study Participants
| Healthy Controls |
||||
|---|---|---|---|---|
| Characteristic | Patients With Tuberculosis (n = 200) | Contemporaneous (n = 40) | Previous (n = 40) | Pa |
| Age, y, median (range) | 28 (15–73) | 29 (18–65) | 27 (18–42) | .2 |
| Papuan | 89 (45) | 20 (50) | 44 (92) | <.0001 |
| Female sex | 69 (35) | 9 (23) | 17 (35) | .4 |
| HIV positive | ||||
| Overall | 19/145 (13) | … | … | |
| Papuan | 15/71 (21) | … | … | |
| Non-Papuan | 4/74 (5) | … | … | |
| Weight, kg, mean (range) | 48.5 (26.3–74.0) | 62.5 (41.6–84.0) | 59.8 (45.0–85.0) | <.0001 |
| Body mass index,bmean (range) | 19.2 (12.0–32.5) | 24.3 (17.1–33.6) | … | <.0001 |
| Height, m, mean (range) | 1.58 (1.35–1.79) | 1.61 (1.48–1.73) | … | .08 |
| Smoking status | ||||
| Current | 56 (28) | 18 (45) | 23 (48) | <.0001 |
| Ex-smoker | 53 (27) | 1 (3) | 3 (6) | |
| Never smoked | 91 (46) | 21 (53) | 22 (46) | |
| FENO, ppb, geometric mean (95% CI) | 12.7 (11.6–13.8) | 16.2 (12.2–21.5) | 17.0 (14.1–20.5) | .002 |
| FVC, L, mean (range) | ||||
| Female sex | 1.62 (0.63–2.96) | 1.91 (1.20–2.99) | … | <.0001 |
| Male sex | 2.36 (0.84–4.45) | 3.37 (1.57–4.22) | … | |
| FEV1, L, mean (range) | ||||
| Female sex | 1.43 (0.63–2.47) | 1.71 (1.18–2.52) | … | <.0001 |
| Male sex | 2.03 (0.59–4.00) | 3.20 (2.16–5.30) | … | |
| Percentage of predicted FEV1, mean (range) | ||||
| Female sex | 62.8 (31.0–99.0) | 81.0 (52.6–108.5) | … | <.0001 |
| Male sex | 63.6 (16.5–108.5) | 95.3 (67.8–148.4) | … | |
| SGRQ score, U, median (range) | 40.7 (5.2–91.9) | 0 (0–9.23) | … | <.0001 |
| 6-min walk test, m, median (range) | ||||
| Female | 375 (20–490) | 460 (360–528) | … | <.0001 |
| Male | 425 (40–612) | 511 (370–640) | … | |
| Chest radiograph finding | ||||
| Cavity size in cm | ||||
| 0 | 69 (44) | … | … | |
| ≤4 | 53 (34) | … | … | |
| >4 | 35 (22) | … | … | |
| Percentage of lung affected, median (IQR) | 40 (23–62) | … | … | |
| Radiography score, median (IQR) | 68 (34–94) | … | … | |
| Sputum acid fast bacilli density | ||||
| 0 or scanty | 55 (28) | … | … | |
| 1+ | 57 (29) | … | … | |
| 2+ | 50 (25) | … | … | |
| 3+ | 38 (19) | … | … | |
| Sputum culture result at diagnosis | ||||
| No growth, contaminated, or no result unavailable | 36 (18) | … | … | |
| M. tuberculosis identified | 164 (82) | … | … | |
| M. tuberculosis susceptibility, proportion tested (%)c | ||||
| Fully susceptible | 126/149 (85) | … | … | … |
| Monoresistance or other (not MDR) | 23/149 (15) | … | … | |
| INH and RIF resistant (MDR) | 2 /149 (1) | … | … | |
Data are no. of subjects with the characteristic/no. tested (%) or no. (%) of subjects, unless otherwise indicated.
Abbreviations: CI, confidence interval; FENO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 minute; FVC, forced vital capacity; HIV, human immunodeficiency virus; INH, isoniazid; IQR, interquartile range; MDR, multidrug resistant; M. tuberculosis, Mycobacterium tuberculosis; ppb, parts per billion; RIF, rifampin; SGRQ, St George's Respiratory Questionnaire.
a Patients with tuberculosis versus all available controls.
b Calculated as the weight in kilograms divided by the height in meters squared.
c Reasons for missing culture results at baseline and the 2-month follow-up visit included specimen contamination, specimen loss during transit, power outage in the laboratory, or participant loss to follow-up <2 months after baseline.
FENO Measurement
The average difference between paired FENO measurements obtained within 15 minutes by an individual using a single NiOX MINO analyzer/sensor was −0.4 ppb (95% limits of agreement, −6.2 to 5.5) and by an individual using 2 different analyzers/sensors was 0.8 ppb (95% limits of agreement, −4.3 to 5.8; Figure 2A and B). FENO values obtained from the NiOX MINO portable analyzers were highly correlated with those from the NiOX FLEX gold standard analyzer (Pearson R, 0.95) but consistently lower (Figure 2C). Rather than applying a correction factor, we used raw FENO results because NiOX MINO devices are now commonly used by ourselves and others in field research [26, 34].
Figure 2.
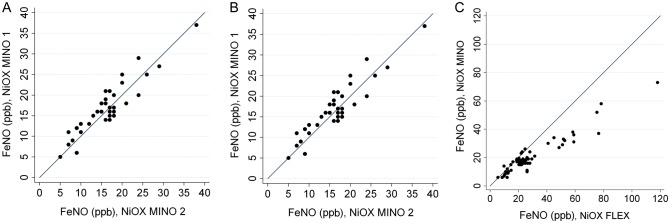
A, Repeated fractional exhaled nitric oxide (FENO) measurements using 1 NiOX MINO analyzer. Dots show individual data points, and the line represents y = x. B, Paired FENO measurements using 2 NiOX MINO analyzers. Dots show individual data points, and the line represents y = x. C, Paired FENO measurements using NiOX MINO (portable analyzer) and NiOX FLEX (gold standard analyzer). Dots show individual data points, and the line represents y = x. Abbreviation: ppb, parts per billion.
Patients With Tuberculosis Versus Healthy Controls
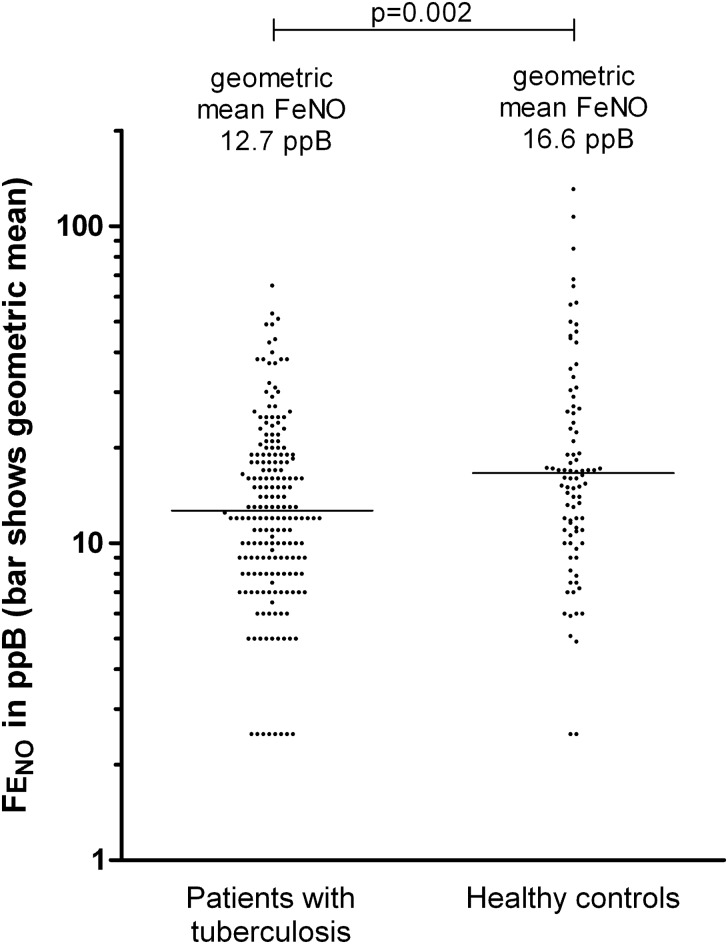
The FENO was significantly lower in patients with tuberculosis (geometric mean FENO, 12.7 ppb; 95% confidence interval [CI], 11.6–13.8) than in controls (geometric mean FENO, 16.6 ppb; 95% CI, 14.2–19.5; P = .002; Figure 3). The relationship between weight and FENO seen in patients with pulmonary tuberculosis at baseline was different from that observed in the controls (P = .02 for test of interaction): weight was directly proportional to FENO in patients with pulmonary tuberculosis, but this finding was not observed among healthy volunteers (Figure 4A). The difference in FENO between patients and controls was unchanged when controlling for covariables or when restricting analyses to only contemporaneously enrolled controls.
Figure 3.
Fractional exhaled nitric oxide (FENO) in healthy controls and patients with pulmonary tuberculosis at diagnosis. Horizontal lines indicate geometric mean values. Abbreviation: ppb, parts per billion.
Figure 4.
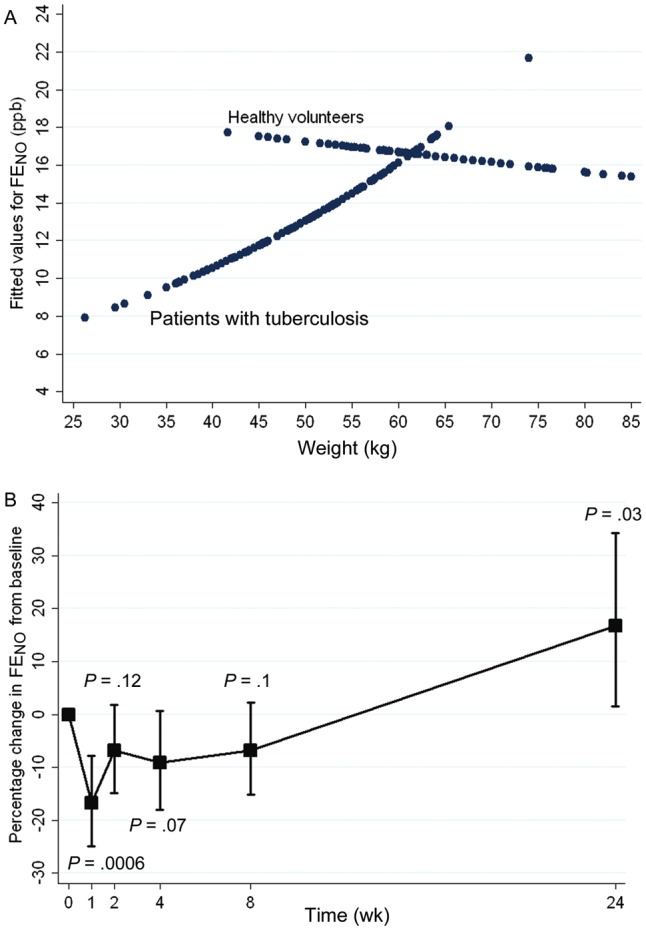
A, Fitted values for fractional exhaled nitric oxide (FENO), by weight, in patients with pulmonary tuberculosis and healthy controls. B, Change in FENO between diagnosis and treatment completion in patients with tuberculosis. Mean values (squares) and 95% confidence intervals (whiskers) are shown. P values relate to differences in FENO, compared with baseline. Abbreviation: ppb, parts per billion.
Determinants of Baseline FENO
Associations between clinical measures and FENO in controls and patients with pulmonary tuberculosis at baseline are shown in Table 3; regression coefficients were calculated for continuous variables according to groups as shown. Among patients with pulmonary tuberculosis, measures signifying greater tuberculosis severity, such as lower weight (or lower body mass index [BMI; calculated as the weight in kilograms divided by the height in meters squared), worse pulmonary function measures, lower hemoglobin level, and higher radiograph score, were each associated with lower FENO. However, these factors only accounted for a small proportion of the overall variation in FENO (eg, for each 10-kg weight increment, FENO increased by a factor of 1.25; Table 3). When controlling for weight (or BMI), the only remaining significant association with FENO, apart from weight/BMI, was FVC. FENO was not significantly different between HIV-positive patients with tuberculosis (geometric mean FENO, 11.2 ppb; 95% CI, 7.8–16.2) and HIV-negative patients with tuberculosis (geometric mean FENO, 13.3 ppb; 95% CI, 11.8–15.0; P = .3), but ascertainment of HIV status was incomplete (Table 2). Among healthy controls, no predictors of FENO were identified. The difference in FENO between smokers (geometric mean FENO, 15.1 ppb; 95% CI, 11.9–19.3) and nonsmokers (geometric mean FENO, 18.3 ppb; 95% CI, 14.7–22.9) was not statistically significantly different (P = .2).
Table 3.
Univariate Analyses of Associations Between Fractional Exhaled Nitric Oxide (FENO) and Clinical Variables
| Patients With Tuberculosis |
Healthy Controls |
|||
|---|---|---|---|---|
| Variable | GMR (95% CI) | P | GMR (95% CI) | P |
| Female sex (vs male sex) | 0.82 (.75–.91) | .05 | 1.22 (.86–1.73) | .3 |
| Age (per 10 y) | 1.06 (.97–1.15) | .2 | 0.98 (.79–1.21) | .8 |
| Weight (per 10 kg) | 1.24 (1.15–1.28) | <.001 | 0.97 (.81–1.15) | .7 |
| Height (per 10 cm)a | 1.13 (1.07–1.20) | .03 | 1.06 (.67–1.69) | .8 |
| Smoking status | ||||
| Current smoker | 1.01 (.81–1.26) | .9 | 0.83 (.60–1.14) | |
| Ex-smoker | 1.11 (.89–1.39) | .4 | NAb | .2 |
| Nonsmoker | 1 (reference) | 1 (reference) | ||
| Papuan (vs non-Papuan) | 1.00 (.83–1.20) | 1.0 | 1.39 (.98–1.99) | .07 |
| HIV positive (vs HIV negative) | 0.85 (.60–1.18) | .3 | … | … |
| FVC (per 1 L)a | 1.24 (1.10–1.39) | .001 | 0.86 (.56–1.33) | .5 |
| FEV1 (per 1 L)a | 1.23 (1.07–1.40) | .003 | 1.02 (.66–1.57) | .9 |
| 6-minute walk test (per 200 m)a | 1.15 (.96–1.38) | .1 | 0.56 (.23–1.39) | .2 |
| SGRQ score (per 50 U)a | 0.78 (.60–1.03) | .08 | 0.43 (.00–84.6) | .7 |
| WBC count (per 10 × 109/L)a | 0.70 (.53–.94) | .02 | 1.04 (.35–3.10) | .9 |
| Hemoglobin level (per 10 g/dL)a | 1.74 (1.16–2.62) | .007 | 0.76 (.31–1.87) | .5 |
| Timika Tuberculosis X-ray score (per 50 U) | 0.86 (.76–.98) | .03 | … | … |
| Sputum acid fast bacillus density (per 1 grade) | 1.00 (.91–1.09) | .9 | … | … |
Data are factor increases in FENO. For example, among patients with tuberculosis, for each 10-y age increment, the FENO increases by a factor of 1.06.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 minute; FVC, forced vital capacity; GMR, geometric mean ratio; HIV, human immunodeficiency virus; NA, not available; SGRQ, St George's Respiratory Questionnaire; WBC, white blood cell.
a Data are only available for 40 healthy controls. Other values are available are available for all 88 controls.
b Too few values were available for comparison.
FENO During Follow-up
After starting tuberculosis treatment, the low baseline FENO fell further to 10.7 ppb (95% CI, 9.4–12.1), with a nadir at 1 week (P = .0006, compared with baseline FENO), and then gradually recovered by treatment completion, to 15.1 ppb (95% CI, 13.3–17.2; P = .03), creating a J-shaped curve (Figure 4B). The FENO at tuberculosis treatment completion was not statistically significantly different from that obtained from healthy controls (16.6 ppb; 95% CI, 14.2–19.5). The longitudinal trend in FENO among patients with tuberculosis was not observed in the quality control measures involving healthy staff members.
Association With Microbiological Outcomes
The proportion of study participants whose 2-month sputum specimen was culture negative for M. tuberculosis was 78.9% (97/123) [27]. Change in FENO from baseline to 2 months (ΔFENO) was significantly associated with sputum culture conversion by 2 months. Specifically, people whose FENO remained unchanged or increased were approximately twice as likely to have achieved conversion to M. tuberculosis–negative sputum cultures by 2 months, compared with individuals whose FENO decreased (odds ratio, 2.72; 95% CI, 1.05–7.12; P = .04). The mean absolute change in 2-month FENO was +1.6 ppb in those achieving culture negativity and –4.7 ppb in those remaining culture positive (P = .02).
The diagnostic usefulness of ΔFENO compared with sputum smear conversion time for predicting 2-month culture conversion is shown in Supplementary Figure 1. The ROC score for ΔFENO (AUC, 0.64) was lower than desirable for a reliable diagnostic test and lower than that for sputum smear conversion (AUC, 0.77), but the difference in these AUCs was not statistically significant (P = .1, by the χ2 test).
In addition to the association with culture conversion, people in whom the FENO stayed the same or increased had a slightly shorter time to sputum smear conversion than those whose FENO decreased (OR, 1.06; 95% CI, 1.00–1.12; P = .06). Changes achieved in FENO were unrelated to baseline weight (P = .9), weight at week 8 (P = .8), or change in weight between weeks 0 and 8 (P = .3), according to regression analyses. Inclusion of these weight variables in multivariable models had a negligible effect on the relationship between ΔFENO and culture or microscopy conversion.
DISCUSSION
In this study, the largest investigation to date of the FENO in patients with pulmonary tuberculosis, we provide the first human in vivo evidence that pulmonary NO bioavailability is significantly associated with both microbiological outcomes and reduced disease severity. Additionally, we show that FENO is low in patients with tuberculosis, compared with controls, lower still in patients with more-severe disease, and recovers over time. This provides clinical support for in vitro data implicating NO as an important component of the human antimycobacterial immune response. Because NO kills M. tuberculosis bacilli and ameliorates immunopathology [3, 4, 11], it is biologically plausible that a low pulmonary NO level, due to impaired production or increased NO clearance, would be associated with more severe disease and worse outcomes, as we have shown.
FENO shows promise as a biomarker of tuberculosis treatment response; however, further work is required, given the importance of FENO determinants other than tuberculosis [23, 35] (Supplementary Panel), the wide overlap with values obtained from healthy controls, and the modest increments seen in ΔFENO. The diagnostic usefulness of ΔFENO in predicting culture conversion is limited (Supplementary Figure 1), but interestingly is not statistically significantly worse than sputum conversion time, a widely used measure of response to tuberculosis treatment.
NO kills M. tuberculosis by direct bacterial cell damage and induction of apoptosis of M. tuberculosis–harboring macrophages [4]. NO is capable of killing tuberculosis bacilli in vitro with a molar potency comparable to that of antibiotics [19]. At low concentrations, NO also plays a role in driving M. tuberculosis into a nonreplicating persister state, resistant to antibiotics [36], through upregulation of a dormancy regulon [37]. Thus, high NO concentrations are mycobactericidal, but subtoxic NO levels contribute to intracellular M. tuberculosis persistence. Impaired NO production as seen in this study may thus be doubly disadvantageous to the host.
As we have reviewed elsewhere [3], animal studies demonstrate that impaired NO production is associated with greater degrees of lung damage [4]. NO ameliorates inflammatory tissue damage in pulmonary tuberculosis by inhibiting NLR3 inflammasome-dependent processing of interleukin [11] and production of tumor necrosis factor α (TNF-α), a mediator of caseating necrosis of lung tissue and weight loss in tuberculosis. Although the pulmonary pathology of tuberculosis in murine models differs from that in humans, results from our study and others [21, 26, 34] suggest a disease-protective role for NO in people, not just mice. Importantly, lung function (FVC) was independently associated with FENO in our patients with tuberculosis in a multivariable model.
An early study of pulmonary NO in tuberculosis found that alveolar macrophage NOS2 gene expression correlated significantly with exhaled NO level [21]. FENO was significantly higher in 19 people with newly diagnosed pulmonary tuberculosis, compared with 14 control subjects, but the FENO measurement technique involved a now-obsolete method (exhalation at an uncontrolled flow rate of around 200 mL/s through an open tube, with a probe sampling from the stream of expired breath). The FENO was inversely associated with tuberculosis severity, as we have also shown, and normalized by 3 months of treatment [21]. A decade later, Idh and colleagues reported lower FENO in patients with pulmonary tuberculosis, compared with controls, using a chemiluminescent analyzer and an exhalation flow rate of 50 ± 5 mL/seconds [26], as used here. In further work by the same group, the median baseline FENO was approximately 15 ppb in patients with pulmonary tuberculosis overall, which did not significantly change during 8 weeks of follow-up, and also was unaffected by supplementation with an arginine-rich food (peanuts) [34]. In this study, post hoc subgroup analyses detected increased cure rates in the HIV-positive patients given arginine-rich food, but in the comparative arm of 32 HIV-positive patients randomly assigned to the non–arginine-rich food, the cure rate was anomalously low, at 53%. Notably, they reported that low baseline eNO (<10 ppb) in HIV-positive patients with tuberculosis was associated with a decreased cure rate. Including our data, the cumulative evidence suggests that poorer pulmonary NO bioavailability during active tuberculosis is detrimental to the host.
Adjunctive therapy with inhaled NO has been tested in 8 patients with tuberculosis and 10 controls [38]. Inhaled NO (80 ppb for 72 hours, from day 2 after starting tuberculosis treatment) was well tolerated but did not have a significant impact on microbiological outcomes. Mean times to sputum culture conversion were 35.5 days in the NO group and 37.2 days in controls. Cystic fibrosis (CF) is another lung disease associated with a low FENO [39] and impaired NOS2 expression in bronchial epithelial cells [40]. Pulmonary NO deficiency may be an important factor in CF patients’ susceptibility to pulmonary bacterial colonization [23]. A clinical trial of inhaled NO in 13 CF patients also demonstrated safety but failed to show benefits [41]. Inhaled L-arginine offers a potentially longer-acting solution to the challenge of enhancing pulmonary NO production, compared with inhaled NO itself, given the short half-life of NO and requirement for prolonged administration regimens. A clinical trial of inhaled L-arginine (1.3 g) versus placebo (normal saline) in CF showed that FENO increased significantly for several hours, and a significant improvement in FEV1 was sustained for over 24 hours after inhalation of L-arginine, but not placebo [39].
It is likely that a combination of factors results in impaired ability to produce NO, since NO bioavailability depends on substrate (L-arginine) availability, arginase production, NOS2 expression, and NOS2 function, as well as the presence of NO-quenching molecules. L-arginine, the main NO source in humans, is a conditionally essential amino acid. Thus, hypoargininemia can develop when catabolism exceeds supply and has been demonstrated in tuberculosis [42] and other infectious diseases [3]. Micro- and macronutrient deficiencies are well-recognized in tuberculosis; it is plausible that a vicious cycle could develop between increasing tuberculosis severity, escalating nutritional deficiencies, decreased NO formation, and impaired NO-dependent macrophage antituberculosis effects. Arginase overproduction is also recognized in tuberculosis [12, 42]. Intracellular pathogens including M. tuberculosis can promote macrophage arginase production as a pathogen-induced immune-evasion strategy [18]. Additionally, circulating or local asymmetric dimethylarginine, an endogenous NOS inhibitor, might play a role in tuberculosis, as in other infections (eg, sepsis and malaria), in limiting NO bioavailability and thereby being associated with worse clinical outcomes [43, 44].
Body weight had an important association with FENO in patients with tuberculosis but not in healthy controls. Weight distributions in the healthy and tuberculosis study populations were clearly different. Weight is a well-recognized measure of tuberculosis severity and treatment response [45]. Other severity markers, including radiography score [31], pulmonary function, and hemoglobin level also predicted FENO in patients with tuberculosis in this study. There are multifactorial explanations for anemia in this environment (eg, malaria and iron deficiency), but chronic disease is a likely contributor here. We hypothesize that the association between weight and FENO in tuberculosis occurs because decreased NO bioavailability allows unchecked immunopathology and more-severe disease.
A limitation of this study is that FENO may imprecisely reflect intracellular alveolar macrophage NO concentrations; however, the correlation shown previously between alveolar macrophage NOS2 expression and FENO in tuberculosis [21] supports the validity of FENO as a measure of macrophage NO production in this disease. FENO measures the total lung NO production; therefore, the real extent of NO impairment at sites of tuberculosis pathology may be underestimated. Our study was designed to investigate association rather than causality. Therefore, since determinants of FENO are varied and incompletely understood, we cannot exclude the possibility that other factors contribute to both impaired FENO and poorer clinical outcomes. Differences in FENO concentration between analyzer types were noted. The handheld devices systematically underestimated FENO, compared with the gold standard, which has not been reported previously. This difference was small in the usual FENO range. Notably, our median FENO among controls (16.6 ppb) is the same as that considered normal (16 ppb) [23]. Discrepancies between devices may have been due to ambient conditions in the tuberculosis clinic (high temperatures, often ≥30°C, and humidity levels of ≥75%). We made concerted efforts to shield the NiOX MINO from these conditions (eg, storage in a 19°C refrigerator between patients and in an air-conditioner overnight). Downward drift in NiOX MINO performance over time has been recognized [46], but we did not observe this, making longitudinal comparisons legitimate. FENO researchers in tropical locations should be cognizant of the susceptibility of NiOX devices to ambient conditions and of the possibility of systematic underestimation of FENO when using NiOX MINO, compared with the gold standard.
We found a marked fall in the FENO in the first week after commencing tuberculosis treatment, before eventual recovery. This could be attributable to longitudinal changes in the predominant macrophage phenotype. Specifically, M2 responses (characterized by a high arginase 1 level and a low NOS2 level [ie, a low NO level]) might predominate in advanced tuberculosis at the time of treatment commencement, become exaggerated during the initial inflammatory cascade occurring in response to treatment initiation, then become gradually superseded by M1 responses (characterized by a TNF-α/interferon γ [IFN-γ]/NOS2 phenotype [ie, a high NO level]) during recovery. This hypothesis is supported by a model proposed by Lugo-Villarino et al, describing (1) the predominance of M1 responses soon after infection with M. tuberculosis, in which appropriate NOS2 upregulation occurs and the TNF-α/IFN-γ phenotype predominates; (2) subsequent transition to an M2-predominant phenotype during the course of untreated illness, characterized by poorly microbicidal responses and progressive disease; and (3) restoration of M1 predominance after successful treatment [47]. Concentrations of both M2 cytokines (interleukin 10 and interleukin 13) and M1 cytokines (interleukin 12p40, macrophage inflammatory protein-1α and -1β, and TNF-α) have been shown to increase at week 1 or other time points shortly after tuberculosis treatment initiation, before returning to pretreatment levels [48]. This would support our hypothesis for the longitudinal FENO trend, assuming that M2 responses initially outweigh M1 responses [48].
Our findings support further research into adjunctive treatments for pulmonary tuberculosis to increase pulmonary NO production. Systemic administration of oral L-arginine (1–6 g) has thus far been disappointing in this regard [27, 34, 49] (Ralph et al, unpublished data), but inhaled L-arginine or alternative NO-donors require investigation. Adjunctive treatments to support NO production might be particularly valuable during the early period of treatment response, to improve M. tuberculosis killing, to reduce inflammatory pulmonary tissue damage, and to prevent the low intracellular NO environments that promote the development of the nonreplicating, antibiotic-resistant state among M. tuberculosis strains. Overall, the clinical data presented here support the in vitro evidence of the importance of NO in human antimycobacterial immune responses, describe the usefulness of FENO as a biomarker of the immune response to tuberculosis and response to treatment, and provide the basis for future studies of adjunctive therapy to augment pulmonary NO production.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the following individuals and organizations for their support and assistance: M. Okoseray, E. Meokbun, and the Timika District Health Authority; D. B. Lolong, M. Girsang, and the National Institute of Health Research and Development, Jakarta; P. Penttinen, M. Bangs, and M. Stone, Public Health and Malaria Control and International SOS; Bapak Istanto and PHMC laboratory staff; J. Lempoy and Timika tuberculosis clinic staff; P. Sugiarto, E. Malonda, and Mimika Community Hospital; D. Lampah, Prayogo, Ferryanto Chalfein, N. D. Haryanti, S. Hasmunik, S. Rahayu, G Bellatrix, and clinical and laboratory staff, Timika Research Facility; R. Soemanto and Y. Rukminiati, University of Indonesia's Faculty of Microbiology; and K. Piera and E. Curry, MSHR.
Disclaimer. The views expressed in this publication are those of the authors and do not reflect the views of National Health and Medical Research Council of Australia.
Financial support. This work was supported by the National Health and Medical Research Council of Australia (grants 605806 and 496600; scholarship to A. P. R.; and fellowships to A. P. R., T. W. Y., P. M. K., N. M. A., G.P.M.), (the Australian Respiratory Council, a Royal Australasian College of Physicians Covance Award (to A. P. R.), a Wellcome Trust Senior Research Fellowship (to R. N. P.) and the Margaret Ross Chair in Indigenous Health (G.P.M).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Onyebujoh P, Rodriguez W, Mwaba P. Priorities in tuberculosis research. Lancet. 2006;367:940–2. doi: 10.1016/S0140-6736(06)68385-2. [DOI] [PubMed] [Google Scholar]
- 2.Wallis RS, Doherty TM, Onyebujoh P, et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis. 2009;9:162–72. doi: 10.1016/S1473-3099(09)70042-8. [DOI] [PubMed] [Google Scholar]
- 3.Ralph AP, Kelly PM, Anstey NM. L-arginine and vitamin D: novel adjunctive immunotherapies in tuberculosis. Trends Microbiol. 2008;16:336–44. doi: 10.1016/j.tim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–40. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson S, Bonecini-Almeida Mda G, Lapa e Silva JR, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med. 2002;166:178–86. doi: 10.1164/rccm.2201023. [DOI] [PubMed] [Google Scholar]
- 7.Kwon OJ. The role of nitric oxide in the immune response of tuberculosis. J Korean Med Sci. 1997;12:481–7. doi: 10.3346/jkms.1997.12.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CH, Lin HC, Liu CY, et al. Upregulation of inducible nitric oxide synthase and cytokine secretion in peripheral blood monocytes from pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2001;5:283–91. [PubMed] [Google Scholar]
- 9.Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect Immun. 1997;65:3644–7. doi: 10.1128/iai.65.9.3644-3647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rich EA, Torres M, Sada E, Finegan CK, Hamilton BD, Toossi Z. Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tuber Lung Dis. 1997;78:247–55. doi: 10.1016/s0962-8479(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 11.Mishra BB, Rathinam VA, Martens GW, et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat Immunol. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pessanha AP, Martins RA, Mattos-Guaraldi AL, Vianna A, Moreira LO. Arginase-1 expression in granulomas of tuberculosis patients. FEMS Immunol Med Microbiol. 2012;66:265–68. doi: 10.1111/j.1574-695X.2012.01012.x. [DOI] [PubMed] [Google Scholar]
- 13.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis. 2003;3:578–90. doi: 10.1016/s1473-3099(03)00741-2. [DOI] [PubMed] [Google Scholar]
- 14.Davis AS, Vergne I, Master SS, Kyei GB, Chua J, Deretic V. Mechanism of inducible nitric oxide synthase exclusion from mycobacterial phagosomes. PLoS Pathog. 2007;3:e186. doi: 10.1371/journal.ppat.0030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman CR, Quinn GC, Kreiswirth BN, et al. Widespread dissemination of a drug-susceptible strain of Mycobacterium tuberculosis. J Infect Dis. 1997;176:478–84. doi: 10.1086/514067. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien L, Carmichael J, Lowrie DB, Andrew PW. Strains of Mycobacterium tuberculosis differ in susceptibility to reactive nitrogen intermediates in vitro. Infect Immun. 1994;62:5187–90. doi: 10.1128/iai.62.11.5187-5190.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idh J, Mekonnen M, Abate E, et al. Resistance to first-line anti-TB drugs is associated with reduced nitric oxide susceptibility in Mycobacterium tuberculosis. PLoS One. 2012;7:e39891. doi: 10.1371/journal.pone.0039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Kasmi KC, Qualls JE, Pesce JT, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darwin KH, Ehrt S, Gutierrez-Ramos JC, Weich N, Nathan CF. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science. 2003;302:1963–6. doi: 10.1126/science.1091176. [DOI] [PubMed] [Google Scholar]
- 20.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A. 2000;97:8841–8. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J. 1998;11:809–15. doi: 10.1183/09031936.98.11040809. [DOI] [PubMed] [Google Scholar]
- 22.Gill M, Graff GR, Adler AJ, Dweik RA. Validation study of fractional exhaled nitric oxide measurements using a handheld monitoring device. J Asthma. 2006;43:731–4. doi: 10.1080/02770900601031045. [DOI] [PubMed] [Google Scholar]
- 23.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngamtrakulpanit L, Yu Y, Adjei A, Amoah G, Gaston B, Hunt J. Identification of intrinsic airway acidification in pulmonary tuberculosis. Glob J Health Sci. 2010;2:106–10. doi: 10.5539/gjhs.v2n1p106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syhre M, Manning L, Phuanukoonnon S, Harino P, Chambers ST. The scent of Mycobacterium tuberculosis–part II breath. Tuberculosis. 2009;89:263–6. doi: 10.1016/j.tube.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Idh J, Westman A, Elias D, et al. Nitric oxide production in the exhaled air of patients with pulmonary tuberculosis in relation to HIV co-infection. BMC Infect Dis. 2008;8:146. doi: 10.1186/1471-2334-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralph AP, Waramori G, Pontororing GJ, et al. L-arginine and Vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS ONE. 2013 doi: 10.1371/journal.pone.0070032. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pontororing GJ, Kenangalem E, Lolong DB, et al. The burden and treatment of HIV in tuberculosis patients in Papua Province, Indonesia: a prospective observational study. BMC Infect Dis. 2010;10:362. doi: 10.1186/1471-2334-10-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 31.Ralph AP, Ardian M, Wiguna A, et al. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. 2010;65:863–9. doi: 10.1136/thx.2010.136242. [DOI] [PubMed] [Google Scholar]
- 32.Handojo T, Anstey N, Kelly P, et al. Normal spirometry, gas transfer and lung volume values in Papua, Indonesia. Southeast Asian J Trop Med Public Health. 2006;37:571–7. [PubMed] [Google Scholar]
- 33.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 34.Schon T, Idh J, Westman A, et al. Effects of a food supplement rich in arginine in patients with smear positive pulmonary tuberculosis–a randomised trial. Tuberculosis (Edinb) 2011;91:370–7. doi: 10.1016/j.tube.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Olin AC, Bake B, Toren K. Fraction of exhaled nitric oxide at 50 mL/s: reference values for adult lifelong never-smokers. Chest. 2007;131:1852–6. doi: 10.1378/chest.06-2928. [DOI] [PubMed] [Google Scholar]
- 36.Connolly L, Edelstein P, Ramakrishnan L. Why is long-term therapy required to cure tuberculosis? PLoS Medicine. 2007;4 doi: 10.1371/journal.pmed.0040120. e120 (435–42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voskuil MI, Schnappinger D, Visconti KC, et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med. 2003;198:705–13. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long R, Jones R, Talbot J, et al. Inhaled nitric oxide treatment of patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob Agents Chemother. 2005;49:1209–12. doi: 10.1128/AAC.49.3.1209-1212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grasemann H, Kurtz F, Ratjen F. Inhaled L-arginine improves exhaled nitric oxide and pulmonary function in patients with cystic fibrosis. Am J Respir Crit Care Med. 2006;174:208–12. doi: 10.1164/rccm.200509-1439OC. [DOI] [PubMed] [Google Scholar]
- 40.Meng QH, Springall DR, Bishop AE, et al. Lack of inducible nitric oxide synthase in bronchial epithelium: a possible mechanism of susceptibility to infection in cystic fibrosis. J Pathol. 1998;184:323–31. doi: 10.1002/(SICI)1096-9896(199803)184:3<323::AID-PATH2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Ratjen F, Gartig S, Wiesemann HG, Grasemann H. Effect of inhaled nitric oxide on pulmonary function in cystic fibrosis. Respir Med. 1999;93:579–83. doi: 10.1016/s0954-6111(99)90158-0. [DOI] [PubMed] [Google Scholar]
- 42.Zea AH, Culotta KS, Ali J, et al. Decreased expression of CD3zeta and nuclear transcription factor kappa B in patients with pulmonary tuberculosis: potential mechanisms and reversibility with treatment. J Infect Dis. 2006;194:1385–93. doi: 10.1086/508200. [DOI] [PubMed] [Google Scholar]
- 43.Davis JS, Darcy CJ, Yeo TW, et al. Asymmetric dimethylarginine, endothelial nitric oxide bioavailability and mortality in sepsis. PLoS One. 2011;6:e17260. doi: 10.1371/journal.pone.0017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo TW, Lampah DA, Tjitra E, et al. Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS pathogens. 2010;6:e1000868. doi: 10.1371/journal.ppat.1000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med. 2006;174:344–8. doi: 10.1164/rccm.200511-1834OC. [DOI] [PubMed] [Google Scholar]
- 46.Taylor DR, Palmay R, Cowan JO, Herbison GP. Long term performance characteristics of an electrochemical nitric oxide analyser. Respir Med. 2011;105:211–7. doi: 10.1016/j.rmed.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Lugo-Villarino G, Verollet C, Maridonneau-Parini I, Neyrolles O. Macrophage polarization: convergence point targeted by mycobacterium tuberculosis and HIV. Front Immunol. 2011;2:43. doi: 10.3389/fimmu.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Djoba Siawaya JF, Beyers N, van Helden P, Walzl G. Differential cytokine secretion and early treatment response in patients with pulmonary tuberculosis. Clin Exp Immunol. 2009;156:69–77. doi: 10.1111/j.1365-2249.2009.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schon T, Elias D, Moges F, et al. Arginine as an adjuvant to chemotherapy improves clinical outcome in active tuberculosis. Eur Respir J. 2003;21:483–8. doi: 10.1183/09031936.03.00090702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.