Abstract
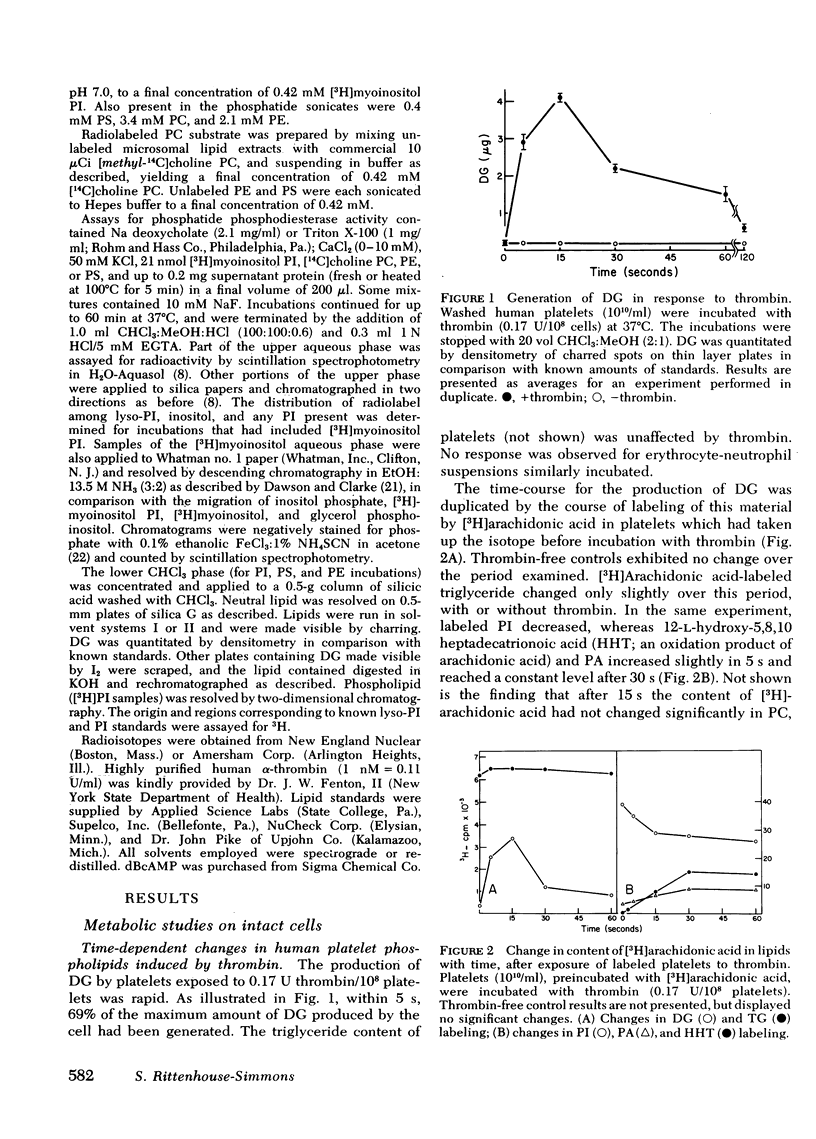
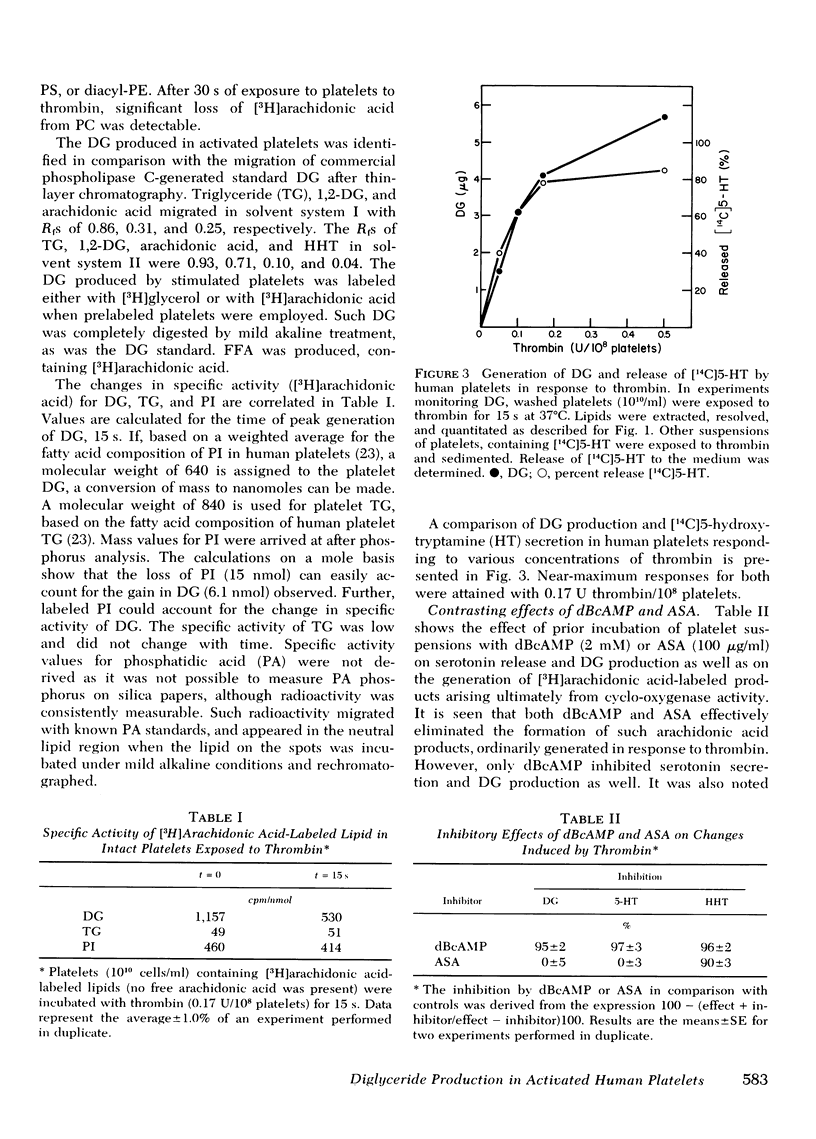
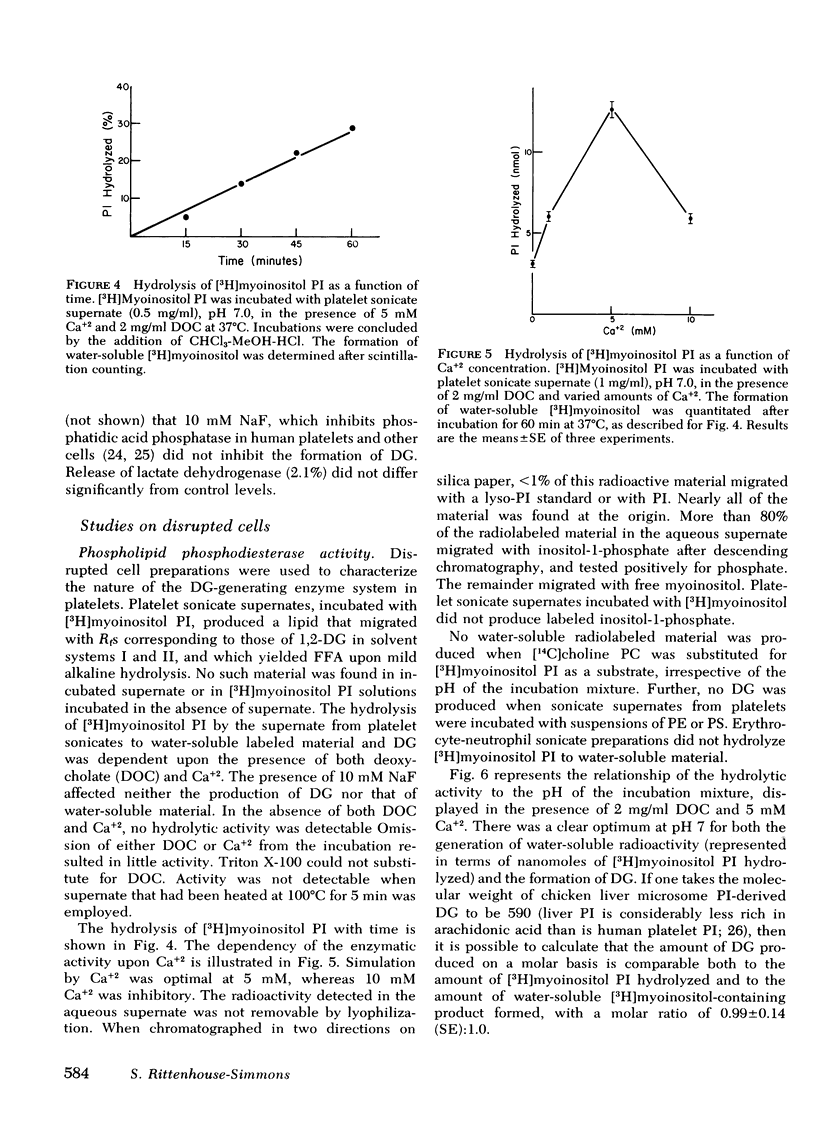
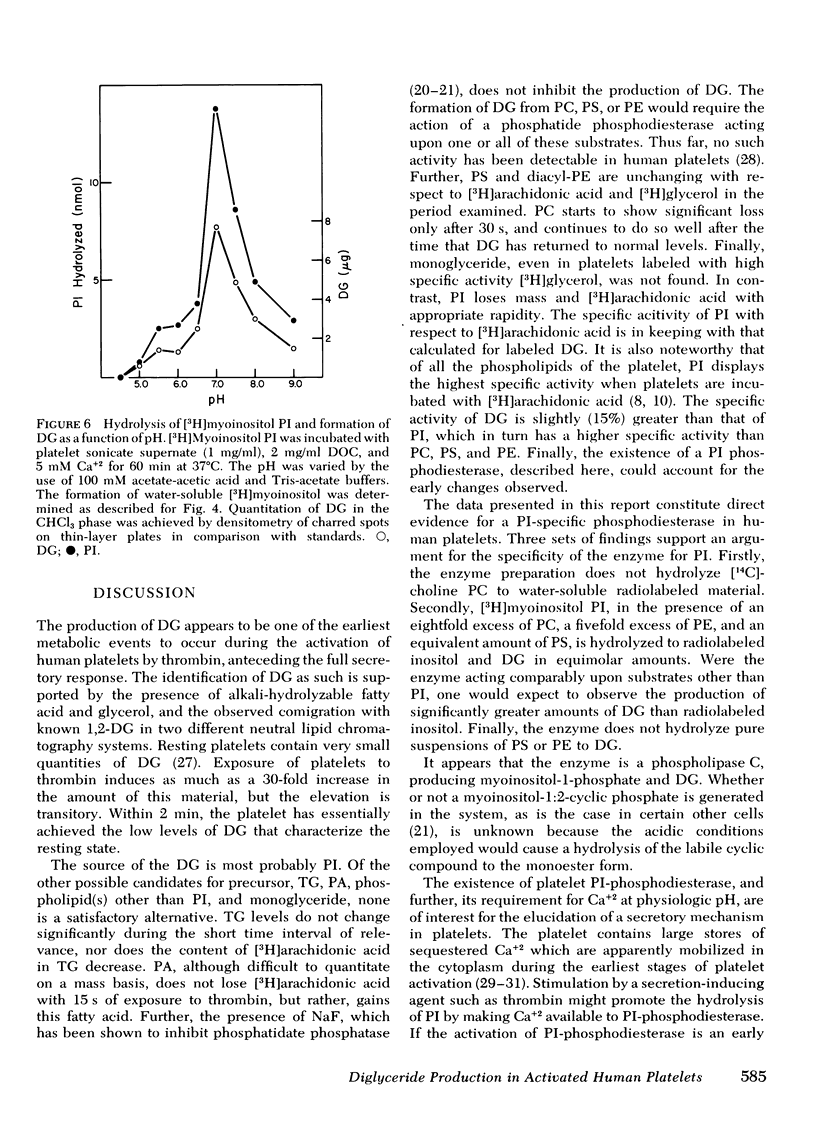
Human platelets generate diglyceride within 5 s of exposure to thrombin. Production of diglyceride is transient. 15 s after the addition of thrombin, the levels of diglyceride have increased up to 30-fold, but decrease thereafter. Prior incubation of platelets with 2 mM dibutyryl cyclic AMP prevents both the generation of diglyceride and the secretion of serotonin. Acetylsalicylic acid (100 microgram/ml), which completely inhibits prostaglandin endoperoxide synthesis, does not block diglyceride production and serotonin secretion induced by thrombin. Based on studies examining the incorporation of [3H]arachidonic acid into diglyceride of prelabeled platelets exposed to thrombin, it is concluded that neither phosphatidic acid nor triglyceride is the source of the diglyceride. Phosphatidylinositol appears to be the most likely source, both because its loss of radiolabel is sizable and rapid enough to account for the appearance of radiolabel in diglyceride, and because a phosphatidylinositol-specific phosphodiesterase, described in this report, exists in platelets. The phosphatidylinositol-phosphodiesterase, which produces diglyceride and inositol phosphate, requires Ca+2 and shows optimal activity at pH 7. The enzyme does not act upon phosphatidylcholine, phosphatidylethanolamine, or phosphatidylserine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. The fusion of erythrocytes by fatty acids, esters, retinol and alpha-tocopherol. Biochem J. 1973 Sep;136(1):147–155. doi: 10.1042/bj1360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Billah M. M., Finean J. B., Michell R. H. Release of diacylglycerol-enriched vesicles from erythrocytes with increased intracellular (Ca2+). Nature. 1976 May 6;261(5555):58–60. doi: 10.1038/261058a0. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Calcium ion-dependent diacylglycerol accumulation in erythrocytes is associated with microvesiculation but not with efflux of potassium ions. Biochem J. 1977 Sep 15;166(3):495–499. doi: 10.1042/bj1660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Metabolism of phosphatidate at the plasma membrane. Biochem Soc Trans. 1977;5(1):55–59. doi: 10.1042/bst0050055. [DOI] [PubMed] [Google Scholar]
- Banschbach M. W., Geison R. L., Hokin-Neaverson M. Acetylcholine increases the level of diglyceride in mouse pancreas. Biochem Biophys Res Commun. 1974 Jun 4;58(3):714–718. doi: 10.1016/s0006-291x(74)80476-6. [DOI] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Metabolism of [14C]arachidonic acid by human platelets. Biochim Biophys Acta. 1976 Feb 23;424(2):303–314. doi: 10.1016/0005-2760(76)90198-3. [DOI] [PubMed] [Google Scholar]
- COLEMAN R., HUEBSCHER G. Metabolism of phospholipids. V. Studies of phosphatidic acid phosphatase. Biochim Biophys Acta. 1962 Jan 29;56:479–490. doi: 10.1016/0006-3002(62)90600-5. [DOI] [PubMed] [Google Scholar]
- Chap H. J., Zwaal R. F., van Deenen L. L. Action of highly purified phospholipases on blood platelets. Evidence for an asymmetric distribution of phospholipids in the surface membrane. Biochim Biophys Acta. 1977 Jun 2;467(2):146–164. doi: 10.1016/0005-2736(77)90192-4. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Feinman R. D., Detwiler T. C. Interrelations of platelet aggregation and secretion. J Clin Invest. 1977 Oct;60(4):866–873. doi: 10.1172/JCI108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Clarke N. D-myoinositol 1:2-cyclic phosphate 2-phosphohydrolase. Biochem J. 1972 Mar;127(1):113–118. doi: 10.1042/bj1270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., Geurts van Kessel W. S., Zwaal R. F., Roelofsen B., van Deenen L. L. Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim Biophys Acta. 1975 Sep 16;406(1):97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Feinman R. D., Detwiler T. C. Letter to the editors-in-chief: Absence of a requirement for extracellular calcium for secretion from platelets. Thromb Res. 1975 Oct;7(4):677–679. doi: 10.1016/0049-3848(75)90113-9. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin-Neaverson M. Acetylcholine causes a net decrease in phosphatidylinositol and a net increase in phosphatidic acid in mouse pancreas. Biochem Biophys Res Commun. 1974 Jun 4;58(3):763–768. doi: 10.1016/s0006-291x(74)80483-3. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Functional activity in glands and synaptic tissue and the turnover of phosphatidylinositol. Ann N Y Acad Sci. 1969 Oct 17;165(2):695–709. [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. Breakdown of phosphatidylinositol provoked by muscarinic cholinergic stimulation of rat parotid-gland fragments. Biochem J. 1974 Sep;142(3):583–590. doi: 10.1042/bj1420583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Breton G. C., Dinerstein R. J. Effect of the calcium antagonist TMB-6 on intracellular calcium redistribution associated with platelet shape change. Thromb Res. 1977 Mar;10(3):521–523. doi: 10.1016/0049-3848(77)90161-x. [DOI] [PubMed] [Google Scholar]
- Leathwood P. D., Gilford M. K., Plummer D. T. Enzymes in rat urine: lactate dehydrogenase. Enzymologia. 1972 Apr 28;42(4):285–301. [PubMed] [Google Scholar]
- Lloyd J. V., Mustard J. F. Changes in 32P-content of phosphatidic acid and the phosphoinositides of rabbit platelets during aggregation induced by collagen or thrombin. Br J Haematol. 1974 Feb;26(2):243–253. doi: 10.1111/j.1365-2141.1974.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Lunt G. G., Pickard M. R. The subcellular localization of carbamylcholine-stimulated phosphatidyl inositol turnover in rat cerebral cortex in vivo. J Neurochem. 1975 Jun;24(6):1203–1208. doi: 10.1111/j.1471-4159.1975.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Ullman H. L., Safier L. B. Lipid composition of subcellular particles of human blood platelets. J Lipid Res. 1969 Jan;10(1):108–114. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Miller O. V., Johnson R. A., Gorman R. R. Inhibition of PGE1-stimulated cAMP accumulation in human platelets by thromboxane a2. Prostaglandins. 1977 Apr;13(4):599–609. doi: 10.1016/0090-6980(77)90231-3. [DOI] [PubMed] [Google Scholar]
- Oberleas D. The determination of phytate and inositol phosphates. Methods Biochem Anal. 1971;20:87–101. doi: 10.1002/9780470110393.ch3. [DOI] [PubMed] [Google Scholar]
- Otnaess A. B., Holm T. The effect of phospholipase C on human blood platelets. J Clin Invest. 1976 Jun;57(6):1419–1425. doi: 10.1172/JCI108411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULUS H., KENNEDY E. P. The enzymatic synthesis of inositol monophosphatide. J Biol Chem. 1960 May;235:1303–1311. [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Deykin D. The activation by Ca2+ of platelet phospholipase A2. Effects of dibutyryl cyclic adenosine monophosphate and 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate. Biochim Biophys Acta. 1978 Nov 1;543(4):409–422. doi: 10.1016/0304-4165(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Deykin D. The mobilization of arachidonic acid in platelets exposed to thrombin or ionophore A23187. Effects of adenosine triphosphate deprivation. J Clin Invest. 1977 Aug;60(2):495–498. doi: 10.1172/JCI108801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Russell F. A., Deykin D. Mobilization of arachidonic acid in human platelets. Kinetics and Ca2+ dependency. Biochim Biophys Acta. 1977 Sep 28;488(3):370–380. doi: 10.1016/0005-2760(77)90196-5. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Russell R. A., Deykin D. Transfer of arachidonic acid to human platelet plasmalogen in response to thrombin. Biochem Biophys Res Commun. 1976 May 3;70(1):295–301. doi: 10.1016/0006-291x(76)91141-4. [DOI] [PubMed] [Google Scholar]
- Russell F. A., Deykin D. The effect of thrombin on the uptake and transformation of arachidonic acid by human platelets. Am J Hematol. 1976;1(1):59–70. doi: 10.1002/ajh.2830010107. [DOI] [PubMed] [Google Scholar]
- White J. G., Rao G. H., Gerrard J. M. Effects of the lonophore A23187 on blood platelets I. Influence on aggregation and secretion. Am J Pathol. 1974 Nov;77(2):135–149. [PMC free article] [PubMed] [Google Scholar]
- Wuthier R. E. Two-dimensional chromatography on silica gel-loaded paper for the microanalysis of polar lipids. J Lipid Res. 1966 Jul;7(4):544–550. [PubMed] [Google Scholar]


