Abstract
Background. Mismatch between circulating influenza B viruses (Yamagata and Victoria lineages) and vaccine strains occurs frequently.
Methods. In a randomized controlled trial, immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate (QIV) versus trivalent inactivated influenza vaccine (TIV)-Victoria(Vic) and TIV-Yamagata(Yam) in children 3–17 years of age was evaluated. In an open-label study arm, QIV only was assessed in children 6–35 months of age.
Results. A total of 3094 children (932 QIV, 929 TIV-Vic, 932 TIV-Yam, and 301 QIV only) were vaccinated. QIV was noninferior to the TIVs for shared strains (A/H3N2 and A/H1N1) based on hemagglutination-inhibition (HI) antibodies 28 days after last vaccination, and superior for the unique B strains Victoria and Yamagata (geometric mean titer ratios 2.61, 3.78; seroconversion rate differences 33.96%, 44.63%). Among children in the randomized trial, adverse event rates were similar except for injection site pain (dose 1: 65.4% QIV, 54.6% TIV-Vic, 55.7% TIV-Yam).
Conclusion. QIV elicited superior HI responses to the added B strains compared to TIV controls, potentially improving its effectiveness against influenza B. HI responses were similar between QIV and TIV controls for the shared strains. QIV had an acceptable safety profile relative to TIVs.
Clinical Trials Registration. NCT01198756.
Keywords: influenza vaccine, immunogenicity, children
(See the editorial commentary by Dolin on pages 539–40.)
The choice of influenza virus strains to be included in each year's vaccine is a complex process in which worldwide surveillance data is used to predict the strains likely to circulate the next season, while taking into account ongoing antigenic drift [1]. Since 1978, there have been 3 strains in the annual vaccine [2]: 2 influenza A strains, A/H3N2 and H1N1; and 1 influenza B strain. However, the emergence of 2 lineages of B virus, Yamagata and Victoria [3], which may circulate simultaneously, has led to mismatch between the predominant B virus and the vaccine virus in at least 5 of 10 seasons from 2001 to 2010 [4]. Because the B virus lineages are antigenically distinct [3], little or no cross-lineage protection is expected to occur, resulting in reduced vaccine protection [5].
The burden of illness associated with influenza B in children is substantial. Surveillance in the United States, for example, identified that during the 2010–2011 season, 38% of all influenza-associated pediatric deaths were attributed to influenza B, with half of these children being previously healthy [6]. Influenza B may also preferentially affect children and young adults [7], and has been associated with higher hospitalization rates in children than influenza A [8] and with particular clinical syndromes, including myocarditis [9–10]. Quadrivalent influenza vaccines containing both B virus lineages are estimated to reduce illness, hospitalization, and death due to influenza [11].
In this Phase III randomized study of children and adolescents 3 through 17 years of age, we assessed the immunogenicity, reactogenicity, and safety of an inactivated quadrivalent influenza vaccine (QIV) containing influenza B strains from both lineages versus 2 inactivated trivalent influenza vaccines (TIV) containing the same influenza A virus strains and a B strain from either the B/Victoria lineage or the B/Yamagata lineage. In a concurrent open-label study arm, the immunogenicity and safety of QIV was assessed in children 6–35 months of age.
METHODS
This was a randomized (1:1:1), controlled, double-blind comparison of QIV and TIV in 3- to 17-year-old children to determine safety and immunogenicity (Figure 1). In an open-label study arm, the reactogenicity, safety, and immunogenicity of QIV in children 6–35 months of age was described.
Figure 1.
Participant flow. Abbreviations: ATP, according-to-protocol; QIV, quadrivalent influenza vaccine; TIV-Vic, trivalent influenza vaccine Victoria lineage B strain; TIV-Yam, trivalent influenza vaccine Yamagata lineage B strain.
The study was undertaken in compliance with Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable regulatory requirements, and was approved by local, regional, or national Institutional Review Boards at each study site.
Participants
Eligible children were in stable health, between 6 months and 17 years of age, and not pregnant if female. Children were excluded if febrile (temperature ≥38.0°C), immunocompromised, receiving aspirin, allergic to any vaccine component, known to have a coagulation disorder, or had received influenza vaccine in the prior 6 months, immunoglobulins or blood products within 3 months, or an investigational product within 30 days. A parent/guardian provided written informed consent for each participant. The study was conducted in 32 centers in 5 countries (Canada, United States, Mexico, Spain, and Taiwan).
Vaccines
The TIVs contained either a Victoria lineage B strain (TIV-Vic), or a Yamagata lineage B strain (TIV-Yam). Both TIVs contained 15 µg each of hemagglutinin antigen (HA) from each of A/H1N1 (A/California/7/2009) and A/H3N2 (A/Victoria/210/2009), TIV-Vic contained 15 µg of B/Brisbane/60/2008, and TIV-Yam contained 15 µg of B/Brisbane/3/2007. The QIV contained 60 µg of HA: 15 µg of the same 2 influenza A strains as the TIVs, as well as 15 µg of each of the B lineage strains.
All vaccines were manufactured by GlaxoSmithKline Vaccines using a thimerosal-free formulation and were opalescent off-white to grayish suspensions provided in prefilled 0.5 mL syringes. The TIVs were manufactured in Dresden, Germany, and the QIV in Quebec City, Canada. Vaccines used in the 3- to 17-year-old study were labeled with the treatment number but no identifying information. Vaccines administered in the QIV-only open-label study of 6- to 35-month-olds were labeled as to contents.
Study Procedures
Following the consent process, study staff determined treatment allocation using an Internet-based central randomization system. The randomization sequence was generated using MATEX, a software program developed for use in SAS (Cary, NC) by GlaxoSmithKline (Rixensart, Belgium). Randomization was stratified equally between groups by age (3–8 years and 9–17 years). A minimization procedure accounted for previous receipt of influenza vaccine (“priming” status), country, and age subgroups (3–4 years and 5–8 years). Neither the parents/guardians, participants, or study staff were aware of treatment allocation for participants ≥3 years of age. In the open-label study, parents/guardians and study staff were not blinded.
Prior to the first vaccination, a brief history-directed physical examination was performed and blood drawn. Children who were ≥9 years of age or had prior receipt of ≥2 doses of an influenza vaccine at least 1 month apart were considered “primed.” Primed children received one 0.5 mL dose of study vaccine on day 0 and had blood collected on day 28 (visit 2). Unprimed participants received a second 0.5 mL dose of vaccine on day 28, and had blood collected on day 56. Vaccines were administered intramuscularly in the anterolateral region of the left thigh (age 6–11 months) or deltoid region of the left arm (age ≥12 months). Children were observed at the study site for 30 minutes after administration of study vaccine.
Parents/guardians were instructed on the use of a diary card for recording any solicited injection site or general adverse events (AEs) for 7 days, and any unsolicited AEs for 28 days, and to bring the diary to study visits. Parents/guardians of children <5 years of age were asked to contact study staff immediately if a seizure or fever ≥39°C occurred within 2 days of vaccination. On day 180, parents/guardians were asked if changes in health or AEs had occurred since the last vaccination.
Participant flow is shown in Figure 1.
Outcomes
Immunogenicity
The primary confirmatory objective was to determine if QIV was noninferior to TIV-Vic and TIV-Yam for the shared vaccine strains in children 3–17 years of age, at 28 days after completion of dosing (day 28 for primed children and day 56 for unprimed children). Immunogenic noninferiority was assessed between shared viral strains in terms of the geometric mean titer (GMT) ratio and seroconversion rate (SCR) difference. A secondary confirmatory objective was to assess the immunogenic superiority of QIV versus TIV-Vic for the B/Yamagata strain and TIV-Yam for the B/Victoria strain (strains absent from the TIV) in children 3–17 years of age at 28 days after dosing. Exploratory objectives were to describe GMT, SCR, seroprotection rate (SPR), and seroconversion factor (SCF) for all strains in each vaccine in children 3–17 years and for QIV in children 6–35 months of age.
Antibody titers against the vaccine strains were measured in serum samples by hemagglutination-inhibition (HI) assay performed at GlaxoSmithKline Vaccines' laboratory in Laval, Canada, using standardized procedures [12].
Reactogenicity
Reactogenicity analyses for participants ≤5 years of age were the frequency of solicited injection site reactions (pain, redness, swelling) and solicited general AEs (fever, irritability/fussiness, drowsiness, loss of appetite) in the 7 days following each vaccination. Fever was defined as a temperature ≥38.0°C measured by any method. For children >5 years, the same injection site reactions were collected, but the solicited general reactions were fever, headache, fatigue, and gastrointestinal symptoms; and joint pain, muscle aches, and shivering. Intensity scales were used for the description of each symptom (Supplementary Table 1). Unsolicited AEs were recorded for 28 days.
Safety was further assessed by consideration of serious adverse events (SAEs), medically attended adverse events (MAEs), and potentially immune-mediated diseases (pIMDs), which were recorded during the entire study period (to day 180). MAEs were defined as events for which the child was hospitalized, visited the emergency room, or had a visit with a physician for any complaint. All injection-site reactions were considered as vaccine-related events. The causality of all other AEs was assessed by investigators. All AEs were classified according to the Medical Dictionary for Regulatory Activities.
Sample Size and Statistical Analysis
The sample size for the randomized controlled trial (RCT) was 2175 evaluable children (725 per group) age 3–17 years, to obtain an overall power of 90% to demonstrate the primary objectives of noninferiority of QIV to TIV-Yam and TIV-Vic for the alternate-lineage B strain. A target of 2700 children (900 per group) was set to account for an attrition rate of 20%. In the open-label QIV-only study, the target sample size was set at 300 infants age 6–35 months, and was not based on a power calculation.
The immunogenicity analyses were performed using the According to Protocol (ATP) cohort for immunogenicity, defined as children who did not meet elimination or exclusion criteria during the study, who had assay results for at least 1 study vaccine antigen, and who complied with study time requirements. In the randomized study of 3- to 17-year-olds, immunogenic noninferiority was concluded if the upper limit of the 2-sided 95% confidence interval (CI) of the GMT ratio (TIV/QIV) after the final vaccine did not exceed 1.5 for the 3 strains (A/H3N2, A/H1N1, and shared B strains), and if the upper limit of the 2-sided 95% CI for the difference in SCR (TIV minus QIV) did not exceed 10% for the 3 strains contained in TIV-Yam and TIV-Vic. Immunogenic superiority was confirmed if the lower limit of the 2-sided 95% CI of the GMT ratio (QIV/TIV) was greater than 1.5 and the difference in SCR (QIV minus TIV) was greater than 10%.
GMT, SCR, SPR, and SCF were tabulated with 95% CIs, and SCR and SPR were evaluated according to Center for Biologics Evaluation and Research (CBER) licensure criteria for immunogenicity of influenza vaccines in adults <65 years of age: the criteria were fulfilled if the lower limit of the 2-sided 95% CI on the SCR was ≥40%, and the lower limit of the 2-sided 95% CI on the SPR was ≥70% [13].
Reactogenicity and safety were assessed in the Total Vaccinated Cohort (TVC). The frequency of AEs after each vaccine dose and overall were tabulated. SAEs and MAEs were described by tabulating the percentage of subjects with at least 1 event in each of these categories, with a 95% CI.
RESULTS
The study was conducted from 1 October 2010 until 6 July 2011. A total of 3109 children were enrolled. The TVC comprised 3094 children (Figure 1); 15 children were not vaccinated. The ATP immunogenicity cohort had a total of 2886 children enrolled. The 6-month safety follow-up was completed by 2960 participants (2685/2793; 96.1%) in the randomized study and 275/301 (91.4%) children in the open study. The median age in the RCT was 8.9 years and in the open study 1.2 years; other demographic characteristics at enrollment are given in Table 1. Age, sex, and ethnicity were similar across groups. Stable health was required for enrollment. Overall, 12.4% (370/2793) of participants had stable asthma (QIV 121/932; TIV-Vic 130/929; TIV-Yam 119/932), 2% had another chronic respiratory disease, and 1.5% had cardiovascular disease.
Table 1.
Demographic Characteristics at Enrollment: Total Vaccinated Cohort
| Age 3–17 y |
Age 6–35 mo |
|||
|---|---|---|---|---|
| QIV (N = 932) |
TIV-Vic (N = 929) | TIV-Yam (N = 932) | QIV (N = 301) |
|
| Mean age in years (SD; median; range) | 8.9 (4.21; 8.0; 3–17) | 8.9 (4.23; 9.0; 2–17) | 8.9 (4.17; 9.0; 3–17) | 1.2 (0.73; 1.0; 0–2) |
| Mean age in months (SD; median; range) | 112.4 (51.06; 107.0; 36–215) | 111.8 (51.10; 108.0; 34–215) | 111.8 (50.08; 108.5; 36–215) | 21.0 (8.68; 21.0; 6–35) |
| Male, n (%) | 498 (53.4) | 474 (51.0) | 468 (50.2) | 158 (52.5) |
| Female, n (%) | 434 (46.6) | 455 (49.0) | 464 (49.8) | 143 (47.5) |
| Hispanic/Latino ethnicity | 235 (25.2) | 235 (25.3) | 245 (26.3) | 56 (18.6) |
| Not Hispanic/Latino ethnicity | 697 (74.8) | 694 (74.7) | 687 (73.7) | 245 (81.4) |
| Heritage/race | ||||
| European heritage/Caucasian | 598 (64.2) | 580 (62.4) | 575 (61.7) | 206 (68.4) |
| Asian | 119 (12.8) | 125 (13.6) | 125 (13.4) | 18 (5.9) |
| African heritage/African American | 83 (8.9) | 85 (9.1) | 87 (9.3) | 48 (15.9) |
| American Indian/native Alaskan | 6 (0.6) | 1 (0.1) | 1 (0.1) | 6 (2.0) |
| Pacific Islander/native Hawaiian | 3 (0.3) | 1 (0.1) | 4 (0.4) | 0 |
| Other | 123 (13.2) | 137 (14.7) | 140 (15.0) | 23 (7.6) |
Abbreviations: QIV, quadrivalent influenza vaccine; SD, standard deviation; TIV-Vic, trivalent influenza vaccine Victoria lineage B strain; TIV-Yam, trivalent influenza vaccine Yamagata lineage B strain.
Immunogenicity
GMT, SCR, SPR, and SCF immunogenicity outcomes for each strain and vaccine in the ATP cohort are given in Table 2. In children 3–17 years of age, the QIV group was shown to be noninferior to both TIVs in terms of GMT ratios and SCR differences to all vaccine strains (Table 3). The upper limit of the 2-sided 95% CI for the adjusted GMT ratio of HI antibody titers did not exceed 1.5 for any comparison between TIV and QIV for shared vaccine strains. The upper limit of the 2-sided 95% CI for the SCR difference did not exceed 10% for any comparisons between TIV and QIV for shared strains. Immunogenicity of QIV was superior versus TIV-Vic for the B/Yamagata strain, and versus TIV-Yam for the B/Victoria strain based on the GMT ratio (QIV/TIV) and the SCR difference (QIV minus TIV) (Table 4).
Table 2.
Immunogenicity of QIV and TIV-Yam and TIV-Vic in Children 3–17 Years of Age and of QIV in Children 6 to 35 Months of Age: ATP Immunogenicity Cohort
| GMT | SCRa | SPRa | SCF | |||
|---|---|---|---|---|---|---|
| Group | Timing | N | Value (95% CI) | % (95% CI) | % (95% CI) | Value (95% CI) |
| A/California/7/2009 (H1N1) | ||||||
| QIV | Pre | 876 | 29.4 (26.8, 32.2) | … | 54.8 (51.4, 58.1) | … |
| Post | 878 | 362.7 (335.3, 392.3) | 84.4 (81.8, 86.7) | 96.8 (95.4, 97.9) | 12.3 (11.3, 13.4) | |
| TIV-Vic | Pre | 870 | 32.2 (29.4, 35.3) | … | 57.0 (53.6, 60.3) | … |
| Post | 871 | 429.1 (396.5, 464.3) | 86.8 (84.3, 89.0) | 97.4 (96.1, 98.3) | 13.3 (12.3, 14.4) | |
| TIV-Yam | Pre | 877 | 29.1 (26.6, 31.8) | … | 54.4 (51.0, 57.7) | … |
| Post | 878 | 420.2 (388.8, 454.0) | 85.5 (83.0, 87.8) | 96.6 (95.2, 97.7) | 14.4 (13.3, 15.7) | |
| QIV-only (6–35 mo) | Pre | 259 | 16.8 (13.9, 20.3) | … | 33.6 (27.9, 39.7) | … |
| Post | 259 | 200.9 (166.6, 242.2) | 84.9 (80.0, 89.1) | 89.6 (85.2, 93.0) | 12.0 (10.5, 13.6) | |
| A/Victoria/210/2009 (H3N2) | ||||||
| QIV | Pre | 876 | 18.1 (16.7, 19.7) | … | 33.7 (30.5, 36.9) | … |
| Post | 878 | 143.7 (134.2, 153.9) | 70.1 (66.9, 73.1) | 92.9 (91.0, 94.5) | 7.9 (7.3, 8.6) | |
| TIV-Vic | Pre | 870 | 19.0 (17.4, 20.6) | … | 34.6 (31.4, 37.9) | … |
| Post | 871 | 139.6 (130.5, 149.3) | 67.8 (64.6, 70.9) | 92.8 (90.8, 94.4) | 7.4 (6.8, 8.0) | |
| TIV-Yam | Pre | 876 | 19.4 (17.8, 21.1) | … | 37.0 (33.8, 40.3) | … |
| Post | 878 | 151.0 (141.0, 161.6) | 69.6 (66.5, 72.7) | 93.3 (91.4, 94.8) | 7.8 (7.2, 8.5) | |
| QIV-only | Pre | 259 | 5.6 (5.3, 6.0) | … | 2.70 (1.1, 5.5) | … |
| Post | 259 | 61.4 (53.8, 70.0) | 73.0 (67.1, 78.3) | 74.5 (68.8, 79.7) | 10.9 (9.6, 12.4) | |
| B/Brisbane/60/2008 (Victoria) | ||||||
| QIV | Pre | 876 | 24.8 (22.5, 27.3) | … | 44.3 (41.0, 47.7) | … |
| Post | 878 | 250.5 (230.8, 272.0) | 74.5 (71.5, 77.4) | 95.4 (93.8, 96.7) | 10.1 (9.2, 11.1) | |
| TIV-Vic | Pre | 870 | 25.8 (23.5, 28.4) | … | 46.4 (43.1, 49.8) | … |
| Post | 871 | 245.4 (226.9, 265.4) | 71.5 (68.4, 74.5) | 96.3 (94.9, 97.5) | 9.5 (8.6, 10.5) | |
| TIV-Yam | Pre | 877 | 25.8 (23.5, 28.4) | … | 45.6 (42.3, 49.0) | … |
| Post | 877 | 68.1 (61.9, 74.9) | 29.9 (26.9, 33.1) | 73.3 (70.3, 76.2) | 2.6 (2.5, 2.8) | |
| QIV-only | Pre | 259 | 8.7 (7.5, 10.0) | … | 10.8 (7.3, 15.2) | … |
| Post | 259 | 127.3 (109.4, 148.1) | 84.6 (79.6, 88.7) | 88.0 (83.4, 91.7) | 14.6 (12.8, 16.6) | |
| B/Florida/4/2006 (Yamagata) | ||||||
| QIV | Pre | 876 | 57.9 (52.0, 64.4) | … | 66.0 (62.7, 69.1) | … |
| Post | 878 | 512.5 (477.6, 549.9) | 75.2 (72.2, 78.1) | 99.0 (8.1, 99.5) | 8.9 (8.1, 9.7) | |
| TIV-Vic | Pre | 870 | 58.4 (52.6, 64.9) | … | 67.0 (63.8, 70.1) | … |
| Post | 871 | 197.0 (180.7, 214.8) | 41.3 (38.0, 44.6) | 92.4 (90.5, 94.1) | 3.4 (3.1, 3.6) | |
| TIV-Yam | Pre | 877 | 65.9 (59.3, 73.2) | … | 70.9 (67.8, 73.9) | … |
| Post | 878 | 579.0 (541.2, 619.3) | 73.4 (70.4, 76.3) | 99.4 (98.7, 99.8) | 8.8 (8.1, 9.6) | |
| QIV-only | Pre | 259 | 7.7 (7.0, 8.6) | … | 8.5 (5.4, 12.6) | … |
| Post | 259 | 192.7 (172.1, 215.7) | 93.8 (90.2, 96.4) | 96.5 (93.5, 98.4) | 24.9 (22.0, 28.3) | |
Abbreviations: ATP, according to protocol; CI, confidence interval; GMT, geometric mean titer; Post, postvaccination; Pre, prevaccination; QIV, quadrivalent influenza vaccine; SCF, seroconversion factor (defined as the geometric mean of the within-subjects ratios of the postvaccination reciprocal HI titer to the day 0 reciprocal HI titer); SCR, seroconversion rate (defined as the proportion of vaccinees with either a prevaccination titer <1:10 and a postvaccination titer ≥1:40, or a prevaccination titer ≥1:10 and at least a 4-fold increase in postvaccination titer); SPR, seroprotection rate (defined as the percentage of subjects who had a serum anti-HI antibody titer ≥1:40); TIV-Vic, trivalent influenza vaccine Victoria lineage B strain; TIV-Yam, trivalent influenza vaccine Yamagata lineage B strain.
a Each of the 4 strains contained in the QIV met Center for Biologics Evaluation and Research licensure criteria for immunogenicity (lower limit of 95% CI for SCR of at least 40% and a postvaccination SPR of at least 70%).
Table 3.
Immunogenic Noninferiority Comparison of QIV to TIV-Yam and TIV-Vic in Children 3–17 Years of Age: ATP Immunogenicity Cohort
| TIV-Vic + TIV-Yam N (adjusted GMT) | QIV N (adjusted GMT) | GMT ratio GMT ratio (95% CI)a | |
|---|---|---|---|
| A/California/7/2009 (H1N1) | 1747 (421.4) | 876 (366.3) | 1.15 (1.06, 1.25)b |
| A/Victoria/210/2009 (H3N3) | 1746 (144.3) | 876 (145.8) | 0.99 (.92, 1.07)b |
| B/Brisbane/60/2008 (Victoria) | 870 (243.4) | 876 (252.5) | 0.96 (.87, 1.07)c |
| B/Florida/4/2006 (Yamagata) | 877 (564.6) | 876 (525.2) | 1.08 (.99, 1.16)d |
| TIV-Vic + TIV-Yam n/N seroconverted (SCR)e |
QIV n/N seroconverted (SCR)e |
SCR difference % difference (95% CI)a |
|
| A/California/7/2009 (H1N1) | 1505/1747 (86.1%) | 739/876 (84.4%) | 1.79% (−1.04, 4.77)f |
| A/Victoria/210/2009 (H3N3) | 1200/1746 (68.7%) | 614/876 (70.1%) | −1.36% (−5.05, 2.41)f |
| B/Brisbane/60/2008 (Victoria) | 622/870 (71.5%) | 653/876 (74.5%) | −3.05% (−7.21, 1.12)g |
| B/Florida/4/2006 (Yamagata) | 644/877 (73.4%) | 659/876 (75.2%) | −1.80% (−5.89, 2.30)h |
Abbreviations: ATP, according to protocol; CI, confidence interval; GMT, geometric mean titer; N, number; QIV, quadrivalent influenza vaccine; SCR, seroconversion rate; TIV-Vic, trivalent influenza vaccine Victoria lineage B strain; TIV-Yam, trivalent influenza vaccine Yamagata lineage B strain .
a Immunogenic noninferiority demonstrated if the upper limit of the 2-sided 95% CI of the adjusted GMT was ≤1.5 and the upper limit of the 2-sided 95% CI of the difference between SCR was ≤10% for all vaccine strains.
b (TIV-Vic + TIV-Yam) divided by QIV.
c TIV-Vic divided by QIV.
d TIV-Yam divided by QIV.
e SCR was defined as the proportion of vaccinees with either a prevaccination titer <1:10 and a postvaccination titer ≥1:40, or a prevaccination titer ≥1:10 and at least a 4-fold increase in postvaccination titer.
f (TIV-Vic + TIV-Yam) minus QIV.
g TIV-Vic minus QIV.
h TIV-Yam minus QIV.
Table 4.
Immunogenic Superiority Comparison of QIV to TIV-Yam and TIV-Vic in Children 3–17 Years of Age: ATP Immunogenicity Cohort
| TIV-Vic | TIV-Yam | QIV | GMT ratio (95% CI)a | |
|---|---|---|---|---|
| N (adjusted GMT) | N (adjusted GMT) | N (adjusted GMT) | ||
| B/Florida/4/2006 (Yamagata) | 870 (196.5) | … | 876 (513.8) | 2.61 (2.41, 2.84)b |
| B/Brisbane/60/2008 (Victoria) | … | 876 (67.2) | 876 (253.7) | 3.78 (3.43, 4.16)c |
| TIV-Vic n/N seroconverted (SCR)d |
TIV-Yam n/N seroconverted (SCR)d |
QIV n/N seroconverted (SCR)d |
SCR difference % difference (95% CI)a |
|
| B/Florida/4/2006 (Yamagata) | 359/870 (41.3%) | … | 659/876 (75.2%) | 33.96% (29.55, 38.24)e |
| B/Brisbane/60/2008 (Victoria) | … | 262/876 (29.9%) | 653/876 (74.5%) | 44.63% (40.35, 48.72)f |
Abbreviations: ATP, according-to-protocol; CI, confidence interval; GMT, geometric mean titer; QIV, quadrivalent influenza vaccine; SCR, seroconversion rate; TIV-Vic, trivalent influenza vaccine Victoria lineage B strain; TIV-Yam, trivalent influenza vaccine Yamagata lineage B strain.
a Immunogenic superiority demonstrated if the lower limit of the 2-sided 95% CI of the adjusted GMT was >1.5 and the lower limit of the 2-sided 95% CI of the difference between SCRs was >10% with respect to each B strain in the QIV compared with corresponding TIV lacking the same B strain.
b QIV divided by TIV-V.
c QIV divided by TIV-Yam.
d SCR was defined as the proportion of vaccinees with either a prevaccination titer <1:10 and a postvaccination titer ≥1:40, or a prevaccination titer ≥1:10 and at least a 4-fold increase in postvaccination titer.
e QIV minus TIV-Vic.
f QIV minus TIV-Yam.
The QIV-fulfilled CBER criteria for immunogenicity. Seroconversion rates in the 3- to 17-year-old group, for strains contained in the vaccine, ranged from 92% to 99%. The TIV controls did induce HI antibodies directed to the alternate B lineage strain not in the vaccine, but with reduced seroconversion rates (29.9%–41.3%) relative to the response of the B strain contained in the vaccines (71.5%–75.2%).
GMTs for each of the 4 strains prior to vaccination and 28 days after the final dose in primed compared to unprimed children 3–8 years of age are seen in Supplementary Figure 1. In general, vaccination in unprimed children (who received 2 doses) resulted in as high or higher GMTs than in primed children (who received 1 dose). GMTs to the B/Yamagata strain in recipients of QIV and TIV-Yam were twice as high compared with the B/Victoria in recipients of QIV or TIV-Vic. In the QIV-only 6- to 35-month-old group, HI antibody titers fulfilled CBER criteria for immunogenicity for the strains contained in the QIV with the exception of SPR for the A/Victoria/210/2009 (H3N2) strain (74.5, 95% CI, 68.8, 79.9). Postvaccination SPRs in this age group ranged from 73% to 96%.
Reactogenicity
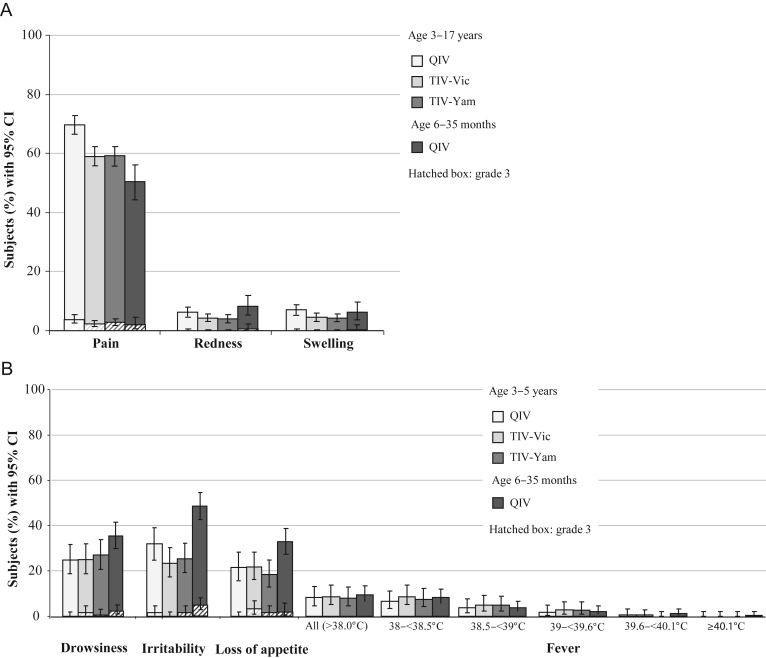
The frequency of injection site and general solicited AEs overall during 7 days after vaccination in children ≤5 years of age is shown in Figure 2. Pain was the most common injection site AE in children 3–17 years of age and in children 6–35 months of age receiving QIV. Grade 3 pain was reported by <4% of participants. The most common solicited general AEs in children 3–5 years of age were drowsiness, irritability, and loss of appetite; in children 6–35 months of age, the most common solicited general AE was irritability (Figure 2). In children >5 year of age, muscle ache, fatigue, and headache were the most frequent general solicited AEs (Supplementary Table 2). Any fever >38°C occurred in less than 10% of children, and grade 3 solicited AEs (including fever ≥39°C [≥102.2°F]) occurred in <3.2% of 3- to 5-year-olds, and less than 1.8% of 5- to 17-year-olds.
Figure 2.
Solicited injection site adverse events (A) and general adverse events (B) in the 7 days after vaccination; Total Vaccinated Cohort.
Abbreviations: CI, confidence interval; QIV, quadrivalent influenza vaccine; TIV-Vic, trivalent influenza vaccine Victoria lineage B strain; TIV-Yam, trivalent influenza vaccine Yamagata lineage B strain.
At least 1 unsolicited AE during the 28-day postvaccination period was reported by 283/932 (30.4%), 291/929 (31.3%), and 275/932 (29.5%) of children in the QIV, TIV-Vic, and TIV-Yam groups (3–17 years), respectively, and reported by 160/301 (53.2%) of the 6–35 months QIV group. In all children, cough was the most frequently reported unsolicited AE in this period (4.1%–11.3%).
At least 1 MAE was reported during the 6-month follow-up by 346/932 (37.1%), 335/929 (36.1%), and 350/932 (37.6%) of children in the randomized study in the QIV, TIV-Vic, and TIV-Yam groups, respectively, and reported by 147/301 (48.8%) of children in the QIV-only arm. Among all participants, upper-respiratory-tract infection was the most common MAE. Thirty-five SAEs were reported in 21 children overall during the 6-month follow-up (Supplementary Table 3); there were neither deaths nor withdrawals due to a SAE. Among children age 3–17 years, 3 (0.3%) children from the QIV group, 6 (0.6%) children from the TIV-Vic group, and 5 (0.5%) children from the TIV-Yam group reported 4, 12, and 9 SAEs, respectively, over the 6-month follow-up. Among children age 6–35 months in the QIV-only arm, 7 children (2.3%) reported a total of 10 SAEs. Four SAEs in 3 children were considered by the investigator to be related to the study vaccines: 2 SAEs (angioedema and acute conjunctivitis), which resolved within 7 days, were reported for a 12-year-old boy given TIV-Yam. A 1-year-old girl had a generalized seizure on the day after QIV receipt, and a 2-year-old boy had a febrile seizure 18 days after QIV receipt; both recovered within 1 day.
DISCUSSION
In this large multicenter, randomized, controlled trial in children 3–17 years of age, the immunogenicity of a candidate QIV was noninferior to TIV for shared vaccine strains, and was superior to TIV with respect to the added B strains. Inclusion of a fourth strain in QIV did not interfere with immune responses to the other 3 strains, which met all CBER criteria. Immune responses to all strains contained in the 3 vaccines were robust, with the highest responses to the B Yamagata and A/California/7/2009 (H1N1) strains. In the superiority analysis, the ratio of the GMT of QIV to TIV for the B strains showed that QIV elicited an immune response 2.5- to 3.7-fold higher than that of TIV. Similarly, the seroconversion rates of QIV were 32 to 40 percentage points higher than TIV. In brief, the immune responses to each B component of the quadrivalent vaccine exceed the cross-reactive immune responses to the B component not contained in each trivalent vaccine. The superior QIV immunogenicity is expected to correspond with superior protection against influenza B relative to TIV in a season when there is lineage mismatch or cocirculation of 2 influenza B lineages.
These immunogenicity results suggest that the candidate QIV can improve protection against influenza B without compromising protection against influenza A. Because influenza B exacts a special toll on the young and particularly those with comorbidities (increasing their risk for complicated influenza), and mismatch of vaccine and circulating B strain has been frequent with TIVs, the option of a QIV for children is particularly relevant.
An open-label study arm of children 6–35 months of age was included in this study. Vaccines for this age group were given at the same dosage as older children, 0.5 mL, which is considered the “full” or “adult” dose. Practice regarding dose for young children varies around the world, with some jurisdictions routinely using a “half dose” for children up to 35 months of age and a 0.5 mL dose for older children. Public health in Canada first recommended the 0.5 mL dose for the 2011–2012 season [14]; recent evidence supports that this dose results in moderate improvement in antibody response without increase in reactogenicity [15–16]. HI antibody responses in 6- to 35-month-olds were lower than in older children, but fulfilled CBER criteria [13] except for the SPR for the A/H3N2 strain. Because no TIV comparator arm was included for the youngest age group, no conclusions about relative immunogenicity or reactogenicity differences can be made. Based on the results of this open-label study, a randomized controlled study of this population is underway (NCT01711736). We speculate that copresentation of antigens from 2 lineages of influenza B virus to young children might result in improved responses to both B lineage strains. Priming with 1 influenza B lineage has been shown to affect immunogenicity to subsequent doses of the other lineage [17–18], without affecting humoral responses to influenza A antigens. In the open-label QIV-only arm, the candidate QIV elicited notably high SCRs of 94.6% and 93.8% for B/Victoria and B/Yamagata strains, respectively, in children 6–35 months old. Because there was no comparator arm for this component of the study, no conclusions can be made, but this possibility warrants further investigation.
Quadrivalent influenza vaccine contains 60 µg of HA antigen, and it is therefore plausible that reactogenicity could be higher than with a vaccine containing 45 µg of HA antigen. Injection site pain was modestly more common with QIV than TIV controls in 3- to 17-year-old recipients after the first dose (65.4% in QIV recipients, 54.6% in TIV-Vic recipients, and 55.7% in the TIV-Yam group), although redness and swelling were not. Pain was also the most common injection site symptom in 6- to 35-month-olds, but less frequent than in older participants. The overall percentage of participants presenting with at least 1 AE during the 7 days following their vaccination ranged from 69.0% to 77.3% in the 3–17 year age group, and was 74.8% in the 6–35 month age group. Other than an increase in injection site pain, the AE profile of the candidate QIV was similar to that of the TIV controls.
There are several limitations to this study. The intermediate outcome of immunogenicity was assessed; therefore, clinical protection cannot be predicted with accuracy. As well, the 6- to 35-month-old group had no TIV comparator, and their outcomes cannot be compared to older children. Finally, we do not know the duration of antibody responses to the 4 strains, as immunogenicity was only assessed to 28 days after final vaccination.
In summary, the evolution of influenza B viruses has led to 2 cocirculating, antigenically distinct B lineages, and immune responses to one confers limited or no cross-lineage protection. A live attenuated QIV has received regulatory approval for use in persons 2–49 years of age in the United States [19] as has an injected QIV [20], and the World Health Organization made its first recommendations for constituents of QIVs for the southern hemisphere for the coming season [21]. The inactivated QIV assessed in this study can potentially provide protection against both B lineages, as well as to the 2 influenza A strains, and thus reduce influenza morbidity in children.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. All authors participated in the implementation of the study, including substantial contributions to conception and design, the gathering of the data, or analysis and interpretation of the data. The corresponding author drafted the manuscript; all authors were involved in revising it critically for important intellectual content, and for final approval of the manuscript. All authors had full access to data, and the corresponding author had final responsibility for submission of the publication. The authors are indebted to the participating study volunteers and their parents, clinicians, nurses, and laboratory technicians at the study sites, as well as to the sponsor's project staff for their support and contributions throughout the study. In particular, we thank W. Seger, D. Connor, L. Wadsworth, T. Araki, P. Bonin, A. Greenspoon, L. Harris-Ford, A. Moskow, B. Muram, K. Sitz, P. Qaqundah, A. Amanullah, and L. Turner. We are grateful to all teams at GlaxoSmithKline Vaccines for their contribution to this study, especially A. Ammanuhla for writing support of the clinical study report, M. Dunaway for clinical study management, and P. Boutet from the clinical and serological laboratory teams, and W. Jiang (Clinical Safety Representative); and V. Dodeur for data management. Finally, the authors thank A. Moon (Moon Medical Communications Ltd, on behalf of GlaxoSmithKline Vaccines) for editorial assistance, and L. Gibbs, J. Dedessus Le Moutier and B. Dumont (Business and Decision Life Sciences, on behalf of GlaxoSmithKline Vaccines) for editorial assistance and manuscript coordination.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA; GlaxoSmithKline SA was involved in all stages of the study conduct and analysis. GlaxoSmithKline SA also bore the costs associated with the development and the publishing of the present manuscript.
Potential conflicts of interest. B. I., V. J., V. C., W. D., and A. L. are employees of GlaxoSmithKline group of companies. B. I., W. D., A. L., and V. J. report ownership of stock options. J. L. reports support for travel to meetings for the study received from GlaxoSmithKline Biologicals SA. S. H. reports payments received from GlaxoSmithKline Biologicals SA for advisory board participation and grants not related to this study. L. M. H. reports payments received from GlaxoSmithKline Biologicals SA for advisory board participation, congress travel costs, grants related or not to this study, support for travel to meetings for the study, and domestic lectures. J. G. S. reports payments received from GlaxoSmithKline Biologicals SA for advisory board participation, grants, and development of educational presentations. A. C. reports grants as payments received from GlaxoSmithKline Biologicals SA for lectures and grants to institution. S. M. reports payments received from Pfizer for lectures, grants, and consultancy; from Merck for lectures and development of educational presentations; and from GlaxoSmithKline Biologicals SA for lectures and grants. M. A. R. W. reports payments received from GlaxoSmithKline Biologicals SA to his institution. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ampofo WK, Baylor N, Cobey S, et al. Improving influenza vaccine virus selection: report of a WHO informal consultation held at WHO headquarters, Geneva, Switzerland, 14–16 June 2010. Influenza Other Respi Viruses. 2012;6:142–52. doi: 10.1111/j.1750-2659.2011.00277.x. e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiore AE, Bridges CB, Katz JM, Cox N. In: Inactivated influenza vaccines. Plotkin S, Orenstein W, Offit P, editors. 2012. pp. 257–93. Vaccines. 6th ed. Elsevier Saunders. [Google Scholar]
- 3.Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990;175:59–68. doi: 10.1016/0042-6822(90)90186-u. [DOI] [PubMed] [Google Scholar]
- 4.Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine. 2010;28(suppl 4):D45–53. doi: 10.1016/j.vaccine.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010;28:2149–56. doi: 10.1016/j.vaccine.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 6.Influenza-associated pediatric deaths—United States, September 2010–August 2011. MMWR. 2011;60:1233–8. [PubMed] [Google Scholar]
- 7.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR. 2010;59:1–62. [PubMed] [Google Scholar]
- 8.Esposito S, Cantarutti L, Molteni CG, et al. Clinical manifestations and socio-economic impact of influenza among healthy children in the community. J Infect. 2011;62:379–87. doi: 10.1016/j.jinf.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Paddock CD, Liu L, Denison AM, et al. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis. 2012;205:895–905. doi: 10.1093/infdis/jir861. [DOI] [PubMed] [Google Scholar]
- 10.Glezen WP, Couch RB, Taber LH, et al. Epidemiologic observations of influenza B virus infections in Houston, Texas, 1976–1977. Am J Epidemiol. 1980;111:13–22. doi: 10.1093/oxfordjournals.aje.a112865. [DOI] [PubMed] [Google Scholar]
- 11.Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30:1993–8. doi: 10.1016/j.vaccine.2011.12.098. [DOI] [PubMed] [Google Scholar]
- 12.Hehme NW, Künzel W, Petschke F, et al. Ten Years of Experience with the Trivalent Split-Influenza Vaccine, Fluarix™. Clin Drug Invest. 2002;22:751–69. [Google Scholar]
- 13.US Food and Drug Administration. Guidance for Industry. Clinical data needed to support the licensure of seasonal inactivated influenza vaccines. 2007. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091990.pdf . Accessed 27 March 2013. [Google Scholar]
- 14.Public Health Agency of Canada National Advisory Committee on Immunization. Statement on Influenza for 2011–2012. 2011;37:1–55. Can Comm Dis Report http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/11vol37/acs-dcc-5/ [Google Scholar]
- 15.Skowronski DM, Hottes TS, Chong M, et al. Randomized controlled trial of dose response to influenza vaccine in children aged 6 to 23 months. Pediatrics. 2011;128:e276–89. doi: 10.1542/peds.2010-2777. [DOI] [PubMed] [Google Scholar]
- 16.Langley JM, Vanderkooi OG, Garfield H, et al. Immunogenicity and safety of 2 dose levels of a thimerosal-free trivalent seasonal influenza vaccine in children aged 6–35 months: a randomized, controlled trial. J Pediatric Infect Dis Soc. 2012;1:55–63. doi: 10.1093/jpids/pis012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skowronski DM, Hottes TS, De Serres G, et al. Influenza Beta/Victoria antigen induces strong recall of Beta/Yamagata but lower Beta/Victoria response in children primed with two doses of Beta/Yamagata. Pediatr Infect Dis J. 2011;30:833–9. doi: 10.1097/INF.0b013e31822db4dc. [DOI] [PubMed] [Google Scholar]
- 18.Gilca V, De Serres G, Hamelin ME, et al. Antibody persistence and response to 2010–2011 trivalent influenza vaccine one year after a single dose of 2009 AS03-adjuvanted pandemic H1N1 vaccine in children. Vaccine. 2011;30:35–41. doi: 10.1016/j.vaccine.2011.10.062. [DOI] [PubMed] [Google Scholar]
- 19.Food and Drug Administration. FDA approves first quadrivalent vaccine to prevent seasonal influenza. 2012. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm294057.htm . Accessed 17 March 2013. [Google Scholar]
- 20.US Food and Drug Administration. US Food and Drug Administration. 2013. Fluarix quadrivalent. US Department of Health and Human Services, Washington, DC. [Google Scholar]
- 21.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2013 southern hemisphere influenza season. World Health Organization, Geneva, Switzerland. http://www.who.int/influenza/vaccines/virus/recommendations/201209_recommendation.pdf. Accessed 3 October 2012. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.