Abstract
Objectives
The relative roles of liver resection (LR) and liver transplantation (LT) in the treatment of a solitary hepatocellular carcinoma (HCC) remain unclear. This study was conducted to provide a retrospective intention-to-treat comparison of these two curative therapies.
Methods
Records maintained at the study centre for all patients treated with LR or listed for LT for hepatitis C-associated HCC between January 2002 and December 2007 were reviewed. Inclusion criteria required: (i) an initial diagnosis of a solitary HCC lesion measuring ≤ 5 cm, and (ii) Child–Pugh class A or B cirrhosis. The primary endpoint analysed was intention-to-treat survival.
Results
A total of 75 patients were listed for transplant (LT-listed group) and 56 were resected (LR group). Of the 75 LT-listed patients, 23 (30.7%) were never transplanted because they were either removed from the waiting list (n = 13) or died (n = 10). Intention-to-treat median survival was superior in the LR group compared with the LT-listed group (61.8 months vs. 30.6 months), but the difference did not reach significance. Five-year recurrence was higher in the LR group than in the 52 LT patients (71.5% vs. 30.5%; P < 0.001).
Conclusions
In the context of limited donor organ availability, partial hepatectomy represents an efficacious primary approach in properly selected patients with hepatitis C-associated HCC.
Introduction
For patients with solitary hepatocellular carcinoma (HCC) and preserved liver function, partial hepatectomy is often considered the treatment of choice. With careful patient selection and modern surgical techniques, resection can be accomplished with low operative mortality1–3 and can achieve 5-year survival rates in the range of 61–78%.4–8 Nevertheless, intrahepatic recurrence affects over 50% of patients at 5 years6,8–10 and relates to both unrecognized intrahepatic dissemination and multicentric carcinogenesis.
Total hepatectomy with liver transplantation (LT) eliminates both unrecognized intrahepatic dissemination and the underlying parenchymal disease that drives neocarcinogenesis. Since patient selection strategies were refined according to the Milan Criteria to include only patients with either one tumour measuring ≤ 5 cm or up to three tumours all measuring < 3 cm,11 many groups have reported dramatically improved recurrence and survival rates following transplant.12–15 However, as a result of donor organ scarcity, many patients placed on the transplant waiting list do not undergo transplantation because they are subject to either tumour progression or hepatic decompensation. As a result, survival rates fall when outcomes are analysed on an intention-to-treat basis.16
The nature of the underlying liver disease is an important consideration in a comparison of resection and transplantation. In the USA, as in Western Europe and Japan, hepatitis C is the most common disease underlying HCC development.17,18 Survival after either resection or transplantation is lower in patients with HCC in the setting of hepatitis C compared with patients with HCC of other aetiologies. Hepatitis C virus (HCV) infection is an independent predictor of local failure and impaired survival following HCC resection,19–21 and recurrent hepatitis C after transplantation may lead to accelerated parenchymal injury, fibrosis and graft failure.22–25
This study provides an intention-to-treat comparison of resection and transplantation as primary curative therapies of solitary hepatitis C-associated HCC measuring ≤ 5 cm.
Materials and methods
Patient selection and study design
The study was approved by the Mount Sinai Medical Center Institutional Review Board. Subsequently, records for all patients with documented HCV-associated HCC (HCV-HCC) listed at the Recanati/Miller Transplantation Institute, Mount Sinai Medical Center and the Division of Surgical Oncology, Department of Surgery, Mount Sinai Medical Center, New York, who were either treated with partial hepatectomy or listed for orthotopic LT between January 2002 and December 2007 were retrospectively reviewed. Criteria for inclusion in the study required: (i) an initial radiographic diagnosis of a solitary HCC lesion of ≤ 5 cm, and (ii) Child–Pugh class A or B cirrhosis. The two groups were compared on the primary endpoint of overall survival. Comparison was carried out in an intention-to-treat fashion16 considering time zero as the date of resection or date of listing for transplantation. Patient outcomes were collected during August 2011.
Diagnosis
Hepatitis C virus infection was documented by anti-HCV serology or evidence of HCV RNA by polymerase chain reaction analysis. The diagnosis of HCC was based on at least one cross-sectional imaging study [computed tomography (CT) or magnetic resonance imaging (MRI)] showing the characteristic vascular enhancement pattern.26 Chest scans by CT were performed routinely to rule out pulmonary metastases. Pathological confirmation of HCC was ultimately obtained for patients who underwent resection or transplantation.
Resection as primary treatment
Patients with solitary HCC without evidence of extrahepatic spread on imaging and with Child–Pugh class A cirrhosis and no clinical or radiographic evidence of portal hypertension were considered for partial hepatectomy.
Resection commenced with intraoperative ultrasound examination of the entire liver to assess the extent of the main tumour and search for additional tumours. Surgery was performed using low central venous pressure anaesthesia and intermittent inflow occlusion (Pringle manoeuvre).3
Surveillance following resection consisted of the monitoring of serum alpha-fetoprotein (AFP) levels and CT (or MRI) scans of the chest and abdomen every 3 months during the first year, every 4 months during the second year, and biannually thereafter. Solitary intrahepatic recurrence was treated with repeat resection provided liver function remained preserved. Solitary pulmonary or intra-abdominal recurrences were also preferentially resected. Resected patients with multinodular intrahepatic recurrence or with compromised liver function were treated with radiofrequency ablation (RFA) or transarterial chemoembolization (TACE) depending on the sizes and number of recurrent tumours; patients in this group without prohibitive comorbidities were also referred for salvage transplant evaluation. From 2008, patients with recurrence who were not eligible for a curative treatment were treated with sorafenib.
Transplant listing as primary treatment
Patients with HCC were placed on the transplant waiting list when impaired liver function or oligonodularity precluded resection. Patients with radiographic evidence of gross vascular invasion were not listed. Patients with tumours that fell within the Milan Criteria11 (one tumour measuring ≤ 5 cm or up to three tumours all of < 3 cm in size) were accorded tumour-related priority according to the policy of the United Network for Organ Sharing (UNOS).27 In this study, only data for patients with solitary HCC lesions of ≤ 5 cm were analysed. Marginal grafts (such as those from donors aged > 65 years, non-beating heart donors, donors with multiple comorbidities and HCV-positive donors, and grafts with hepatic macrosteatosis of > 10% or cold ischaemic time of > 10 h) were generally not used in this population. Listed patients who developed gross vascular invasion, tumour progression outside the Milan Criteria or prohibitive comorbidities were removed from the list. Patients who received transplants from living donors were not excluded from this study.
Post-transplant immunosuppression consisted of tacrolimus, mycophenolate mofetil and corticosteroid. Surveillance of HCC following transplantation consisted of the monitoring of serum AFP and CT (or MRI) scans of the chest and abdomen every 3 months in the first year, every 6 months in the second year, and yearly thereafter until year 5.
Prognostic variables
Variables collected and analysed included age, gender, race, albumin, total bilirubin, creatinine, international normalized ratio (INR), platelets, Child–Pugh class, calculated Model for End-stage Liver Disease (MELD) score, AFP, radiographic tumour size, waiting list exclusion, pathological tumour size and number, microscopic and gross vascular invasion, satellite nodules, tumour grade, UNOS modified tumour–node–metastasis (TNM) stage,27 American Joint Committee on Cancer (AJCC) pathological TNM stage,28 and primary treatment modality (i.e. resection vs. transplant listing).
Statistical analysis
Categorical variables were expressed as valid percentages and compared using the chi-squared test or Fisher's exact test as appropriate. Continuous variables were expressed as the mean ± standard deviation and compared using Student's t-test; alternatively, they were expressed as the median and range and compared using the Mann–Whitney test. Survival and recurrence were estimated using the Kaplan–Meier method and groups were compared using the log-rank test. Those patients receiving salvage LT were censored on the date of death or last follow-up for the survival analyses in this study. Univariate analyses were performed to determine which clinical and pathological variables were associated with increased recurrence and impaired survival. Factors deemed significant on univariate analysis were entered into a multivariate analysis based on the Cox regression model. Continuous variables were dichotomized at the median value or using cut-offs determined by receiver operator characteristic analyses. P-values of < 0.05 were considered to indicate statistical significance. Analysis was carried out using spss Version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
A total of 271 consecutive patients with documented primary HCV-HCC were treated with resection (n = 93) or placed on the transplant waiting list (n = 178) at Mount Sinai Medical Center. Of these 271 patients, 135 fulfilled the aforementioned criteria of a single HCC of ≤ 5 cm at initial diagnosis and Child–Pugh class A or B cirrhosis; four of these patients were excluded because ultimate pathological analysis revealed a non-HCC tumour. The present study group comprised the remaining 131 patients, who were divided according to whether they underwent liver resection (the LR group, n = 56) or were listed for LT (the LT-listed group, n = 75). A comparison of the demographic and clinical data is presented in Table 1.
Table 1.
Clinical and demographic data for patients with hepatitis C-associated hepatocellular carcinoma listed for liver transplantation (LT-listed) or treated with liver resection (LR)
| LT-listed group (n = 75) | LR group(n = 56) | P-value | |
|---|---|---|---|
| Age, years, mean ± SD | 57.4 ± 6.3 | 60.7 ± 8.8 | 0.020 |
| Male gender, n (%) | 60 (80.0) | 41 (73.2) | 0.361 |
| Hepatitis B virus co-infection, n (%) | 1 (1.3) | 1 (1.8) | 0.891 |
| Alcoholic liver disease, n (%) | 15 (20.0) | 3 (5.4) | 0.020 |
| Ethnicity, n (%) | |||
| White | 39 (52.0) | 24 (42.9) | 0.051 |
| African-American | 12 (16.0) | 13 (23.2) | |
| Hispanic | 21 (28.0) | 10 (17.9) | |
| Asian | 1 (1.3) | 7 (12.5) | |
| Other | 2 (2.7) | 2 (3.6) | |
| Child–Pugh class, n (%) | |||
| A | 28 (37.3) | 55 (98.2) | < 0.001 |
| B | 47 (62.7) | 1 (1.8) | |
| C | 0 | 0 | |
| Calculated MELD score, mean ± SD | 12.1 ± 3.8 | 7.8 ± 1.9 | < 0.001 |
| Bilirubin, mg/dl, mean ± SD | 2.32 ± 3.59 | 0.66 ± 0.25 | 0.001 |
| Albumin, g/dl, mean ± SD | 3.14 ± 0.52 | 3.93 ± 0.50 | < 0.001 |
| INR, mean ± SD | 1.35 ± 0.25 | 1.08 ± 0.14 | < 0.001 |
| Creatinine, mg/dl, mean ± SD | 0.88 ± 0.25 | 0.89 ± 0.33 | 0.747 |
| Platelets ≥ 100 000/μl, n (%) | 24 (32.0) | 48 (85.7) | < 0.001 |
| Median AFP, ng/ml, median (range) | 10.5 (2.2–6301.0) | 21.0 (2.5–5312.6) | 0.011 |
| Radiographic tumour sizea, cm, mean ± SD | 2.28 ± 1.03 | 3.13 ± 1.08 | < 0.001 |
Measured at time of listing or resection.
SD, standard deviation; MELD, Model for End-stage Liver Disease; INR, international normalized ratio; AFP, alpha-fetoprotein.
Transplant waiting list
Median waiting time on the transplant list was 5.7 months (range: 0.1–44.6 months). Of the 75 LT-listed patients, 59 (78.7%) received one or more pre-transplant locoregional therapies (mean of 1.80 ± 0.94 treatments each) to prevent tumour progression. These included: TACE (n = 28); RFA (n = 15); percutaneous ethanol ablation (PEI) (n = 3), and a combination of TACE and RFA/PEI (n = 13). Fifty-two (69.3%) patients underwent LT; the remaining 23 patients were either removed from the waiting list (n = 13) or died while listed (n = 10). Causes of removal from the waiting list were tumour progression (n = 8), non-compliance (n = 2) and complete response to neoadjuvant treatment (n = 3). Causes of death while on the waiting list were hepatic decompensation (n = 3), sepsis (n = 2), respiratory failure (n = 1), complications of myasthenia gravis (n = 1) and subdural haematoma (n = 1). The cause of death was unknown in two patients.
Perioperative and pathological data
All 52 transplants were performed using organs procured from deceased donors, three of which were partial livers (all right lobe). The average donor age was 51.6 ± 17.1 years. Mortality within 90 days of transplant was observed in five (9.6%) patients. The causes of death were primary graft non-function (n = 1), biliary sepsis (n = 1), aspiration (n = 1), aplastic anaemia (n = 1) and graft-vs.-host disease (n = 1).
Of the 56 resections performed, 31 (55.4%) were non-anatomic; the remaining 25 (44.6%) procedures were anatomic resections, specifically: segmentectomy (n = 8); bisegmentectomy (n = 11); left lobectomy (n = 3); right lobectomy (n = 2), and central hepatectomy (n = 1). Mortality within 90 days of resection was observed in two (3.6%) patients secondary to intestinal infarction and aspiration, respectively.
Pathological data for the two groups are compared in Table 2.
Table 2.
Pathological data for patients with hepatitis C-associated hepatocellular carcinoma treated with liver transplantation (LT) or liver resection (LR)
| LT group (n = 52) | LR group (n = 56) | P-value | |
|---|---|---|---|
| Tumour size, cm, mean ± SD | 3.30 ± 1.86 | 3.35 ± 1.31 | 0.871 |
| Tumour number, n (%) | |||
| Solitary | 27 (51.9) | 49 (89.1) | < 0.001 |
| Multinodular | 25 (48.1) | 6 (10.9) | |
| Vascular invasion, n (%) | |||
| None | 30 (57.7) | 22 (40.0) | 0.120 |
| Microscopic | 19 (36.5) | 25 (45.5) | |
| Gross | 3 (5.8) | 8 (14.5) | |
| Satellite nodules, n (%) | 7 (13.5) | 12 (21.8) | 0.258 |
| Differentiation, n (%) | |||
| Good | 17 (32.7) | 15 (27.3) | 0.156 |
| Moderate | 22 (42.3) | 28 (50.9) | |
| Poor | 7 (13.5) | 11 (20.0) | |
| Necrotic (treated) | 6 (11.5) | 1 (1.8) | |
| AJCC TNM stagea, n (%) | |||
| I | 14 (26.9) | 19 (34.5) | 0.451 |
| II | 32 (61.5) | 28 (50.9) | |
| III | 5 (9.6) | 8 (14.5) | |
| IV | 1 (1.9) | 0 | |
| UNOS TNM stagea, n (%) | |||
| I | 6 (11.5) | 3 (5.5) | 0.201 |
| II | 27 (51.9) | 39 (70.9) | |
| III | 8 (15.4) | 4 (7.3) | |
| IV | 11 (21.2) | 9 (16.4) | 0.871 |
Based on pathological analysis.
SD, standard deviation; AJCC, American Joint Committee on Cancer; TNM, tumour–node–metastasis; UNOS, United Network for Organ Sharing.
Longterm outcomes: LT-listed group
Median follow-up in the LT-listed group was 30.1 months (range: 0.0–108.7 months). Survivors were followed for a median of 74.3 months (range: 1.4–108.7 months). Of the 52 patients transplanted, tumour recurrence was observed in 12 (23.1%) cases. Actuarial 5-year recurrence following transplant was 30.5%; the corresponding median time to recurrence was not reached.
At the time of final analysis, 44 patients had died, including the 19 patients who did not undergo transplantation. Of the 25 patients who died following transplantation, five succumbed to hepatic decompensation from HCV-associated graft cirrhosis and four of these died within 24 months of transplant. Another nine patients died of recurrent tumour. Intention-to-treat survival rates at 1, 3 and 5 years from the date of listing were 74.3%, 48.6% and 44.1%, respectively; the corresponding median ± standard error (SE) intention-to-treat survival was 30.6 ± 16.6 months. In the 52 patients who underwent LT, survival rates at 1, 3 and 5 years from the date of transplantation were 75.0%, 59.4% and 51.3%, respectively; the corresponding median survival was 62.7 months.
Longterm outcomes: LR group
Median follow-up in the LR group was 46.6 months (range: 0.1–101.0 months). Survivors were followed for a median of 58.3 months (range: 0.4–101.1 months). Tumour recurrence was observed in 34 (60.7%) patients. Actuarial 5-year recurrence following resection was 71.5%; the corresponding median ± SE time to recurrence was 27.3 ± 5.0 months. Recurrence was solely intrahepatic in 32 of 34 patients. Twenty-nine of these 32 patients were treated for recurrence with the following modalities: salvage LT (n = 11); RFA (n = 7); repeat resection (n = 6), and TACE (n = 5). Recurrence in the LR group is compared with that in the 52 patients who received primary transplantations in Fig. 1.
Figure 1.
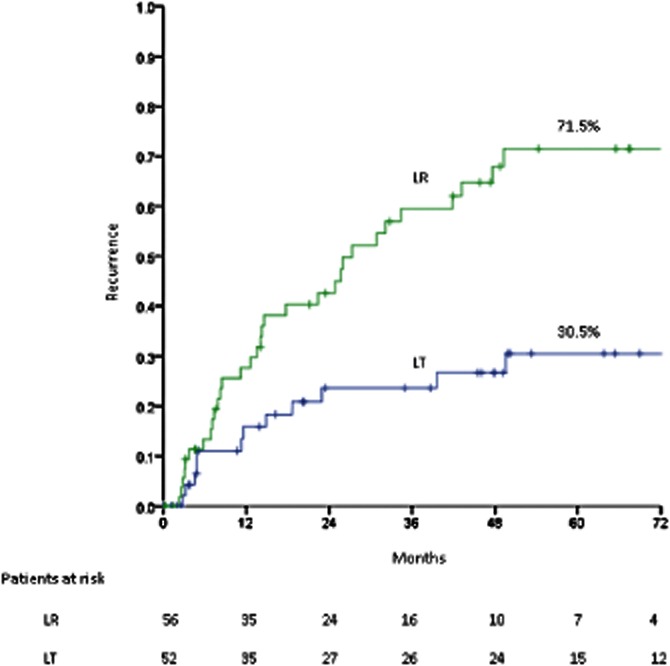
Recurrence rates in resected patients (n = 56) and transplanted patients (n = 52); P < 0.001. LR, liver resection; LT, liver transplant
At the time of final analysis, there had been 29 deaths, 14 of which were secondary to recurrent HCC. Survival rates at 1, 3 and 5 years from the date of resection were 85.1%, 65.4% and 51.6%, respectively; the corresponding median ± SE survival was 61.8 ± 9.8 months. Median ± SE survival following treatment of recurrence (n = 23) was 27.5 ± 3.1 months. Intention-to-treat survival rates in the two groups are compared in Fig. 2; a clear trend favouring resection over listing for LT is demonstrated. This finding persisted despite stratification of the cohort to those treated before and after each of the three dates on which UNOS modified the allocation of MELD exception points (February 2003, April 2004, March 2005)27 (curves not shown). Post-surgical survival in the LR group is compared with that in the 52 patients primarily transplanted in Fig. 3.
Figure 2.
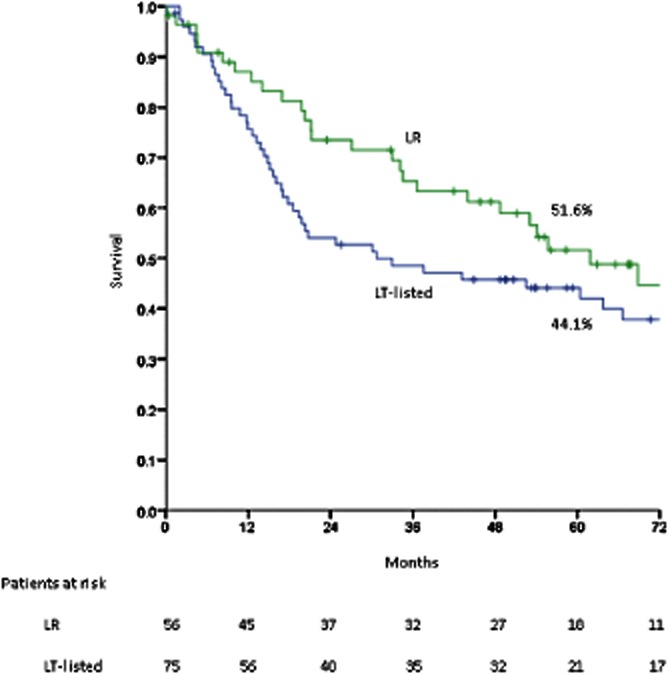
Intention-to-treat survival in resected patients (n = 56) vs. patients listed for transplant (n = 75); P = 0.311. LR, liver resection; LT-listed, liver transplant-listed
Figure 3.
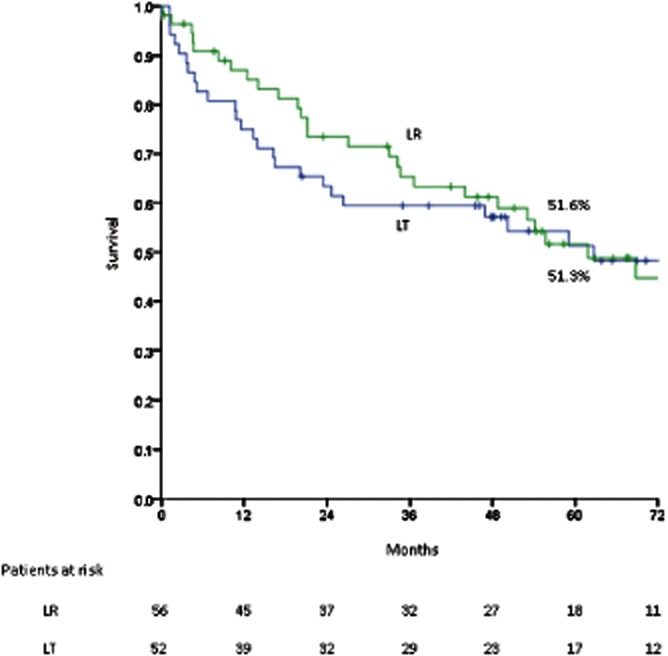
Post-surgical survival in resected patients (n = 56) vs. transplanted patients (n = 52); P = 0.244. LR, liver resection; LT, liver transplant
Prognostic factors: survival
For the entire cohort, factors associated with inferior survival on univariate analysis are presented in Table 3. Of note, primary treatment modality (resection vs. LT listing) was not a significant factor on univariate analysis. The following factors retained significance as independent predictors on multivariate analysis: radiographic tumour size of > 2.5 cm [hazard ratio (HR) 2.25, 95% confidence interval (CI) 1.36–3.70; P = 0.001] and albumin ≤ 3.5 g/dl (HR 2.07, 95% CI 1.08–4.00; P = 0.03).
Table 3.
Univariate analysis of factors for survival in patients with hepatitis C-associated hepatocellular carcinoma who were resected or listed for transplant (n = 131)
| n (%) | Survival, months, median ± SEM | P-value | 5-year survival, % | |
|---|---|---|---|---|
| Radiographic tumour size | ||||
| ≤ 2.5 cm | 77 (58.8) | 92.2 ± 18.0 | 0.006a | 56.7 |
| > 2.5 cm | 54 (41.2) | 27.1 ± 8.3 | 34.2 | |
| Albumin level | ||||
| ≤ 3.5 gm/dl | 75 (57.3) | 20.7 ± 6.4 | 0.002a | 32.8 |
| > 3.5 gm/dl | 56 (42.7) | 92.1 ± 21.1 | 65.0 | |
| INR level | ||||
| ≤ 1.2 | 79 (60.3) | 68.7 ± 14.7 | 0.009 | 49.5 |
| > 1.2 | 52 (39.7) | 20.7 ± 7.5 | 30.8 | |
| MELD score | ||||
| ≤ 9.0 | 63 (48.1) | 68.7 ± 14.3 | 0.027 | 59.5 |
| > 9.0 | 68 (51.9) | 24.7 ± 7.5 | 36.3 | |
Retained significance in multivariate analysis.
INR, international normalized ratio; MELD, Model for End-stage Liver Disease; SEM, standard error of the mean.
Prognostic factors: recurrence
For the entire cohort (excluding patients listed for but not receiving LT), factors associated with increased recurrence on univariate analysis are presented in Table 4. On multivariate analysis, only vascular invasion (microscopic or gross) retained significance as an independent predictor (HR 3.86, 95% CI 1.91–7.79; P < 0.001).
Table 4.
Univariate analysis of factors associated with recurrence in patients with hepatitis C-associated hepatocellular carcinoma treated with liver resection or transplantation (n = 108)
| n (%) | Recurrence, months, median ± SEM | P-value | 5-year recurrence, % | |
|---|---|---|---|---|
| Treatment type | ||||
| Liver transplant | 52 | Not reached | < 0.001 | 30.5 |
| Liver resection | 56 | 27.3 ± 5.0 | 71.5 | |
| AFP level | ||||
| ≤ 10 mg/dl | 44 | 99.6 ± NA | < 0.001 | 28.2 |
| > 10 mg/dl | 64 | 25.9 ± 5.4 | 69.1 | |
| Pathological tumour size | ||||
| ≤ 3 cm | 58 | 99.6 ± 43.1 | 0.012 | 44.0 |
| > 3 cm | 49 | 25.9 ± 10.2 | 63.2 | |
| Vascular invasiona | ||||
| Present | 55 | 17.7 ± 5.4 | < 0.001b | 87.5 |
| Absent | 52 | Not reached | 27.8 | |
| Tumour grade | ||||
| Good | 32 | 99.6 ± 13.0 | 0.005 | 30.1 |
| Moderate–poor | 68 | 27.3 ± 9.3 | 76.8 | |
| Satellites | ||||
| Present | 19 | 14.0 ± 2.2 | < 0.001 | 92.0 |
| Absent | 88 | 99.6 ± 32.8 | 44.4 | |
| AJCC TNM staging | ||||
| I | 33 | 85.1 ± NA | 0.016 | 38.9 |
| II | 60 | 47.6 ± 15.8 | 55.0 | |
| III | 13 | 14.5 ± 5.5 | 79.8 | |
| IV | 1 | – | – | |
Microscopic or gross.
Retained significance in multivariate analysis.
AFP level, alpha-fetoprotein level; AJCC, American Joint Committee on Cancer; NA, not available; SEM, standard error of the mean; TNM, tumour–node–metastasis.
Child–Pugh class A cirrhosis subgroup comparison
An intention-to-treat survival comparison of Child–Pugh class A patients is presented in Fig. 4. There was no significant difference in intention-to-treat survival between the 28 patients listed for transplant (median survival 63.8 ± 15.0 months) and the 55 patients resected (median survival 61.9 ± 9.5 months).
Figure 4.
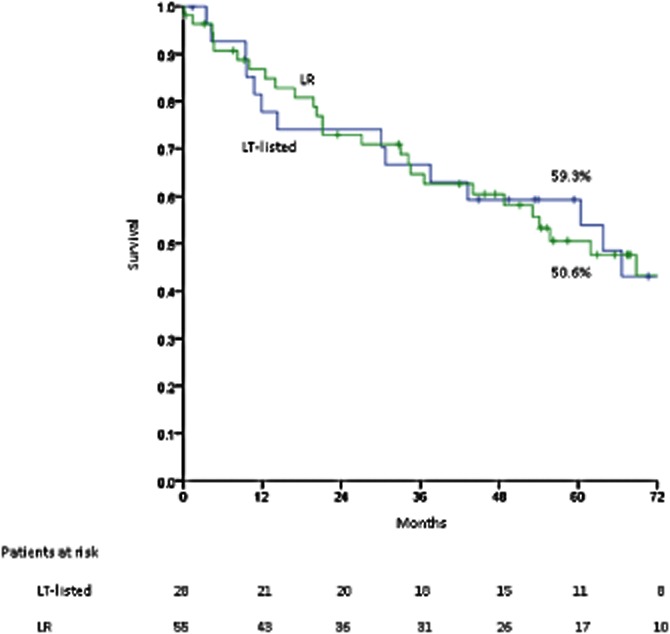
Intention-to-treat survival in patients with Child–Pugh class A cirrhosis who underwent resection (n = 55) or who were listed for transplant (n = 28); P = 795. LR, liver resection; LT-listed, liver transplant-listed
Discussion
Partial hepatectomy and LT are competing potentially curative treatments for early-stage HCC. Underlying HCV infection introduces an additional layer of complexity to patient management and has a strong influence on outcomes. A single-institution intention-to-treat comparison of these two primary approaches is the focus of this report.
Similarly to previous studies,14,16,29 this analysis demonstrated no significant survival difference between transplantation and resection; median and 5-year survival were essentially equivalent in patients undergoing resection and those undergoing LT. However, there was a clear trend favouring resection over transplant from an intention-to-treat perspective. The basis for this trend is the 30% rate of dropout from the waiting list, which leads to an early decline in the survival curve for LT-listed patients. Shah et al.29 showed a 21% dropout rate after a median waiting list time of 7.7 months; the rate identified in the present study was less encouraging despite a shorter waiting time (5.7 months) and the liberal use of preoperative locoregional therapies. Tumour-related exclusion accounted for only part of this deficit; a significant proportion of the present patients died while awaiting transplant as a result of a variety of medical causes including hepatic decompensation. Liver injury from locoregional treatment may have been a contributing factor in these cases of hepatic decompensation.
Early post-transplant mortality from sepsis and immunosuppression-related haematologic disorders also contributed to the early divergence of the intention-to-treat (and post-surgical) survival curves in favour of the LR group. In addition, accelerated HCV-related graft cirrhosis and ultimate hepatic decompensation led to death in four LT patients. The rate at which HCV-associated graft failure was observed in the present study was similar to those reported in other transplant series,30–32 and underscores the relevance of this problem in the HCV-infected transplant population as a whole.
With regard to recurrence, the high 5-year recurrence rate (71.5%) following resection is in line with those cited in previous reports describing the resection of small HCV-associated HCC,6,20,21 and recurrence was the most frequent cause of death in the LR group. The emergence of vascular invasion as an independent predictor for recurrence in the present cohort was also consistent with previous reports describing outcomes of resection as well as those of transplant,8,9,13,15,20,30 and was associated in the present LR group with early (within the first 2 years) intrahepatic recurrence. Later intrahepatic recurrence was also common in the LR group and was presumably related to de novo carcinogenesis.33 Regardless of their aetiopathogenesis, the overwhelming majority of recurrences following resection were intrahepatic. Accordingly, a large proportion of these recurrences (> 90%) were amenable to curative treatments including RFA, repeat resection and salvage LT, and an additional median survival from the date of recurrence of 27.5 months was achieved. The present study group has previously shown that repeat resection for recurrence provides an additional median survival of 59 months.34
By contrast, the 5-year recurrence rate of 30.5% following transplant was much higher than expected. With the use of the Milan Criteria,11 most groups5,14,15,29 have reported rates of post-transplant HCC recurrence lower than those observed here, including in reports focused solely on HCC arising in the context of HCV infection.12,30,31,35 It is likely that the high prevalence of vascular invasion in the transplanted patients (42.3%) in the present series underlies this high rate of recurrence; other transplant series in which the Milan Criteria have been employed for case selection have reported rates of vascular invasion of 6–20%.5,15,29,36 Although the present study group has previously reported a higher incidence of vascular invasion in HCV-HCC than in hepatitis B-associated HCC,37 other series specifically reporting on transplant outcomes in HCV-HCC have noted lower rates of vascular invasion than that observed in the current series.30,31,35 Vascular invasion is an aspect of the natural progression of HCC over time. Patients in the present series were maintained within the Milan Criteria over extended waiting periods by the application of locoregional treatments and although progression as judged by imaging may not have been observed in those patients who reached transplant, progression in terms of vascular invasion may have nonetheless occurred in a proportion of patients.
The aim of this study was to compare resection and transplantation as competing alternatives for the primary treatment of HCV-HCC. The study population was therefore limited to HCC patients who could reasonably be treated with either modality: they were required to exhibit a solitary HCC lesion measuring ≤ 5 cm because multinodularity is a well-recognized predictor of poor outcome after resection and because waiting list priority for the receipt of a donor organ is unavailable to patients with HCC of > 5 cm in size. Patients with Child–Pugh class A or B cirrhosis were included. There is clearly an imbalance between the LR and LT-listed groups in this regard: only patients with Child–Pugh class A cirrhosis and no portal hypertension were submitted to resection, whereas patients with portal hypertension or with Child–Pugh class B cirrhosis were listed for transplant. Although the present authors attempted to lessen this imbalance by making a Child–Pugh class A subgroup comparison, inequity persists. This flaw can only be truly overcome in a randomized trial.
Neither resection nor transplant is an optimal treatment for HCV-HCC. Resected patients are plagued by a high incidence of tumour recurrence and resection is applicable in only a minority of patients. Conversely, transplant results are dependent on organ availability, and even those patients fortunate enough to receive a donor liver in the present series were no more likely to survive than appropriate candidates who underwent resection, largely as a result of the impact of HCV recurrence. This survival comparison does, however, require cautious interpretation, given the aforementioned differences in baseline liver function between the two groups and the unfortunately high prevalence of vascular invasion in transplanted patients. Nevertheless, in a setting of donor organ scarcity, the present authors believe that these data support resection as a safe and reasonable option for patients with single HCC lesions of ≤ 5 cm against a background of Child–Pugh class A cirrhosis without portal hypertension.
Conflicts of interest
None declared.
References
- 1.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 2.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Merchant NB, Didolkar MS. Hepatic resection using intermittent vascular inflow occlusion and low central venous pressure anaesthesia improves morbidity and mortality. J Gastrointest Surg. 2000;4:162–167. doi: 10.1016/s1091-255x(00)80052-9. [DOI] [PubMed] [Google Scholar]
- 4.Cha CH, Ruo L, Fong Y, Jarnagin WR, Shia J, Blumgart LH, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg. 2003;238:315–321. doi: 10.1097/01.sla.0000086548.84705.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margarit C, Escartin A, Castells L, Vargas V, Allende E, Bilbao I. Resection of hepatocellular carcinoma is a good option in Child–Turcotte–Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11:1242–1251. doi: 10.1002/lt.20398. [DOI] [PubMed] [Google Scholar]
- 6.Taura K, Ikai I, Hatano E, Nakajima A, Tada M, Seo S, et al. Influence of coexisting cirrhosis on outcome after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery. 2007;142:685–694. doi: 10.1016/j.surg.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, et al. Liver resection for transplantable hepatocellular carcinoma: longterm survival and role of secondary liver transplantation. Ann Surg. 2009;250:738–746. doi: 10.1097/SLA.0b013e3181bd582b. [DOI] [PubMed] [Google Scholar]
- 8.Fan ST, Poon RT, Yeung C, Lam CM, Lo CM, Yuen WK, et al. Outcome after partial hepatectomy for hepatocellular cancer within the Milan criteria. Br J Surg. 2011;98:1292–1300. doi: 10.1002/bjs.7583. [DOI] [PubMed] [Google Scholar]
- 9.Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–543. doi: 10.1097/01.SLA.0000059988.22416.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham SC, Tsai S, Marques HP, Mira P, Cameron A, Barroso E, et al. Management of early hepatocellular carcinoma in patients with well-compensated cirrhosis. Ann Surg Oncol. 2009;16:1820–1831. doi: 10.1245/s10434-009-0364-1. [DOI] [PubMed] [Google Scholar]
- 11.Mazzaferro V, Regalio E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinoma in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 12.Figueras J, Ibanez L, Ramos E, Jaurrieta E, Ortiz-de-Urbina J, Pardo F, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: result of a multicentre study. Liver Transpl. 2001;7:877–883. doi: 10.1053/jlts.2001.27856. [DOI] [PubMed] [Google Scholar]
- 13.Hemming AW, Cattral MS, Reed AI, Van der Werf WJ, Greig PD, Howard RJ. Liver transplantation for hepatocellular carcinoma. Ann Surg. 2001;233:652–659. doi: 10.1097/00000658-200105000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Gaudio M, Ercolani G, Ravaioli M, Cescon M, Lauro A, Vivarelli M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant. 2008;8:1177–1185. doi: 10.1111/j.1600-6143.2008.02229.x. [DOI] [PubMed] [Google Scholar]
- 15.Bellavance EC, Lumpkins KM, Mentha G, Marques HP, Capussotti L, Pulitano C, et al. Surgical management of early-stage hepatocellular carcinoma: resection or transplantation? J Gastrointest Surg. 2008;12:1699–1708. doi: 10.1007/s11605-008-0652-2. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 17.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 18.Leong TY, Leong AS. Epidemiology and carcinogenesis of hepatocellular carcinoma. HPB. 2005;7:5–15. doi: 10.1080/13651820410024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagasue N, Ono T, Yamanoi A, Kohno H, El-Assal ON, Taniura H, et al. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg. 2001;88:515–522. doi: 10.1046/j.1365-2168.2001.01732.x. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki Y, Yamada T, Tanaka H, Ohigashi H, Eguchi H, Yano M, et al. Risk of recurrence in a longterm follow-up after surgery in 417 patients with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Ann Surg. 2006;244:771–780. doi: 10.1097/01.sla.0000225126.56483.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Li H, Qin Y, Wang PP, Hao X. Comparison of surgical outcomes for small hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a Chinese experience. J Gastroenterol Hepatol. 2007;22:1936–1941. doi: 10.1111/j.1440-1746.2006.04619.x. [DOI] [PubMed] [Google Scholar]
- 22.Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673–684. doi: 10.1016/s0168-8278(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 23.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz M. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(Suppl. 1):268–276. doi: 10.1053/j.gastro.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 25.Futagawa Y, Terasaski PI, Waki K, Cai J, Gjertson DW. No improvement in longterm liver transplant graft survival in the last decade: an analysis of the UNOS data. Am J Transplant. 2006;6:1398–1406. doi: 10.1111/j.1600-6143.2006.01256.x. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 27.Organ Procurement and Transplantation Network/United Network for Organ Sharing. 2010. Policy 3.6. Available at http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_8.pdf (last accessed 28 July 2011)
- 28.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th edn. New York, NY: Springer-Verlag; 2010. [Google Scholar]
- 29.Shah SA, Cleary SP, Tan JCC, Wei AC, Gallinger S, Grant DR, et al. An analysis of resection vs. transplantation for early hepatocellular carcinoma: defining the optimal therapy at a single institution. Ann Surg Oncol. 2007;14:2608–2614. doi: 10.1245/s10434-007-9443-3. [DOI] [PubMed] [Google Scholar]
- 30.Moya A, Berenguer M, Aguilera V, Juan FS, Nicolás D, Pastor M, et al. Hepatocellular carcinoma: can it be considered a controversial indication for liver transplantation in centres with high rates of hepatitis C? Liver Transpl. 2002;8:1020–1027. doi: 10.1053/jlts.2002.35664. [DOI] [PubMed] [Google Scholar]
- 31.Shimoda M, Ghobrial RM, Carmody IC, Anselmo DM, Farmer DG, Yersiz H, et al. Predictors of survival after liver transplantation for hepatocellular carcinoma associated with hepatitis C. Liver Transpl. 2004;10:1478–1486. doi: 10.1002/lt.20303. [DOI] [PubMed] [Google Scholar]
- 32.Firpi RJ, Clark V, Soldevila-Pico C. The natural history of hepatitis C cirrhosis after liver transplantation. Liver Transpl. 2009;15:1063–1071. doi: 10.1002/lt.21784. [DOI] [PubMed] [Google Scholar]
- 33.Bilimoria MM, Lauwers GY, Doherty DA, Nagorney DM, Belghiti J, Do KA, et al. Underlying liver disease, not tumour factors, predicts longterm survival after resection of hepatocellular carcinoma. Arch Surg. 2001;136:528–535. doi: 10.1001/archsurg.136.5.528. [DOI] [PubMed] [Google Scholar]
- 34.Roayaie S, Bassi D, Tarchi P, Labow D, Schwartz M. Second hepatic resection for recurrent hepatocellular cancer: a Western experience. J Hepatol. 2011;55:346–350. doi: 10.1016/j.jhep.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Bozorgzadeh A, Orloff M, Abt P, Tsoulfas G, Younan D, Kashyap R, et al. Survival outcomes in liver transplantation for hepatocellular carcinoma, comparing impact of hepatitis C versus other aetiology of cirrhosis. Liver Transpl. 2007;13:807–813. doi: 10.1002/lt.21054. [DOI] [PubMed] [Google Scholar]
- 36.Bigourdan JM, Jaeck D, Meyer N, Meyer C, Oussoultzoglou E, Bachellier P, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl. 2003;9:513–520. doi: 10.1053/jlts.2003.50070. [DOI] [PubMed] [Google Scholar]
- 37.Roayaie S, Haim MB, Emre S, Fishbein TM, Sheiner PA, Miller CM, et al. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a western experience. Ann Surg Oncol. 2000;7:764–770. doi: 10.1007/s10434-000-0764-8. [DOI] [PubMed] [Google Scholar]


