Abstract
To identify and characterize anti-citrullinated glucose-6-phosphate isomerase (GPI) peptide antibodies in patients with rheumatoid arthritis (RA). Nine GPI arginine-bearing peptides in human GPI protein were selected and cyclic citrullinated GPI peptides (CCG-1–9) were constructed. Samples were obtained from RA (n = 208), systemic lupus erythematosus (SLE) (n = 101), Sjögren's syndrome (SS; n = 101) and healthy controls (n = 174). Antibodies against CCG-1–9 were measured, and anti-citrullinated α-enolase-1 (CEP-1), -cyclic citrullinated peptides (CCP) and -GPI proteins antibodies were also examined. Patients with RA were genotyped for HLA-DRB1. The numbers of shared epitope (SE) alleles were counted and compared with those of the autoantibodies. Rabbit GPI was citrullinated with rabbit peptidylarginine deiminase and immunoblot analysis of RA sera performed. The levels of autoantibodies were compared before and after treatment with TNF antagonists in 58 RA patients. Anti-CCG-2, -4 and -7 antibodies were detected in 25·5, 33·2 and 37·0% patients with RA, respectively, and these antibodies were very specific for RA (specificity, 98·1–99·7%). Altogether, 44·2, 86·1 and 13·9% of RA sera were positive for anti-CEP-1, -CCP and -GPI protein antibodies, respectively. Anti-CCG-2, -4 and -7 antibodies were correlated with anti-CCP and anti-CEP-1 antibodies and with the presence of HLA-DRB1 SE alleles. Citrullinated GPI protein was detected using RA sera. Treatment with tumour necrosis factor antagonists reduced significantly the levels of anti-CCG-2 and -7 but not of anti-CEP-1 antibodies. This is the first report documenting the presence of anti-CCG antibodies in RA. Anti-CCG-2 and -7 antibodies could be considered as markers for the diagnosis of RA and its disease activity.
Keywords: anti-citrullinated peptide antibody, autoantibody, citrullinated antigen, glucose-6-phosphate isomerase, rheumatoid arthritis
Introduction
Autoantibodies are the hallmark of rheumatoid arthritis (RA), although their pathogenic role in the disease remains controversial. Recently, several studies have reported that citrullinated proteins are RA-specific autoantigens, and enzyme-linked immunosorbent assays (ELISA) using cyclic citrullinated peptides (CCP) are used frequently for the diagnosis [1]. The citrullinated autoantigen in anti-CCP antibody-positive individuals seems to be mixed and cross-reactive [2]. Candidate citrullinated autoantigens that have been identified in RA include fibrinogen, vimentin, collagen type II and α-enolase [3–6]. For example, positivity of autoantibodies to citrullinated α-enolase peptide-1 (CEP-1) shows high association with the HLA-DRB1 shared epitope (SE) and smoking, suggesting its importance as a marker for RA [7]. However, the pathogenic roles of anti-citrullinated protein antibodies remain elusive, owing partly to the lack of association with disease activity [8].
Glucose-6-phosphate isomerase (GPI), a major glycolytic enzyme, was first described as an arthritogenic target in the K/B×N T cell receptor transgenic mouse model, and arthritis was sustained almost completely by autoantibodies to GPI [9,10]. Recently, immunization with human GPI was reported to provoke arthritis in the DBA/1 mouse, suggesting that autoimmunity to GPI plays a direct role in arthritis in genetically unaltered mice [11,12]. In humans, several groups have described the up-regulated expression of autoantigen GPI in sera of patients with RA [13,14], as well as in the joint synovium [15,16]. Conversely, the first report on anti-GPI antibodies in humans showed a high frequency of such antibodies in the sera of RA patients [15], although their frequency is still debated [17–20]. Using our in-house anti-GPI antibody assay, which employs two different GPIs (recombinant human GPI and rabbit native GPI), we reported that only 15% of patients with RA were positive for anti-GPI antibodies and that the severity of arthritis correlated with the serum anti-GPI antibody levels [17]. Others have also reported that extra-articular complications in RA correlated with serum anti-GPI antibody levels [18].
The present study is an extension of our previous investigation [17]. We have assumed a hypothesis that antibodies against citrullinated part of GPI protein exist in a subset of patients with RA specifically the same as other anti-citrullinated protein antibodies (ACPA), and attempt to further characterize antibodies against citrullinated GPIs in patients with RA.
Nine cyclic citrullinated peptides spanning the whole GPI sequence were constructed (CCG-1–9) and the levels of anti-CCG antibodies measured by ELISA. The antibodies were also compared with anti-CEP-1, -CCP and anti-GPI protein antibodies. HLA-DRB1 genotyping was performed and the numbers of SE alleles were counted. In addition, we focused on highly specific and SE-related anti-CCGs such as anti-CCG-2, -4 and -7 and anti-CEP-1 antibodies, and compared the levels of these antibodies in patients with RA before and after they received treatment with tumour necrosis factor (TNF) antagonists. We further investigated the association between decreased levels of these antibodies and disease activity.
Materials and methods
Serum samples from patients and healthy controls
Serum, plasma and whole blood samples were collected from 208 Japanese patients with RA, diagnosed by rheumatologists according to the criteria of the American College of Rheumatology (ACR) in 1987 [21]. The mean age of the patients was 54 years (range 16–84 years); 76% were female. Serum samples were also obtained from 174 healthy control subjects (HS) (mean age, 27 years; range 18–55 years; 48% female). Disease control samples were also collected from patients with systemic lupus erythematosus (SLE; n = 101; mean age 40 years; range 15–67 years; 88% female) and Sjögren's syndrome (SS; n = 101; mean age 55 years; range 21–84 years; 97% female). All patients with SLE fulfilled the 1997 ACR classification criteria [22], and all patients with SS satisfied the Japanese Ministry of Health criteria for the diagnosis of SS. The criteria of SS included four clinicopathological findings, while the diagnosis of SS was based on the presence of two or more of the following conditions: presence of anti-SS-A or SS-B antibodies, keratoconjunctivitis sicca, salivary dysfunction and lymphocytic infiltration of the salivary or lacrimal glands. None of the patients with SLE or SS had overlapping RA. All samples were collected at the University of Tsukuba Hospital after informed consent was obtained from all patients. Samples were also collected from 58 patients (at least one sample positive for anti-CCG-2, -4 and -7 or anti-CEP-1 antibodies) with RA before and 6 months after treatment with TNF antagonists (infliximab, n = 41; etanercept, n = 15; adalimumab, n = 2). All antibody-positive patients were grouped into four (anti-CCG-2, 4, 7 and CEP-1-positive) groups. All patients were positive for antibodies at baseline (before treatment) in each group. This study was reviewed and approved by the ethics committee of the University of Tsukuba.
Synthetic peptides
Nine 19-mer peptides were selected from a human GPI amino acid sequence containing one, two, three or four arginine residues. The amino acid numbers of the peptide region were 12–30, 70–88, 91–109, 131–149, 264–282, 337–355, 435–453, 457–475 and 540–558 from the N terminal. In the peptides, arginine was replaced with citrulline residues and cysteine residues were added to the amino and carboxyl terminal for circularization (Table 1), and CEP-1 peptide (CKIHAXEIFDSXGNPTVEC, in which X represents citrulline) [6] was synthesized at the Medical and Biological Laboratories in Nagano, Japan. Five unmodified 19-mer peptides were also synthesized from human GPI (G-1: KLQQWYREHRSELNLRRLF; G-2: DLAKSRGVEAARERMFNGE; G-3: NYTEGRAVLHVALRNRSNT; G-4: SFCQRVRSGDWKGYTGKTI; G-7: ALMRGKSTEEARKELQAAG).
Table 1.
Antibody response to citrullinated peptides in patients with rheumatoid arthritis (RA) and in controls*
| Antigen | Sequence | RA (n = 208) | SLE (n = 101) | SS (n = 101) | HS (n = 174) | RA versus controls (SLE, SS, HS) |
|---|---|---|---|---|---|---|
| CCG-1 | CKLQQWYXEHXSELNLXXLFC | 8·7 | 0 | 0 | 0 | 100 |
| CCG-2 | CDLAKSXGVEAAXEXMFNGEC | 25·5 | 0 | 0 | 0·6 | 99·7 |
| CCG-3 | CNYTEGXAVLHVALXNXSNTC | 14·4 | 6·0 | 15·8 | 1·7 | 93·4 |
| CCG-4 | CSFCQXVXSGDWKGYTGKTIC | 33·2 | 2·0 | 4·0 | 0·6 | 98·1 |
| CCG-5 | CFEFWDWVGGXYSLWSAIGLC | 3·4 | 2·0 | 0 | 1·7 | 98·7 |
| CCG-6 | CAMLPYDQYLHXFAAYFQQGC | 0·5 | 0 | 1·0 | 0·6 | 99·5 |
| CCG-7 | CALMXGKSTEEAXKELQAAGC | 37·0 | 2·0 | 0 | 1·2 | 98·9 |
| CCG-8 | CEDLEXLLPHKVFEGNXPTNC | 25·0 | 8·9 | 2·0 | 1·7 | 96·3 |
| CCG-9 | CASTNGLINFIKQQXEAXVQC | 5·8 | 5·0 | 0 | 1·7 | 97·9 |
| CEP-1 | CKIHAXEIFDSXGNPTVEC | 44·2 | 2·0 | 0 | 1·2 | 98·9 |
| CCP | 86·1 | 6·9 | 3·4 | 1·2 | 97·3 |
Values represent the percentages of positivity (RA, systemic lupus erythematosus (SLE), Sjögren's syndrome (SS) and healthy control subjects (HS)] or specificity (RA versus controls). RA versus controls: specificity of the negative reaction rate compared with those of the HS and disease controls. X = citrulline.
GPI proteins
Recombinant human GPI (rhGPI) was prepared as described previously [17]. Briefly, human GPI complementary DNA (cDNA) was inserted into the plasmid pGEX-4T3 (Pharmacia, Uppsala, Sweden). The resulting clone encoded amino acids with the whole GPI sequence and linked to glutathione S-transferase (GST)-tagged proteins. The resulting solution was purified with a glutathione–sepharose column (Pharmacia). Rabbit native GPI (rabGPI) from rabbit muscle was used (Sigma, St Louis, MO, USA).
ELISA for peptides
CCG-1–9 and CEP-1 were used at 10 μg/ml (diluted in 50 mM Na2CO3; pH 9·6) to coat 96-well plates (MaxiSorp; Nunc, Roskilde, Denmark) and incubated overnight at 4°C. After three washes with phosphate-buffered saline (PBS), 5% bovine serum albumin (BSA) in PBS was used for blocking non-specific binding (1 h at room temperature). After three washes with washing buffer (0·05% Tween-20 in PBS), sera from patients with RA (n = 208), HS (n = 174) and disease controls (diluted 1:100) was added. The plates were then incubated for 1 h at room temperature. After washing, alkaline phosphatase (AP)-conjugated anti-human immunoglobulin (Ig)G (Fc fragment-specific; American Qualex International, San Clemente, CA, USA) was added to the plate (diluted 1:2000) and then incubated for 1 h at room temperature. After washing, the colour was developed with AP reaction solution (containing 9·6% diethanolamine, 0·25 mM MgCl2; pH 9·8) with AP substrate tablets (Sigma; 1 AP tablet per 5 ml AP reaction solution). The plates were incubated for 30 min at room temperature, and optical density (OD) was measured by plate spectrophotometry at 405 nm. The primary OD reading was subtracted from the OD readings of the control wells (coated with 50 mM Na2CO3; pH 9·6) to eliminate non-specific reactions. Measurements were performed in duplicate. All OD data values shown were the subtracted OD. The cut-off OD [mean value ± 3 standard deviations (s.d.)] was calculated from the ELISA reactions of HS. Anti-CCP antibodies were measured by a commercial anti-CCP2 ELISA kit (Cosmic Corporation, Tokyo, Japan).
ELISA for GPI proteins
To measure anti-GPI antibodies, both rhGPI and rabGPI were used at 5 μg/ml (diluted in PBS) for coating 96-well plates (Sumilon; Sumitomo Bakelite, Tokyo, Japan). The plates were then incubated overnight at 4°C. After three washes with washing buffer (0·05% Tween-20 in PBS), Block Ace (diluted 1:4 in PBS; Dainippon Pharmaceuticals Companys, Osaka, Japan) was used for blocking non-specific binding (1 h at room temperature). After three washes, the sera of patients with RA (n = 158) and of healthy controls (n = 71) (diluted 1:500) was added, and the plates were incubated for 2 h at room temperature. After washing, AP-conjugated anti-human IgG was added to the plates (diluted 1:1000), and the plates were incubated for 1 h at room temperature. After three washes, the colour was developed with the AP reaction solution with AP substrate tablets. The plates were incubated for 30 min at room temperature and the OD was measured by plate spectrophotometry at 405 nm. The primary OD reading was subtracted from the OD readings of the control wells to eliminate non-specific reactions. The control wells were coated with GST for recombinant human GPI–GST and with PBS for rabbit native GPI. Measurements were performed in triplicate. The cut-off OD (mean value + 2 s.d.) was calculated from the ELISA reactions of HS.
HLA-DRB1 genotyping
Genomic DNA was extracted from peripheral blood leucocytes of patients with RA (n = 147) using QuickGene (Fujifilm, Tokyo, Japan). HLA-DRB1 typing was performed by a sequence-specific oligonucleotide probe polymerase chain reaction (PCR) assay (PCR–SSOP) using a WAKFlow human leucocyte antigen (HLA) typing kit (Wakunaga, Hiroshima, Japan). The PCR products were hybridized and measured using a Luminex-200 multi-analyte profiling system (xMAP; Luminex Corporation, Austin, TX, USA). Results were analysed using the typing software provided by the manufacturer.
In-vitro deimination
Rabbit native GPI was diluted to a concentration of 0·3 mg/ml in peptidylarginine deiminase (PAD) buffer (0·1 M Tris HCl, pH 7·6, 10 mM CaCl2, 5 mM dithiothreitol) and incubated for 3 h with rabbit skeletal muscle PAD (Sigma) at a concentration of 7 units/mg protein at 37°C in an incubator. Citrullination was terminated by the addition of 20 mM ethylenediamine tetraacetic acid (EDTA). Deimination of GPI was confirmed by use of an anti-citrulline (modified) detection kit (Millipore, Temecula, CA, USA).
Immunoblot analysis
Citrullinated rabbit GPI (cit rabGPI) and rabGPI were confirmed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were stained either with Gelcode Blue stain reagent (Thermo Fisher Scientific, Rockford, IL, USA) or transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Jupiter, CA, USA) for immunoblot analysis. After blocking with Block Ace for 1 h at room temperature, the membranes were cut into strips. Each strip was incubated with 3 ml of sera from 15 patients with RA or from five HS, diluted 1:100 in Block Ace overnight at 4°C. Horseradish peroxidase (HRP)-conjugated anti-human IgG (Fc fragment-specific; American Qualex International) diluted to 1:4000 was allowed to react with PVDF membranes for 1 h at room temperature. HRP-conjugated secondary antibody was visualized using Amersham ECL prime Western blotting detection reagent (GE Healthcare UK, Little Chalfont, Bucks, UK).
Statistical analysis
Data were analysed using the Mann–Whitney U-test, t-test and paired t-test and Pearson's product–moment coefficient of correlation to evaluate the distribution and correlation of antibodies. The χ2 test and Fisher's exact test were used to evaluate differences in the antibody positivity rates. When one or more of the variables in the 2 × 2 tables was ≤5, Fisher's exact test was used. A P-value < 0·05 was considered statistically significant.
Results
Identification of specific anti-citrullinated GPI peptide antibodies in patients with RA
To examine the presence of anti-citrullinated GPI peptide antibodies in RA sera, we selected nine peptides from the GPI sequence. The levels of anti-CCG-1, -2, -3, -4, -7 and -8 antibodies were significantly different between patients with RA and HS (P < 0·01, P < 0·001, P < 0·001, P < 0·001, P < 0·001, P < 0·001, respectively, t-test) (Fig. 1a,b). To determine the disease specificity of the anti-CCGs, we also screened the sera of other autoimmune diseases, such as SLE and SS. The summarized data are shown in Table 1. Among these six peptides, antibodies to CCG-2, -4 and -7 were both highly sensitive compared to the remaining anti-CCGs and highly specific in patients with RA. The positivity of anti-CCG-2, -4 and -7 antibodies in RA were 25·5, 33·2 and 37·5%, respectively. The specificities of anti-CCG-2, -4 and -7 antibodies in RA were 99·7, 98·1 and 98·9% compared with the healthy and disease controls, respectively (Table 1). Anti-CCG-1 antibodies seemed to have low sensitivity and anti-CCG-3 and -8 antibodies were sometimes detected in the sera of the disease controls, suggesting weak specificity. Reactivity to CCG-2, -4 and -7 (at least one of CCG-2, -4 and -7) was detected in 57·2% of the RA samples. Anti-CEP-1 antibody was detected in 44·2% of the RA samples and the level was significantly different in the RA and control samples (P < 0·001, t-test) (Table 1, Fig. 1a,b). Of the 208 patients with RA, 86·1% were positive for anti-CCP antibodies (Table 1).
Figure 1.
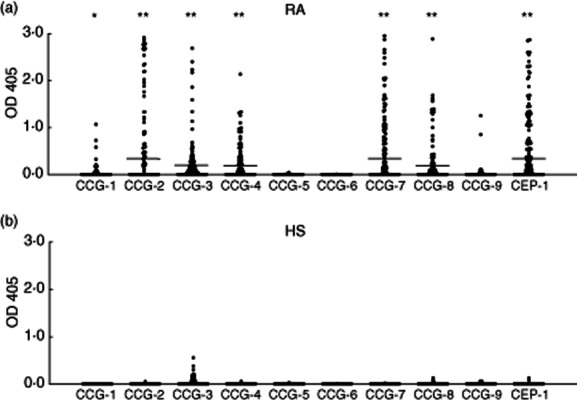
(a) Antibody levels in patients with rheumatoid arthritis (RA). Anti-cyclic citrullinated glucose-6-phosphate isomerase peptide-1–9 (CCG-1–9) antibodies and anti-citrullinated α-enolase peptide (CEP-1) antibodies were measured in the sera of patients with RA (n = 208) by enzyme-linked immunosorbent assay (ELISA). (b) Antibody levels in healthy control subjects (HS). Anti-CCG-1–9 and anti-CEP-1 antibodies were measured in sera of HS (n = 174) by ELISA. Data represent the optical density values at 405 nm (OD 405). Each datum point represents an individual subject. Bar indicates the mean value. *P < 0·01; **P < 0·001.
Antibodies to non-modified peptides (G-1, -2, -3, -4 and -7) were detected rarely in the sera of the RA patients or the HS by ELISA (1·9, 3·8, 1·4, 2·4 and 0% of the RA sera and 1·7, 1·7, 2·3, 0·6 and 0% of HS sera, respectively; Supporting Fig. S1). These findings suggest that anti-CCG antibodies, especially anti-CCG-2, -4 and -7 antibodies, are highly sensitive and specific for RA.
Comparison of anti-GPI protein antibodies and anti-CCG antibodies
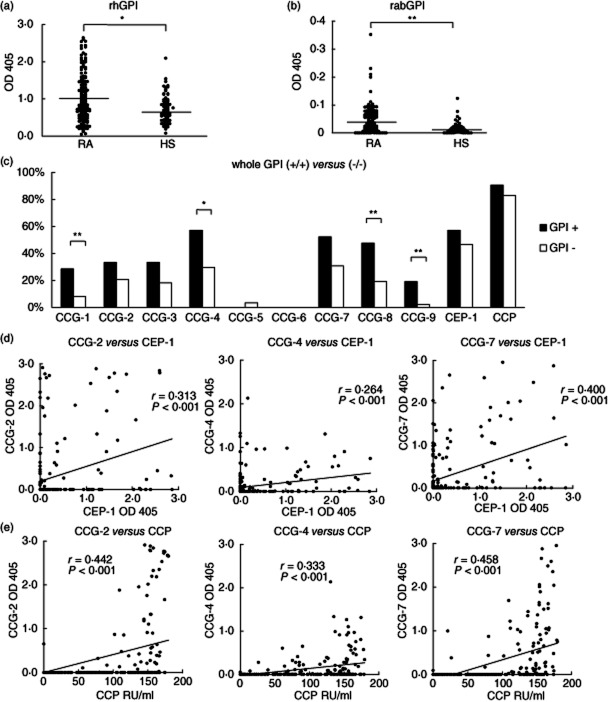
The positivity and specificity of anti-GPI protein antibodies is still being debated. Thus, to determine the association between anti-GPI protein antibodies and anti-CCG-1–9 antibodies, we used ELISA to detect anti-rhGPI and anti-rabGPI protein antibodies in the RA (n = 158) and HS (n = 71) sera. The level of anti-rhGPI antibody was significantly different between RA (OD mean = 1·038) and HS (OD mean = 0·736) (P < 0·01, Mann–Whitney U-test), although a weaker reaction was evident in the control sera (Fig. 2a). The level of anti-rabGPI antibody was also significantly different between the RA (OD mean = 0·041) and HS (OD mean = 0·015) sera (P < 0·001, Mann–Whitney U-test; Fig. 2b). The proportion of RA samples positive for anti-rhGPI antibody was 24·1%, and antibody for rabGPI was 37·1%, while the double-positive rate (both anti-rhGPI and rabGPI antibodies) was 13·9% in RA, consistent with the findings of our previous study [17]. Next, we compared the anti-CCG positivity in anti-GPI protein antibody-positive and -negative individuals. The positive rates for anti-CCG-1–9 antibodies in patients positive for both anti-rhGPI and rabGPI antibodies (n = 22) tended to be higher than in those negative for both (n = 88) (Fig. 2c). Among anti-CCGs, the positivity of anti-CCG-1, -4, -8 and -9 antibodies in the anti-GPI protein antibody positive for both groups was significantly higher than in the anti-GPI negative for both groups (Fig. 2c), whereas the remaining anti-CCGs, CEP-1 and CCP antibody was not. These findings suggest that anti-CCG antibodies are linked weakly to anti-GPI protein antibodies.
Figure 2.
(a) Anti-recombinant human glucose-6-phosphate isomerase (rhGPI) antibody levels. Enzyme-linked immunosorbent assay (ELISA) was performed for rhGPI in rheumatoid arthritis (RA) (n = 158) and healthy control subjects (HS) (n = 71) sera. (b) Anti-rabGPI antibody levels. ELISA assays were also performed for native rabGPI. Data represent the optical density values at 405 nm (OD 405). Each datum point represents an individual subject. Bar indicates the mean value. *P < 0·01; **P < 0·001. (c) Positivity for anti-citrullinated peptide antibodies (ACPA) in anti-whole GPI antibody-positive and -negative patients. Positivity for anti-citrullinated glucose-6-phosphate isomerase peptide-1–9 (CCG-1–9), anti-citrullinated α-enolase peptide (CEP)-1 and anti-cyclic citrullinated peptides (CCP) antibodies was evaluated in anti-rhGPI/anti-rabGPI antibody-positive (GPI +/+; n = 22) and -negative (GPI –/–; n = 88) patients. Positivity of anti-CCG-1, -4, -8 and -9 antibodies in the GPI-positive group was significantly higher than in the GPI-negative group. Data represent the positive percentage of each antibody. *P < 0·05; **P < 0·01. (d,e) Relationship among anti-CCG-2, -4 and -7 antibodies and anti-CEP-1 and anti-CCP antibodies. Anti-CCG-2, -4 and -7 antibodies correlated significantly with anti-CEP-1 antibodies (r = 0·313, P < 0·001; r = 0·264, P < 0·001; and r = 0·400, P < 0·001, respectively) (d). Anti-CCG-2, -4 and -7 antibodies also correlated significantly with anti-CCP antibodies (r = 0·442, P < 0·001; r = 0·333, P < 0·001; and r = 0·458, P < 0·001, respectively) (e). Data represent the OD 405 (anti-CCG-2, -4 and -7 and anti-CEP-1) or RU/ml (anti-CCP). Each datum point represents an individual subject. Solid line indicates the correlation among antibodies.
Correlation between anti-CCG-2, -4 and -7 antibodies and anti-CEP-1 and CCP antibodies
Next, we investigated the association between anti-CCG and anti-CEP-1, -CCP antibodies. Among CCGs, anti-CCG-2, -4 and -7 antibodies were correlated significantly with anti-CEP-1 antibodies (r = 0·313, P < 0·001; r = 0·264, P < 0·001; and r = 0·400, P < 0·001, respectively; Pearson's product–moment coefficient of correlation; Fig. 2d). Anti-CCG-2, -4 and -7 antibodies were also correlated significantly with anti-CCP antibodies (r = 0·442, P < 0·001; r = 0·333, P < 0·001; and r = 0·458, P < 0·001, respectively; Pearson's product–moment coefficient of correlation; Fig. 2e). In the anti-CCP antibody-negative population, anti-CCG-2, -4, -7 and anti-CEP-1 antibodies were positive for 3·5, 3·5, 3·5 and 10·3% patients with RA, respectively. These findings suggest that anti-CCG-2, -4 and -7 antibodies are associated with anti-CEP-1 and -CCP antibodies.
HLA-DRB1 shared epitope alleles correlate with anti-CCG-2, -4 and -7 and anti-CEP-1 antibodies
HLA-DRB1 genotyping was performed in 147 patients with RA to examine the relationship between anti-CCGs and anti-CEP-1 antibodies and HLA-DRB1 SE alleles. Six alleles bearing HLA-DRB1 SE (DRB1*01:01, *04:01, *04:05, *04:10, *10:01, *14:06) were identified. However, the DRB1*04:04 allele, which is the major SE allele in Caucasians, was not identified, because it is rare in the Japanese population. The number of SE alleles in each patient was zero in 41 patients (27·9%), one allele in 75 patients (51·0%) and two SE alleles in 31 patients (21·1%). The presence of anti-CCG-2, -4 and -7, anti-CEP-1 and anti-CCP antibodies was associated clearly with HLA-DRB1 SE (P < 0·01, P < 0·05, P < 0·001, P < 0·001 and P < 0·001, respectively; Fisher's exact test and χ2 test; Fig. 3a). Anti-CCG-2 and -7 and anti-CEP-1 antibody-positive patients were highly likely to have one or two HLA-DRB1 SE alleles (Fig. 3b). These findings suggest that anti-CCG-2, -4 and -7 and anti-CEP-1 antibodies are associated strongly with HLA-DRB1 SE alleles in this Japanese cohort.
Figure 3.
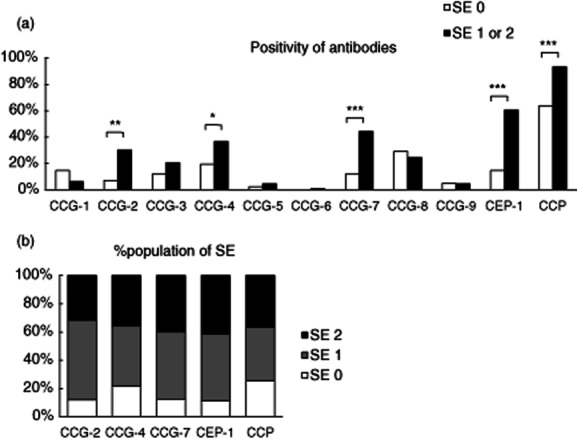
(a) Association between the HLA-DRB1 shared epitope (SE) allele and anti-citrullinated glucose-6-phosphate isomerase peptide-1–9 (CCG-1–9), anti-citrullinated α-enolase peptide (CEP)-1 and anti-cyclic citrullinated peptides (CCP) antibodies. Patients with rheumatoid arthritis (RA) were divided according to the HLA-DRB1 SE genotype into two groups and positivity for anti-CCG-1–9, anti-CEP-1 and anti-CCP antibodies was evaluated for each antibody. Anti-CCG-2, -4 and -7, anti-CEP-1 and anti-CCP antibodies were associated with the presence of HLA-DRB1 SE alleles. SE 0: patients with null SE alleles (n = 41); SE 1: patients with 1 SE alleles (n = 75); SE 2: patients with 2 SE alleles (n = 31); *P < 0·05; **P < 0·01; ***P < 0·001. (b) Percentage of the population of SE in RA patients with anti-CCG-2, -4 and -7, anti-CEP-1 and anti-CCP antibodies. A high proportion of anti-CCG-2, -7 and anti-CEP-1 antibody-positive patients carried one or two HLA-DRB1 SE alleles.
Identification of specific reaction to citrullinated GPI protein in RA patients by Western blot analysis
To confirm the antibody reaction to citrullinated GPI protein, we performed immunoblot analysis using native (glycosylated, 93% homology to human GPI) and citrullinated rabbit GPI (cit rabGPI) by PAD. After citrullination, Gelcode Blue stain bands were identified at approximately 60 kDa protein, suggesting rabGPI and cit rabGPI (Fig. 4a, left). The presence of citrullinated protein at 60 kDa was identified specifically by anti-modified citrulline antibodies (Fig. 4a, right). We also examined the reaction to native and citrullinated GPI using 20 serum samples from RA patients and HS (Fig. 4b). Interestingly, the same reaction appeared in the native and cit GPI in RA group 1 (RA1; n = 5). However, the reaction to cit GPI in RA2 (n = 1) and RA3 (n = 1) was stronger, suggesting a positive reaction to the citrullinated epitope of GPI protein. Neither band was detected in the sera of RA group 4 (RA4; n = 8) and HS (n = 5).
Figure 4.
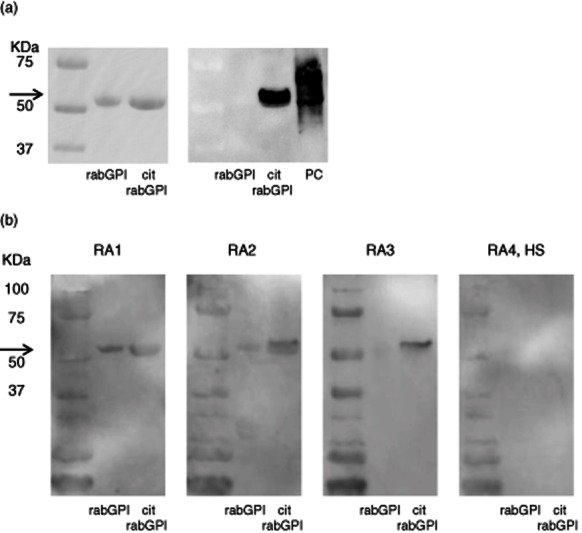
(a) Immunoblotting of native rabbit glucose-6-phosphate isomerase (GPI) and citrullinated rabbit GPI (cit rabGPI). Bands from Gelcode Blue stain after sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) were identified as rabGPI and cit rabGPI (left). Anti-modified citrulline antibodies demonstrated the presence of citrullinated protein at 60 kDa only in cit rabGPI well (right). Arrow shows 60 kDa. (b) Identification of anti-rabGPI and anti-cit rabGPI antibodies by Western blotting. The same reaction was shown to rabGPI and cit rabGPI in rheumatoid arthritis (RA) group 1 (RA1). However, a stronger reaction was shown to citrullinated GPI in RA2 and RA3. Neither of them was detected in RA4 and healthy control subjects (HS). Arrow shows 60 kDa.
Treatment with TNF antagonists specifically down-regulates anti-CCG-2 and -7 antibodies
Whether treatment with TNF antagonists induces changes in anti-CCP antibody levels remains controversial [23]. Anti-GPI antibodies are pathogenic antibodies in K/B×N mice [10], and their relationship with RA activity has already been evaluated in humans [16,17]. In the last step of this study, we examined the effect of 6-month treatment with TNF antagonists on the levels of anti-CCG-2, -4 and -7 and anti-CEP-1 antibodies in 58 patients with RA. Before treatment, anti-CCG-2, -4 and -7 and anti-CEP-1 antibodies were detected in 23, 30, 26 and 29 patients, respectively. Six-month treatment with TNF antagonists resulted in a significant decrease in anti-CCG-2 antibodies from OD 1·237–0·906 (P < 0·001, paired t-test; Fig. 5a) and in anti-CCG-7 antibodies (OD 0·785–0·623, P < 0·05, paired t-test; Fig. 5b). However, TNF antagonists had no effect on the levels of anti-CCG-4 antibodies (OD 0·515–0·469; P = 0·532, paired t-test; Fig. 5c) or anti-CEP-1 antibodies (OD 0·866–0·861; P = 0·957, paired t-test; Fig. 5d). In each group, the mean value of the disease activity score (DAS)-28 C-reactive protein (CRP) decreased significantly after treatment. In the anti-CCG-2, -4 and -7 and anti-CEP-1-positive groups, the treatment reduced the DAS-28 CRP from 4·25 to 3·03 (P < 0·001, paired t-test), from 4·27 to 2·70 (P < 0·001), from 4·42 to 3·17 (P < 0·001) and from 4·28 to 3·23 (P < 0·001), respectively. Only the change in anti-CCG-7 antibodies was correlated significantly with the change in the DAS-28 CRP (r = 0·500, P < 0·05; Pearson's product–moment coefficient of correlation Fig. 5e). These findings suggest that treatment with TNF antagonists down-regulates anti-CCG-2 and -7 antibodies, and this effect seems to be linked to disease activity.
Figure 5.
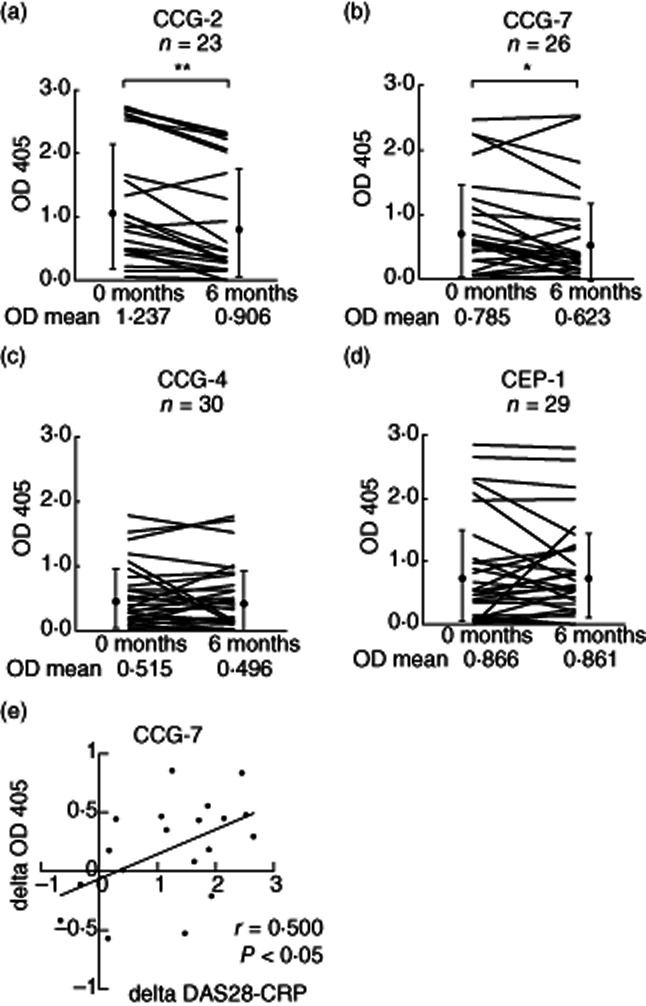
Effect of 6-month treatment with tumour necrosis factor (TNF) antagonists on anti-citrullinated glucose-6-phosphate isomerase peptide-2 (CCG-2 (a), CCG-4 (b), CCG-7 (c) and anti-cyclic citrullinated α-enolase peptide (CEP)-1 (d) antibodies. The treatment decreased anti-CCG-2 antibodies (n = 23) [optical density (OD) 1·237–0·906, P < 0·001, paired t-test] and anti-CCG-7 antibodies (n = 26) (OD 0·785–0·623, P < 0·05, paired t-test). The same treatment had no effect on anti-CCG-4 antibodies (n = 30) and anti-CEP-1 antibodies (n = 29). Data represent the optical density values measured at 405 nm (OD 405). Each data line represents an individual subject. Dots and vertical lines represent the mean ± standard deviation values. *P < 0·05; **P < 0·001. (e) Correlation of the changing of anti-CCG-7 antibodies versus disease activity score (DAS)-28 C-reactive protein (CRP). The decreased levels of anti-CCG-7 antibodies (delta OD 405) correlated with the decrease level of DAS28-CRP (delta DAS28-CRP) (r = 0·500, P < 0·05). Each data point represents an individual subject. Solid line indicates the correlation.
Discussion
To our knowledge, this is the first study to identify anti-citrullinated GPI peptide and protein antibodies in patients with RA. Specifically, anti-CCG-2 and -7 antibodies were highly specific to RA, and their levels correlated with the presence of HLA-DRB1 SE alleles and with RA disease activity.
Our analysis also indicated that anti-CCG-2, -4 and -7 antibody levels correlated with anti-CCP and anti-CEP-1 antibodies. The reported sensitivity and specificity of anti-CCP antibodies for RA are 68 and 96–98% [24]. We measured anti-CCP antibodies with an anti-CCP-2 ELISA kit in our Japanese cohort and the results showed similar detection rates to those reported previously [24]. In the anti-CCP2 kit, the substrates are selected from a large panel of randomly generated citrulline-containing peptides; in other words, the reaction occurs with several citrullinated proteins. Several studies have indicated that anti-CCP antibodies appear before the development of clinically apparent RA [25–27], although their relationship to disease activity remains controversial [8].
Fibrinogen, vimentin, collagen type II and α-enolase have been confirmed as citrullinated autoantigens in RA [3–6]. The glycolytic enzyme α-enolase was first reported by Saulot et al. [28]. Subsequently, Lundberg et al. reported that 37% of patients with RA were positive for anti-CEP-1 (circulating and citrullinated α-enolase peptide) antibodies, although a smaller proportion of patients (13·9%) was positive for antibodies against a linear peptide for CEP-1 [6]. In our study, anti-CEP-1 antibodies were detected in 44·2% of RA patients, which is almost consistent with the findings of the above study. Like α-enolase, GPI is an ubiquitous glycolytic enzyme; thus, a similar protocol can be applied for GPI. The results of our study indicate that anti-CCG-2, -4 and -7 antibodies are highly specific and sensitive to RA, as well as a clear correlation between anti-CCG-2, -4 and -7 and anti-CEP-1 antibody levels. In our immunoblot analysis, we also detected specific reaction to citrullinated GPI protein in RA serum.
Anti-GPI protein antibodies were detected previously in RA, although their prevalence and disease specificity was not confirmed [16–19] because the sera of patients with certain control autoimmune diseases (e.g. SLE and SS) and of healthy controls also react to GPI proteins. In this report, we examined the correlation between anti-CCGs and anti-GPI protein antibodies. Most of the anti-CCG antibodies were very specific to RA patients and frequently detected in anti-GPI-protein antibody-positive individuals, but the correlation was significant only in CCG-1, -4, -8 and -9 antibodies owing to the low sensitivity of anti-GPI protein antibodies. These findings suggest that post-translational modification of GPI, in addition to other autoantigens, is important for the reactivity of RA sera. Also, it is possible that different pathogenetic mechanisms might be involved in the presence of anti-GPI and anti-CCG antibodies.
The present study confirmed that anti-CCG-2, -4 and -7 and anti-CEP-1 antibodies are associated with HLA-DRB1 SE alleles in a Japanese cohort. Several recent reports have indicated the association between HLA-DRB1 SE and anti-citrullinated protein antibodies (ACPA) [5,7,29–33]. With regard to anti-GPI protein antibodies, we have reported that the majority of anti-GPI antibody-positive patients were also anti-CCP-positive and carried HLA-DRB1 SE alleles [34] and that the HLA-DRB1*04:05/09:01 genotype is associated specifically with GPI-reactive T cells in RA patients [35]. Hill et al. [36] reported recently that immunization of HLA-DRB1*04:01 transgenic mice with citrullinated fibrinogen caused arthritis with 35% penetrance, strongly supporting the notion that SE primarily determines the reaction to citrullinated antigen in the presence of ACPA. Furthermore, this association with SE was not confined to CEP-1 [7], but was noted in most of the citrullinated antigens, including GPI.
In the present study, we evaluated the levels of anti-CCG-2, -4 and -7 and anti-CEP-1 antibodies before and after 6-month treatment with TNF antagonists. Surprisingly, the treatment reduced only the anti-CCG-2 and -7, not the CCG-4 and anti-CEP-1 antibodies levels. We also evaluated the levels of anti-CCG-2, -4 and -7 and anti-CEP-1 antibodies in the treatment with disease-modifying anti-rheumatic drugs (DMARDs) (not using TNF antagonists; n = 13) as a control. However, treatment with non-biological DMARDs showed no significant decrease in the levels of anti-CCG-2, -4 and -7 and anti-CEP-1 antibodies. Similarly, several studies have reported that treatment with TNF antagonists did not decrease significantly the level of anti-CCP antibodies despite a significant fall in the level of rheumatoid factor and other markers of disease activity [37–39]. Anti-GPI antibodies in K/B×N mice play a pathogenic role in arthropathy and disease activity; hence, it is possible that anti-CCG-2 and -7 antibodies are also associated with the pathogenesis of RA. Further studies are needed to characterize anti-CCG-2 and -7 antibodies and their role in RA diseases course and status of RA. The changes in levels of antibodies need to be analysed in treatment with non-biological DMARDs in a larger number of samples and with other biologics [for example, anti-interleukin (IL)-6 receptor antibody and cytotoxic T lymphocyte antigen 4-Ig] in the future.
In conclusion, we have identified anti-CCG antibodies in patients with RA. In particular, anti-CCG-2, -4 and -7 antibodies are highly specific and correlate with SE alleles. Moreover, the levels of anti-CCG-2 and -7 antibodies are down-regulated by treatment with TNF antagonists. These findings suggest that anti-CCG antibodies are potentially suitable markers for the diagnosis of RA and assessment of its disease activity.
Acknowledgments
This work was supported in part by a grant from The Japanese Ministry of Science and Culture (to I.M., N.T. and T.S.) and the Bristol-Myers RA Research Fund (I.M.).
Disclosure
The authors declare no competing interests.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1. (a) The levels of anti-G-1, -2, -3, -4 and -7 antibodies. Anti-unmodified glucose-6-phosphate isomerase peptide-1–4 and -7 (G-1–4 and -7) antibodies were measured in sera of patients with rheumatoid arthritis (RA) (n = 208) and HS (n = 174) by enzyme-linked immunosorbent assay (ELISA). Antibodies to G-1, -2, -3, -4 and -7 were rarely detected. Data represent the optical density values at 405 nm [optical density (OD) 405]. Each data point represents an individual subject.
References
- 1.Van Venrooij WJ, Zendman AJ. Anti-CCP2 antibodies: an overview and perspective of the diagnostic abilities of this serological marker for early rheumatoid arthritis. Clin Rev Allergy Immunol. 2008;34:36–39. doi: 10.1007/s12016-007-8029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ioan-Facsinay A, El-Bannoudi H, Scherer HU, et al. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann Rheum Dis. 2011;70:188–3. doi: 10.1136/ard.2010.131102. [DOI] [PubMed] [Google Scholar]
- 3.Wegner N, Lundberg K, Kinloch A, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233:34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 4.Vander Cruyssen B, Cantaert T, Nogueira L, et al. Diagnostic value of anti-human citrullinated fibrinogen ELISA and comparison with four other anti-citrullinated protein assays. Arthritis Res Ther. 2006;8:R122. doi: 10.1186/ar2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snir O, Widhe M, von Spee C, et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann Rheum Dis. 2009;68:736–743. doi: 10.1136/ard.2008.091355. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg K, Kinloch A, Fisher BA, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58:3009–3019. doi: 10.1002/art.23936. [DOI] [PubMed] [Google Scholar]
- 7.Mahdi H, Fisher BA, Källberg H, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009;41:1319–1324. doi: 10.1038/ng.480. [DOI] [PubMed] [Google Scholar]
- 8.Landmann T, Kehl G, Bergner R. The continuous measurement of anti-CCP-antibodies does not help to evaluate the disease activity in anti-CCP-antibody-positive patients with rheumatoid arthritis. Clin Rheumatol. 2010;29:1449–1453. doi: 10.1007/s10067-010-1557-5. [DOI] [PubMed] [Google Scholar]
- 9.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoreactivity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 11.Schubert D, Maier B, Morawietz L, Krenn V, Kamradt T. Immunization with glucose-6-phosphate isomerase induces T cell-dependent peripheral polyarthritis in generally unaltered mice. J Immunol. 2004;172:4503–4509. doi: 10.4049/jimmunol.172.7.4503. [DOI] [PubMed] [Google Scholar]
- 12.Iwanami K, Matsumoto I, Tanaka-Watanabe Y, et al. Crucial role of the interleukin-6/interleukin-17 cytokine axis in the induction of arthritis by glucose-6-phosphate isomerase. Arthritis Rheum. 2008;58:754–763. doi: 10.1002/art.23222. [DOI] [PubMed] [Google Scholar]
- 13.Dai L, Zhu LJ, Zheng DH, et al. Elevated serum glucose-6-phosphate isomerase correlates with histological disease activity and clinical improvement after initiation of therapy in patients with rheumatoid arthritis. J Rheumatol. 2010;37:2452–2461. doi: 10.3899/jrheum.100157. [DOI] [PubMed] [Google Scholar]
- 14.Fan LY, Zong M, Wang Q, et al. Diagnostic value of glucose-6-phosphate isomerase in rheumatoid arthritis. Clin Chim Acta. 2010;411:2049–2053. doi: 10.1016/j.cca.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Schaller M, Burton DR, Ditzel HJ. Autoantibodies to GPI in rheumatoid arthritis: linkage between an animal model and human disease. Nat Immunol. 2001;8:746–753. doi: 10.1038/90696. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto I, Maccioni M, Lee DM, et al. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol. 2002;3:360–365. doi: 10.1038/ni772. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto I, Lee DM, Goldbach-Mansky R, et al. Low prevalence of antibodies to glucose-6-phosphate isomerase in patients with rheumatoid arthritis and a spectrum of other chronic autoimmune disorders. Arthritis Rheum. 2003;48:944–954. doi: 10.1002/art.10898. [DOI] [PubMed] [Google Scholar]
- 18.van Gaalen FA, Toes RE, Ditzel HJ, et al. Association of autoantibodies to glucose-6-phosphate isomerase with extraarticular complications in rheumatoid arthritis. Arthritis Rheum. 2004;50:395–399. doi: 10.1002/art.20028. [DOI] [PubMed] [Google Scholar]
- 19.Kassahn D, Kolb C, Solomon S, Bochtler P, Illges H. Few human autoimmune sera detect GPI. Nat Immunol. 2002;3:411–412. doi: 10.1038/ni0502-411b. [DOI] [PubMed] [Google Scholar]
- 20.Jouen F, Vittecoq O, Leguillou F, et al. Diagnostic and prognostic values of anti glucose-6-phosphate isomerase antibodies in community-recruited patients with very early arthritis. Clin Exp Immunol. 2004;137:606–611. doi: 10.1111/j.1365-2249.2004.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 23.Bobbio-Pallavicini F, Caporali R, Alpini C, Moratti R, Montecucco C. Predictive value of antibodies to citrullinated peptides and rheumatoid factors in anti-TNF-alpha treated patients. Ann N Y Acad Sci. 2007;1109:287–295. doi: 10.1196/annals.1398.034. [DOI] [PubMed] [Google Scholar]
- 24.Schellekens GA, Visser H, de Jong BA, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 26.Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 27.van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 2004;50:709–715. doi: 10.1002/art.20044. [DOI] [PubMed] [Google Scholar]
- 28.Saulot V, Vittecoq O, Charlionet R, et al. Presence of autoantibodies to the glycolytic enzyme alpha-enolase in sera from patients with early rheumatoid arthritis. Arthritis Rheum. 2002;46:1196–1201. doi: 10.1002/art.10252. [DOI] [PubMed] [Google Scholar]
- 29.van Gaalen FA, van Aken J, Huizinga TW, et al. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum. 2004;50:2113–2121. doi: 10.1002/art.20316. [DOI] [PubMed] [Google Scholar]
- 30.Irigoyen P, Lee AT, Wener MH, et al. Regulation of anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis: contrasting effects of HLA-DR3 and the shared epitope alleles. Arthritis Rheum. 2005;52:3813–3818. doi: 10.1002/art.21419. [DOI] [PubMed] [Google Scholar]
- 31.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54:1117–1121. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- 32.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 33.van der Woude D, Alemayehu WG, Verduijn W, et al. Gene–environment interaction influences the reactivity of autoantibodies to citrullinated antigens in rheumatoid arthritis. Nat Genet. 2010;42:814–816. doi: 10.1038/ng1010-814. [DOI] [PubMed] [Google Scholar]
- 34.Furuya T, Hakoda M, Ichikawa N, et al. Differential association of HLA-DRB1 alleles in Japanese patients with early rheumatoid arthritis in relationship to autoantibodies to cyclic citrullinated peptide. Clin Exp Rheumatol. 2007;25:219–224. [PubMed] [Google Scholar]
- 35.Kori Y, Matsumoto I, Zhang H, et al. Characterisation of Th1/Th2 type, glucose-6-phosphate isomerase reactive T cells in the generation of rheumatoid arthritis. Ann Rheum Dis. 2006;65:968–969. doi: 10.1136/ard.2005.045286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill JA, Bell DA, Brintnell W, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med. 2008;205:967–979. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruns A, Nicaise-Roland P, Hayem G, et al. Prospective cohort study of effects of infliximab on rheumatoid factor, anti-cyclic citrullinated peptide antibodies and antinuclear antibodies in patients with long-standing rheumatoid arthritis. Joint Bone Spine. 2009;76:248–253. doi: 10.1016/j.jbspin.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 38.De Rycke L, Verhelst X, Kruithof E, et al. Rheumatoid factor, but not anti-cyclic citrullinated peptide antibodies, is modulated by infliximab treatment in rheumatoid arthritis. Ann Rheum Dis. 2005;64:299–302. doi: 10.1136/ard.2004.023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bos WH, Bartelds GM, Wolbink GJ, et al. Differential response of the rheumatoid factor and anticitrullinated protein antibodies during adalimumab treatment in patients with rheumatoid arthritis. J Rheumatol. 2008;35:1972–1977. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.