Abstract
Splenectomy has been used in patients with common variable immunodeficiency disorders (CVID), mainly in the context of refractory autoimmune cytopenia and suspected lymphoma, but there are understandable concerns about the potential of compounding an existing immunodeficiency. With increasing use of rituximab as an alternative treatment for refractory autoimmune cytopenia, the role of splenectomy in CVID needs to be re-examined. This retrospective study provides the largest cohesive data set to date describing the outcome of splenectomy in 45 CVID patients in the past 40 years. Splenectomy proved to be an effective long-term treatment in 75% of CVID patients with autoimmune cytopenia, even in some cases when rituximab had failed. Splenectomy does not worsen mortality in CVID and adequate immunoglobulin replacement therapy appears to play a protective role in overwhelming post-splenectomy infections. Future trials comparing the effectiveness and safety of rituximab and splenectomy are needed to provide clearer guidance on the second-line management of autoimmune cytopenia in CVID.
Keywords: autoimmune cytopenia, common variable immunodeficiency, splenectomy, splenomegaly
Introduction
Common variable immunodeficiency disorders (CVID) is a heterogeneous group of disorders characterized by recurrent infections, hypogammaglobulinaemia and poor response to vaccination. Splenomegaly is a common feature in CVID, but neither its causes nor its consequences are well understood [1]. This clinical observation is associated closely with certain clinical presentations such as lymphadenopathy, granulomatous inflammation and autoimmune cytopenia [1,2], and is more prevalent among those with reduced class-switched memory B cells (CD19+CD27+IgD–IgM–) and increased CD21lo B cells [3–5].
The spleen is the largest secondary lymphoid organ and plays vital roles in the immune response. Due to its unique structure and vascularization, the spleen is essential in the removal of various blood-borne pathogens [6]. The innate and adaptive immune systems co-operate closely in the marginal zone of the spleen where the highly specialized monocyte–macrophage system captures pathogens by a set of unique surface receptors [7,8]. In humans, the severe reduction of marginal zone-like B cells in the peripheral blood following splenectomy indicates an important role for the spleen in the development or maintenance of this B cell population [9]. These B cells are thought to be crucial for the immune response to polysaccharides of encapsulated bacteria; therefore, CVID patients with decreased numbers of CD27+IgM+IgD+ marginal zone-like B cells may have an increased burden of infections [10]. Thus the spleen, via several complementary mechanisms, supports defence especially against encapsulated bacteria. These unique functions of the spleen are illustrated clearly by the increased life-long risk of severe infections such as meningitis and septicaemia after splenectomy [11].
Despite the potential of compounding an existing immunodeficiency, splenectomy has been used in CVID patients mainly in the context of refractory autoimmune cytopenia and suspected lymphoma. There are understandable concerns about this approach, but unfortunately there are limited published data to guide these clinical decisions. Resnick and colleagues have reported recently that splenectomy was not associated with reduction in survival in CVID patients [12], but significant post-operative complications and infections have been shown in earlier reports [1,13]. With the increasing use of rituximab as an alternative treatment for refractory autoimmune cytopenia in immunocompetent individuals [14,15], the role of splenectomy needs to be re-examined in immune-incompetent patients.
Therefore, on behalf of the European Society for Immunodeficiencies (ESID) Clinical working party and EUROpean-Primary Antibody Deficiency network (EUROPADnet), a multi-centre retrospective clinical survey was set up to investigate the prevalence, indications for and outcomes of splenectomy in patients with CVID.
Patients and methods
Data collection
A retrospective survey was distributed among the European Society for Immunodeficiencies (ESID) Clinical Working Party and EUROPADnet between June and December 2010. The survey was also available for download on the ESID website during that period. Other centres were contacted based on a literature search of published case reports and through personal communications. Further details and clarifications were obtained by e-mail communication following submission of the survey.
Data of all splenectomized CVID patients, both alive and deceased, were collected from 16 centres in seven European countries: the Czech Republic, Italy, Germany, Spain, Sweden, Turkey and the United Kingdom. CVID patients were identified by individual centres in accordance with the ESID criteria (http://www.esid.org). Where splenectomy was performed > than 3 years prior to the formal diagnosis of CVID, the co-existing diagnosis of CVID was verified by the presence of (i) laboratory evidence of hypogammaglobulinaemia at the time of splenectomy, or (ii) a significant infection history predating splenectomy and a clinical history compatible with CVID. Patients who were diagnosed with a haematological malignancy during this window period were excluded from the study.
We defined splenomegaly as a palpable spleen on clinical examination, ultrasound caudal measurement of greater than 13 cm [16] or an actual weight greater than 400 g. Granulomatous complication was defined if granulomas were identified at any site, including the spleen, in a given patient. Autoimmune cytopenia included idiopathic thrombocytopenic purpura (ITP), autoimmune haemolytic anaemia (AIHA), Evan's syndrome (including trilineage cytopenia) and autoimmune neutropenia.
Criteria for response in autoimmune cytopenia to splenectomy or other treatments
Attempts to determine the therapeutic response of autoimmune cytopenia according to the recent international workshop [17] failed, due mainly to the loss of historical data. Therefore, treatment response was divided broadly into responder and non-responder based on the treating physician's judgement and the need for further therapies. Responders included those who were described as having complete or partial responses and who required no further treatment except for low-dose maintenance corticosteroid. Non-responders included patients who were described as having poor and no responses or who required subsequent alternative treatment. A partial response with multiple relapses following splenectomy was classified as a non-responder.
Defining severe infection after splenectomy
Meningitis and bacterial septicaemia were defined as overwhelming post-splenectomy infections (OPSI). Opportunistic infections and any infections resulting in death were also noted. The follow-up time in months was used as time denominator to calculate the rate of infection. Due to the potential confounding effects of lymphoma and chemotherapy on morbidity and mortality, patients (n = 5, patients 1, 5, 6, 21 and 31) with lymphoma were not included in this analysis.
Results
Patient characteristics
Forty-eight splenectomies were reported among 1211 CVID patients (4·0%; 12 deceased) between 1972 and 2010 (Fig. 1). No details other than alive/deceased status were available from three patients, so these were excluded from further evaluation. The indications for splenectomy are shown in Fig. 2.
Figure 1.
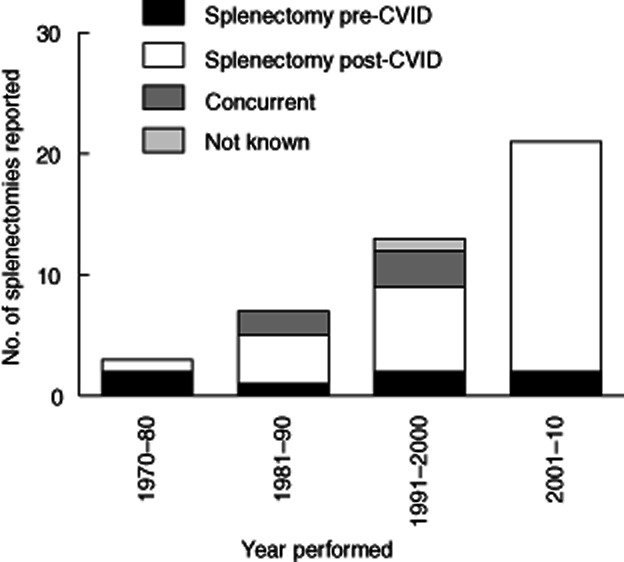
Number of splenectomies performed on common variable immunodeficiency disorders (CVID) patients within Europe between 1972 and 2010 in relation to the timing of CVID diagnosis.
Figure 2.
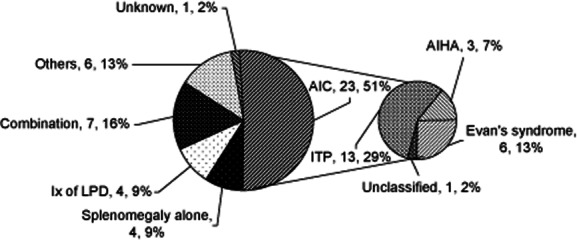
Reasons for splenectomy. LPD: lymphoproliferative disorder; AIC: autoimmune cytopenia; ITP: idiopathic thrombocytopenic purpura; AIHAL autoimmune haemolytic anaemia.
The characteristics of the remaining 45 patients (male/female = 18/27) are summarized in Table 1 (also see Fig. 2). The ages at diagnosis of CVID ranged from 5 to 68 years [mean ± standard deviation (s.d.) = 30·5 ± 17·5]; patient 24 was excluded from the calculation because no data in this field were provided. The ages at splenectomy ranged from 8 to 74 years (32·8 ± 16·7) (patient 30 excluded). Thirteen splenectomies were performed prior to or at the diagnosis of CVID, 31 splenectomies with the knowledge of CVID and one unknown (year of CVID diagnosis not clear).
Table 1.
Patients' characteristics
| Patient no. | M/F | Age of CVID diagnosis | Age of splenectomy | Timing of splenectomy (before/after CVID diagnosis) | Splenomegaly | Other clinical phenotypes | Reason for splenectomy | Alive / deceased |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 22 | 44 | After | Yes | Granulomatous, NHL | Splenomegaly, splenitis, atypical mycobacterium | A |
| 2 | F | 12 | 14 | After | Yes | – | Evan's syndrome | A |
| 3 | M | 18 | 8 | Before | Yes | Evan's syndrome, GI symptoms | – | A |
| 4 | M | 53 | 53 | Concurrent | Yes | Granulomatous, gastric carcinoma, pancreatic insufficiency | Part of gastrectomy | A |
| 5 | F | 42 | 52 | After | Yes | Pancreatic insufficiency, lymphoma | Evan's syndrome, suspected lymphoma | D |
| 6 | F | 45 | 48 | After | Yes | Chronic diarrhoea, liver disease, lymphadenopathy, lymphoma | AIC | D |
| 7 | M | 35 | 36 | After | Yes | Granulomatous, chronic diarrhoea | Suspected lymphoma | A |
| 8 | M | 11 | 13 | After | Yes | Granulomatous, lymphadenopathy, enteropathy, ILD | Evan's syndrome | A |
| 9 | F | 47 | 49 | After | Yes | Lymphadenopathy, malabsorption, ILD, liver disease | Evan's syndrome | A |
| 10 | F | 17 | 16 | After | Yes | Lymphadenopathy | Evan's syndrome | A |
| 11 | F | 8 | 9 | After | Yes | Lymphadenopathy, liver disease | Suspected lymphoma | A |
| 12 | F | 30 | 43 | After | Yes | Lymphadenopathy, gastritis with intestinal metaplasia, basal cell carcinoma | Suspected lymphoma | A |
| 13 | F | 68 | 74 | After | Yes | Granulomatous, lymphadenopathy, ILD, liver disease | Splenomegaly | A |
| 14 | F | 62 | 66 | After | Yes | Granulomatous, lymphadenopathy, ITP, chronic diarrhoea, liver disease | Splenomegaly | A |
| 15 | F | 30 | 39 | After | Yes | GI symptoms. ITP, liver disease | Trauma | A |
| 16 | F | 17 | 22 | After | Yes | Granulomatous, lymphadenopathy, enteropathy, liver disease | ITP | A |
| 17 | F | 28 | 40 | After | Yes | Granulomatous, lymphadenopathy, gastritis, liver disease | ITP | A |
| 18 | M | 25 | 25 | Concurrent | Yes | Granulomatous, lymphadenopathy, liver disease | ITP | A |
| 19 | F | 22 | 23 | After | No | Granulomatous, lymphadenopathy | ITP | A |
| 20 | M | 31 | 31 | Concurrent | No | Granulomatous, lymphadenopathy, ILD | ITP | A |
| 21 | M | 65 | 61 | After | Yes | Lymphadenopathy, ITP, ILD, liver disease, lymphoma | Splenomegaly, suspected lymphoma | A |
| 22 | F | 5 | 20 | After | Yes | Granulomatous, lymphadenopathy, ITP, ILD, liver disease, iron deficiency | Splenomegaly | A |
| 23 | F | 35 | 35 | Concurrent | No | Granulomatous, liver disease | ITP | A |
| 24 | M | – | 18 | Unknown | No | Lymphadenopathy, liver disease | Evan's syndrome | A |
| 25 | F | 41 | 33 | Before | No | Chronic diarrhoea, AIHA, autoimmune diabetes | ITP | A |
| 26 | F | 43 | 20 | Before | Yes | – | ITP | A |
| 27 | M | 11 | 11 | After | Yes | Granulomatous, lymphadenopathy, ILD | Evan's syndrome | A |
| 28 | M | 13 | 15 | After | Yes | Lymphadenopathy | ITP | A |
| 29 | F | 22 | 35 | After | Yes | Lymphadenopathy, malabsorption, liver disease | AIHA | A |
| 30 | M | 35 | – | After | Yes | Lymphadenopathy, AIHA, malabsorption, liver disease | Lymphoproliferative syndrome | D |
| 31 | F | 37 | 48 | After | Yes | Granulomatous, lymphadenopathy | T cell lymphoma | D |
| 32 | F | 59 | 57 | Before | Yes | Lymphadenopathy, ILD | AIHA | D |
| 33 | M | 10 | 15 | After | Yes | Chronic diarrhoea | ITP | A |
| 34 | F | 20 | 26 | After | Yes | Granulomatous, chronic diarrhoea | Evan's syndrome, splenomegaly | D |
| 35 | M | 15 | 12 | Before | Yes | Lymphadenopathy, enteropathy, AIN | ITP | A |
| 36 | F | 28 | 31 | After | Yes | Granulomatous, lymphadenopathy, liver disease | ITP, splenomegaly, suspected lymphoma | A |
| 37 | F | 10 | 16 | After | Yes | Lymphadenopathy, colitis, scleroderma | Splenomegaly | A |
| 38 | F | 8 | 11 | After | Yes | Granulomatous, lymphadenopathy, Evan's syndrome | Retroperitoneal bleeding following splenic biopsy | A |
| 39 | F | 51 | 70 | After | Yes | Lymphadenopathy, Evan's syndrome | Suspected lymphoma | A |
| 40 | M | 7 | 17 | After | Yes | – | ITP | A |
| 41 | M | 61 | 68 | After | Yes | Liver disease | AIHA | D |
| 42 | M | 28 | 27 | Before | Yes | Lymphadenopathy, granulomatous, enteropathy, iron deficiency, liver disease | Hepatosplenomegaly, AIHA | D |
| 43 | M | 32 | 35 | After | Yes | Liver disease. | Splenomegaly, AIHA | D |
| 44 | F | 38 | 30 | Before | No | Iron deficiency, liver disease | ITP | A |
| 45 | F | 45 | 51 | After | Yes | Lymphadenopathy, granulomatous, enteropathy, ILD, iron deficiency, liver disease | Systemic granulomatous disease | D |
NHL: non-Hodgkin's lymphoma; AIC: unclassified autoimmune cytopenia; ITP: idiopathic thrombocytopenic purpura; AIHA: autoimmune haemolytic anaemia; AIN: autoimmune neutropenia; ILD: interstitial lung disease; CVID: common variable immunodeficiency disorders; GI: gastrointestinal; M/F: male/female.
Of the CVID patients, 39 (86·7%) patients had splenomegaly, 36 (80%) autoimmune cytopenia, 29 (64·4%) lymphadenopathy, 20 (44·4%) liver disease, 20 (44·4%) gastrointestinal disease excluding iron deficiency, 20 (44·4%) granulomata and nine (20%) interstitial lung disease. Five lymphomas, including one T cell lymphoma, were reported. Organ-specific autoimmune diseases reported were scleroderma (one) and autoimmune diabetes (one). One patient had basal cell carcinoma.
Effectiveness of splenectomy in CVID
Splenectomy in autoimmune cytopenia
Splenectomy appears to be an effective treatment in autoimmune cytopenia in CVID. Evaluable data were available from 24 patients with autoimmune cytopenia (11 ITP, seven Evan's syndrome, five AIHA and one unclassified; 20 of 23 were splenectomized for refractory autoimmune cytopenia, four of seven for a combination of reasons). All patients except for patients 18, 19, 24, 40 and 42 had diseases refractory to corticosteroids, high-dose intravenous immunoglobulin (IVIG) or both. A summary is shown in Table 2. Eighteen of 24 patients (75%) were classified as responders and six patients (25%) were classified as non-responders. The use of both splenectomy and rituximab was noted in five patients. Patient 6 initially achieved a partial response after rituximab and then went into complete remission after splenectomy. Patient 17 failed to respond to splenectomy and cyclosporin, but achieved a partial response to combined therapy with rituximab, vincristine and cyclophosphamide. Patient 24 did not respond to splenectomy, but was treated successfully with rituximab and maintenance prednisolone 15 years later. Patient 25 had no response to rituximab, but went into complete remission after splenectomy. Finally, patient 26 went into complete remission for 23 years following splenectomy, and was treated successfully with rituximab upon relapse.
Table 2.
Outcome of splenectomy in autoimmune cytopenia
| Patient no. | Autoimmune cytopenia | Previous treatment | Outcome of splenectomy | Final treatment |
|---|---|---|---|---|
| 6 | Unclassified | Corticosteroid, rituximab | Responder | – |
| 8 | Evan's syndrome | Corticosteroid, high-dose IVIG | Responder | – |
| 9 | Evan's syndrome | Corticosteroid | Responder | – |
| 10 | Evan's syndrome | Corticosteroid, high-dose IVIG | Responder | – |
| 17 | ITP | Corticosteroid, cyclosporin | Non-responder | Rituximab, vincristine, cyclophosphamide |
| 18 | ITP | Unknown | Responder | – |
| 19 | ITP | Unknown | Responder | – |
| 20 | ITP | Corticosteroid, high-dose IVIG | Responder | – |
| 24 | ITP | Unknown | Non-responder | Rituximab |
| 25 | Evan's syndrome | Corticosteroid, high-dose IVIG, rituximab | Responder | – |
| 26 | ITP | Corticosteroid, high-dose IVIG, azathioprine, cyclosporin, cyclophosphamide | Responder | Rituximab (23 years later) |
| 27 | Evan's syndrome | Corticosteroid, high-dose IVIG | Responder | – |
| 28 | ITP | Corticosteroid, high-dose IVIG | Non-responder | Unknown |
| 29 | AIHA | Corticosteroid, high-dose IVIG | Responder | – |
| 32 | AIHA | Corticosteroid, high-dose IVIG, other immunosuppressants | Responder | – |
| 33 | ITP | Corticosteroid | Responder | – |
| 34 | Evan's syndrome | Corticosteroid, high-dose IVIG | Responder | – |
| 35 | ITP | Corticosteroid, high-dose IVIG | Responder | – |
| 36 | ITP | Corticosteroid | Responder | – |
| 40 | Evan's syndrome | None | Responder | – |
| 41 | AIHA | Corticosteroid, deoxycoformicin, azathioprine, chlorambucil | Non-responder | Corticosteroid, azathioprine |
| 42 | AIHA | None | Responder | – |
| 43 | AIHA | Corticosteroid | Non-responder | Corticosteroid |
| 44 | ITP | Corticosteroid | Non-responder | Bleomycin, azathioprine |
AIHA: autoimmune haemolytic anaemia; ITP: idiopathic thrombocytopenic purpura; IVIG: intravenous immunoglobulin.
Splenectomy for suspected lymphoma
The diagnosis of lymphoma by splenectomy in CVID was low in this cohort (two of seven). Including those with a combination of reasons, seven patients underwent splenectomy as a diagnostic procedure for suspected lymphoma. Only patients 5 and 21 were diagnosed with lymphoma by splenectomy. Patient 5 had isolated splenic high-grade non-Hodgkin's lymphoma.
Adverse effect of splenectomy
Severe infection following splenectomy
When patients with lymphoma were excluded (n = 5), nine episodes of OPSI including eight cases of bacterial meningitis (two meningococcal, two pneumococcal, one Haemophilus influenzae and three not stated) and one case of pneumococcal sepsis were reported among 40 patients. IgG trough levels were available for 36 of 40 patients (mean = 8·46 g/l). Six episodes of OPSI occurred prior to Ig replacement therapy, as CVID was not yet diagnosed; one patient made a personal choice not to commence replacement therapy until a later date. Seven of the nine (77·8%) episodes of OPSI occurred within 3 years of splenectomy, two (22·2%) occurred between 4–6 years and none beyond. The annual risk of OPSI was calculated at 2·47% (nine episodes × 12/4375 months of follow-up × 100%).
Six episodes of cytomegalovirus (CMV) reactivation, five fungal infections, one enteroviral meningoencephalitis, one septic arthritis, one enterococcal peritonitis, one Proteus mirabilis septicaemia and one Escherichia coli pneumonia were also reported.
Surgical complication following splenectomy
Surgical complications were uncommon in this cohort. Two of 45 patients (patients 4 and 9) experienced non-fatal surgical complications (one subdiaphragmatic abscess and one portal vein thrombosis).
Mortality of splenectomized patients
A total of 10 deaths were reported in this cohort, of which the cause of death was available for nine patients. Four patients died from unusual infections (E. coli septicaemia, fungal septicaemia, disseminated CMV and Pneumocystis jirovecii pneumonia). Two of those four patients had received long-term immunosuppression for autoimmune cytopenia. The remaining deaths are thought to be unrelated to splenectomy. Three patients died from a combination of lymphoma, chemotherapy and secondary infections, one died from a sustained seizure secondary to existing neurological disease (no infection mentioned) and one died from uncontrolled bleeding following a lung biopsy. There were no reported deaths from OPSI. The overall risk of mortality, excluding patients with lymphoma, was 1·6% per patient year (6 × 12/4375 × 100% = 1·6%).
Splenic histology
Histological descriptions of spleen were available from 26 patients and are summarized in Table 3. More than one finding was noted in some reports. Granulomas were reported in 13 (50%) patients, while congestive red pulp, lymphoid malignancy, follicular hyperplasia and atrophic germinal centres/white pulp were noted in eight (30·1%), four (15·3%), three (11·5%) and three (11·5%) patients, respectively.
Table 3.
Summary of 26 histological reports
| Histological findings | Frequency (of 26) |
|---|---|
| Granulomatous inflammation | 13 |
| Congestive red pulp | 8 |
| Lymphoma/lymphoma-like | Highly malignant B cell NHL × 1 |
| Plasmoblastic lymphoma × 1 | |
| Low-grade B cell lymphoma × 1 | |
| Castleman's disease-like × 1 | |
| Follicular hyperplasia | 3 |
| Atrophic germinal centres/white pulp | 3 |
NHL: non-Hodgkin's lymphoma.
Discussion
This report represents the largest cohort to date describing the outcome of splenectomy in CVID including treatment response in autoimmune cytopenia (Table 4). Splenectomy in CVID was described in a few reports, but detailed descriptions of outcomes were not always available [1,18–22]. The majority of splenectomies in our cohort were performed for refractory autoimmune cytopenia in the presence of splenomegaly, followed by investigational splenectomy for suspected lymphoma.
Table 4.
Summary of literature on splenectomy in common variable immunodeficiency
| Reports | No. of splenectomies | Surgical complication | Severe infection | Response for refractory AIC | Mortality in splenectomized patients |
|---|---|---|---|---|---|
| Hermaszewski & Webster 1993 | 14 | 1 Pulmonary embolism | Total = 5 2 meningitis, 3 pneumococcal infection resulting in death | Unknown | 3 from infections, 1 from pulmonary embolism, 1 from lymphoma |
| Cunningham-Rundles & Bodian 1999 | 16 | 1 LUQ abscess and bowel obstruction, 1 draining fistula, 1 collection of fluid | Total = 2 2 pneumococcal sepsis | 7/8 responses | None |
| Michel et al. 2004 | 6 | None | Total = 1 1 Toxoplasmosis gondii uveitis (but also received multiple immunosuppressants) | 4/6 responses | None |
| Seve et al. 2005 | 12 | None | Total = 6 4 life-threatening pneumococcal infections, 2 Neisseria meningitidis | 7/7 responses (4 relapses after 14 years) | None |
| Wang & Cunningham-Rundles 2005 | 11 | Not mentioned | Not mentioned | 5/7 responses (Evan's syndrome not included) | Not mentioned |
| Resnick et al. 2012 | 39 | 2 portal hypertension 2 fistulae | Total = 1 1 post-operative bacterial sepsis | 15/19 responses (2 after rituximab) | Not applicable |
| ESID Clinical Working Party 2012 | 45 | 2/45 | Total = 24 8 meningitis, 1 pneumococcal septicaemia, 5 fungal infections, 5 CMVs, 1 septic arthritis, 1 bacterial peritonitis, 1 enteroviral meningoencephalitis, 1 Escherichia coli pneumonia and 1 Proteus sepsis | 18/24 responses (2 after rituximab) | 4 from infections, 3 from lymphoma + chemotherapy + sepsis, 2 from unrelated causes, 1 unknown |
| Approximate estimation | 143 | 10/132 = 7·6% | Not applicable | 56/71 = 78·9% | Not applicable |
LUQ: left upper quadrant; CMV: cytomegalovirus; ESID: European Society for Immunodeficiencies.
Effectiveness of splenectomy in CVID
Splenectomy is regarded as ‘the most effective and best-evaluated’ second-line therapy for AIHA that is refractory to corticosteroids [17]. However, while similar success was demonstrated in some reports of CVID-associated autoimmune cytopenia [1,13], the results from other studies were less convincing and relapses were common even if the initial responses were adequate (Table 4) [18,19,23]. Within our cohort, sustained responses of >1 year were seen in at least 17 of the 18 responders (17 of 24 = 70·8%), and nine of 16 (56·3%) remained in remission for 8 years or more. Our finding clearly supports previous studies reporting a high success rate of splenectomy in refractory autoimmune cytopenia [1,16]. Of note, a number of patients still required long-term oral corticosteroids (prednisolone 4–15 mg) following their splenectomies, but were able to avoid critical levels of cytopenia (data not shown). One patient remained on prednisolone 20–30 mg for granulomatous lung disease.
Rituximab is being used increasingly as an alternative in refractory autoimmune cytopenia in CVID [23–27]. A recent study reported an 85% success rate at 1 year and 50% at 39 ± 30 months, including four patients treated for relapse post-splenectomy. At the same time, three rituximab non-responders were treated successfully with splenectomy [23]. Another study also reported two patients requiring splenectomy after rituximab [12]. While in most autoimmune diseases rituximab use is followed by restoration of B cell populations [28,29], it is uncertain whether this is also true of its use in hypogammaglobulinaemic patients, and deaths have been reported following rituximab in these patients [23,30].
Both splenectomy and rituximab appear to be equivalent in their efficacy in autoimmune cytopenia in CVID. The follow-up after splenectomy has been longer than for rituximab, although splenectomy is irreversible. Therefore, decisions regarding these therapies should be made on an individual basis following careful risk/benefit assessments.
CVID patients carry an up to 12-fold increase risk of B cell lymphoma [31,32]. The diagnosis requires histological verification, which in some patients may lead to a splenectomy. In this survey, lymphoma was identified in only two of seven patients, and one patient also had lymph node involvement. Isolated splenic lymphoma was rare in this series, and in this case splenectomy is the diagnostic and therapeutic procedure of choice. However, all other means, including positron emission tomography (PET) scanning, should be exploited before resorting to splenectomy.
Histological findings
Splenic granulomata were noted in half the histological reports in this series. While splenic granulomata are rare and have been described mainly in sarcoidosis, lymphoma and tropical infections [33–35], the spleen is the third most common site after the lungs and lymph nodes in CVID [21,36–38].
Adverse effects of splenectomy in CVID
The risk of procedure-related early complications following splenectomy in CVID is low and comparable to those in non-CVID cohorts (Table 4) [39,40]. The two reported complications in our cohort were non-fatal and treatable.
None of our patients suffered from septicaemia or meningitis prior to their splenectomies, suggesting that these infections were related, in part, to their procedures. Despite being treated adequately with Igs, the annual OPSI risk (2·47%) in our cohort is higher than that reported in non-CVID splenectomized patients (0·23–2·3%) [41,42]. However two-thirds of our OPSIs occurred when patients were not receiving Igs, suggesting that Ig replacement therapy may play a role in preventing these infections in CVID. This protective role of Ig replacement therapy is supported by the low infection rate also reported by Resnick and colleagues, in which all except one patient were on Ig replacement therapy at the time of their splenectomies [12]. As reported in non-CVID patients [43], the highest risk of OPSI lies within the first 3 years of splenectomy, as nearly 80% of the episodes occurred during this period. However, the life-long risk of OPSI as suggested by Waghorn was not demonstrated, but this could be secondary to the limited size of our cohort.
A fifth of our cohort suffered from unusual infections, such as CMV reactivation and fungal infections. Our result may be skewed by the high number of patients receiving maintenance corticosteroids and other immunosuppressants for autoimmune cytopenia. Unfortunately, we could not collect enough immunophenotypical data for any meaningful analyses, and the impact of splenectomy on the manifestation of opportunistic infection remains to be understood.
Data from the Northern European Database showed that the annual mortality rate associated with CVID patients with no disease-related complications, with autoimmune cytopenia, with lymphadenopathy or with enteropathy were 0·6, 2·3, 2·4 and 3·1%, respectively [44]. In agreement with other previously published data [2,12], non-infectious complications of CVID are associated with higher mortality. Given the high prevalence of non-infectious complications within our cohort, a mortality rate of 1·6% per patient year is lower than one would expected and does not suggest a higher mortality among splenectomized CVID patients.
Limitations of this study
These data are observational, and no direct comparison can be made with other published data in CVID or other diseases. The data were submitted voluntarily, and there is a risk that this would bias towards a higher apparent rate of splenectomy in CVID. Older data may be less complete because of the difficulty in capturing events from older records, and long-deceased patients may not be identified. Data returns were often incomplete, and the submitting centres were contacted to obtain clarification wherever possible. Where incomplete data were analysed, this is noted in the text.
Conclusion
Our data suggest that splenectomy represents an effective treatment for autoimmune cytopenia in most CVID patients who are refractory to steroids, and even rituximab. As in AIHA, there are currently no directly comparable data on the long-term efficacy and side effects of rituximab and splenectomy. Both therapies appear to be equivalent in their efficacy, but given the invasive and irreversible character of splenectomy, the sequential use of rituximab before splenectomy seems appropriate. Ig replacement therapy may have a protective role in overwhelming post-splenectomy infections in CVID and therefore, if possible, CVID patients should aim to achieve stable therapeutic IgG trough levels prior to splenectomy. Future trials investigating the role of prophylactic antibiotics, in particular during the early years, and a head-to-head comparison between splenectomy and rituximab would be invaluable for the care of CVID patients with refractory autoimmune cytopenia.
Acknowledgments
We would like to express our gratitude to all our collaborators who helped us gather this invaluable data and all those who had contributed to the discussion during the ESID Clinical working party workshop at Istanbul in October 2010. This work was supported by the 7th European framework grant EUROPAD.net (HEALTH-F2-2008-201549 to K.W. and B.G. and BMBF grant no. 01 EO 0803 to K.W.). The authors are responsible for the contents of this publication.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 2.Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 3.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27+IgM–IgD–) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 4.Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 5.Al Kindi M, Mundy J, Sullivan T, et al. Utility of peripheral blood B cell subsets analysis in common variable immunodeficiency. Clin Exp Immunol. 2012;167:275–281. doi: 10.1111/j.1365-2249.2011.04507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 7.Lanoue A, Clatworthy MR, Smith P, et al. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J Exp Med. 2002;200:1383–1393. doi: 10.1084/jem.20040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oehen S, Odermatt B, Karrer U, Hengartner H, Zinkernagel R, López-Macías C. Marginal zone macrophages and immune responses against viruses. J Immunol. 2002;169:1453–1458. doi: 10.4049/jimmunol.169.3.1453. [DOI] [PubMed] [Google Scholar]
- 9.Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carsetti R, Rosado MM, Donnanno S, et al. The loss of IgM memory B cell correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115:412–417. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Hazlewood M, Kumararatne DS. The spleen? Who needs it anyway? Clin Exp Immunol. 1992;89:327–329. doi: 10.1111/j.1365-2249.1992.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119:1650–1657. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermaszewski RA, Webster ADB. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med. 1993;86:31–42. [PubMed] [Google Scholar]
- 14.Lechner K, Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831–1838. doi: 10.1182/blood-2010-03-259325. [DOI] [PubMed] [Google Scholar]
- 15.Michel M. Classification and therapeutic approaches in autoimmune haemolytic anemia: an update. Expert Rev Hematol. 2011;4:607–618. doi: 10.1586/ehm.11.60. [DOI] [PubMed] [Google Scholar]
- 16.Fevang B, Yndestad A, Sandberg WJ, et al. Low numbers of regulatory T cells in common variable immunodeficiency: association with chronic inflammation in vivo. Clin Exp Immunol. 2007;147:521–525. doi: 10.1111/j.1365-2249.2006.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adult and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 18.Michel M, Chanet V, Galicier L, et al. Autoimmune thrombocytopenic purpura and common variable immunodeficiency: analysis of 21 cases and review of the literature. Medicine (Balt) 2004;83:254–263. doi: 10.1097/01.md.0000133624.65946.40. [DOI] [PubMed] [Google Scholar]
- 19.Sève P, Bourdillon L, Sarrot-Reynauld F, et al. Autoimmune hemolytic anaemia and common variable immunodeficiency: a case control study of 18 patients. Medicine (Balt) 2008;87:177–184. doi: 10.1097/MD.0b013e31817a90ba. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Cunningham-Rundles C. Treatment and outcome of autoimmune hematological disease in common variable immunodeficiency (CVID) J Autoimmun. 2005;25:57–62. doi: 10.1016/j.jaut.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Ardeniz O, Cunnungham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clin Immunol. 2009;133:198–207. doi: 10.1016/j.clim.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moulliot G, Carmagnat M, Gérard L, et al. B-cell and T-cell phenotypes in CVID patients correlate with clinical phenotype of the disease. J Clin Immunol. 2010;30:746–755. doi: 10.1007/s10875-010-9424-3. [DOI] [PubMed] [Google Scholar]
- 23.Gobert D, Bussell JB, Cunningham-Rundles C, et al. Efficacy and safety of rituximab in common variable immunodeficiency associated immune cytopenias: a retrospective multicentre study on 33 patients. Br J Hematol. 2011;155:498–508. doi: 10.1111/j.1365-2141.2011.08880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone J, Escudero A, Mayayo M, et al. Partial response to anti-CD20 monoclonal antibody treatment of severe immune thrombocytopenic purpura in a patient with common variable immunodeficiency. Ann NY Acad Sci. 2005;1051:666–671. doi: 10.1196/annals.1361.111. [DOI] [PubMed] [Google Scholar]
- 25.El-Shanawany TM, Williams PE, Jolles S. Response of refractory immune thrombocytopenic purpura in a patient with common variable immunodeficiency to treatment with rituximab. J Clin Pathol. 2007;60:715–716. doi: 10.1136/jcp.2006.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahévas M, Le Page L, Salle V, et al. Efficiency of rituximab in the treatment of autoimmune thrombocytopenic purpura associated with common variable immunodeficiency. Am J Hematol. 2006;81:645–646. doi: 10.1002/ajh.20619. [DOI] [PubMed] [Google Scholar]
- 27.Al-Ahmad M, Al-Rasheed M, Al-Muhani A. Successful use of rituximab in refractory idiopathic thrombocytopenic purpura in a patient with common variable immunodeficiency. J Invest Allergol Clin Immunol. 2010;20:259–262. [PubMed] [Google Scholar]
- 28.Roll P, Dorner T, Tony HP. Anti-CD20 therapy in patients with rheumatoid arthritis: predictors of response and B cell subset regeneration after repeated treatment. Arthritis Rheum. 2008;58:1566–1575. doi: 10.1002/art.23473. [DOI] [PubMed] [Google Scholar]
- 29.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–2386. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 30.Diwakar L, Gorrie S, Richter A, et al. Does rituximab aggravate pre-existing hypogammaglobulinaemia? J Clin Pathol. 2010;63:275–277. doi: 10.1136/jcp.2009.068940. [DOI] [PubMed] [Google Scholar]
- 31.Mellemkjaer L, Hammarstrom L, Andersen V, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. 2002;130:495–500. doi: 10.1046/j.1365-2249.2002.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gompels MM, Hodges E, Lock RJ, et al. Lymphoproliferative disease in antibody deficiency: a multi-centre study. Clin Exp Immunol. 2003;134:314–320. doi: 10.1046/j.1365-2249.2003.02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer-vermfs LM, Burger E, Calich VLG, et al. Pathogenicity and immunogenicity of Paracoccidioides brasiliensis isolates in the human disease and in an experimental murine model. Clin Exp Immunol. 1994;97:113–119. doi: 10.1111/j.1365-2249.1994.tb06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neiman RS. Incidence and importance of splenic sarcoid-like granuloma. Arch Pathol Lab Med. 1997;101:518–521. [PubMed] [Google Scholar]
- 35.Scolfaro C, Leunga GG, Bezzio S, et al. Prolonged follow up of seven patients affected by hepatosplenic granulomata due to cat-scratch disease. Eur J Pediatr. 2008;167:471–473. doi: 10.1007/s00431-007-0500-5. [DOI] [PubMed] [Google Scholar]
- 36.Fasano MB, Sullivan KE, Sarpong SB, et al. Sarcoidosis and common variable immunodeficiency. Report of 8 cases and review of the literature. Medicine (Balt) 1996;75:251–261. doi: 10.1097/00005792-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Mechanic LJ, Dikman S, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Ann Intern Med. 1997;127:613–617. doi: 10.7326/0003-4819-127-8_part_1-199710150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Kanathur N, Byrd JRP, Fields CL, Roy TM. Noncaseating granulomatous disease in common variable immunodeficiency. South Med J. 2000;93:631–633. [PubMed] [Google Scholar]
- 39.Pomp A, Gagner M, Salky B, et al. Laparoscopic splenectomy: a selected retrospective review. Surg Laparosc Endosc Percutan Tech. 2005;15:139–143. doi: 10.1097/01.sle.0000166990.66980.78. [DOI] [PubMed] [Google Scholar]
- 40.Rosen M, Brody F, Walsh RM, Tarnoff M, Malm J, Ponsky J. Outcome of laparoscopic splenectomy based on hematologic indication. Surg Endosc. 2002;16:272–279. doi: 10.1007/s00464-001-8150-6. [DOI] [PubMed] [Google Scholar]
- 41.Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect. 2001;7:657–660. doi: 10.1046/j.1198-743x.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 42.Ejstrud P, Kristensen B, Hansen JB, Madsen KM, Schønheyder HC, Sørensen HT. Risk and pattern of bacteraemia after splenectomy: a population-based study. Scand J Infect Dis. 2000;32:521–525. doi: 10.1080/003655400458811. [DOI] [PubMed] [Google Scholar]
- 43.Waghorn DJ. Overwhelming infection in asplenic patients: current best practice preventive measures are not being followed. J Clin Path. 2001;54:214–218. doi: 10.1136/jcp.54.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapel H, Lucas M, Patel S, et al. Confirmation and improvement of criteria for clinical phenotyping in common variable immunodeficiency disorders in replicate cohorts. J Allergy Clin Immunol. 2012;130:1197–1198. doi: 10.1016/j.jaci.2012.05.046. [DOI] [PubMed] [Google Scholar]


