Abstract
Chronic fatigue syndrome (CFS) is a heterogeneous disorder of unknown aetiology characterized by disabling fatigue, headaches, sleep disturbance and several other symptoms. The onset of CFS may follow a viral infection or period of stress. Patients with CFS do not have hypogammaglobulinaemia, predisposition to recurrent bacterial infections or symptoms of autoimmunity. To date, defects in B cell numbers or function have not been shown in the literature. However, treatment with anti-B cell therapy using Rituximab has recently shown benefit to CFS patients. We therefore postulated that patients with CFS had a subtle humoral immune dysfunction, and performed extended B cell immunophenotyping. We undertook a detailed characterization of the proportions of the different B cell subsets in 33 patients with CFS fulfilling the Canadian and Fukada criteria for CFS and compared these with 24 age- and gender-matched healthy controls (HC). CFS patients had greater numbers of naive B cells as a percentage of lymphocytes: 6·3 versus 3·9% in HC (P = 0·034), greater numbers of naive B cells as a percentage of B cells: 65 versus 47% in controls (P = 0·003), greater numbers of transitional B cells: 1·8 versus 0·8% in controls (P = 0·025) and reduced numbers of plasmablasts: 0·5 versus 0·9% in controls (P = 0·013). While the cause of these changes is unclear, we speculate whether they may suggest a subtle tendency to autoimmunity.
Keywords: B cells, chronic fatigue syndrome, flow cytometry/FACS, myalgic encephalitis (ME)
Introduction
When strictly defined, the present illness, called chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME), has a prevalence of approximately 0·2% [1]. The diagnosis of CFS is based currently on patients fulfilling one or more of several criteria [2–5] and in the absence of an alternative medical or psychiatric cause of the fatigue. The clinical assessment is often supplemented by tests of haematological, biochemical, endocrine and immunological dysfunction as well as investigations checking for inflammation and gluten sensitivity. These tests are almost always normal or negative.
The aetiology of CFS is still far from understood [6]. Clinicians remain divided as to whether the disease has a physical or psychological cause [7]. Different aetiological hypotheses include viral, immune dysfunction, neurological disease, neuroendocrine disorder, metabolic or autonomic disturbances, ion channel dysfunction and exposure to toxins or vaccinations, reviewed in [8]. It is, of course, possible that CFS represents the clinical manifestations of an illness caused by one or more of these factors.
During the last decade we have observed an elevated prevalence of persistent fatigue in our patients with primary antibody deficiency and speculated on subtle B cell dysfunction in CFS. Recently, three patients with CFS were treated with a total of three separate Rituximab infusions which resulted in symptomatic benefit after each infusion [9]. A subsequent larger double-blind, placebo-controlled clinical trial showed symptomatic benefit in 67% of CFS patients receiving two infusions of Rituximab versus 13% of CFS patients receiving placebo [10]. These results suggest the involvement of B cells in the pathology of CFS in at least a subset of patients. Importantly, Rituximab does not simply deplete CD20+ cells (B cells) but has many mechanisms, including down-regulating CD40L and CD80 on B cells, decreasing CD4 effector cells, reducing natural killer (NK) cell numbers and activation, inducing macrophage maturation and reducing tumour necrosis factor (TNF)-α secretion and increasing the suppressive function of T regulatory cells [11]. However, the onset of B cell depletion correlated with a reduction in symptoms and with the expected appropriate lag phase. Possible explanations for the improvement of CFS symptoms as a result of B cell depletion include allowing repopulation of the B cell compartment with normalized proportions of the subset population; in other words, ‘resetting’ the B cell compartment. An alternative explanation includes the removal of autoreactive B cells that could be responsible for the pathology of CFS. Additionally, depletion of B cells may have resulted in the removal of the niche and reservoir of one or more lymphotrophic B cell viruses, such as Epstein–Barr virus (EBV). It is tempting to favour the removal of autoreactive B cells, as the female preponderance of CFS is also seen commonly in many autoimmune diseases. In addition, the relapsing/remitting course of CFS is similar to several autoimmune diseases. Moreover, the raised frequency of patient-reported lymphadenopathy, sore throat, myalgia and arthralgia that are seen in CFS suggest an inflammatory process [12].
CFS patients do not suffer recurrent bacterial infections such as those seen with primary immune deficiency, but it is possible that subtle defects of B cell function may underlie CFS. We aimed to characterize the phenotype of B cell populations in the peripheral blood of CFS patients, and compare that with healthy gender- and age-matched subjects. This approach aimed to determine whether depletion of abnormal B cells by Rituximab constitutes a mechanism for symptom relief in CFS patients [9,10]. We also determined serum immunoglobulin concentrations as a basic overall screen of B cell function. Antibodies are required in an immune response to neutralize and opsonize pathogens and toxic products, and to activate the classical complement system. Defective antibody class-switching and antibody production would result in recurrent infection, as seen in primary immunodeficiency states; however, a milder defect may lead to inappropriate immune response and possibly autoimmunity.
Materials and methods
Subject selection and ethical approval
All participants were informed verbally about the study and given information sheets with written informed consent (obtained by A.S.B.). Consecutive patients diagnosed with CFS and fulfilling the Fukada, Canadian and Oxford criteria were selected for study. The Chalder Fatigue Scale was used to assess tiredness to assign severity. The Hospital Anxiety Depression Scale (HADS) and clinical history were used to determine the presence of significant depression and anxiety. Clinically depressed patients (HADS depression > 7) and those with significant anxiety (HADS anxiety > 7) were excluded, as both are known to affect the immune system. While low mood may be evident in those with CFS, owing to the chronic nature of the symptoms, it is distinct from major depression, as CFS patients do not generally exhibit the classic symptoms of depression: guilt, anhedonia and low motivation [13–15]. Of 56 eligible CFS patients, 23 were excluded as they were on medication that affected mood, had possible obstructive sleep apnoea, declined participation owing to needle phobia or had CFS with very mild symptoms.
The selection of an appropriate control group in CFS studies is extremely difficult. Those with depression are often on one or more medications and have increased stress and sleep disturbance that is different to that in CFS. In contrast, patients with impaired mobility often have an underlying illness that affects immune function and are also on a diverse range of medications. We therefore chose healthy controls (HCs) who were selected to match the age and gender of our CFS patients. Any volunteer who suffered from chronic illness, depression, anxiety or was on medication was not included in the study. The investigator who performed the sample process was unaware if samples were collected from patients or HCs.
This research project was granted ethical approval by the Central London Rec. 1 Research Ethics Committee (Rec. no: 09/H0718/54) and approved by the research and development department at St Helier hospital (R&D no: 015/2009/DPH).
B cell immunophenotyping method
Five ml of whole blood was collected into ethylenediamine tetraacetic acid (EDTA) from each subject and stained immediately following the whole blood method for immunophenotyping B cell functional subsets, as described previously [16]. In brief, 1 ml of whole blood was washed three times in phosphate-buffered saline (PBS) with azide and resuspended in PBS/azide/bovine serum albumin (BSA) to a volume of 1 ml. The blood was stained with antibody panels (Table 1) for 20 min, lysed with fluorescence activated cell sorter (FACS) easy lyse, washed twice in PBS/azide and resuspended in Cell Fix (Becton Dickinson, Oxford, UK). Samples were acquired on a FACSCalibur cytometer using CellQuest software (Becton Dickinson) (Figs 1 and 2).
Table 1.
The antibody panel used to stain the whole blood
| Tube | Antibody combinations | |||
|---|---|---|---|---|
| 1 | CD19-Pcy5 | CD27-FITC | IgD-PE | IgM-Cy5 |
| 2 | CD19-Pcy5 | CD21-FITC | CD38-PE | IgM-Cy5 |
| 3 | CD19-Pcy5 | IgG1/2A-FITC | ||
Cy5: cyanin 5; FITC: fluorescein isothiocyanate; Ig: immunoglobulin; PE: phycoerythrin.
Figure 1.
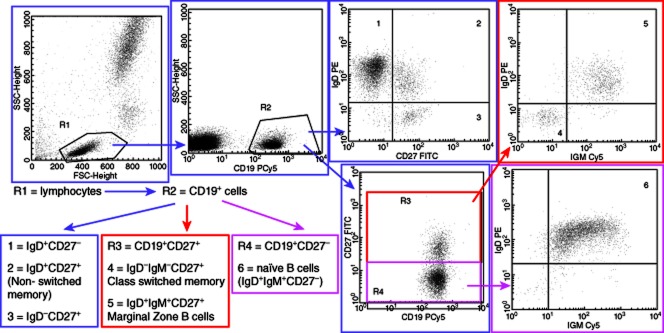
B cell gating strategy. The first gate, R1, selects lymphocytes based on side-scatter versus forward-scatter. These cells are analysed further for CD19 expression and the second gate, R2, selects B cells (CD19+ cells). These B cells (R6) are analysed further to determine proportions of non-switched memory, class-switched memory, marginal zone and naive B cells.
Figure 2.
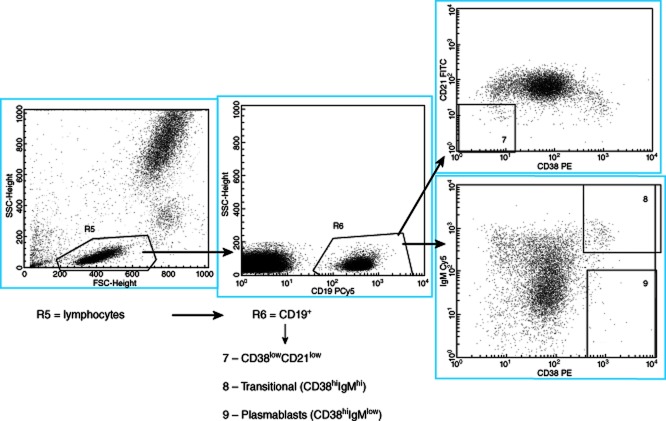
B cell gating strategy for determining CD38lowCD21low, transitional B cells (CD38hiIgMhi) and plasmablast (CD38hiIgMlow) subpopulations. The first gate, R5, selects lymphocytes based on side-scatter versus forward-scatter. CD19 expression is used to select B cells (CD19+ cells) in the second gate, R6. The B cells (R6) are analysed further for CD21 and CD38 expression to identify gate 7, CD38lowCD21low cells. Alternatively, the B cells (R6) are analysed for immunoglobulin (Ig)M versus CD38 expression to identify gate 8, transitional B cells (CD38hiIgMhi) and gate 9, plasmablasts (CD38hiIgMlow).
Measurement of immunoglobulin concentrations
Clotted samples were also taken and serum was separated and stored at –80°C. Sera were thawed and immunoglobulin concentrations were measured for all the samples using a rate nephelometer (BN2 supplied by Siemens AG, Munich, Germany) on the same day.
Data and statistical analysis
All the data were determined to be distributed non-parametrically during analysis using the Kolmogorov–Smirnov normality test, except the B cells, as a percentage of lymphocytes. The data were therefore analysed using the non-parametric Mann–Whitney rank sum test. The data were analysed statistically on two separate occasions using two software packages: SigmaStat version 3·11 and spss version 20. Identical statistical results were obtained using both packages. The P-values were not modified.
Results
The demographics of the participants are summarized in Table 2. The majority of the CFS patients were of moderate severity (23 patients), as assessed by the Chalder Fatigue Scale. Two patients were affected severely and the rest were mild (eight patients). For most B cell populations measured, the ranges of both patients and controls were relatively wide. This was expected, as B cells are a dynamic immune population. B cell populations fluctuate in response to many parameters, such as infection and stress, and so tight clusters of data were not expected. The majority of B cell subset populations showed no significant difference in patients versus controls; subsets that were significantly different are shown in italic type (Table 3) and displayed in (Fig. 3). CFS patients had greater numbers of naive B cells as a percentage of lymphocytes: 6·3 versus 3·9% in HC (P = 0·034), greater numbers of naive B cells as a percentage of B cells: 65 versus 47% in controls (P = 0·003), greater numbers of transitional B cells: 1·8 versus 0·8% in controls (P = 0·025) and reduced numbers of plasmablasts: 0·5 versus 0·9% in controls (P = 0·013).
Table 2.
Demographics of patients and healthy controls (HC) enrolled in the study
| CFS patients (n = 33) | HC (n = 24) | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Mean | Range | s.d. | Mean | Range | s.d. | P-value |
| Age (years) | 35 | 20–66 | 11·8 | 40 | 22–63 | 12·2 | 0·183 |
| % Female | 79% | 86% | n.a. | ||||
CFS: chronic fatigue syndrome; n.a.: not applicable; s.d.: standard deviation.
Table 3.
Functional B cell subsets in chronic fatigue syndrome (CFS) patients versus healthy controls (HC)
| CFS patients (n = 33) | HCs (n = 24) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell population compared | Min–max | Med. | 25% quartile | 75% quartile | Min–max | Med. | 25% quartile | 75% quartile | % diff | P-value |
| B cells (% lymphocytes) | 3·4–23·2 | 9·5 | 6·5 | 12·8 | 1·8–19 | 10·3 | 6·4 | 13·9 | −8 | 0·802 |
| Switched-memory B cells (% lymphocytes) | 0·0–5·6 | 0·4 | 0·0 | 0·9 | 0·0–2·9 | 0·5 | 0·2 | 1·0 | −20 | 0·167 |
| Switched-memory B cells (% B cells) | 0·0–28·0 | 5·2 | 0·0 | 7·7 | 0·0–29·0 | 6·9 | 3·6 | 11·9 | −25 | 0·082 |
| Marginal zone B cells (% lymphocytes) | 0·2–2·6 | 0·99 | 0·7 | 1·6 | 0·1–5·7 | 1·2 | 0·8 | 1·4 | −18 | 0·853 |
| Marginal zone B cells (% B cells) | 3·3–25·8 | 11·0 | 8·1 | 16·2 | 3·0–34·3 | 11·4 | 7·5 | 17·3 | −4 | 0·740 |
| Naive B cells (% lymphocytes) | 1·7–16·3 | 6·3 | 4·2 | 7·9 | 0·1–12·9 | 3·9 | 2·0 | 6·7 | 138 | 0·034 |
| Naive B cells (% B cells) | 33·8–85·0 | 65·0 | 52·8 | 71·5 | 0·3–77·6 | 47·0 | 32·8 | 62·9 | 128 | 0·003 |
| CD38low CD21low cells (% B cells) | 0·6–20·3 | 2·7 | 1·1 | 4·4 | 0·1–68·3 | 3·1 | 2·4 | 4·1 | −13 | 0·193 |
| Transitional B cells (% B cells) | 0·0–8·3 | 1·8 | 0·5 | 4·1 | 0·0–7·3 | 0·8 | 0·3 | 1·2 | 155 | 0·025 |
| Plasmablasts (% B cells) | 0·0–8·6 | 0·5 | 0·3 | 1·1 | 0·1–24·3 | 0·9 | 0·6 | 2·7 | −44 | 0·013 |
| CD27+ cells (% B cells) | 12·6–45·8 | 25·2 | 19·5 | 37·5 | 13·6–97·0 | 27·4 | 19·3 | 46·0 | −8 | 0·268 |
| Non-switched memory B cells (% B cells) | 5·0–27·6 | 13·3 | 9·5 | 18·0 | 7·1–97·0 | 14·5 | 11·6 | 24·8 | −8 | 0·162 |
Results of the statistical analysis of B cell functional subsets using the non-parametric Mann–Whitney rank sum test. P < 0·05 represent a statistically significant result; these results are shown in bold type. The statistical data have not been modified. Med: median.
Figure 3.
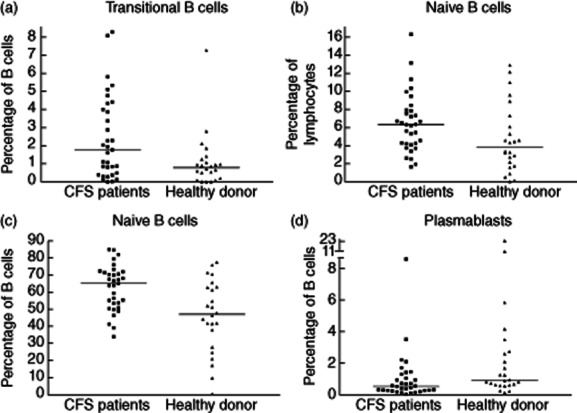
Graphs summarizing B cell subsets which are significantly different between 33 chronic fatigue syndrome (CFS) patients and 24 donors. (a) Percentage of B cells that are transitional B cells. CFS patients had 1·8 versus 0·8% in healthy controls (P = 0·025). (b) Percentage of lymphocytes that are naive B cells. CFS patients had 6·3 versus 3·9% in healthy controls (P = 0·034). (c) Percentage of B cells that are naive B cells. CFS patients had 65 versus 47% in controls (P = 0·003). (d) Percentage of B cells that are plasmablasts. CFS patients had 0·5 versus 0·9% in controls (P = 0·013).
Immunoglobulin concentrations
The immunoglobulin concentrations of the CFS patients and controls were normal, as measured by rate nephelometry (Table 4). This was expected, as neither of the groups reported recurrent infections.
Table 4.
Immunoglobulin (Ig) concentrations of chronic fatigue syndrome (CFS) patients and controls
| CFS patients (n = 33) (g/l) | HCs (n = 24) (g/l) | ||||||
|---|---|---|---|---|---|---|---|
| Ig | Min–max | Mean | s.d. | Min–max | Mean | s.d. | P-value |
| IgG | 7·3–16·1 | 11·1 | 2·22 | 7·6–15·3 | 11·1 | 1·85 | 0·94 |
| IgM | 0·5–3·7 | 1·4 | 0·6 | 0·5–2·3 | 1·3 | 0·5 | 0·57 |
| Min–max | Median | 25 and 75% quartiles | Min–max | Median | 25 and 75% quartiles | P-value | |
|---|---|---|---|---|---|---|---|
| IgA | 0·8–5·5 | 1·8 | 1·4, 2·3 | 0·8–4·1 | 2·2 | 1·5, 2·5 | 0·251 |
HC: healthy controls; s.d.: standard deviation. IgG and IgM were distributed parametrically, so were analysed using a t-test; IgA was distributed non-parametrically and was analysed using a Mann–Whitney U-test.
Application of the common variable immunodeficiency (CVID) classification to CFS patients
Owing to their tendency to ‘recurrent infections’, some patient groups have suggested that those with CFS have some sort of immune deficiency syndrome. With this in mind, the B cell characterization data were used to determine classifications of CFS patients and controls along the lines of those with CVID using the Warnatz [17], Paris [18] and EUROclass [19] definitions (Table 5). These definitions are used to assign CVID patients into groups that are thought to be more likely to develop complications such as lymphadenopathy, splenomegaly, granulomas and autoimmunity. CFS patients do not suffer classically from splenomegaly, granulomas or significant autoimmunity, so it was expected that this analysis would show no difference between the CFS patients and controls. Indeed, there was no significant difference between the CFS patients and controls regarding classification, as determined by χ2 test analysis.
Table 5.
The classification of the chronic fatigue syndrome (CFS) patients and controls using the Warnatz, Paris and EUROclass definitions
| Warnatz | Paris | EUROclass | |
|---|---|---|---|
| CFS patients (n = 33) | II = 15 (45%) | MB1 = 25 (76%) | SmB(+)21(norm) = 19 (58%) |
| Ib = 18 (55%) | MB2 = 8 (24%) | SmB(–)Tr(norm)21(norm) = 13 (39%) | |
| SmB(+)21(low) = 1 (3%) | |||
| HCs (n = 24) | II = 15 (63%) | MB1 = 14 (58%) | SmB(+)21(norm) = 18 (79%) |
| Ib = 9 (38%) | MB2 = 10 (42%) | SmB(–)Tr(norm)21(norm) = 4 (13%) | |
| SmB(+)21(low) = 2 (8%) |
HC: healthy controls.
Discussion
We selected a well-defined group of CFS patients and a group of age-, sex- and ethnicity-matched controls. We detected no difference in the immunoglobulin concentrations. However, compared to the HC, the CFS cohort had significantly greater proportions of transitional B cells, naive B cells and reduced proportions of plasmablasts expressed as a percentage of B cells.
CFS and B cells
B cells produce antibodies and are potent antigen-presenting cells. Impairment of B cell function or development leads to recurrent infections, or a propensity to autoimmunity or allergy. The majority of early studies on CFS patients found no difference in B cell numbers or immunoglobulin concentrations compared with HCs [20,21]. Interestingly, one study found increased B cell lymphocyte numbers in CFS patients, with a subset of these patients (37%) having an expansion of CD5+CD19+ B cells. These latter cells have been considered to be producers of IgA, with increased numbers involved putatively in autoimmune disease [22]. However, all these studies were published before the new CFS case definitions were proposed, thus our CFS cohort is not directly comparable to these studies. Nevertheless, CFS patients do not suffer from significant recurrent upper or lower respiratory tract bacterial infections, so it is unlikely that CFS patients have profound B cell abnormalities. However, immune abnormalities have been suggested by the results of a large epidemiological study, which looked at 1·2 million cancer cases and 100 000 controls [23]. This study found that CFS was associated with an increased risk of non-Hodgkin lymphoma (NHL) in elderly patients [23]. The authors speculated that the increased risk of NHL attributable to CFS may indicate chronic immune activation or infection. Specifically, the NHL subtype most associated with CFS was large B cell lymphoma, which may indicate problems with B cell development, but more research is required in this area. Moreover, patients with CFS have been shown to benefit from treatment with repeated infusions with Rituximab [9,10]. While the exact mechanism of the delayed symptomatic benefit from the infusions is unclear, it is possible that it is related to the elimination of dysfunctional B cell populations [10]. Thus, CFS patients may have some unusual, unrecognized autoimmune disease, or it is possible that CFS patients are unable to control lymphotrophic viral infections due to some defect of B cell memory or T cell dysfunction. To our knowledge, extended B cell immunophenotyping has not been reported in the CFS patients who received Rituximab. Our earlier observation of persistent fatigue in those with primary antibody deficiency, supported by the beneficial effect of Rituximab in CFS, prompted us to search specifically for a subtle defect in B cell subsets, which would not have been detected previously by a simple analysis of B cell numbers.
B cell development and transitional B cells
B cell development in the bone marrow is antigen-independent, and is a tightly regulated process [24]. After several rounds of expansion, functional light chains replace the surrogate light chain and pair with the μ heavy chain, resulting in cell surface IgM expression and forming the B cell receptor (BCR). BCR expression allows negative selection of autoreactive B cells and their elimination by apoptosis. Deficiency of negative selection that eliminates cells with autoreactive heavy chains may allow the later development of systemic autoimmunity [25]. Surviving B cells subsequently become transitional B cells (CD19+CD38++IgM+IgD+) and migrate via the peripheral blood into secondary lymphoid organs such as the spleen, where full maturation occurs [26]. Only 10–20% of immature B cells produced in the bone marrow reach the spleen; a large proportion are deleted due to the expression of autoreactive BCRs. Our CFS patients had increased transitional B cells, so it is tempting to speculate if this reflects a defective negative selection checkpoint. If so, the consequence of this defect may encourage self-reactive B cells to escape the bone marrow. Importantly, increased numbers of transitional B cells have been reported in several patient groups with defective humoral immunity, including patients with systemic lupus erythematosus (SLE) [27,28], X-linked lymphoproliferative disease, CVID, patients recovering from haemopoietic transplantation and neonates [29].
B cell development in the spleen and naive B cells
In the spleen, transitional B cells mature into long-lived naive B cells (CD19+IgM+IgD+CD27–); an important survival factor is B cell-activating factor (BAFF) (Blys) and its receptor BAFF-R. The CFS patients had greater proportions of naive B cells compared to controls, which is not surprising as they have greater proportions of transitional B cells which are naive B cell progenitors. None the less, it is also possible that CFS patients may be producing more BAFF to allow the survival of these increased proportions of naive B cells. Once through this transitional stage of development, B cells develop into either follicular B cells (CD19+CD27– CD38+mIgD+mIgMhi) or marginal zone (MZ) B cells (CD19+CD27+CD38–mIgD+mIgMhi).
Plasmablasts
Antigen encounter stimulates affinity maturation of the BCR by somatic hypermutation within germinal centres. During this process, follicular B cells can differentiate into memory B cells (CD19+CD27+CD38+mIgM–mIgD–) or plasmablasts (CD19+CD27++CD38hi), while MZ B cells only differentiate into plasmablasts. Memory B cells can be subdivided into non-switched memory B cells (CD19+CD27+IgM+IgD+) or class-switched memory B cells (CD19+CD27+IgM+IgD–) depending, in part, on the cytokine milieu. In CFS patients, memory B cell subsets were comparable to controls, but plasmablasts were reduced. One explanation for reduced plasmablasts in the CFS cohort is that increased numbers of transitional B cells and naive B cells may overwhelm the B cell maturation process, which may consequently become suboptimal. Alternatively T cell help provided by cytokines may not support naive B cells to develop into plasmablasts.
CFS patients and altered B cell subsets
In patients with CFS it is possible that there are one or more alterations of B cell maturation which may lead to an increased tendency to autoimmunity and a subtle humoral immune dysfunction. There are no studies detailing B cell populations in patients with depression and significant anxiety. B cell negative selection requires appropriate integration of complex signals that are also key for clonal expansion, isotype-switching, affinity maturation, long-lived memory and maintaining tolerance to self-antigens. At least two broad categories of genetic defects promote loss of B cell tolerance and promote autoreactivity. If B cell thresholds for cellular signalling, activation or proliferation are altered this will increase the risk of self-reactive B cells escaping the checkpoints and the potential of autoimmune disease. Additionally, defective apoptotic genes increase B cell life-spans, allowing survival of self-reactive B cell clones, leading to the potential of autoantibody production and possible autoimmune disease. A small number of studies show an increased frequency of low-level autoantibodies directed against nuclear, thyroid and other antigens in CFS patients [30,31]. Others have proposed autoimmunity to neuropeptide antigens [32]. Alternatively, depletion of B cells may have resulted in the removal of the reservoir of lymphotrophic B cell viruses such as EBV. However, the clinical improvement of patients with CFS after Rituximab treatment suggests an active process by which the dysfunctional B cell maturation process contributes to symptomatology. It would be interesting to monitor B cell subsets of CFS patients before and after Rituximab treatment and monitor repopulation of the different B cell subsets with the return of symptoms.
CFS patients do not experience splenomegaly or granulomatous disease, but some complain of lymphadenopathy. Thus the B cell characterization of the CFS patients was used to assess whether CFS patients exhibit similar B cell subset abnormality patterns to the CVID patients, and whether there is a significant difference with controls (Tables 3 and 5). Intriguingly, the CFS patients showed increased transitional B cells compared to controls (1·9% of B cells in the CFS group compared to 0·8% of B cells in the HC group, P = 0·025). In the CVID patient cohort, increased transitional B cells (≥ 9% of CD19+ B cells) are associated with lymphadenopathy, a pathology commonly experienced by CFS patients. A reported normal range of transitional B cells as a percentage of B cells is 0·6–3·4% of B cells [33], so the CFS cohort in this study had only a modest expansion of transitional B cells compared to controls. However, the CFS patients examined in our cohort were mainly moderate sufferers with intermittent but not persistent lymphadenopathy. It is tempting to speculate whether a group of severe CFS patients suffering from more frequent or significant lymphadenopathy may, perhaps, have an even greater increase of their transitional B cells, similar to that in the CVID cohort.
In conclusion, we have observed patients with moderate CFS to have increased proportions of transitional and naive B cells and reduced plasmablasts. The precise basis for these findings is unclear, and our work does not allow clarification of whether or not these changes are the cause of the CFS symptoms or the result of patient inactivity, sleep disturbance or raised stress. The therapeutic response to Rituximab suggests that B cells are somehow involved in the pathogenesis or perpetuation of CFS symptoms.
Acknowledgments
The authors thank Sree Bhaskaran for assistance with technical aspects of the flow cytometry. This work was made possible by funding from the ME solutions charity and the estate of Abraham Goudsmit.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Nacul LC, Lacerda EM, Pheby D, et al. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: a repeated cross-sectional study in primary care. BMC Med. 2011;9:1–12. doi: 10.1186/1741-7015-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carruthers BM. Definitions and aetiology of myalgic encephalomyelitis: how the Canadian consensus clinical definition of myalgic encephalomyelitis works. J Clin Pathol. 2007;60:117–119. doi: 10.1136/jcp.2006.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387–389. doi: 10.7326/0003-4819-108-3-387. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Sharpe MC, Archard LC, Banatvala JE, et al. A report – chronic fatigue syndrome: guidelines for research. J R Soc Med. 1991;84:118–121. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyall M, Peakman M, Wessely S. A systematic review and critical evaluation of the immunology of chronic fatigue syndrome. J Psychosom Res. 2003;55:79–90. doi: 10.1016/s0022-3999(02)00515-9. [DOI] [PubMed] [Google Scholar]
- 7.Chew-Graham CA, Cahill G, Dowrick C, Wearden A, Peters S. Using multiple sources of knowledge to reach clinical understanding of chronic fatigue syndrome. Ann Fam Med. 2008;6:340–348. doi: 10.1370/afm.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bansal AS, Bradley AS, Bishop KN, Kiani-Alikhan S, Ford B. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav Immun. 2012;26:24–31. doi: 10.1016/j.bbi.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Fluge O, Mella O. Clinical impact of B-cell depletion with the anti-CD20 antibody rituximab in chronic fatigue syndrome: a preliminary case series. BMC Neurol. 2009;9:1–7. doi: 10.1186/1471-2377-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fluge O, Bruland O, Risa K, et al. Benefit from B-lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study. PLOS ONE. 2011;6:e26358. doi: 10.1371/journal.pone.0026358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessel A, Rosner I, Toubi E. Rituximab: beyond simple B cell depletion. Clin Rev Allergy Immunol. 2008;34:74–79. doi: 10.1007/s12016-008-8074-1. [DOI] [PubMed] [Google Scholar]
- 12.Klimas NG, Koneru AO. Chronic fatigue syndrome: inflammation, immune function, and neuroendocrine interactions. Curr Rheumatol Rep. 2007;9:482–487. doi: 10.1007/s11926-007-0078-y. [DOI] [PubMed] [Google Scholar]
- 13.Wessely S, Chalder T, Hirsch S, Wallace P, Wright D. Psychological symptoms, somatic symptoms, and psychiatric disorder in chronic fatigue and chronic fatigue syndrome: a prospective study in the primary care setting. Am J Psychiatry. 1996;153:1050–1059. doi: 10.1176/ajp.153.8.1050. [DOI] [PubMed] [Google Scholar]
- 14.Powell R, Dolan R, Wessely S. Attributions and self-esteem in depression and chronic fatigue syndromes. J Psychosom Res. 1990;34:665–673. doi: 10.1016/0022-3999(90)90111-g. [DOI] [PubMed] [Google Scholar]
- 15.Johnson SK, DeLuca J, Natelson BH. Depression in fatiguing illness: comparing patients with chronic fatigue syndrome, multiple sclerosis and depression. J Affect Disord. 1996;39:21–30. doi: 10.1016/0165-0327(96)00015-8. [DOI] [PubMed] [Google Scholar]
- 16.Ferry BL, Jones J, Bateman EA, et al. Measurement of peripheral B cell subpopulations in common variable immunodeficiency (CVID) using a whole blood method. Clin Exp Immunol. 2005;140:532–539. doi: 10.1111/j.1365-2249.2005.02793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(–)IgD(–)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 18.Piqueras B, Lavenu-Bombled C, Galicier L, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23:385–400. doi: 10.1023/a:1025373601374. [DOI] [PubMed] [Google Scholar]
- 19.Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 20.Mawle AC, Nisenbaum R, Dobbins JG, et al. Immune responses associated with chronic fatigue syndrome: a case–control study. J Infect Dis. 1997;175:136–141. doi: 10.1093/infdis/175.1.136. [DOI] [PubMed] [Google Scholar]
- 21.Natelson BH, LaManca JJ, Denny TN, et al. Immunologic parameters in chronic fatigue syndrome, major depression, and multiple sclerosis. Am J Med. 1998;105:43S–49. doi: 10.1016/s0002-9343(98)00165-x. [DOI] [PubMed] [Google Scholar]
- 22.Tirelli U, Marotta G, Improta S, Pinto A. Immunological abnormalities in patients with chronic fatigue syndrome. Scand J Immunol. 1994;40:601–608. doi: 10.1111/j.1365-3083.1994.tb03511.x. [DOI] [PubMed] [Google Scholar]
- 23.Chang CM, Warren JL, Engels EA. Chronic fatigue syndrome and subsequent risk of cancer among elderly US adults. Cancer. 2012;118:5929–5936. doi: 10.1002/cncr.27612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 25.Keenan RA, De Riva A, Corleis B, et al. Censoring of autoreactive B cell development by the pre-B cell receptor. Science. 2008;321:696–699. doi: 10.1126/science.1157533. [DOI] [PubMed] [Google Scholar]
- 26.Verma S, Waldschmidt TJ. Characterization of splenic CD21hi T2 B cells. Immunol Res. 2007;39:240–248. doi: 10.1007/s12026-007-0072-5. [DOI] [PubMed] [Google Scholar]
- 27.Dorner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13:243–283. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182:4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 29.Cuss AK, Avery DT, Cannons JL, et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176:1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 30.Maes M, Mihaylova I, Leunis JC. Increased serum IgM antibodies directed against phosphatidyl inositol (Pi) in chronic fatigue syndrome (CFS) and major depression: evidence that an IgM-mediated immune response against Pi is one factor underpinning the comorbidity between both CFS and depression. Neuro Endocrinol Lett. 2007;28:861–867. [PubMed] [Google Scholar]
- 31.Skowera A, Stewart E, Davis ET, et al. Anti-nuclear autoantibodies (ANA) in Gulf War-related illness and chronic fatigue syndrome (CFS) patients. Clin Exp Immunol. 2002;129:354–358. doi: 10.1046/j.1365-2249.2002.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staines DR. Postulated vasoactive neuropeptide autoimmunity in fatigue-related conditions: a brief review and hypothesis. Clin Dev Immunol. 2006;13:25–39. doi: 10.1080/17402520600568252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warnatz K, Schlesier M. Flowcytometric phenotyping of common variable immunodeficiency. Cytometry B Clin Cytom. 2008;74:261–271. doi: 10.1002/cyto.b.20432. [DOI] [PubMed] [Google Scholar]