Abstract
Allergen-specific immunotherapy (SIT) is the only treatment for allergic diseases that targets allergen-specific T helper type 2 (Th2) cells, which are the cause of the disease. There is an unmet requirement for adjuvants that increase the clinical efficacy of SIT allowing application of lower doses of the allergen, thereby reducing the risk of anaphylactic reactions. Cytotoxic T lymphocyte antigen 4–immunoglobulin (CTLA-4–Ig) has been shown to induce immunological tolerance in autoimmunity and allograft transplantation by blocking T cell co-stimulation and induction of the immunoregulatory enzyme indoleamine 2,3 dioxygenase (IDO). Previously, we showed that CTLA-4–Ig treatment at the time of allergen inhalation induced tolerance to subsequent allergen exposure in a mouse model of asthma. In this study, we test the hypothesis that CTLA-4–Ig acts as an adjuvant for experimental SIT. We evaluated the adjuvant effects of CTLA-4–Ig on SIT in a mouse model of ovalbumin-driven asthma. We used both wild-type and IDO-deficient mice to assess the role of IDO in the adjuvant effects of CTLA-4–Ig. Co-administration of CTLA-4–Ig strongly increased SIT-induced suppression of airway hyperreactivity (AHR), specific IgE in serum, airway eosinophilia and Th2 cytokine levels. Moreover, we found that CTLA-4–Ig, as an adjuvant for SIT, is equally effective in IDO-deficient and wild-type mice, demonstrating that the effect of CTLA-4–Ig is independent of IDO expression. We show that CTLA-4–Ig acts as a potent adjuvant to augment the therapeutic effects of SIT. As the adjuvant activity of CTLA-4–Ig is independent of IDO, we conclude that it acts by blocking CD28-mediated T cell co-stimulation.
Keywords: adjuvant, airway hyperreactivity, allergen immunotherapy, CTLA-4-Ig in allergy
Introduction
Atopic T helper type 2 (Th2) immune responses against innocuous environmental antigens are the cause of allergic diseases that impair the quality of life of a significant proportion of the world's population [1,2]. Currently, allergen-specific immunotherapy (SIT) is the only remedy for allergic diseases that modifies the dominant Th2 response and causes long-lasting relief of symptoms [3]. Classically, SIT is performed by repeated administration of high doses of the sensitizing allergen for a period of 3–5 years, after an initial gradual increase of administered allergen to avoid anaphylaxis [3]. SIT not only induces a sustained relief of allergic symptoms; it can also prevent the development of new allergen sensitizations [4,5] and the progression of allergic rhinitis to allergic asthma [6]. Currently, there are concerns about the safety of using high doses of allergen and the required long-term duration of treatment [7,8]. Therefore, improvement of SIT is highly required by using clinically applicable adjuvants that achieve optimal efficacy at lower doses of allergen and lead to a safer therapy in possibly a shorter time-frame [9].
Cytotoxic T lymphocyte antigen 4 (CTLA-4) is an inhibitory co-stimulatory molecule expressed by naturally occurring regulatory and activated T cells [10,11]. T cell receptor signalling upon antigen presentation results in T cell activation or inhibition when accompanied by CD28 or CTLA-4 co-stimulation, respectively [11,12].
CTLA-4–immunoglobulin (Ig) is a fusion molecule of the extracellular domain of CTLA-4 and the heavy chain of human or mouse IgG [13,14]. This molecule has been shown to exhibit tolerogenic properties towards self- and allograft antigens in human patients and in animal models [15–17]. CTLA-4–Ig is a US Food and Drug Administration (FDA)-approved compound that has been used in the treatment of rheumatoid arthritis and prevention of allograft rejection [18,19]. Interestingly, we have shown previously that CTLA-4–Ig treatment at the time of allergen inhalation in sensitized mice induced long-term tolerance to subsequent allergen-induced airway eosinophilia, but not airway hyperreactivity (AHR), in a mouse model of experimental asthma [20].
CTLA-4–Ig shows tolerogenic properties through two mechanisms: (i) sequestration of B7 and thereby inhibition of CD28 signalling [11,21] and (ii) reverse signalling into dendritic cells (DC) through B7 and subsequent activation of the alternative nuclear factor (NF)κB pathway leading to expression of the immunoregulatory enzyme indoleamine 2,3 dioxygenase (IDO) [22]. Interestingly, we have shown previously that IDO contributes to SIT-induced tolerance induction in our model [23]. Recently, an early induction of IDO has been observed after venom SIT, suggesting a role for IDO in SIT-induced allergen tolerance in human patients [24].
In this study, we tested whether CTLA-4–Ig can act as an adjuvant for experimental SIT. To this aim we administered CTLA-4–Ig with SIT in an ovalbumin (OVA)-driven mouse model of asthma. We show that co-administration of CTLA-4–Ig with SIT highly enhances the SIT-induced suppression of AHR, airway eosinophilia and OVA-specific IgE levels in serum. Furthermore, we show that the effect of CTLA-4–Ig is independent of IDO, indicating that CTLA-4–Ig in our model acts by blocking the CD28-mediated T cell co-stimulatory signal.
Methods
Animals
Specific pathogen-free 6–8-week-old BALB/cByJ mice (Charles River Laboratories, L'Arbresle, France) and IDO-knock-out (IDO-KO; C.129X1(B6)-Ido1tm1Alm) on a BALB/c background (kindly provided by Dr A.L. Mellor, GA, USA), were used according to the guidelines of the institutional animal care and use committee of the University of Groningen.
Experimental allergic asthma, SIT
Experimental allergic asthma was induced and SIT was performed according to the previously described protocol [25]. Concisely, as shown in Fig. 1, mice were sensitized by intraperitoneal (i.p.) injection of 10 μg endotoxin-free/low (<5 EU/mg) OVA (Seikagaku Kogyo, Tokyo, Japan) and 2·25 mg alum (Pierce, Rockford, IL, USA) in 100 μl of pyrogen-free saline. Two weeks later, they either received 100 μg OVA in 200 μl saline per injection as OVA-SIT or 200 μl saline as placebo through three subcutaneous (s.c.) injections on alternate days. At least 10 days after the third s.c. injection mice were challenged by aerosolized OVA 1% in phosphate-buffered saline three times every third day. Airway responsiveness to increasing doses of methacholine was measured 24 h after the last challenge; thereafter, mice were dissected, bronchoalveolar lavage was performed and blood and lung samples were taken.
Figure 1.
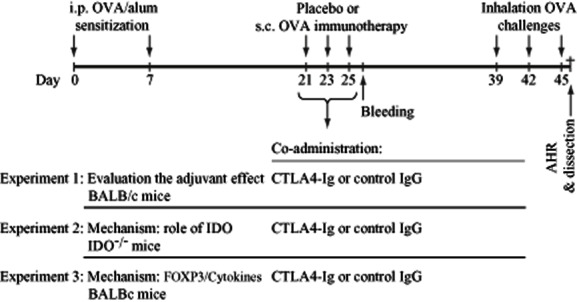
Experimental layout: In experiment 1, to evaluate that whether cytotoxic T lymphocyte antigen 4-immunoglobulin (CTLA-4–Ig) can enhance the suppressive effects of allergen-specific immunotherapy (SIT), it was combined with ovalbumin (OVA)-SIT (100 μg/mouse) in BALB/c mice. In experiment 2, CTLA-4–Ig is combined with OVA)-SIT in indoleamine 2,3 dioxygenase (IDO–/–) mice to examine whether the effect of CTLA-4–Ig is mediated by IDO. In experiment 3, to analyse the effects of CTLA-4–Ig on regulatory T cells the frequency of CD4+CD25+ forkhead box protein 3 (FoxP3)+ regulatory T cells (Treg) cells and Th2 cells were analysed 24 h after the last SIT injection and 24 h after inhalation challenges.
Administration of CTLA-4–Ig as adjuvant for SIT
Clinical grade CTLA-4–Ig (Abatacept; Bristol-Myers, Woerden, the Netherlands) was used in the experiment using IDO-KO mice. In other experiments CTLA-4–Ig was obtained as described previously [26,27]. CTLA–Ig (280 μg/injection) or control IgG (280 μg/injection) were mixed with OVA-SIT (100 μg/injection) and injected s.c.
Measurement of lung function
Airway reactivity to methacholine was evaluated by direct measurement of airway resistance in response to increasing doses of methacholine, as explained previously [23]. In brief, anaesthetized mice (by i.p. injection of ketamine 100 mg/kg; Pfizer, New York, NY, USA and medetomidine 1 mg/kg; Pfizer) were tracheotomized (20-gauge intravenous: i.v. cannula; Becton Dickinson, Alphen a/d Rijn, the Netherlands), attached to a computer-controlled small-animal ventilator (Flexivent; Scireq, Montreal, Quebec, Canada), then paralysed (i.v. injection of pancuronium bromide: Pavulon, 50 μg/kg; Merck Sharp & Dohme, Rahway, NJ, USA).Ventilation was adjusted at a breeding frequency of 300 breaths/min and a tidal volume of 10 ml/kg. Tidal volume was pressure limited at 300 mm H2O. An i.v. cannula was inserted through the jugular vein for the administration of methacholine. Airway resistance in response to i.v. methacholine (acetyl-b-methylcholine chloride; Sigma-Aldrich, Dordrecht, the Netherlands) was calculated from the pressure response to a 2-s pseudorandom pressure wave.
Determination of serum levels of specific IgE
Serum levels of OVA-specific IgE were determined by enzyme-linked immunosorbent assay (ELISA), as described previously [28], and results are expressed as experimental unit/ml.
Analyses of bronchoalveolar lavage fluid
Animals were lavaged five times through the tracheal cannulae with 1-ml aliquots of saline. Broncho-alveolar lavage (BAL) cells were pooled, counted, and cell types were identified using flow cytometry, as described elsewhere [29].
Cytokine measurement in the lung tissue
Homogenates were made from the cardiac lobe of lung, as described elsewhere [30]. The levels of interleukin (IL)-4, IL-5, IL-10, interferon (IFN)-γ and transforming growth factor (TGF)-β in the lung homogenates were determined by commercially available ELISA kits, according to the manufacturer's instructions (BD Pharmingen, Franklin Lakes, NJ, USA).
Flow cytometry and antibodies
Peridinin chlorophyll (Per-CP)-anti-CD4 (BD Pharmingen), fluorescein isothiocyanate (FITC)-anti-T1ST2 (also known as IL-33Ra) (MD-Biosciences, Zurich, Switzerland), phycoerythrin (PE)-anti-forkhead box protein 3 (FoxP3) and eFluor450-anti-CD25 (eBioscience, San Jose, CA, USA) were used for fluorescence activated cell sorting (FACS).
Statistical analysis
Data are expressed as mean ± standard error of the mean (s.e.m.). The airway response curves to methacholine were analysed by repeated measurements. All the other data were compared using the Mann–Whitney U-test corrected for multiple comparisons. A P-value of less than 0·05 was considered significant.
Results
CTLA-4–Ig strongly augments the therapeutic effects of SIT
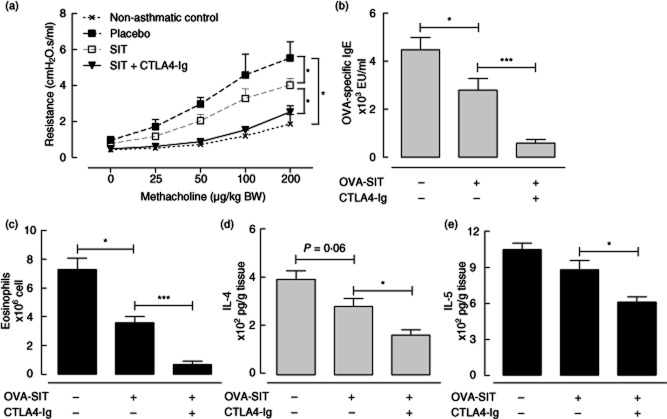
CTLA-4–Ig was combined with SIT to examine whether it augments the suppressive effects of SIT in a mouse model of allergic asthma (Fig. 1). OVA-sensitized placebo-treated mice exhibit a strong OVA-specific IgE response, airway eosinophilia and AHR upon OVA inhalation challenges (Fig. 2a–c). OVA-SIT treatment reduced the level of these three basic manifestations of allergic asthma significantly (P < 0·05, Fig. 2a–c), but did not affect significantly the levels of IL-4 and IL-5 in lung tissue (Fig. 2d,e). Co-administration of CTLA-4–Ig with SIT highly augmented the SIT-induced suppression of AHR (P < 0·05), OVA-specific IgE (P < 0·005) and airway eosinophilia (P < 0·005) compared to SIT alone. Combination of CTLA-4–Ig with SIT also induced a reduction in the levels of IL-4 (P < 0·05) and IL-5 (P < 0·05) in lung tissue, which was not observed with SIT treatment alone (Fig. 2d,e).
Figure 2.
The effects of co-administration of cytotoxic T lymphocyte antigen 4-immunoglobulin (CTLA-4–Ig) with allergen-specific immunotherapy–ovalbumin (OVA-SIT). (a) Airway reactivity to methacholine; (b) OVA-specific immunoglobulin (Ig)E in serum; (c) number of eosinophils in bronchoalveolar lavage; (d) level of interleukin (IL)-4 in lung tissue; (e) level of IL-5 in lung tissue.
CTLA-4–Ig-mediated augmentation of SIT is independent of IDO
Because CTLA-4–Ig has been shown to increase the expression of IDO and thereby induce tolerogenic effects [31], we tested whether the augmenting effect of CTLA-4–Ig on SIT in our model is dependent upon IDO activity. To this aim we compared the effects of co-administration of CTLA-4–Ig with SIT between IDO-KO and wild-type BALB/c mice. OVA-SIT alone suppressed AHR (P < 0·05), specific IgE in serum (P < 0·05) and airway eosinophilia (P < 0·05) in wild-type mice significantly (Fig. 3a,c,d). Co-administration of CTLA-4–Ig with OVA-SIT increased the suppression levels of AHR (P < 0·05), OVA-specific IgE in serum (P < 0·05) and airway eosinophilia (P < 0·05) significantly, compared to OVA-SIT alone in wild-type mice (Fig. 3a,c,d). In IDO-KO mice, OVA-SIT suppressed airway eosinophilia significantly (P < 0·05), but neither AHR nor specific OVA-specific IgE levels were suppressed (Fig. 3b–d). Surprisingly, co-administration of CTLA-4–Ig with OVA-SIT in IDO-KO mice also strongly enhanced SIT-induced suppression of the manifestation of experimental allergic asthma, resulting in significant suppression of OVA-specific IgE and AHR, which was not achieved by the OVA-SIT alone, and significantly augmented suppression of eosinophils (Fig. 3b–d). These data indicate that although SIT treatment is less efficient in IDO-KO mice, CTLA-4–Ig co-administration remains effective in enhancing the suppressive effects of the OVA-SIT.
Figure 3.
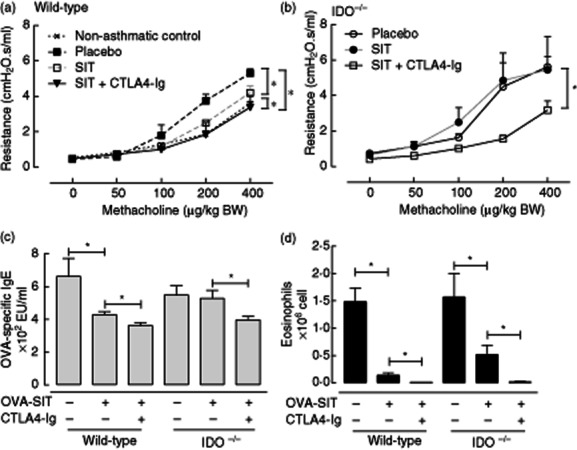
The effects of co-administration of cytotoxic T lymphocyte antigen 4-immunoglobulin (CTLA-4–Ig) with allergen-specific immunotherapy–ovalbumin (OVA-SIT) in indoleamine 2,3 dioxygenase (IDO–/–) mice. (a) Airway reactivity to methacholine in wild-type mice; (b) airway reactivity to methacholine in indoleamine 2,3 dioxygenase (IDO–/–) mice; (c) OVA-specific immunoglobulin (Ig)E in serum; (d) number of eosinophils in bronchoalveolar lavage.
Co-administration of CTLA-4–Ig with SIT reduces peripheral regulatory T (Treg) and Th2 cells
To evaluate whether administration of CTLA-4–Ig results in the induction of Treg cells, which might suppress reactivation of Th2 cells upon allergen inhalation challenge, we analysed the frequency of CD4+CD25+FoxP3+ Treg cells and CD4+T1ST2+ Th2 cells in peripheral blood 24 h after OVA-SIT. Solo treatment of OVA-SIT alters neither the frequency of CD4+CD25+FoxP3+ Treg cells nor the frequency of CD4+T1ST2+ Th2 cells (Fig. 4a,b). Surprisingly, co-administration of CTLA-4–Ig with SIT reduced significantly the percentage of both CD4+CD25+FoxP3+ Treg cells and CD4+T1ST2+ Th2 cells within the CD4+ T cell population (P < 0·05, Fig. 4a,b) compared to OVA-SIT alone. To test whether these effects of CTLA-4–Ig on Treg persist after OVA inhalation challenges, the percentage of CD4+CD25+FoxP3+ Treg cells were analysed in the blood 24 h after the last inhalation challenge. No significant differences in the percentage of CD4+CD25+FoxP3+ Treg cells were observed between the different treatment groups at this time-point (Fig. 4c).
Figure 4.
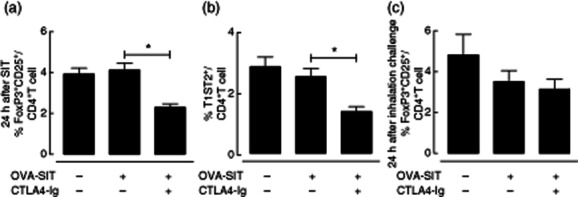
The effects of co-administration of cytotoxic T lymphocyte antigen 4-immunoglobulin (CTLA-4–Ig) with allergen-specific immunotherapy–ovalbumin (OVA-SIT) on the frequency of CD4+CD25+ forkhead box protein 3 (FoxP3)+ regulatory T cells (Treg) cells and T helper type 2 (Th2) cells in the blood. (a) Frequency of CD4+CD25+FoxP3+Treg cells in the blood 24 h after the last SIT injection; (b) frequency of CD4+CD25+FoxP3+Treg cells in the blood 24 h after the last inhalation challenge; (c) frequency of CD4+T1ST2+ [also known as interleukin (IL)-33Ra] T cells in the blood 24 h after the last SIT injection.
CTLA-4–Ig reduces IFN-γ in the lung tissue after inhalation challenge
To further dissect the mechanism of the augmenting effects of CTLA-4–Ig on SIT we tested whether these effects are mediated by enhancing the activity of lung-resident Treg cells or Th1 cells which can suppress Th2 and effector cells upon allergen inhalation challenge. To this end we measured the levels of IL-10, TGF-β and IFN-γ in the lung tissue 24 h after the last OVA inhalation challenge. Remarkably, the levels of IFN-γ in lung tissue were reduced significantly in the group receiving combined CTLA-4–Ig and OVA-SIT compared to the group receiving only OVA-SIT (P < 0·05, Fig. 5c). No differences were observed in the levels of IL-10 and TGF-β in lung tissue between the different experimental groups (Fig. 5a,b).
Figure 5.
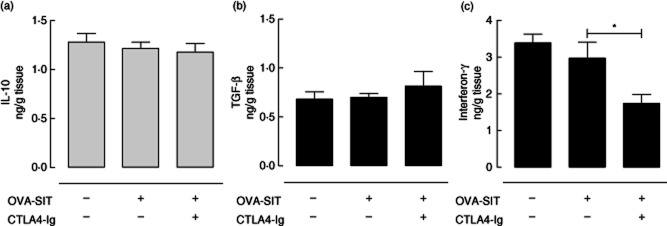
The effects of co-administration of cytotoxic T lymphocyte antigen 4-immunoglobulin (CTLA-4–Ig) with allergen-specific immunotherapy–ovalbumin (OVA-SIT) on the levels of cytokines in the lung tissue 24 h after the last inhalation challenge. (a) Interleukin (IL)-10; (b) transforming growth factor (TGF)-β; (c) interferon (IFN)-γ.
Discussion
In this study we demonstrate that CTLA-4–Ig acts as a potent adjuvant for SIT by strongly enhancing SIT-induced suppression of the manifestations of experimental allergic asthma, including the suppression of Th2 cytokine production, which was not achieved by SIT treatment alone. The adjuvant effect of CTLA-4–Ig on SIT is independent of IDO activity, indicating that it is mediated by blocking the CD28-mediated T cell co-stimulatory signal.
The tolerogenic effects of CTLA-4–Ig can be mediated by two mechanisms: (i) signalling into DC through B7 molecules, leading to activation of the non-canonical NF-κB pathway and induction of IDO [32] and (ii) blocking the CD-28-mediated co-stimulatory signal on T cells [12]. Here, we show that the adjuvant effect of CTLA-4–Ig on SIT is independent of IDO. In agreement with our observations, David et al. showed that CTLA-4–Ig inhibits DC-dependent proliferation of human T cells in vitro in an IDO-independent fashion [33]. In contrast, it has also been observed that administration of CTLA-4–Ig is tolerogenic in non-obese diabetic mice in a strictly IDO-dependent fashion [32]. However, as non-obese diabetic (NOD) mice show impaired expression of CTLA-4–Ig and develop autoinflammatory disorders spontaneously [32], these latter observations might not be relevant to our model, in which CTLA-4–Ig has been used in mice without such an impaired expression of CTLA-4. Moreover, IDO can only partially explain the CTLA-4-dependent regulation of T cell responses, as IDO-KO mice do not show the same lymphoproliferative phenotype as CTLA-4-KO mice [34].
In our model, we have demonstrated previously that SIT-induced suppression of the manifestation of allergic asthma are antigen-specific, as treatment with an unrelated antigen, keyhole limpet haemocyanin, does not induce suppression [35] and that IDO contributes to these effects [23]. In the present study, we confirm these observations using IDO-KO mice and show that the suppression of AHR and specific IgE induced by SIT treatment in wild-type mice is absent in IDO-KO mice. Apparently, loss of IDO changes the sensitivity to SIT-mediated suppression of asthmatic manifestations, but remains sensitive to the adjuvant effect of CTLA-4–Ig as CTLA-4–Ig co-administration restores the suppression of AHR and OVA-specific IgE responses in IDO-KO mice to the level observed in wild-type mice.
The adjuvant effect of CTLA-4–Ig might also utilize other tolerogenic mechanisms such as activation of members of the forkhead box O (FoxO) family of transcription factors, or induction of nitric oxide synthesis by so-called reverse signalling in DCs through B7 molecules. Interestingly, FoxO has been implicated in tolerance induction and it has been shown that CTLA-4–Ig induces tolerogenic effects by activating FoxO in DCs [32,36]. Moreover, it has been observed that induction of allograft tolerance by CTLA-4–Ig is dependent upon both IDO and nitric oxide [37]. More studies are needed to unravel the role of other pathways induced by reverse signalling in the adjuvant effect of CTLA-4–Ig towards SIT.
Although we cannot yet exclude all reverse signalling pathways, it appears very likely that CTLA-4–Ig acts by blocking CD28-mediated T cell co-stimulation during SIT treatment. Antigen presentation in the absence of proper co-stimulation leads to T cell anergy or induction of inducible regulatory T cells (iTreg cells) [38]. Because we found that CTLA-4–Ig co-administration suppresses the frequency of both CD4+CD25+FoxP3+ Treg and CD4+ST2+ Th2 cells in blood, we speculate that the augmented suppression induced by CTLA-4–Ig is mediated by a FoxP3-negative Treg cell subset or the direct induction of anergy in Th2 cells. Alternatively, the reduced percentage of CD4+CD25+FoxP3+ T cells in the blood could be due to migration of these cells to the lymph nodes, as has been seen in venom SIT in human [39].
After inhalation challenges, when SIT-induced tolerance suppresses the manifestation of experimental asthma, we observed no increased production of TGF-β or IL-10. In fact, at this time-point, we observed suppression of both Th1 (IFN-γ) and Th2 (IL-4, IL-5) cytokines in the lung tissue. This may indicate that co-administration of CTLA-4–Ig with SIT leads to an increased function of Treg cells which are capable of suppressing both Th1 and Th2 cell activity. Such an enhanced Treg cell function, however, appears to be independent of the production of the immunoregulatory cytokines TGF-β or IL-10, as their levels were not elevated. An alternative mode of action might entail suppression of Th1 and Th2 effector cells mediated by direct cell–cell contact [40]. However, we cannot exclude that these immunoregulatory cytokines are produced at an earlier time-point or are produced locally in the lung, and this is not reflected in increased levels in BAL fluid.
In conclusion, we found that CTLA-4–Ig acts as an adjuvant for SIT by highly enhancing its suppressive effects on manifestations of experimental allergic asthma. Adjuvant effects of CTLA-4–Ig appear to be mediated by blocking CD28-mediated T cell co-stimulation, as they are independent of IDO function. It is tempting to speculate that using CTLA-4–Ig might allow a safer SIT treatment regimen with lower doses of allergen.
Interestingly, CTLA-4–Ig (Abatacept) has been approved for clinical use by the US FDA and European Medicines Agency [41], and has been used safely in clinical trials as a treatment for rheumatoid arthritis [18] and to prevent transplant rejection [19]. Therefore, it is feasible to design clinical studies using CTLA-4–Ig in combination with SIT in allergic patients to achieve enhanced efficacy of the treatment.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010;65:152–167. doi: 10.1111/j.1398-9995.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 3.Frew AJ. Allergen immunotherapy. J Allergy Clin Immunol. 2010;125(2 Suppl. 2):S306–S313. doi: 10.1016/j.jaci.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 4.Reha CM, Ebru A. Specific immunotherapy is effective in the prevention of new sensitivities. Allergol Immunopathol (Madr) 2007;35:44–51. doi: 10.1157/13101337. [DOI] [PubMed] [Google Scholar]
- 5.Pajno GB, Barberio G, De LF, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31:1392–1397. doi: 10.1046/j.1365-2222.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- 6.Niggemann B, Jacobsen L, Dreborg S, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61:855–859. doi: 10.1111/j.1398-9995.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- 7.Ownby DR, Adinoff AD. The appropriate use of skin testing and allergen immunotherapy in young children. J Allergy Clin Immunol. 1994;94:662–665. doi: 10.1016/0091-6749(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 8.Casale TB. Status of immunotherapy: current and future. J Allergy Clin Immunol. 2004;113:1036–1039. doi: 10.1016/j.jaci.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Francis JN, Durham SR. Adjuvants for allergen immunotherapy: experimental results and clinical perspectives. Curr Opin Allergy Clin Immunol. 2004;4:543–548. doi: 10.1097/00130832-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 11.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 12.Carreno BM, Bennett F, Chau TA, et al. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165:1352–1356. doi: 10.4049/jimmunol.165.3.1352. [DOI] [PubMed] [Google Scholar]
- 13.Alegre ML, Fallarino F. Mechanisms of CTLA-4-Ig in tolerance induction. Curr Pharm Des. 2006;12:149–160. doi: 10.2174/138161206775193046. [DOI] [PubMed] [Google Scholar]
- 14.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigby MR, Trexler AM, Pearson TC, Larsen CP. CD28/CD154 blockade prevents autoimmune diabetes by inducing nondeletional tolerance after effector T-cell inhibition and regulatory T-cell expansion. Diabetes. 2008;57:2672–2683. doi: 10.2337/db07-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariyan C, Salvalaggio P, Fecteau S, et al. Cutting edge: transplantation tolerance through enhanced CTLA-4 expression. J Immunol. 2003;171:5673–5677. doi: 10.4049/jimmunol.171.11.5673. [DOI] [PubMed] [Google Scholar]
- 17.Guo Z, Wang J, Dong Y, et al. Long-term survival of intestinal allografts induced by costimulation blockade, busulfan and donor bone marrow infusion. Am J Transplant. 2003;3:1091–1098. doi: 10.1034/j.1600-6143.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Quiroga C, Abud-Mendoza C, Doniz-Padilla L, et al. CTLA-4-Ig therapy diminishes the frequency but enhances the function of treg cells in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:588–595. doi: 10.1007/s10875-011-9527-5. [DOI] [PubMed] [Google Scholar]
- 19.Furuzawa-Carballeda J, Lima G, Uribe-Uribe N, et al. High levels of IDO-expressing CD16+ peripheral cells, and Tregs in graft biopsies from kidney transplant recipients under belatacept treatment. Transplant Proc. 2010;42:3489–3496. doi: 10.1016/j.transproceed.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 20.Deurloo DT, van Oosterhout AJ. Role of T cell co-stimulation in murine models of allergic asthma. Clin Exp Allergy. 2004;34:17–25. doi: 10.1111/j.1365-2222.2004.01847.x. [DOI] [PubMed] [Google Scholar]
- 21.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 22.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 23.Taher YA, Piavaux BJ, Gras R, et al. Indoleamine 2,3-dioxygenase-dependent tryptophan metabolites contribute to tolerance induction during allergen immunotherapy in a mouse model. J Allergy Clin Immunol. 2008;121:983–991. doi: 10.1016/j.jaci.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy. 2010;65:1558–1565. doi: 10.1111/j.1398-9995.2010.02430.x. [DOI] [PubMed] [Google Scholar]
- 25.Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1Alpha,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-beta. J Immunol. 2008;180:5211–5221. doi: 10.4049/jimmunol.180.8.5211. [DOI] [PubMed] [Google Scholar]
- 26.Deurloo DT, van Esch BCAM, Hofstra CL, Nijkamp FP, van Oosterhout AJM. CTLA4-IgG reverses asthma manifestations in a mild but not in a more ‘severe’ ongoing murine model. Am J Respir Cell Mol Biol. 2001;25:751–760. doi: 10.1165/ajrcmb.25.6.4607. [DOI] [PubMed] [Google Scholar]
- 27.Grohmann U, Volpi C, Fallarino F, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 28.Shirinbak S, Taher YA, Maazi H, et al. Suppression of Th2-driven airway inflammation by allergen immunotherapy is independent of B cell and Ig responses in mice. J Immunol. 2010;185:3857–3865. doi: 10.4049/jimmunol.0903909. [DOI] [PubMed] [Google Scholar]
- 29.van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immunol Methods. 2004;288:111–121. doi: 10.1016/j.jim.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Maazi H, Shirinbak S, Bloksma N, Nawijn MC, van Oosterhout AJ. Iron administration reduces airway hyperreactivity and eosinophilia in a mouse model of allergic asthma. Clin Exp Immunol. 2011;166:80–86. doi: 10.1111/j.1365-2249.2011.04448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 32.Fallarino F, Bianchi R, Orabona C, et al. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med. 2004;200:1051–1062. doi: 10.1084/jem.20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis PM, Nadler SG, Stetsko DK, Suchard SJ. Abatacept modulates human dendritic cell-stimulated T-cell proliferation and effector function independent of IDO induction. Clin Immunol. 2008;126:38–47. doi: 10.1016/j.clim.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 35.Van Oosterhout AJ, Van Esch B, Hofman G, et al. Allergen immunotherapy inhibits airway eosinophilia and hyperresponsiveness associated with decreased IL-4 production by lymphocytes in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1998;19:622–628. doi: 10.1165/ajrcmb.19.4.3112m. [DOI] [PubMed] [Google Scholar]
- 36.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 37.Hill M, Zagani R, Voisine C, Usal C, Anegon I. Nitric oxide and indoleamine 2,3-dioxygenase mediate CTLA4Ig-induced survival in heart allografts in rats. Transplantation. 2007;84:1060–1063. doi: 10.1097/01.tp.0000285293.75911.56. [DOI] [PubMed] [Google Scholar]
- 38.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerstan A, Albert C, Klein D, Brocker EB, Trautmann A. Wasp venom immunotherapy induces activation and homing of CD4(+)CD25(+) forkhead box protein 3-positive regulatory T cells controlling T(H)1 responses. J Allergy Clin Immunol. 2011;127:495–501. doi: 10.1016/j.jaci.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 40.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 41.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229:307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]