Abstract
Mast cells have been implicated in the first line of defence against parasites and bacteria, but less is known about their role in anti-viral responses. Allergic diseases often exacerbate during viral infection, suggesting an increased activation of mast cells in the process. In this study we investigated human mast cell response to double-stranded RNA and viral infection. Cultured human mast cells were incubated with poly(I:C), a synthetic RNA analogue and live Sendai virus as a model of RNA parainfluenza virus infection, and analysed for their anti-viral response. Mast cells responded to intracellular poly(I:C) by inducing type 1 and type 3 interferons and TNF-α. In contrast, extracellular Toll-like receptor 3 (TLR)-3-activating poly(I:C) failed to induce such response. Infection of mast cells with live Sendai virus induced an anti-viral response similar to that of intracellular poly(I:C). Type 1, but not type 3 interferons, up-regulated the expression of melanoma differentiation–associated gene 5 (MDA-5) and retinoic acid-inducible gene-1 (RIG-1), and TLR-3, demonstrating that human mast cells do not express functional receptors for type 3 interferons. Furthermore, virus infection induced the anti-viral proteins MxA and IFIT3 in human mast cells. In conclusion, our results support the notion that mast cells can recognize an invading virus through intracellular virus sensors and produce high amounts of type 1 and type 3 interferons and the anti-viral proteins human myxovirus resistance gene A (MxA) and interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) in response to the virus infection.
Keywords: IL-29, inflammation, interferon, mast cells, virus
Introduction
The innate immune system plays a critical role in the recognition of viral infections and mounting anti-viral responses. An important type of innate effector cell is the mast cell (MC) [1]. MCs are tissue-resident cells associated closely with blood vessels, thus allowing them a sentinel role in host defence, particularly in the skin and mucosal membranes of the lungs and the gut [2]. Upon activation, mast cells produce and secrete a wide variety of biologically active mediators such as heparin, histamine, tryptase and cytokines [3]. Because MCs are in the first line to encounter invading organisms, they have been implicated in the defence against pathogens, both parasites and bacteria [1,2,4–6]. However, the role of MCs in the defence against viruses is less well characterized.
Induction of type 1 interferons (IFNs), including IFN-α and IFN-β, by viruses and other pathogens is of crucial importance for innate immunity [7]. In addition to type 1 IFNs, type 3 IFNs, interleukin (IL)-28 and IL-29 also contribute to the anti-viral response during viral infections. Production of types 1 and 3 IFNs is activated by the pattern-recognition receptors (PRRs) of the innate immune system. These receptors include membrane-bound Toll-like receptors (TLRs) and intracellular RNA helicases, such as MDA-5 (melanoma differentiation-associated gene 5) and RIG-1 (retinoic acid-inducible gene-1) [8,9]. RIG-I detects intracellular viral products such as genomic RNA to signal IFN production in virus-infected cells [10]. TLR-3 and MDA-5 are receptors for viral double-stranded RNA, which is produced during the replication cycle of many different viruses.
Viral upper respiratory tract infections can lead to exacerbation of allergic asthma [11,12], suggesting an increased activation of MCs during infections. Specific immunoglobulin (Ig)E antibodies can be produced in response to infection by pathogens, including a variety of viruses. Previous studies have also suggested a role for virus-specific IgE in airway responsiveness [13,14], but the significance of this type of activation during viral infections is less clear. MCs have been shown to respond to many viruses, including respiratory syncytial virus, human immunodeficiency virus (HIV), dengue virus and Sendai virus [5,15,16]. Moreover, MC interactions with viruses have been implicated in the pathogenesis of chronic viral myocarditis [17].
The present study demonstrates that cultured human MCs elicit a strong anti-viral response to intracellular viral dsRNA and live Sendai virus by expressing tumour necrosis factor (TNF)-α and IFNs, including IL-29. We also show that MCs possess RIG-1 and MDA-5 as sensor elements for cytosolic viral RNA, and are able to induce the anti-viral proteins human myxovirus resistance gene A (MxA) and IFN-induced protein with tetratricopeptide repeats 3 (IFIT3) in response to virus infection. MCs may therefore be involved in the modulation of the pathogenesis of allergic inflammation during viral infections.
Materials and methods
Cell culture
Mature human MCs were generated as described previously [18]. Briefly, CD34+ progenitor cells were isolated from human peripheral blood-derived buffy coats (Finnish Red Cross Blood Transfusion Service, Helsinki, Finland), following a gradient centrifugation with Ficoll-Paque (GE Healthcare, Uppsala, Sweden) and subsequent immunomagnetic separation using magnetic activated cell sorting (MACS) affinity columns (Miltenyi Biotec, Auburn, CA, USA). The isolated CD34+ cells were cultured at 37°C in Iscove's modified Dulbecco's medium (IMDM) supplemented with HEPES (25 mM), L-glutamine (2 mM), 2-mercaptoethanol (0·1 mM), 1% bovine serum albumin (BSA), recombinant human insulin (10 μg/ml) and human iron-saturated transferrin (200 μg/ml), penicillin (100 U/ml) and streptomycin (100 μg/ml), and recombinant human stem cell factor and cytokines (PeproTech, Rocky Hill, NJ, USA). Cells were used for experiments after 8–12 weeks of culture.
Cell stimulation
A synthetic RNA polymer consisting of annealing strands of inosine and cytosine, poly(I:C), was from Sigma-Aldrich (Steinheim, Germany), and used at 10 μg/ml final concentration. Mast cells were treated with poly(I:C) at 1 × 106 cells per ml in the above-described cell culture medium. To introduce poly(I:C) into MC cytoplasm, Lipofectamine 2000 was used according to the manufacturer's instructions (Life Technologies, Gaithersburg, MA, USA). Murine Sendai virus (strain Cantell) was grown as described previously [19]. To infect MCs, 150 haemagglutination units/ml of the virus was used and allowed to adsorb for 1 h, followed by a wash with phosphate-buffered saline (PBS), and resuspended in the above-described cell culture medium. The cells and cell-free culture supernatants were harvested 1–24 h after the infections, and samples were prepared for enzyme-linked immunosorbent assay (ELISA), β-hexosaminidase and quantitative polymerase chain reaction (PCR) analyses. In some cell stimulation experiments recombinant human IL-29 and IFN-β (both from PeproTech) were used at 50 ng/ml and 200 IU/ml concentrations, respectively.
Real-time PCR
Total cellular RNA was isolated with RNeasy columns and RNase-Free DNase set (Qiagen, Valencia, CA, USA), and converted to cDNA by using Moloney murine leukaemia virus (MMLV) reverse transcriptase with random primers (both from Promega, Madison, WI, USA). The cDNAs were amplified on an ABI Prism 7500 Sequence Detection System with TaqMan Gene Expression Master Mix (both from PE Applied Biosystems, Foster City, CA, USA), gene-specific primers and fluorescein amidite (FAM)-labelled fluorogenic probes for IFN-α (detecting all forms), IFN-β, IL-29, IL-6, TNF-α, MCP-1, MDA-5, MxA, IFIT3, TLR-3 and RIG-1 using the standard TaqMan PCR program. Ribosomal protein S18 was used as an endogenous control for data normalization.
Immunoassay for protein secretion
Cell culture supernatants were analysed for IL-29 secretion with ELISA according to the manufacturer's instructions (eBioscience, San Diego, CA, USA).
Western blot
MCs were lysed in a buffer containing 10 mM Tris (pH 7·4), 150 mM NaCl and 25% ethylene glycol supplemented with Complete Mini Protease Inhibitor Mixture (Roche Diagnostics, Indianapolis, IN, USA), and homogenized by ultrasound sonication. Ten milligrams of whole cell lysate was separated on a 15% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on Immobilon-P membranes (Millipore, Bedford, MA, USA) and stained with Ponceau S to confirm equal loading and transfer. Next, the membranes were blocked in PBS containing 5% non-fat milk and stained with rabbit anti-human MxA and rabbit anti-human IFIT3 antibodies (Imgenex, San Diego, CA, USA) and goat polyclonal actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as a loading control for 18 h at +4°C. After this, the membranes were incubated for 1 h at room temperature (RT) with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Dako A/S, Glostrup, Denmark) and visualized by enhanced chemiluminescence (ECL) (PerkinElmer™ Life Sciences, Zaventem, Belgium).
β-hexosaminidase release
The amount of β-hexosaminidase in cell culture supernatants was analysed by an enzymatic colorimetric assay [20] with hydrolysis of p-nitrophenyl N-acetyl-β-D-glucosamide (Sigma, St Louis, MO, USA) in 100 mM sodium citrate (pH 4·5), registering the absorbance at 405 nm.
Statistics
Comparisons were made with Student's t-test or one-way analysis of variance (anova), when appropriate. Statistical significance was considered at P < 0·05. Data are reported as mean ± standard deviation of at least three independent experiments.
Results
Cytosolic dsRNA induces strong expression of IFNs and TNF-α by mast cells
Viral infections typically involve the presence of virus replication intermediates, such as dsRNA, which are detected by receptors of the innate immunity. Multiple signalling cascades are activated during viral infection by the host cell, leading to the production of IFNs and other cytokines, and they often initiate apoptosis of the host cell. To investigate the effect of extracellular and cytoplasmic dsRNA on IFN and cytokine responses, including their kinetics in MCs, a synthetic dsRNA analogue, poly(I:C), was introduced to the cell culture medium to activate the TLR-3 pathway or transfected into cytosol to trigger MDA-5 signalling. As demonstrated in Fig. 1, extracellular poly(I:C) had no effect on MC IFN-α, IFN-β, IL-29 or TNF-α mRNA levels. However, when poly(I:C) was transfected into the MC cytosol, a pronounced time-dependent expression of these mRNAs was induced (Fig. 1).
Figure 1.
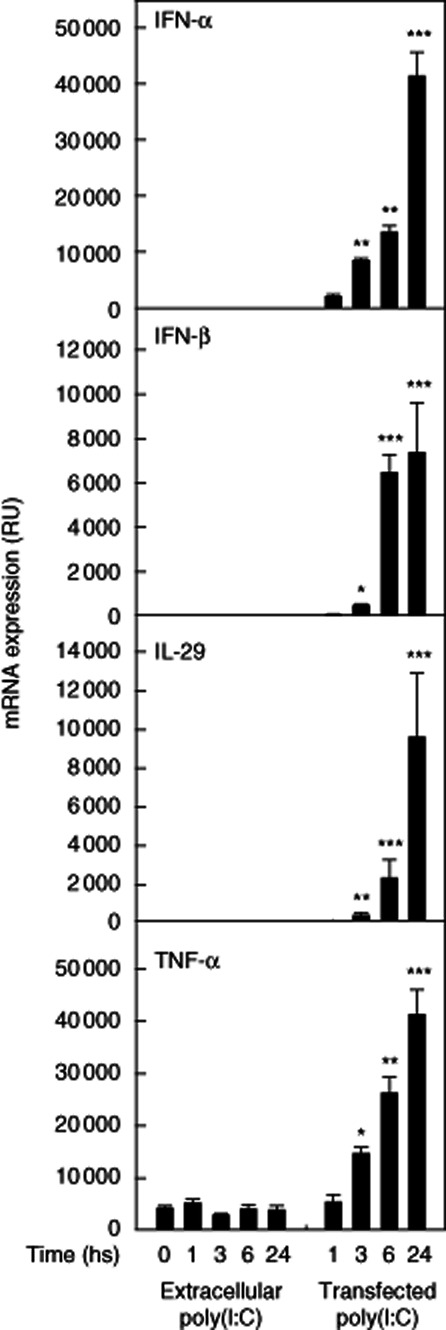
Cytosolic dsRNA induces interferon (IFN) and tumour necrosis factor (TNF)-α expression in human mast cells. Cultured human mast cells were incubated in the presence 10 μg/ml of poly(I:C) for 1–24 h. Poly(I:C) was either added to cell culture media or transfected into cytoplasm. The cells were then sedimented, and total cellular RNA was extracted and analysed for mRNA expression of IFN-α, IFN-β, interleukin (IL)-29 and TNF-α by quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR). The data show relative units (RU), i.e. fold change in gene expression normalized to 18S as an endogenous reference gene, and is calculated relative to a no-template control calibrator. Data are mean ± standard deviation from cells derived from three donors. *P < 0·05; **P < 0·01; ***P < 0·001 compared to non-treated cells at 0 h.
Virus infection induces transient expression of IFNs and TNF-α by mast cells
Upon RNA virus infection, dsRNA is generated for replication in host cell by the virus. As with the synthetic analogue of viral dsRNA, live Sendai virus also induced strong activation of IFN-α, IFN-β, IL-29 and TNF-α mRNA expression in human MCs (Fig. 2). Furthermore, the expression levels peaked 8 h after infection, whereas TNF-α mRNA expression had already increased rapidly at 4 h after infection followed by a gradual decrease. At 24 h after infection the expression levels remained low, although they were clearly elevated compared to the respective non-infected MCs (Fig. 2).
Figure 2.

Exposure to live Sendai virus induces anti-viral response in human mast cells. Cultured human mast cells were incubated in the presence of Sendai virus or left untreated for 4–20 h. Thereafter, the cells were sedimented and total cellular RNA was extracted and analysed for interferon (IFN)-α, IFN-β, interleukin (IL)-29 and tumour necrosis factor (TNF)-α mRNA expression by quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR). The data show relative units (RU) as in Fig. 1. Data are mean ± standard deviation from cells derived from three donors. **P < 0·01; ***P < 0·001 compared to non-treated cells at 0 h.
Mast cells secrete high levels of IL-29 in response to virus infection
We next studied whether Sendai virus infection of human MCs results in substantial production of the IL-29 protein. As shown in Fig. 3, MCs had already secreted IL-29 at 4 h after Sendai infection, and the levels were increased further at 8 and 24 h from the onset of infection. Furthermore, Sendai infection did not trigger a significant release of the granule component β-hexosaminidase, revealing that the MCs do not degranulate significantly in response to Sendai virus infection (Fig. 3).
Figure 3.

Human mast cells secrete high levels of interleukin (IL)-29 in response to Sendai virus exposure. Cultured human mast cells were incubated in the presence of Sendai virus or left untreated for 4–20 h. Thereafter, the cells were sedimented, the supernatants were collected and analysed for IL-29 and β-hexosaminidase release by enzyme-linked immunosorbent assay (ELISA) and a colorimetric assay, respectively. Data are mean ± standard deviation from cells derived from three donors. ***P < 0·001 compared to non-treated cells at 0 h.
Sendai virus infection of human mast cells results in the production of anti-viral proteins
Type 1 IFN stimulation and viral infection are both known to induce expression of IFN-stimulated genes (ISGs), including the anti-viral proteins MxA and IFIT3. To study whether the expression of these genes is activated and whether the respective proteins are produced in human MCs in response to Sendai infection, we performed quantitative real-time reverse transcription (RT)–PCR and Western blot analyses. As shown in Fig. 4a,b, both MxA and IFIT3 mRNAs and proteins were produced at high levels in human MCs following Sendai virus infection.
Figure 4.

Human mast cells produce the anti-viral proteins myxovirus resistance gene A (MxA) and interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) as a response to Sendai virus infection. Human mast cells were cultured in the presence of Sendai virus or left untreated for 4–20 h. Thereafter, the cells were sedimented, the supernatants were collected and analysed for MxA and IFIT3 mRNA expression by quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR) (a). The data show relative units (RU), as defined in Fig. 1. To analyse the protein levels, mast cells were cultured with Sendai virus up to 48 h, and the MxA and IFIT3 proteins were analysed by Western blot (b). Actin was used as a loading control. *P < 0·05; ***P < 0·001 compared to non-treated cells at 0 h.
Expression of MDA-5, RIG-1 and TLR-3 is up-regulated during Sendai virus infection and by type I IFN stimulation of human mast cells
TLR-3, RIG-1 and MDA-5 are PRRs for viral RNA structures, and the expression of these genes is known to be up-regulated during viral infection [10,21]. In accordance with these reports, Sendai virus infection of human MCs resulted in a robust up-regulation of MDA-5, RIG-1 and TLR-3 mRNA expression that was evident already at 4 h, and was increased further at 20 h after the onset of infection (Fig. 5a). Both type 1 and 3 IFNs are known to activate anti-viral responses in human dendritic cells [22]. We therefore treated MCs with type 1 IFN (IFN-β) and type 3 IFN (IL-29) and a combination of both. Stimulation of human MCs by IFN-β strongly up-regulated the expression of MDA-5, RIG-1 and TLR-3 (Fig. 5b). In contrast, IL-29 had no effect on the expression of these genes. In accordance with these results, IFN-β, but not IL-29, up-regulated expression of the anti-viral genes MxA and IFIT3 in human MCs (Fig. 5b). These results indicate that human MCs do not express receptors for type 3 IFNs. Finally, to gain insight into whether or not viral infection of MCs induces mainly anti-viral responses or a more general inflammatory response, MCP-1 and IL-6 mRNA expressions were analysed. No significant expression of IL-6 was observed either at baseline or after Sendai infection (data not shown). Consistent with IL-6 expression, MCP-1 expression was not induced in response to viral infection or type 1 IFN stimulation (Fig. 5a,b). Thus, the results indicate that the MC response is directed mainly towards an anti-viral rather than an inflammatory response (Fig. 5a,b).
Figure 5.

Expression of retinoic acid-inducible gene-1 (RIG-1), melanoma differentiation-associated gene 5 (MDA-5) and Toll-like receptor 3 (TLR-3), but not monocyte chemotactic protein-1 (MCP-1), is up-regulated in human mast cells during Sendai virus infection and by type 1 interferon (IFN) stimulation. Cultured human mast cells were incubated in the presence of Sendai virus or left untreated for 4–20 h (a). Alternatively, mast cells were treated for 3, 6 or 24 h with interleukin (IL)-29, IFN-β or a combination of both (b). The cells were then sedimented, and total cellular RNA was extracted and analysed for RIG-1, MDA-5, TLR-3 and MCP-1 mRNA expression by quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR). The data show relative units (RU), as defined in Fig. 1. Data are mean ± standard deviation from cells derived from three donors. **P < 0·01; ***P < 0·001 compared to non-treated cells at 0 h.
Discussion
Mast cells are resident in peripheral tissues and play a critical role in the defence against bacterial infections, but less is known about their role in anti-viral immunity. The most important pathogen-associated molecular patterns (PAMPs) during viral infections are virus replication intermediates and viral nucleic acids [23].
The results of the present study show that a strong anti-viral response can be induced in human MCs, evidenced by the production of types 1 and 3 IFNs, an up-regulated expression of the cytoplasmic virus detectors RIG-1 and MDA-5 and synthesis of the anti-viral proteins MxA and IFIT3. MCs have been shown to interact with several viruses (see [24]), including dengue [15], HIV [25], influenza [26] and Sendai [16]. Moreover, in response to viral antigens or virus infection, human MCs have been shown to elicit secretion of cytokines and chemokines, e.g. CCL4, CXCL8 and CXCL10 [15,27].
In contrast to extracellular poly(I:C) treatment, the types 1 and 3 IFN responses were observed in MCs only after the poly(I:C) was introduced into the cytosol to activate the MDA-5 pathway. Complementary to these findings, similar results were obtained when the MCs were infected with live Sendai virus. Together, these data suggest that human MCs respond to virus infection and cytoplasmic virus material through the MDA-5/RIG-1 pathway that binds cytoplasmic dsRNA generated during viral replication, rather than TLR-3, which is localized on the cell surface. Others have reported that external poly(I:C) treatment also induces selective type 1 IFN production in human HMC-1 and LAD-2 MC lines [28]. These data, including ours, suggest that primary human MCs may require signalling through the intracellular virus sensors for the full anti-viral response. Furthermore, we found that virus infection or stimulation with dsRNA analogue poly(I:C) did not induce MC degranulation and β-hexosaminidase release, which is also in line with previous reports [15,28]. It has been suggested that, despite cytokine or chemokine release, a direct interaction with cell surface receptors such as TLR-mediated activation does not lead to MC degranulation, whereas an indirect interaction such as via Fc receptor-mediated activation can induce degranulation and synthesis of newly generated mediators [5].
Type 1 IFNs comprise of multiple IFN-α isoforms and single IFN-β, and also other members [29]. The type 3 IFN family is composed of three molecules in humans: IFN-λ1 (IL-29), IFN-λ2 (IL-28A) and IFN-λ3 (IL-28B), which all signal through the same receptor complex and respond to viral infection by generating IFN-related inducible genes. IL-29 is produced by human peripheral blood mononuclear cells [30] and monocyte-derived dendritic cells [31] in response to viral infection [32] and poly(I:C) stimulation [33]. The present study shows for the first time that human MCs express IL-29 upon stimulation with intracellular poly(I:C) and in response to Sendai virus infection.
Viruses, especially the rhinovirus, are common triggers for the exacerbation of asthma [34]. Recently, human rhinovirus infection was shown to be associated with wheezing in asthmatic children [35]. In particular, the IL-29 levels were higher in wheezing than in non-wheezing children and increased with worsening of the symptoms [35]. Rhinovirus is a picornavirus, which is detected by MDA-5 in the infected cells [36]. In this study we show that human MCs possess MDA-5 and produce IL-29. Thus the activation of MCs by viruses, rhinovirus in particular, could lead to production of IL-29 by MCs, suggesting that MCs may play a significant role in the exacerbation of asthma. Furthermore, virus-induced strong activation of MCs could also play a role in autoimmune diseases, such as rheumatoid arthritis, which is often activated during viral infections [37]. Finally, local activation of human MCs by viruses could be a contributing factor in the inflammatory reaction, which takes place in the atherosclerotic arterial wall [38].
We have shown previously that the expression of PRRs MDA-5, RIG-1 and TLR-3 is highly up-regulated in human macrophages and lung epithelial cells in response to type 1 IFNs and viral infection [22,39–41]. While the present report was being prepared, Brown et al. also described expression of the RNA sensors RIG-1 and MDA-5 in human cord blood-derived MCs and in the immature human HMC-1 MC line [42]. In the present report, we show that stimulation with type 1 IFN as well as virus infection result in high expression of MDA-5, RIG-1 and TLR-3 genes in mature human MCs, and postulate that MCs can contribute to anti-viral defence during viral infections. In summary, human MCs possess the intracellular viral sensors RIG-1 and MDA-5, and via this cytoplasmic dsRNA recognition pathway induce a strong expression of types 1 and 3 IFNs and the anti-viral proteins MxA and IFIT3 in MCs to eliminate invading viruses. The intracellular viral sensors in MCs first recognize the foreign material and then trigger the anti-viral interferon responses to eliminate invading viruses. Increased levels of type 1 IFNs may then up-regulate the expression of MDA-5 and RIG-1 in human MCs, which may further augment the anti-viral response. Such MC response could play a role in systemic anti-viral defence and pathological conditions, e.g. in the exacerbation of asthma. The present findings strengthen the notion that, in various tissues, MCs defend the host by participating in the initial innate immune responses to viral infections.
Acknowledgments
Wihuri Research Institute is maintained by the Jenny and Antti Wihuri Foundation. The authors thank Mari Jokinen, Maija Atuegwu and Suvi Sokolnicki for technical assistance.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010;40:1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 4.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 5.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 6.Oksaharju A, Lappalainen J, Tuomainen AM, et al. Pro-atherogenic lung and oral pathogens induce an inflammatory response in human and mouse mast cells. J Cell Mol Med. 2009;13:103–113. doi: 10.1111/j.1582-4934.2008.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Nakhaei P, Genin P, Civas A, Hiscott J. RIG-I-like receptors: sensing and responding to RNA virus infection. Semin Immunol. 2009;21:215–222. doi: 10.1016/j.smim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Yoneyama M, Fujita T. Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem. 2007;282:15315–15318. doi: 10.1074/jbc.R700007200. [DOI] [PubMed] [Google Scholar]
- 11.Corne JM, Holgate ST. Mechanisms of virus induced exacerbations of asthma. Thorax. 1997;52:380–389. doi: 10.1136/thx.52.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston SL. Influence of viral and bacterial respiratory infections on exacerbations and symptom severity in childhood asthma. Pediatr Pulmonol Suppl. 1997;16:88–89. doi: 10.1002/ppul.1950230851. [DOI] [PubMed] [Google Scholar]
- 13.Dakhama A, Lee YM, Ohnishi H, et al. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J Allergy Clin Immunol. 2009;123:138–145. doi: 10.1016/j.jaci.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Dakhama A, Park JW, Taube C, et al. The role of virus-specific immunoglobulin E in airway hyperresponsiveness. Am J Respir Crit Care Med. 2004;170:952–959. doi: 10.1164/rccm.200311-1610OC. [DOI] [PubMed] [Google Scholar]
- 15.King CA, Anderson R, Marshall JS. Dengue virus selectively induces human mast cell chemokine production. J Virol. 2002;76:8408–8419. doi: 10.1128/JVI.76.16.8408-8419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama K. Histamine release from rat mast cells induced by Sendai virus. Nature. 1977;270:614–615. doi: 10.1038/270614a0. [DOI] [PubMed] [Google Scholar]
- 17.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lappalainen J, Lindstedt KA, Kovanen PT. A protocol for generating high numbers of mature and functional human mast cells from peripheral blood. Clin Exp Allergy. 2007;37:1404–1414. doi: 10.1111/j.1365-2222.2007.02778.x. [DOI] [PubMed] [Google Scholar]
- 19.Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol. 1999;162:7322–7329. [PubMed] [Google Scholar]
- 20.Taub D, Dastych J, Inamura N, et al. Bone marrow-derived murine mast cells migrate, but do not degranulate, in response to chemokines. J Immunol. 1995;154:2393–2402. [PubMed] [Google Scholar]
- 21.Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate Toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–355. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- 22.Österlund P, Veckman V, Siren J, et al. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol. 2005;79:9608–9617. doi: 10.1128/JVI.79.15.9608-9617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito T, Gale M., Jr Principles of intracellular viral recognition. Curr Opin Immunol. 2007;19:17–23. doi: 10.1016/j.coi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Marshall JS, King CA, McCurdy JD. Mast cell cytokine and chemokine responses to bacterial and viral infection. Curr Pharm Des. 2003;9:11–24. doi: 10.2174/1381612033392413. [DOI] [PubMed] [Google Scholar]
- 25.Taub DD, Mikovits JA, Nilsson G, et al. Alterations in mast cell function and survival following in vitro infection with human immunodeficiency viruses-1 through CXCR4. Cell Immunol. 2004;230:65–80. doi: 10.1016/j.cellimm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Grunewald SM, Hahn C, Wohlleben G, et al. Infection with influenza A virus leads to flu antigen-induced cutaneous anaphylaxis in mice. J Invest Dermatol. 2002;118:645–651. doi: 10.1046/j.1523-1747.2002.01732.x. [DOI] [PubMed] [Google Scholar]
- 27.Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–5476. doi: 10.1182/blood-2007-10-118547. [DOI] [PubMed] [Google Scholar]
- 28.Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 29.Honda K, Takaoka A, Taniguchi T. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Pekarek V, Srinivas S, Eskdale J, Gallagher G. Interferon lambda-1 (IFN-lambda1/IL-29) induces ELR(–) CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-gamma-independent manner. Genes Immun. 2007;8:177–180. doi: 10.1038/sj.gene.6364372. [DOI] [PubMed] [Google Scholar]
- 31.Megjugorac NJ, Gallagher GE, Gallagher G. Modulation of human plasmacytoid DC function by IFN-lambda1 (IL-29) J Leukoc Biol. 2009;86:1359–1363. doi: 10.1189/jlb.0509347. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler T, Matikainen S, Rönkkö E, et al. Severe acute respiratory syndrome coronavirus fails to activate cytokine-mediated innate immune responses in cultured human monocyte-derived dendritic cells. J Virol. 2005;79:13800–13805. doi: 10.1128/JVI.79.21.13800-13805.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautier G, Humbert M, Deauvieau F, et al. A type I interferon autocrine–paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller EK, Hernandez JZ, Wimmenauer V, et al. A mechanistic role for type III interferon-lambda1 in asthma exacerbations mediated by human rhinoviruses. Am J Respir Crit Care Med. 2012;185:508–516. doi: 10.1164/rccm.201108-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Miller DJ, Bowman ER, et al. MDA5 and TLR3 initiate pro-inflammatory signaling pathways leading to rhinovirus-induced airways inflammation and hyperresponsiveness. PLOS Pathog. 2011;7:e1002070. doi: 10.1371/journal.ppat.1002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9:246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb Haemost. 2011;105:S34–42. doi: 10.1160/THS10-11-0717. [DOI] [PubMed] [Google Scholar]
- 39.Siren J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005;174:1932–1937. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- 40.Tissari J, Siren J, Meri S, Julkunen I, Matikainen S. IFN-alpha enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J Immunol. 2005;174:4289–4294. doi: 10.4049/jimmunol.174.7.4289. [DOI] [PubMed] [Google Scholar]
- 41.Siren J, Sareneva T, Pirhonen J, et al. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J Gen Virol. 2004;85:2357–2364. doi: 10.1099/vir.0.80105-0. [DOI] [PubMed] [Google Scholar]
- 42.Brown MG, McAlpine SM, Huang YY, et al. RNA sensors enable human mast cell anti-viral chemokine production and IFN-mediated protection in response to antibody-enhanced dengue virus infection. PLOS ONE. 2012;7:e34055. doi: 10.1371/journal.pone.0034055. [DOI] [PMC free article] [PubMed] [Google Scholar]


