Abstract
Increased CD8+ T-cell precursor frequency (PF) precludes the requirement of CD4+ helper T (Th) cells for primary CD8+ cytotoxic T-lymphocyte (CTL) responses. However, the key questions of whether unhelped CTLs generated at higher PF are functional effectors, and whether unhelped CTLs can differentiate into functional memory cells at higher PF are unclear. In this study, ovalbumin (OVA) -pulsed dendritic cells (DCOVA) derived from C57BL/6, CD40 knockout (CD40−/−) or CD40 ligand knockout (CD40L−/−) mice were used to immunize C57BL/6, Iab−/−, CD40−/− or CD40L−/− mice, whose PF was previously increased with transfer of 1 × 106 CD8+ T cells derived from OVA-specific T-cell receptor (TCR) transgenic OTI, OTI(CD40−/−) or OTI(CD40L−/−) mice. All the immunized mice were then assessed for effector and memory CTL responses. Following DC immunization, relatively comparable CTL priming occurred without CD4+ T-cell help and Th-provided CD40/CD40L signalling. In addition, the unhelped CTLs were functional effectors capable of inducing therapeutic immunity against established OVA-expressing tumours. In contrast, the functional memory development of CTLs was severely impaired in the absence of CD4+ T-cell help and CD40/CD40L signalling. Finally, unhelped memory CTLs failed to protect mice against lethal tumour challenge. Taken together, these results demonstrate that CD4+ T-cell help at higher PF, is not required for effector CTL priming, but is required for functional memory CTL development against cancer. Our data may impact the development of novel preventive and therapeutic approaches in cancer patients with compromised CD4+ T-cell functions.
Keywords: CD4+ T-cell help, CD40/CD40 ligand signalling, increased precursor frequency, primary and memory CD8+ cytotoxic T lymphocyte responses and anti-tumour immunity
Introduction
A hallmark of cell-mediated immunity is the generation of millions of copies of effector CD8+ cytotoxic T lymphocytes (CTLs) and a small fraction of memory CTLs arising from the proliferation of extremely low numbers of naive CD8+ T-cell precursors (approximately 10 of 3000).1–3 This dynamic event is largely regulated by a multitude of factors during priming and effector phases of CTL responses.4 Among these factors, CD4+ T cells are known to mediate helper effects either indirectly by inducing maturation signals to antigen-presenting cells via CD40 ligand (CD40L) signalling,5–7 or directly by modulating CD8+ T-cell responses via cytokine (interleukin-2; IL-2) and co-stimulatory (CD40L) signals.8,9
Recently, several studies have indicated that natural differences in the size of the CD8+ T-cell precursor population for a given antigenic epitope can affect the magnitude of effector and memory CTL responses.2,10–16 Even in genetically identical twins, variations in CD8+ T-cell repertoires impact the development of autoimmune diseases, such as type 1 diabetes and multiple sclerosis.17 These observations inspired researchers to investigate whether altered precursor frequency (PF) modifies the requirement for specific regulatory factors in the development of CD8+ T-cell responses. Studies in several models have recently suggested that primary CTL responses at higher PF could occur independent of CD4+ T-cell help, even against minor-H antigen.11,12,18 We have recently demonstrated that at higher PF, the primary CTL responses derived from ovalbumin (OVA) -pulsed dendritic cell (DCOVA) stimulation occur in the absence of CD4+ T-cell help (unhelped) in Iab knockout (Iab−/−) mice.19 However, whether these unhelped CTLs are functional effectors is unknown. Although most of the studies have focused on the effect of PF on the primary CTL responses, its potential relation to the requirement of CD4+ T-help and its helper signals mediated by CD40L for functional memory CTL development at higher PF is poorly understood. In addition, the situation is further complicated by observations that show significant differences in the requirement for CD4+ T-cell help in different types of infections or immunizations.5,8,9,11,12,20,21
Hence, in the current study, by employing an adoptive transfer system and various gene knockout and transgenic mice, we investigated whether unhelped CTLs stimulated by DCOVA at higher PF are functional effectors, and whether unhelped CTLs can differentiate into functional memory cells at higher PF.
Materials and methods
Reagents, tumour cells and animals
Biotin- or fluorescence-labelled [FITC or phycoerythrin (PE)] antibodies specific for CD11c (HL3), H-2Kb (AF6-88.5), Iab (KH74), CD80 (16-10A1), CD40 (3/23), CD4 (GK1.5), CD44 (IM7), CD127 (A7R34), CD62L (MEL-14), CD69 (H1.2F3), Vβ5.1,5.2 T-cell receptor (TCR; MR9-4), interferon-γ (IFN-γ; XMG1.2) and IL-2 (JES6-5H4), streptavidin-PE-Cy5 and streptavidin-FITC were purchased from BD-Biosciences (San Diego, CA), and FITC-anti-CD8 (KT15) was obtained from Beckman Coulter (Miami, FL). The recombinant granulocyte–macrophage colony-stimulating factor, IL-2 and anti-IL-4 antibodies were obtained from R&D Systems (Minneapolis, MN). The OVAI (SIINFEKL) and the control 3LL lung carcinoma Mut1 (FEQNTAQP) peptides20 were synthesized by Multiple Peptide Systems (San Diego, CA). The OVA was obtained from Sigma-Aldrich Canada (Oakville, ON, Canada). The OVA-transfected, mouse malignant melanoma (BL6-10OVA)20 cell lines were cultured as described previously.8 The wild-type (WT) C57BL/6 (B6), OVA257–264-specific TCR-transgenic OTI, CD40−/−, CD40L−/− and Iab−/− mice on B6 background were purchased from Jackson Laboratory (Bar Harbor, ME) or bred in the University's animal resource centre (Saskatoon, SK, Canada). The OTI/CD40−/− and OTI/CD40L−/− mice were generated by backcrossing designated knockout mice with OTI mice, and tested as described previously.8 All the animal experiments were performed as per the guidelines approved by the University Committee on Animal Care and Supply, University of Saskatchewan.
Preparation of mature DCOVA
Bone-marrow-derived DCOVA from B6 mice were generated by culturing bone marrow cells for 6 days in medium containing IL-4 (20 ng/ml) and granulocyte–macrophage colony-stimulating factor (20 ng/ml) and pulsing with 0·1 mg/ml OVA overnight at 37° as described previously.20 The DCOVA generated from CD40−/− and CD40L−/− mice were referred to as (CD40−/−) DCOVA and (CD40L−/−) DCOVA, respectively.
Isolation of mononuclear leucocytes from lung
The lungs were finely minced and digested for 30 min with collagenase D (1 mg/ml) at 37°. The cell suspension was incubated with 0·01 m EDTA for 5 min and subjected to gradient centrifugation using Histopaque (Sigma, St Louis, MO). The white buffy coat at the suspension and Histopaque interface was collected for analysis.
Assessment of primary and memory CTL responses
The naive CD8+ T cells were isolated from WT OTI, (OTI) CD40−/− or (OTI) CD40L−/− mouse splenocytes by enriching T lymphocytes in nylon wool columns (C&A Scientific, Manassas, VA), and negative selection using anti-CD4 (L3T4) paramagnetic beads (DYNAL, Lake Success, NY) as previously described.8 The endogenous PF of mice was increased by intravenous transfer of the above different types of OTI CD8+ T cells (1 × 106 cells) 1 day before intravenous immunization of WT B6 or CD40−/− or CD40L−/− mice with DCOVA or (CD40−/−) DCOVA or (CD40L−/−) DCOVA (1 × 106 cells) derived from WT B6 or CD40−/− or CD40L−/− mice. Six or 90 days after DCOVA immunization, the blood samples were collected, re-stimulated with OVAI and subjected to intracellular IFN-γ staining (BD Biosciences) for assessment of primary and memory CTL responses.22 Experiments were also performed to determine the presence of IFN-γ+ CTLs in lung or spleen during effector stage or day 24 of tumour challenge.
In vivo cytotoxicity assay
The targets were prepared as described previously8 by labelling splenocytes differentially with high (3·0 μm) or low (0·6 μm) concentrations of CFSE and by pulsing with OVAI or the control Mut1 peptide, respectively, and co-injected intravenously (2 × 106 cells/mouse) at a 1 : 1 ratio into immunized or unimmunized mice. Sixteen hours later, the relative proportions of target CFSEhigh (H) and CFSElow (L) cells remaining in the spleens were analysed by flow cytometry.
Phenotypic characterization of memory CTLs
To phenotypically characterize memory CTLs, the blood samples were collected 60 days after DCOVA immunization, and stained with PE-tetramer, FITC-anti-CD44, a panel of biotin-conjugated antibodies specific for effector or memory T-cell markers and subsequently with streptavidin-PECy5. The relative expressions of surface markers were analysed in the tetramer+ CD44hi cell population.
Tumour protection studies
All the immunized mice were challenged with BL6-10 (0·5 × 106 cells/mice) on the 90th day of DCOVA immunization as shown in Table 1 and monitored for protection up to 24 days or earlier if the mice became moribund as described previously.8 The tumour grading was carried out depending on mean numbers of metastatic tumour colonies in lungs: –, no tumours; +, 1–24; ++, 25–49; +++, 50–74; ++++, 75–99; +++++, 100–250; ++++++, > 250.
Table 1.
CD4+ T helper signals are required of functional memory cytotoxic T-lymphocyte development at higher precursor frequency
| Mice1 | Adoptive transfer (106) | Immunization | Tumour-bearing mice (%) | Lung tumour scoring |
|---|---|---|---|---|
| (a) In the absence of endogenous CD4+ T cells | ||||
| WT | OTI CD8+ T | – | 8/8 (100) | +++++ |
| Iab−/− | OTI CD8+ T | – | 8/8 (100) | ++++++ |
| WT | OTI CD8+ T | DCOVA | 0/12 (0) | – |
| Iab−/− | OTI CD8+ T | DCOVA | 10/10 (100) | +++++ |
| Iab−/− | (CD40−/−) OTI CD8+ T | DCOVA | BL6-10OVA | 10/10 (100) |
| Iab−/− | (CD40L−/−) OTI CD8+ T | DCOVA | BL6-10OVA | 10/10 (100) |
| (b) In the presence of endogenous CD4+ T cells | ||||
| WT | OTI CD8+ T | DCOVA | 0/12 (0) | – |
| CD40−/− | (CD40−/−) OTI CD8+ T | (CD40−/−) DCOVA | 10/10 (100) | +++ |
| CD40L−/− | (CD40L−/−) OTI CD8+ T | (CD40L−/−) DCOVA | 10/10 (100) | ++++ |
The precursor frequency (PF) of wild-type (WT) B6, Iab−/−, CD40−/− or CD40L−/− mice were increased by transferring OTI CD8+ T cells with or without CD40 or CD40 ligand (CD40L) into mice. All the groups were immunized with ovalbumin (OVA) -pulsed dendritic cells (DCOVA) with or without CD40 or CD40L as indicated. Ninety days later, all these groups were intravenously challenged with highly metastasizing BL6-10OVA tumour cells. Twenty-four days later, the percentage of tumour-bearing mice and the grading of metastasis in lungs of tumour-bearing mice were determined. (a) The impact of CD4+ T helper signals on functional memory cytotoxic T-lymphocyte responses was measured by comparing protective immunity in WT B6 and Iab−/− mice. (b) The impact of CD40/40L signal alone on functional memory CTL responses was assessed in CD40−/− and CD40L−/− mice, which were previously transferred respectively with (CD40−/−) OTI CD8+ T and (CD40L−/−) OTI CD8+ T cells before immunizing with (CD40−/−) DCOVA and (CD40L−/−) DCOVA. The data in (a) and (b) are cumulative of two independent experiments, each with four or six mice per group.
Statistical analysis
The statistical analyses were performed using Student's t-test or Mann–Whitney U-test (Graphpad Prism-3.0, GraphPad Software Inc., San Diego, CA); *P < 0·05 and **P < 0·01.
Results
Unhelped CTLs generated from higher PF retain effector cytokine-secreting and cytotoxic functions
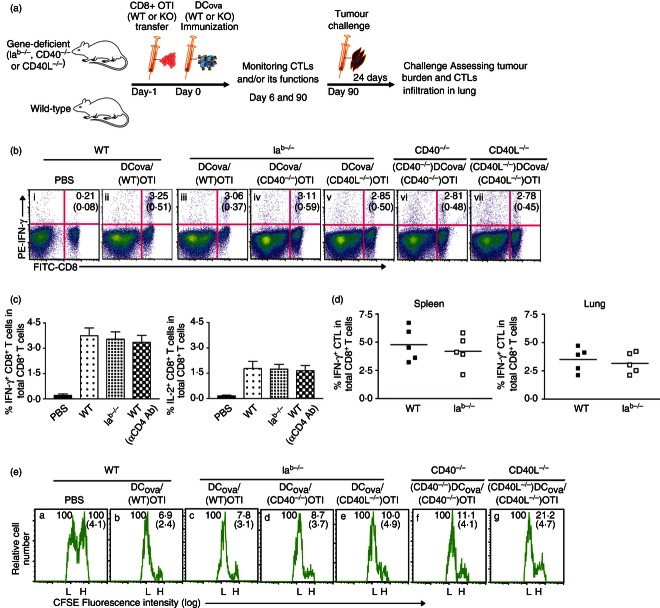
We previously demonstrated CD4+ T-cell-independent CTL responses in Iab−/− mice at higher PF.19 Here, we investigated whether the CD4+ T-cell-independent primary CTLs (unhelped CTLs) generated at higher PF retain effector cytokine (IFN-γ) secretion and cytotoxic functions using OVA-specific intracellular cytokine staining and in vivo cytotoxicity assay, respectively. Two critical experiments were performed as shown in Fig. 1(a,b): one in the absence of CD4+ T-cell help by transferring WT OTI CD8+ T cells to WT or Iab−/− mice before immunizing with DCOVA; and the other in the presence of endogenous CD4+ T cells but in the absence of CD40 or CD40L signalling by transferring (CD40−/−) OTI or (CD40L−/−) OTI CD8+ T cells to CD40−/− or CD40L−/− mice before immunizing with (CD40−/−) DCOVA or (CD40L−/−) DCOVA, respectively. The initial experiment allowed us to determine whether CD4+ T-cell help is required for priming CTL responses, whereas the latter experiment precluded the possible CD40 or CD40L signalling among interactions of CD4+ T, DC and CD8+ T cells.5,9,23–26 Consistent with earlier reports,3,19,27 following DCOVA immunization, we observed a CD4+-independent primary CTL response in Iab−/− mice with transfer of 1 × 106 naive OTI CD8+ T cells (at higher PF). This number was used throughout the experiment unless specified. At higher PF, intracellular IFN-γ staining, rather than tetramer staining, was used because it detects only antigen-experienced CTLs derived from activation of transferred OTI CD8+ T cells as well as the endogenous OVA-specific CD8+ T cells, providing a complete picture of overall CD8+ T-cell repertoire responses. In the absence of CD4+ T-cell help or CD4+ T helper-provided CD40/CD40L co-stimulation (Fig. 1b-iii–v), DCOVA immunization induced considerable proportions of IFN-γ+ effector CTLs relatively comparable to the induction in WT mice (Fig. 1b-ii). Similarly, the CD4+ T-cell-independent IFN-γ+ CTL response was also observed in CD4+ T-cell-depleted WT mice (Fig. 1c, left panel). Interestingly, both Iab−/− and CD4+ T-cell-depleted WT B6 mice also showed the presence of IL-2+ CTLs comparable to their presence in WT B6 mice (Fig. 1c, right panel). The levels of IFN-γ+ CTLs also did not vary considerably in spleens and lungs of WT B6 and Iab−/− mice (Fig. 1d). Furthermore, correlating with the levels of IFN-γ+ CTLs (Fig. 1b,d), the results from the in vivo cytotoxicity assay also showed a substantial loss of the CFSEhigh-labelled OVA-specific target cells in CD4+ T-cell-deficient mice or in mice without CD4+ T helper-provided CD40/CD40L compensatory signalling (Fig. 1e-iii–v), similar to the situation observed in the WT B6 mice (Fig. 1e-ii). These results indicate that unhelped CTLs generated from higher PF are functional effectors.
Figure 1.
Unhelped primary cytotoxic T lymphocytes (CTLs) generated from higher precursor frequency (PF) retain normal effector cytokine-secreting and cytotoxic functions. (a) A schematic protocol. Wild-type (WT) B6 or knockout mice were adoptively transferred with OTI CD8+ T cells [with or without CD40 or CD40 ligand (CDL molecules)] and intravenously immunized with ovalbumin (OVA) -pulsed dendritic cells (DCOVA) (with or without CD40 or CD40L). All the groups were monitored for CTL proliferation, survival and function during priming and memory stages. Ninety days later, all the groups were challenged with BL6-10OVA and assessed for protection. (b, c) After immunizing mice with higher PF, the blood samples were analysed by intracellular interferon-γ (IFN-γ; b, and c, left panel) or interleukin-2 (IL-2; c, right panel) staining assays 6 days later. The values in each figure or bar diagram represent mean % ± SD of IFN-γ+ or IL-2+ CTL in total CD8+ T-cell population, and are cumulative of two independent experiments with five or six mice per group. (d) The infiltration of IFN-γ+ CTLs was also determined in spleens and lungs of WT B6- and Iab−/−-immunized mice. The values represent frequencies of IFN-γ+ CTLs in the total CD8+ T-cell population, and are cumulative of two independent studies with two or three mice per group. The horizontal bars indicate means. (e) In the above immunized groups shown in (b), the proportions of CFSEhigh-OVAI-pulsed target cells lysed by effector CTL were determined in the spleen 7 days later by in vivo cytotoxicity assay. The values in each figure represent mean % ± SD of targets remaining in the spleen relative to the controls.
Unhelped CTLs generated from higher PF have therapeutic effects against early established tumour
As the success of cancer immunotherapy heavily depends upon its ability to induce protection against established tumours, we asked whether the functional CD4+ T-cell-independent primary responses observed at higher PF can be exploited to treat established tumours in DCOVA immunization protocols. We challenged two groups of mice with BL6-10OVA, as shown in Fig. 2(a). In the first group, 3 days after challenge (early tumour burden), PF was increased before immunizing with DCOVA. In the second group, similar procedures were performed on the sixth day of challenge (established tumour burden). When compared with WT B6 and Iab−/− mice with endogenous PF, day 3 tumour-bearing WT B6 and Iab−/− mice with increased PF showed nearly complete protection (Fig. 2b), which also correlated with efficient recruitment of IFN-γ+ CTLs into lungs (P < 0·01; Fig. 2c). Furthermore, even on day 6 after challenge, higher PF significantly decreased the tumour burden and incidence in both WT B6 and Iab−/− mice (Fig. 2d). In contrast, increasing PF (as high as 2 × 106 to 5 × 106 precursor cells/mouse) alone, without DCOVA immunization, failed to provide anti-tumour protection (data not shown). These results indicate that unhelped CTLs generated from higher PF have a therapeutic effect against early established tumour.
Figure 2.
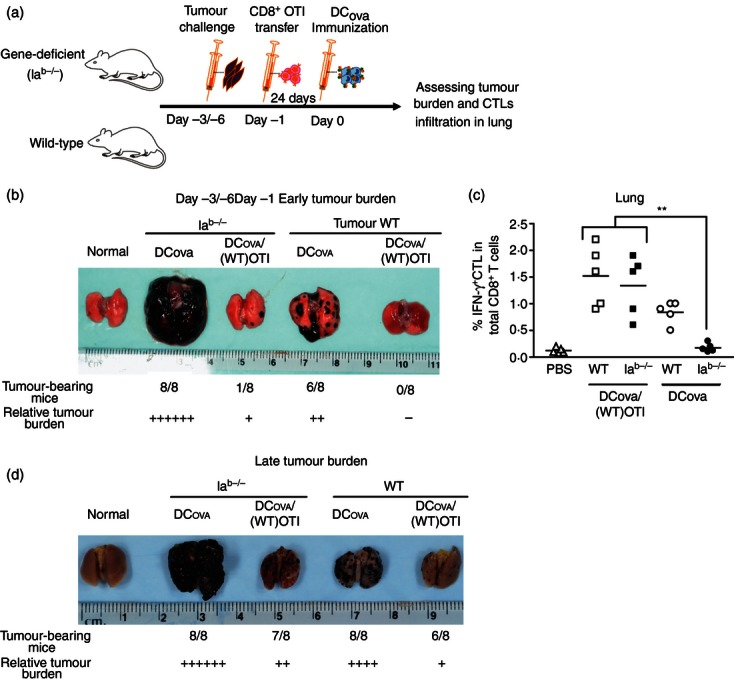
Unhelped primary cytotoxic T lymphocytes (CTLs) generated from higher precursor frequency (PF) have therapeutic effect against early established tumours. (a) An experimental design. Wild-type (WT) B6 or Iab−/− mice were first challenged with BL6-10OVA 3 days (early tumour burden) or 6 days (late tumour burden) before ovalbumin (OVA) -pulsed dendritic cells (DCOVA) immunization. One day before immunizing, the endogenous-PF of all these mice was increased by transferring OTI CD8+ T cells. Twenty-four days after challenge, all the groups were assessed for tumour protection. (b) Impact of higher PF on the efficacy of DCOVA immunization in early established tumours. Gross pathology of lungs showing relative surface tumour burden. (c) To determine whether protection is a result of effector CTL recruitment, the infiltration of interferon-γ-positive (IFN-γ+) CTLs in the lungs of some immunized mice was determined by intracellular staining assay. The values represent frequencies of IFN-γ+ CTL in the total CD8+ T-cell population, and are cumulative of two independent studies with two or three mice per group. The horizontal bars indicate means. **P < 0·01, versus Iab−/− with endogenous-PF. (d) Impact of higher PF on the efficacy of DCOVA immunization in late-established tumours. Gross pathology of lungs showing relative surface tumour burden. The results in (b) and (d) are cumulative of two independent studies with four mice per group.
CD4+ T-cell helper signals are required for generation of memory CTLs at higher PF
In various models, CD4+ T cells have been shown to affect the generation and survival of memory CTLs.8,21,28–30 Hence, the fate of helped and unhelped effector CTLs that generated at higher PF were monitored by intracellular staining assay 90 days after DCOVA immunization. When compared with WT B6 mice, mice missing CD4+ T cells or possible compensatory CD40/CD40L signalling or mice missing CD40/CD40L signalling alone showed significant decrease in the number of IFN-γ+ memory CTLs in the blood (Fig. 3a; P < 0·01). The decrease in the IFN-γ+ memory CTL population was strongly pronounced in Iab−/− compared with the CD40−/− or CD40L−/− mice lacking CD40L signalling alone. As there were considerable differences in memory CTL survival in Iab−/− mice compared with WT B6 mice, we sought to determine whether altered surface-marker expression correlates with survival rates. In both WT B6 and Iab−/− mice, the tetramer+ population expressed high levels of CD44. Furthermore, the analysis of tetramer+ CD44+ population revealed the central memory (TCM) phenotype, showing considerable expression of CD62L and IL-7Rα, but not CD69 (Fig. 3b). However, there was no drastic difference in the expression of these markers between WT B6 and Iab−/− mice, suggesting that other factors might influence the generation of helped versus unhelped memory CTLs.
Figure 3.
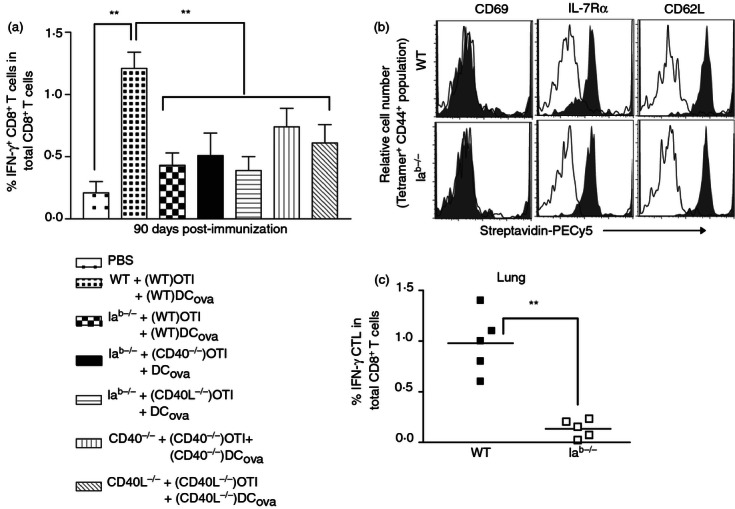
CD4+ T helper signals are required for optimal generation and functions of memory cytotoxic T-lymphocytes (CTLs) at higher precursor frequency (PF). (a) Ninety days after ovalbumin (OVA) -pulsed dendritic cells (DCOVA) immunization, the peripheral blood samples were analysed for interferon-γ-positive (IFN-γ+) memory CTL survival by intracellular staining assay. The values represent mean % ± (SD) of IFN-γ+ CTL in total CD8+ T-cell population, and are cumulative of two independent experiments with five or six mice per group. **P < 0·01, versus wild-type (WT). (b) Sixty days following immunization, the blood samples were stained for triple markers, and the expression of activation (CD69) and memory [CD62 ligand (CD62L) and interleukin 7 receptor α (IL-7Rα)] markers were analysed in tetramer+ CD44hi population (histogram grey-filled overlays). Irrelevant isotype-matched antibodies were used as controls (open black lines). (c) To determine recruitment and expansion of IFN-γ+ memory CTLs in lungs of challenged mice, the WT B6-immunized and Iab−/−-immunized mice were challenged 90 days later, and the lungs were assessed for IFN-γ+ CTLs by intracellular IFN-γ assay after 24 days. The values represent frequencies of IFN-γ+ CTLs in total CD8+ T-cell population, and are cumulative of two independent experiments with two or three mice per group. The horizontal bars indicate means. **P < 0·01, versus WT B6 mice.
CD4+ T-cell helper signals are required for functional memory CTL development at higher PF
To further analyse tumour-killing functions of memory CTLs generated at higher PF under CD4+ T helper influence, all the immunized mice were challenged with BL6-10OVA 90 days after immunization and assessed for anti-tumour protection as shown in Table 1. Interestingly, our preliminary attempts to challenge mice at 30–45 days after immunization induced anti-tumour protection irrespective of CD4+ T-cell help (data not shown), perhaps because of the prolonged maintenance of the transferred unstimulated naive29 and stimulated effector CTLs or effector memory CTLs derived from higher PF. It was previously shown that the presence of pre-existing effector CTLs can boost the responses of other naive CD8+ T cells in certain situations.27,31 Consequently, survived residual DCOVA might receive additional signals to prime remaining naive OTI CD8+ T cells, which escaped priming during the early stage, and so prolong the anti-tumour protective ability of primary CTLs. However, during the later stages, the residual naive OTI CD8+ T cells, which escape DCOVA priming, are unlikely to participate in protection against lethal tumour challenge, as we observed complete failure to protect against tumour challenge in both WT B6 and Iab−/− mice transferred with OTI CD8+ T cells alone (Table 1). Upon challenge, in contrast to WT B6, Iab−/− mice with or without possible CD40/CD40L signalling completely failed to protect against tumour challenge (Table 1, Expt a). Consistent with these results, WT B6 mice displayed much higher recruitment of IFN-γ+ CTLs in lungs, compared with Iab−/− mice (Fig. 3c; P < 0·01), consistent with the above animal study (Table 1, Expt a). Although other CD4+ T-cell signals ensued, all mice missing exclusively CD40L developed tumours (Table 1, Expt b). However, these mice had twofold to fivefold lowered tumour burden, when compared with mice with the complete absence of CD4+ T-helper signals. These results indicate that although CD4+- and CD40L-independent primary responses occur, CD40L-induced signal alone or in concert with other CD4+ T-helper signals appears to be essential for programming of memory CTLs for both development and anti-tumour functions, even at higher PF.
Discussion
It has been demonstrated that altered CD8+ T-cell PF could affect effector and memory CTL responses.2,10–16 We have recently demonstrated that, at higher PF, primary CTL responses derived from DCOVA stimulation occur in the absence of CD4+ T-cell help in Iab−/− mice.19 However, whether unhelped CTLs stimulated by DCOVA at higher PF are functional effectors, and whether unhelped CTLs can differentiate into functional memory cells is still unclear. In this study, we reported that it is possible to achieve the generation of CD4+ T-cell-independent primary CTLs that retain normal phenotype and cytotoxic functions by increasing PF in the DC immunization protocol, which is consistent with some previous observations in other models.12,27 Mintern et al.27 showed that CD40L-deficient CD8+ T cells proliferated well and exhibited cytotoxicity when stimulated with antigen-coated splenocytes. Similarly, functional effector CTLs were observed in a tissue transplantation model, even when CD40L or CD28 co-stimulation was blocked using specific antibodies.11,12 In the present study, we prevented any compensatory mechanisms that may occur in the absence of a single co-stimulatory molecule among DC, CD4+ or CD8+ T cells5,9,23–26 by using gene knockout mice and their immune cells, and confirmed that, even in the complete absence of CD40L co-stimulation, relatively comparable primary CTL responses occur at higher PF. Although not dramatic, it appears that CD40/CD40L signalling also slightly affects acute CTL responses (Fig. 1b). This is possibly because of using DC lacking CD40, the co-stimulatory molecule important in optimal CTL programming by antigen-presenting cells.23 These unhelped CTLs displayed in vivo killing activity against OVA-pulsed target cells as well as a therapeutic effect against 3-day and 6-day established tumours, suggesting that it is possible to considerably enhance anti-tumour therapeutic efficacy of DCOVA immunization by increasing PF even in the absence of CD4+ T-cell help.
To date, factors that govern CTL memory development at higher PF have not been established. We demonstrated here that CD4+ T helper signals, although dispensable for primary CTL responses, are indispensable for the functional memory CTL development even at increased PF. This phenomenon of differential CD4+ T helper requirements for primary and memory CTL responses is also frequently observed in many acute infections.29,30,32–34 Primary CTL responses to live microbial diseases, such as intracellular bacterial and viral infections, occur independent of CD4+ T-cell help. It was suggested that live microbes directly license DCs by providing ‘danger or inflammatory’ signals such as toll-like receptor signalling. Yet, in these circumstances, although dispensable for primary CTL responses, CD4+ T-cell help has been frequently implicated for subsequent memory CTL responses. From these observations, it is apparent that CD4+ T-cell help is indispensable for functional memory CTL responses across a range of infectious or immunization conditions. At higher PF, what determines the generation of functional primary CTLs independent of CD4+ T-cell signals needs further investigation. At least one explanation could be the attainment of threshold levels of CD8+ T-cell-secreted IL-2 signals as we observed considerable proportion of IL-2-secreting primary CTLs at higher PF without CD4+ T help.
The CD4+ T cells contribute to memory CTL generation either indirectly by modulating antigen-presenting cells,5–7,21 or directly by modulating cognate CD8+ T cells8,9,20,35 via CD40L signalling. Consistent with these data, even at higher PF, we observed poor memory CTL responses with an inability to provide anti-tumour protection in the absence of CD40L signalling, although endogenous CD4+ T cells retaining other helper factors were ensured. Nevertheless, the lack of CD40L signal alone resulted in a relatively low tumour burden, compared the situation with the complete absence of CD4+ T cells, suggesting that other CD4+ T helper factors are likely to contribute to memory generation. Whether the reduced protection in Iab−/− mice or in mice without CD40/40L signalling alone is the result of an actual decrease of CTL survival rate as shown previously29 or the loss of memory functions needs further investigation.
DC-based and T-cell-based vaccines have been widely applied to induce therapeutic anti-tumour immunity.36–43 Unfortunately, they often fail in the treatment of malignancies because of inefficient CTL responses, resulting from tolerance induction, inhibitory receptor expression, lower reactive PF and lack of antigen immunodominance.3,44,45 The present results are particularly relevant in the development of effective DC-based vaccines against established malignant tumours. For example, the frequency of pre-existing tumour-specific CTL precursors in mice represents a critical determinant of the quality of anti-tumour responses, in accordance with the already recognized role that initial T-cell numbers have in the functional immune responses against pathogens.14,46 Their cumulative frequency was found to be significantly higher in cancer patients and varies widely in relation to various tumour-antigen peptides.10 The detection of these CTLs in cancer patients is currently applied for evaluating tumour antigens in vaccination.47,48 Recent evidence suggests that antigen presentation by DCs and PF levels also determine the immunodominance, and thereby, protection against foreign pathogens.49,50 In support of this, we also observed an increase in the therapeutic anti-tumour efficacy of DCOVA immunization for prolonged periods in the absence of CD4+ T-cell help, suggesting that sustained CD4+-independent effector functions can be achieved by increasing CTL PF and by immunization with mature DCs.
Taken together, we demonstrate that CD4 T-cell help is not required for effector CTL priming but is required for functional memory CTL development against cancer at higher CD8+ T-cell precursor frequency. Our data may therefore impact the development of novel immune-based therapies and prophylaxis in cancer patients with compromised CD4+ T-cell functions.
Acknowledgments
This study was supported by grants from CIHR and a Bridge Fund of University of Saskatchewan. Dr Umeshappa was supported by the prestigious Dean's scholarship from the University of Saskatchewan and Vanier Canada Graduate Scholarship from the CIHR. The authors wish to thank M. Boyd for help with the flow cytometric analysis.
Disclosures
The authors declare no competing financial interests.
References
- 1.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–64. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–69. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzuto GA, Merghoub T, Hirschhorn-Cymerman D, et al. Self-antigen-specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. J Exp Med. 2009;206:849–66. doi: 10.1084/jem.20081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umeshappa CS, Xiang J. Regulators of T-cell memory generation: TCR signals versus CD4+ help? Immunol Cell Biol. 2011;89:578–80. doi: 10.1038/icb.2011.28. [DOI] [PubMed] [Google Scholar]
- 5.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 6.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 7.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 8.Umeshappa CS, Huang H, Xie Y, Wei Y, Mulligan SJ, Deng Y, Xiang J. CD4+ Th-APC with acquired peptide/MHC class I and II complexes stimulate type 1 helper CD4+ and central memory CD8+ T cell responses. J Immunol. 2009;182:193–206. doi: 10.4049/jimmunol.182.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–3. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 10.Karanikas V, Zamanakou M, Soukou F, Kerenidi T, Gourgoulianis KI, Germenis AE. Naturally occurring tumor-specific CD8+ T-cell precursors in individuals with and without cancer. Immunol Cell Biol. 2011;88:575–85. doi: 10.1038/icb.2010.8. [DOI] [PubMed] [Google Scholar]
- 11.Ford ML, Wagener ME, Hanna SS, Pearson TC, Kirk AD, Larsen CP. A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection. J Immunol. 2008;180:7203–11. doi: 10.4049/jimmunol.180.11.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marzo AL, Sowell RT, Scott B. The role of precursor frequency in the differentiation of memory T cells: memory by numbers. Adv Exp Med Biol. 2010;684:69–78. doi: 10.1007/978-1-4419-6451-9_6. [DOI] [PubMed] [Google Scholar]
- 14.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–9. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jooss J, Eiermann TH, Wagner H, Kabelitz D. Interleukin 2 production by alloantigen-stimulated CD4+ and CD8+ human T cell subsets: frequency of HLA class I or class II-reactive precursor cells and clonal specificity of activated T cells. Immunobiology. 1989;179:366–81. doi: 10.1016/s0171-2985(89)80042-7. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg H, Greuter M, Kraal G, den Haan JM. Different mechanisms regulate CD4+ T cell independent induction of oral and nasal tolerance of CD8+ T cells. Immunobiology. 2010;215:163–71. doi: 10.1016/j.imbio.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Somma P, Ristori G, Battistini L, et al. Characterization of CD8+ T cell repertoire in identical twins discordant and concordant for multiple sclerosis. J Leukoc Biol. 2007;81:696–710. doi: 10.1189/jlb.0906584. [DOI] [PubMed] [Google Scholar]
- 18.Floyd TL, Orr SB, Coley SM, Hanna SS, Wagener ME, Kirk AD, Larsen CP, Ford ML. High-frequency alloreactive T cells augment effector function of low-frequency CD8+ T-cell responses under CD28/CD154 blockade. Transplantation. 2010;89:1208–17. doi: 10.1097/TP.0b013e3181df53dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye Z, Ahmed KA, Huang J, Xie Y, Munegowda MA, Xiang J. T cell precursor frequency differentially affects CTL responses under different immune conditions. Biochem Biophys Res Commun. 2008;367:427–34. doi: 10.1016/j.bbrc.2007.12.149. [DOI] [PubMed] [Google Scholar]
- 20.Xiang J, Huang H, Liu Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol. 2005;174:7497–505. doi: 10.4049/jimmunol.174.12.7497. [DOI] [PubMed] [Google Scholar]
- 21.Wiesel M, Joller N, Ehlert AK, Crouse J, Sporri R, Bachmann MF, Oxenius A. Th cells act via two synergistic pathways to promote antiviral CD8+ T cell responses. J Immunol. 2010;185:5188–97. doi: 10.4049/jimmunol.1001990. [DOI] [PubMed] [Google Scholar]
- 22.Shi M, Hao S, Chan T, Xiang J. CD4+ T cells stimulate memory CD8+ T cell expansion via acquired pMHC I complexes and costimulatory molecules, and IL-2 secretion. J Leukoc Biol. 2006;80:1354–63. doi: 10.1189/jlb.0506321. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez MG, Shen L, Rock KL. CD40 on APCs is needed for optimal programming, maintenance, and recall of CD8+ T cell memory even in the absence of CD4+ T cell help. J Immunol. 2008;180:4382–90. doi: 10.4049/jimmunol.180.7.4382. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S, Zhan Y, Sutherland RM, et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity. 2009;30:218–27. doi: 10.1016/j.immuni.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murugaiyan G, Agrawal R, Mishra GC, Mitra D, Saha B. Differential CD40/CD40L expression results in counteracting antitumor immune responses. J Immunol. 2007;178:2047–55. doi: 10.4049/jimmunol.178.4.2047. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez MG, Shen L, Rock KL. CD40–CD40 ligand interaction between dendritic cells and CD8+ T cells is needed to stimulate maximal T cell responses in the absence of CD4+ T cell help. J Immunol. 2007;178:2844–52. doi: 10.4049/jimmunol.178.5.2844. [DOI] [PubMed] [Google Scholar]
- 27.Mintern JD, Davey GM, Belz GT, Carbone FR, Heath WR. Cutting edge: precursor frequency affects the helper dependence of cytotoxic T cells. J Immunol. 2002;168:977–80. doi: 10.4049/jimmunol.168.3.977. [DOI] [PubMed] [Google Scholar]
- 28.Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179:8243–51. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- 29.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–33. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol. 2011;186:2729–38. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherritt MA, Gardner J, Elliott SL, Schmidt C, Purdie D, Deliyannis G, Heath WR, Suhrbier A. Effect of pre-existing cytotoxic T lymphocytes on therapeutic vaccines. Eur J Immunol. 2000;30:671–7. doi: 10.1002/1521-4141(200002)30:2<671::AID-IMMU671>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Rahemtulla A, Fung-Leung WP, Schilham MW, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–4. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 33.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 34.Jiao L, Han X, Wang S, Fan Y, Yang M, Qiu H, Yang X. Imprinted DC mediate the immune-educating effect of early-life microbial exposure. Eur J Immunol. 2009;39:469–80. doi: 10.1002/eji.200838367. [DOI] [PubMed] [Google Scholar]
- 35.Rapetti L, Meunier S, Pontoux C, Tanchot C. CD4 help regulates expression of crucial genes involved in CD8 T cell memory and sensitivity to regulatory elements. J Immunol. 2008;181:299–308. doi: 10.4049/jimmunol.181.1.299. [DOI] [PubMed] [Google Scholar]
- 36.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas-Kaskel AK, Waller CF, Schultze-Seemann W, Veelken H. Immunotherapy with dendritic cells for prostate cancer. Int J Cancer. 2007;121:467–73. doi: 10.1002/ijc.22859. [DOI] [PubMed] [Google Scholar]
- 38.Jahnisch H, Fussel S, Kiessling A, et al. Dendritic cell-based immunotherapy for prostate cancer. Clin Dev Immunol. 2010;2010:517493. doi: 10.1155/2010/517493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiessling A, Fussel S, Wehner R, Bachmann M, Wirth MP, Rieber EP, Schmitz M. Advances in specific immunotherapy for prostate cancer. Eur Urol. 2008;53:694–708. doi: 10.1016/j.eururo.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 40.Dokouhaki P, Han M, Joe B, Li M, Johnston MR, Tsao MS, Zhang L. Adoptive immunotherapy of cancer using ex vivo expanded human γδ T cells: a new approach. Cancer Lett. 2010;297:126–36. doi: 10.1016/j.canlet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Anraku M, Tagawa T, Wu L, Yun Z, Keshavjee S, Zhang L, Johnston MR, de Perrot M. Synergistic antitumor effects of regulatory T cell blockade combined with pemetrexed in murine malignant mesothelioma. J Immunol. 2010;185:956–66. doi: 10.4049/jimmunol.0900437. [DOI] [PubMed] [Google Scholar]
- 42.Maas RA, Becker MJ, Weimar IS, De Nooy JC, Dullens HF, Den Otter WD. Transfer of tumor immunity by both CD4+ and CD8+ tumor infiltrating T lymphocytes activated in vivo by IL-2 therapy of tumor bearing mice. Immunobiology. 1993;188:281–92. doi: 10.1016/s0171-2985(11)80236-6. [DOI] [PubMed] [Google Scholar]
- 43.Soruri A, Fayyazi A, Gieseler R, Schlott T, Runger TM, Neumann C, Peters JH. Specific autologous anti-melanoma T cell response in vitro using monocyte-derived dendritic cells. Immunobiology. 1998;198:527–38. doi: 10.1016/S0171-2985(98)80076-4. [DOI] [PubMed] [Google Scholar]
- 44.Tatum AM, Mylin LM, Bender SJ, Fischer MA, Vigliotti BA, Tevethia MJ, Tevethia SS, Schell TD. CD8+ T cells targeting a single immunodominant epitope are sufficient for elimination of established SV40 T antigen-induced brain tumors. J Immunol. 2008;181:4406–17. doi: 10.4049/jimmunol.181.6.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umeshappa CS, Xiang J. Tumor-derived HLA-G1 acquisition by monocytes through trogocytosis: possible functional consequences. Cell Mol Life Sci. 2010;67:4107–8. doi: 10.1007/s00018-010-0553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8+ T cell response to infection. Immunity. 2007;26:827–41. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato Y, Shomura H, Maeda Y, et al. Immunological evaluation of peptide vaccination for patients with gastric cancer based on pre-existing cellular response to peptide. Cancer Sci. 2003;94:802–8. doi: 10.1111/j.1349-7006.2003.tb01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt J, Neumann-Haefelin C, Altay T, Gostick E, Price DA, Lohmann V, Blum HE, Thimme R. Immunodominance of HLA-A2-restricted hepatitis C virus-specific CD8+ T cell responses is linked to naive-precursor frequency. J Virol. 2011;85:5232–6. doi: 10.1128/JVI.00093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotturi MF, Scott I, Wolfe T, et al. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 2008;181:2124–33. doi: 10.4049/jimmunol.181.3.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]