Abstract
Compared with adults, the circulating Vγ2Vδ2 T-cell population in cord blood is present at low levels and does not show the strong bias for Vγ2-Jγ1.2 rearrangements. These features may be a result of limited exposure to stimulatory phosphoantigens, lack of T-cell-derived interleukin-2 (IL-2) or both. In cord blood mononuclear cell cultures, a single round of stimulation, using aminobisphosphonates to elevate phosphoantigen levels, resulted in expansion of adult-like Vγ2 chains and accumulation of memory cells with cytotoxic potential. Selection was similar using IL-2 or myeloid-derived IL-15. The Vγ2Vδ2 T cells present in neonates are capable of generating potent immune responses even when relying on IL-15.
Keywords: gammadelta, interleukin-15, neonatal, repertoire
Introduction
Peripheral blood Vγ2Vδ2 T cells (also called Vδ2 cells or Vγ9Vδ2 in an alternative nomenclature) provide rapid, innate-like responses to bacterial (i.e. Mycobacterium tuberculosis), protozoal (i.e. Plasmodium falciparum) and viral infections in humans. These lymphocytes recognize low-molecular-weight intermediates of isoprenoid biosynthesis, termed phosphoantigens (PAg);1–3 PAg are produced by microorganisms or eukaryotic cells and recognition is MHC-unrestricted.4,5 Vδ2 cell proliferation is also triggered by bisphosphonates, inhibitors of isoprenoid biosynthesis6 that cause the accumulation of phosphoantigens to stimulatory levels.7
The subset of γδ cells most reactive to PAg expresses the Vδ2 chain and the Vγ2 gene rearranged with the Jγ1.2 segment (also named JγP).8 In healthy adults 1–5% of all lymphocytes have a Vγ2Vδ2 T-cell receptor (TCR) and up to 70% of these cells express the Jγ1.2 segment.9,10 Among thymocytes11 or in neonates (exemplified by cord blood),12,13 Vγ2Vδ2 cells are present only at low levels and most do not have the Jγ1.2 segment.10,11,14 Vγ2Vδ2 T-cell levels in blood increase13 shortly after birth, mostly as the result of selective growth of Vγ2-Jγ1.2+ cells10 driven by ubiquitous PAg.
Optimal Vγ2Vδ2 T-cell responses to PAg also depend on cytokines;15,16 most studies use the T-cell-derived interleukin-2 (IL-2). This convention contributed to a view that γδ T cells require concomitant CD4+ T-cell responses to supply IL-2.16–19 However, IL-15, a common γ-chain cytokine produced by activated monocyte/macrophages20,21 or dendritic cells,22,23 and found during mycobacterial infection,24–26 sustains proliferation of adult Vδ2 T cells27 and may be required for Vδ2-cell responses to mycobacteria.28,29 The cytokine IL-15 is important for γδ T-cell homeostasis30 and augments responses to Plasmodium in the absence of CD4 T cells,18 alone or in combination with low levels of IL-2.31 Whether IL-15 and IL-2 have distinct roles in γδ T-cell biology is largely unknown. However, the myeloid-derived IL-15 may be important for Vγ2Vδ2-cell responses in neonates, where the CD4 T-cell population, responsible for producing IL-2, is still immature.
We focused on responses in cord blood cells because of increasing evidence that Vδ2 cells might contribute to strengthen resistance to infections in infants by responding directly to pathogens and improving innate or adaptive immunity. The neonatal immune system is immature compared with the adult counterpart.32 Defects in TCR-αβ cells (especially CD4+ T cells),33–37 impaired dendritic cell function38–41 and high levels of regulatory T cells can blunt adaptive immunity.42 Neonatal Vδ2 T cells proliferate and produce cytokines in response to stimuli used to trigger adult cells,43–45 though less efficiently in some experimental conditions.12,46,47 Vδ2 T cells are a significant component of immune responses to the tuberculosis vaccine bacillus Calmette–Guérin (BCG),46,48,49 which is administered routinely to neonates in sub-Saharan Africa, and they are probably important for infant immune responses to P. falciparum.
In this study we tested whether IL-15 can replace IL-2 during neonatal Vδ2 T-cell proliferative and functional responses. A single round of aminobisphosphonate stimulation in vitro was sufficient for selecting a Vγ2 repertoire similar to that found in adults, and IL-15 efficiently substituted for IL-2 in achieving Vγ2 repertoire maturation. When comparing IL-15 and IL-2 effects on neonatal Vδ2 T-cell functions, IL-15 was best for prolonging survival of activated cells with cytotoxic potential. Our study suggests that neonatal Vδ2 T-cells can respond to stimulation efficiently relying either on IL-2 or IL-15.
Materials and methods
Cord blood collection and cord blood mononuclear cell isolation
Women were enrolled at the maternity division of the ‘Hôpital Central de Yaoundé’, before onset of active labour, after signing an informed consent form. The study was approved by the Ethics Committee of the Centre International de Référence Chantal Biya, Yaoundé, and by the Division for Health Operations Research (Division de la Recherche Opérationnelle en Santé, DROS) in Cameroon. Only HIV-negative/P. falciparum-negative women with full-term pregnancies were included in this study.
Cord blood was collected by puncturing the umbilical vein soon after uncomplicated, full-term delivery. Umbilical cord blood (20–30 ml) was collected using a sterile syringe and transferred quickly into 50-ml collection tubes containing anticoagulant. Cord blood was diluted with RPMI-1640 and layered over Ficoll–Hypaque density gradient to isolate cord blood mononuclear cells (CBMC). Viability was assessed by trypan blue exclusion. A fraction of CBMC was reserved for cell culture and 0·5 × 107 to 1 × 107 were lysed for RNA extraction; remaining cells were frozen at 1 × 107 CBMC/ml, in 90% fetal bovine serum (FBS), 10% DMSO freezing medium.
Some CBMC specimens were purchased from Allcells (Emeryville, CA), and stored at −130° before use.
Cell culture
The CBMC were resuspended at 106 cells/ml in complete medium, RPMI-1640 supplemented with 10% FBS (Gibco, Life Technologies, Carlsbad, CA), 2 mm l-glutamine, 100 IU/ml penicillin–streptomycin (Lonza, Walkersville, MD). To expand Vγ2Vδ2 T lymphocytes, cultures were treated with 4-amino-1-hydroxy-1-phosphonobutyl phosphonic acid [alendronate sodium trihydrate (ALN); Sigma, St Louis, MO] at 5 μm, in the presence of 100 IU/ml human recombinant IL-2 (Tecin, NHI reagent program; NIH, Bethesda, MD) or 10 ng/ml human recombinant IL-15 (ThermoScientific, Rockford, IL). Medium with IL-2 or IL-15 represented, respectively, the control treatment. Cells were incubated for 14 days at 37° with 5% CO2 and fresh cytokines were added every 3 days; fresh medium was added on days 7 and 10. After 14 days, cells were harvested and viability was assessed by trypan blue exclusion. A fraction of the lymphocytes was used to determine Vδ2 T-cell frequency and phenotype, 5 × 106 to 10 × 106 cells were lysed for RNA extraction and stored as cells lysates at −20°.
For some specimens, cell culture was prolonged up to day 28 in resting conditions. From day 14, cytokines were added every 3 days (10 U/ml IL-2 or 1 ng/ml IL-15), and medium was replaced (not added) when necessary. On days 21 and 28, cell viability was assessed by trypan blue exclusion.
Vδ2 T-cell frequency, phenotype and degranulation/cytokine production (on selected samples only) were assessed by flow cytometry at different time-points during cell culture (see below). Stimulation index, defined as (no. of Vγ2Vδ2 T cells after ALN + cytokine)/(no. Vγ2Vδ2 T cells after cytokine alone), was calculated 14 days after stimulation.
Flow cytometry
Ex vivo CBMC or expanded Vδ2 lymphocytes were resuspended in PBS/10% FBS and stained at 4° with directly conjugated monoclonal antibodies. After 15 min, cells were washed with PBS/10% FBS and resuspended in PBS/10% FBS with 1% paraformaldehyde. Then, 5 × 104 lymphocytes (gated on the basis of forward and side scatter profiles) were collected for each sample on a FACSCalibur (BD Biosciences, San Jose, CA) and results were analysed with Flowjo software (Tristar, San Jose, CA).
The expression of Ki67 was analysed on day 14 by intracellular staining, using anti-human Ki67-phycoerythrin (clone B56; BD Biosciences) as recommended by the manufacturer. The appropriate isotype control (MOPC-21, mouse IgG1, k) was also purchased from BD Biosciences and 5 × 104 lymphocytes were collected for each sample.
To evaluate perforin and granzyme B production, on days 16 and 28 intracellular staining was performed as follows. After staining of surface markers, cells were permeabilized by incubating for 20 min at 4° with fixation/permeabilization solution (BD Biosciences). Cells were then washed twice with 1× Perm/wash buffer (BD Biosciences). Anti-human perforin-peridinin chlorophyll protein-Cy5.5 (clone dG9; Biolegend, San Diego, CA) and anti-human granzyme B-phycoerythrin (Clone GB12; Invitrogen, Camarillo, CA) were added for 30 min at 4°. Finally, cells were washed once with Perm/wash buffer and 5 × 104 lymphocytes were collected for each sample.
The following monoclonal antibodies, all purchased from BD/Pharmingen (San Jose, CA), were used for four-colour staining: anti-Vδ2 (clone B6), anti-Vγ9 (clone B3), anti-CD3 (clone SP34-2 and UCHT1), anti-CD25 (clone M-A251), anti-CD45-RA (clone HI100), anti-NKG2D (clone 1D11), anti-CD16 (clone 3G8), anti-CD56 (clone B159). Anti-CD56 (clone N901) and anti-NKG2A (clone Z199) were purchased from Beckman-Coulter (Indianapolis, IN). Anti-CD27 (clone O323) was purchased from eBioscience (San Diego, CA), and anti-Vδ1 (clone TS8.2) from Thermo Scientific (Rockford, IL).
Granule mobilization assay
After 16 days in culture, CBMC were resuspended at 2 × 106 cells/ml in fresh complete medium and re-stimulated in 96-well plate pre-coated with anti-TCR-γδ (clone B1.1; eBioscience). Plates were coated overnight at 4° with anti-TCR-γδ (diluted 1 : 500 in PBS, 50 μl/well) or isotype control antibody at the same concentration. The CBMC were plated in triplicate (100 μl/well) with anti-CD107a-FITC (clone H4A3, 5 μl/well) and GolgiPlug (1 μg/ml; BD Biosciences). After 5 hr of incubation, cells were collected, washed once with cold PBS, and stained for membrane markers as well as tumour necrosis factor (TNF-α) production (explained above). Anti-CD107a-FITC, anti-TNF-α-allophycocyanin (clone MAb11), anti CD27-peridinin-Cy7 (clone M-T271) and anti-Vδ2-phycoerythrin (clone B6) were purchased from BD Biosciences.
RNA extraction, RT-PCR, PCR
Total RNA was extracted from 1 × 106 to 10 × 106 cells using the RNeasy mini Kit (Qiagen, Valencia, CA), as described by the manufacturer. Total RNA (1 μg) was converted into cDNA using the reverse transcription system kit (Promega, Madison, WI), as described previously.50 Polymerase chain reactions was performed as described,50 using the following primers for the Vγ2 chain (Vγ9 according to the IMGT nomenclature): oligo-Vγ2 (5′-ATC AAC GCT GGC AGT CC-3′) and oligo-Cγ-1 (5′-GTT GCT CTT CTT TTC TTG CC-3′). The PCR products were separated on 1% agarose/Tris-acetate-EDTA buffer gels containing 0·5 μg/ml ethidium bromide.
Run-off reaction
Primer extension reactions were performed as described elsewhere.9 Each reaction contained 2 μl PCR product, 3 mm MgCl2, 0·2 mm dNTP, 0·1 mm of 6-carboxyfluorescein (6-FAM) -labelled primer (Cg-6: 5′-6-FAM-AAT AGT GGG CTT GGG GGA AAC-3′; Cd: 5′-6-FAM-ACG GAT GGT TTG GTA TGA GG-3′), approximately 0·2 units Taq DNA polymerase (Promega), 10 mm Tris–HCl pH8·8, and 50 mm KCl. Run-off products (4 μl) were diluted with deionized formamide (6 μl) (Applied Biosystems, Foster City, CA), and 0·7 μl GeneScan-500 ROX size standard was added to each sample. After a denaturation step (5 min at 95° followed by immediate quenching on ice) products were loaded on a 3130 genetic analyser (Applied Biosystems) and run on a performance-optimized polymer (POP-7). Molecular size and relative frequency of extension products were determined using Genemapper software (Applied Biosystems). To standardize the data irrespective of the run-off primer position, CDR3 length variation was expressed in terms of the total Vγ2, Vδ2 and Vδ1 coding region lengths. Run-off product lengths were corrected by adding the length of the known mRNA coding region outside the run-off primer-binding site.
Cloning and sequencing of Vγ2 chains
The PCR products for the Vγ2 chain were purified by gel extraction, using QIAquick gel extraction kits (Qiagen) according to the manufacturer's instructions. Purified products were denatured (1 min at 94°), then incubated for 30 min at 72° with 2 mm MgCl2, 0·2 mm dATPs and 2·5 units Amplitaq Gold (Promega), then ligated into a pCR2.1 vector (Invitrogen). Ligated vectors were transfected into TOP 10F′ competent cells (TA cloning kit; Invitrogen), and bacterial colonies representing a library of Vγ2 chain sequences were grown overnight on agar plates containing 50 μg/ml ampicillin, 500 μm Isopropyl β-D-1-thiogalactopyranoside and 80 μg/ml X-Gal (Promega). Colonies containing recombinant plasmids were cultured overnight in LB medium and bacterial suspensions were used as template to amplify the Vγ2 chain inserted in the plasmid. M13 PCR were performed using: 2 μl bacterial suspension as template, 100 nm M13 forward (5′-GTA AAA CGA CGG CCA G-3′) and M13 reverse primer (5′-CAG GAA ACA GCT ATG AC-3′), 0·2 mm dNTPs, 2 mm MgCl2, 10 mm Tris–HCl pH 8·8, 50 mm KCl, 0·1%Triton-X-100 and 1 unit of AmpliTaq Gold (Promega). The PCR were run with the following profile: denaturation: 1 min at 94°; 25 cycles (45 seconds at 94°, 1 min at 60°, 1 min at 72°); extension: 10 min at 72°. PCR products were purified by size exclusion, after running through a bed of Sephacryl S400 (GE Healthcare, Uppsala, Sweden) packed (200 μl/well) into 96-well MultiScreen HTS filtration plates (Millipore, Billerica, MA).
Sequencing reactions were performed with a Big Dye v3.1 fluorescent sequencing kit (Applied Biosystems), and M13F or M13R oligonucleotide primers for each sample. Sequencing reactions were run with the following profile: denaturation: 1 min at 94°; 25 cycles (30 seconds at 96°, 20 seconds at 50°, 4 min at 60°). Sequences were loaded on an automated sequencer ABI3700 and analysed using Sequencher (Gene Codes Corporation, Ann Arbor, MI) and MacClade (Sinauer Associates Inc., Sunderland, MA) softwares.
Statistical analysis
Statistical analyses were performed using the software GraphPad Prism (GraphPad Software, La Jolla, CA). For each measured variable, a D'Agostino and Pearson omnibus normality test was performed to assess whether values were normally distributed. For sequence analysis, where small sample size did not allow normality tests, we assumed that values for the variables of interest were normally distributed, based on spectratyping data (available for n > 10) and sequencing data for larger groups of specimens (n > 10).
Differences between means (for normally distributed variables) or medians (for variables displaying a non-Gaussian distribution) were evaluated using a Student's t-test or Mann–Whitney U-test, when comparing only two groups, and analysis of variance or Kruskal–Wallis when comparing more than two groups. For paired groups, paired Student's t-test or Wilcoxon matched-pairs signed rank test were used, respectively, for parametric or non-parametric tests.
Results
Higher proportions of cord blood Vδ2 T cells survive up to 28 days following ALN plus IL-15 stimulation
We treated CBMC with ALN plus IL-2 or IL-15 and compared Vδ2 T-cell responses. Interleukin-15 was titrated (on peripheral blood mononuclear cells and CBMC) to determine the optimal dose (10 ng/ml); this dose was used for all subsequent experiments. Interleukin-2 was used at 100 U/ml, the optimum for γδ T-cell cultures. As a control, we analysed ALN stimulation of adult peripheral blood mononuclear cells for 14 days. These cultures had similar levels of Vδ2 T cells using ALN with either IL-2 or IL-15 (65·8% + 21·8% versus 60% + 25% of CD3+ cells).
Cord blood mononuclear cell proliferation responses to ALN plus IL-2 or IL-15 were measured at several time-points. The average Vδ2 T-cell frequencies among CD3+ cells by 14 days after stimulation were similar with IL-15 + ALN or IL-2 + ALN, and significantly higher than ex vivo or control values (IL-2 or IL-15 alone) (Fig. 1a). Vδ2 cell frequencies and absolute numbers (see Supplementary material, Fig. S1) after control treatment (IL-2 or IL-15 alone) were higher than ex vivo frequencies (Fig. 1a), indicating that homeostatic proliferation of neonatal Vδ2 cells could be driven by cytokine as was seen with adult Vδ2 T cells.30
Figure 1.
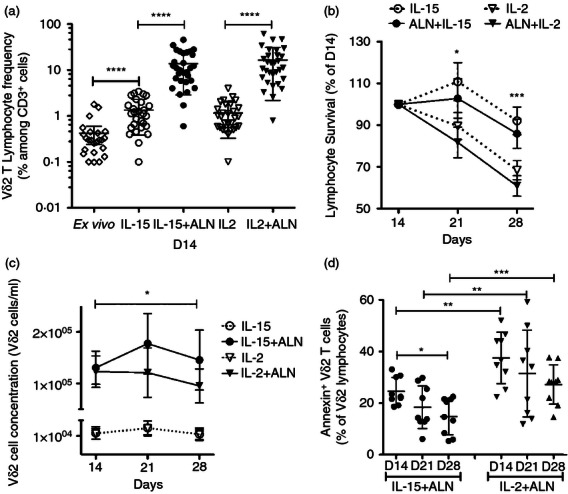
Interleukin-15 (IL-15) sustains the survival of CB Vδ2 cell lines expanded with bisphosphonate. (a) Cord blood mononuclear cells (CBMC) were stimulated with alendronate (5 μm) + IL-2 (100 U/ml), Alendronate + IL-15 (10 ng/ml), or IL-2 or IL-15 alone (as controls). Lymphocyte viability and Vγ2Vδ2 T-cell frequency were assessed after 14 days of culture for 25 specimens. Individual values, means and standard deviations are shown in the scatter plot. (b) Viable lymphocytes were determined on days 14, 21 and 28, and expressed as a percentage of cell numbers on day 14. Mean ± standard deviation values are shown for eight specimens. (c) Concentrations of Vδ2 cells after 14, 21 and 28 days of culture. Mean and standard deviation values are shown for each culture condition. The plot shows mean ± standard deviation for each culture condition (n = 8). (d) The fraction of AnnexinV+ Vδ2 cells was assessed after 14, 21 and 28 days of culture. The scatter plot reports individual values, mean and standard deviation for each culture condition. Differences between mean values were analysed by paired Student's t-test or analysis of variance. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
The proportion of Vδ2 T cells increased until day 14, then remained relatively stable until day 28 (data not shown). For cells treated with ALN + IL-2, lymphocyte viability declined sharply after day 14 (Fig. 1b), and the concentration of Vδ2 T cells decreased after day 21, reaching on day 28 lower levels than on day 14 (Fig. 1c). For cultures stimulated with IL-15 + ALN, lymphocyte viability started to decline only after day 21 (Fig. 1b), and the interval for Vδ2 expansion was extended from 14 to 21 days after stimulation, producing similar Vδ2 T-cell concentrations on days 14 and 28 (Fig. 1c).
We also compared IL-15 and IL-2 for effects on spontaneous Vδ2 T-cell apoptosis. The proportions of Vδ2 lymphocytes positive for Annexin V (on days 14, 21 and 28) were higher for IL-2 + ALN than for IL-15 + ALN cultures (Fig. 1d). The fraction of Annexin V+ Vδ2 lymphocytes decreased between days 14 and 28 for both treatments, becoming significantly lower on day 28 for IL-15 + ALN-stimulated CBMC (Fig. 1d).
CD56 and NKG2 family receptors are up-regulated during cell culture
The neural cell adhesion molecule, CD56, is a marker for cytotoxic Vδ2 T cells.51 After 14 days of culture, the fraction of CD56+ Vδ2 cells was increased significantly compared with fresh CBMC, and was highest when cells were treated with cytokine alone (Fig. 2a). The day 14 fraction of CD56+ Vδ2 cells was similar when using ALN + IL-2 or ALN + IL-15 but by day 28, IL-15 + ALN cultures had significantly higher fractions of CD56+ cells compared with cells expanded with IL-2 + ALN (Fig. 2b). On day 28, Vδ2 T lymphocytes treated with IL-15 alone also contained a fraction of CD56+ cells significantly greater than when cells were treated with IL-2, so CD56 expression was affected even in the absence of ALN.
Figure 2.
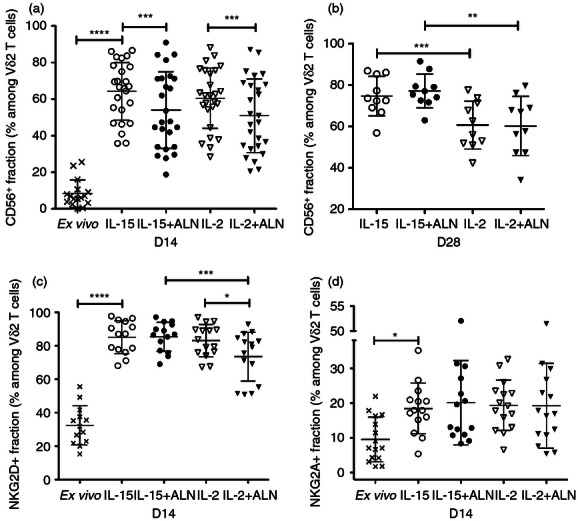
Interleukin-15 (IL-15) or IL-2 up-regulate natural killer (NK) receptors on Vγ2Vδ2 T cells. CD56 expression was assessed (a) 14 or (b) 28 days after stimulation. Expression of (c) NKG2D and (d) NKG2A was analysed 14 days after stimulation. Scatter plots represent individual values, means and standard deviations for each receptor. Differences between groups were analysed by paired Student's t-test or Wilcoxon matched-pairs signed rank test. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
By day 14, most Vδ2 T cells expressed the activating natural killer (NK) receptor NKG2D. IL-2 + ALN stimulation produced fewer NKG2D+ Vδ2 lymphocytes compared with IL-2 alone or IL-15 + ALN (Fig. 2c). The inhibitory NK receptor NKG2A was up-regulated to the same extent in all culture conditions. However, the fraction of NKG2A+ Vδ2 T cells remained low (19%, Fig. 2d) and expression of this receptor was limited to NKG2D+ Vδ2 T cells.
Expression of activation markers CD25 and CD27 is differentially regulated by IL-2 and IL-15
The fraction of CD27+ Vδ2 T cells was greater with IL-15 (with or without ALN) than with IL-2 (data not shown) and the subset of Vδ2+ CD45RA− CD27− effector-memory (EM) cells was lower (Fig. 3a) with IL-15 + ALN than with IL-2 + ALN. The majority of expanded Vδ2 cells were CD45RA− CD27+ central memory phenotype (CM; 75% for IL-15 + ALN and 70% for IL-2 + ALN), whereas CD45RA+ CD27− terminally differentiated effector memory (TEMRA) Vδ2 cells were scarce (typically < 3%, data not shown).
Figure 3.
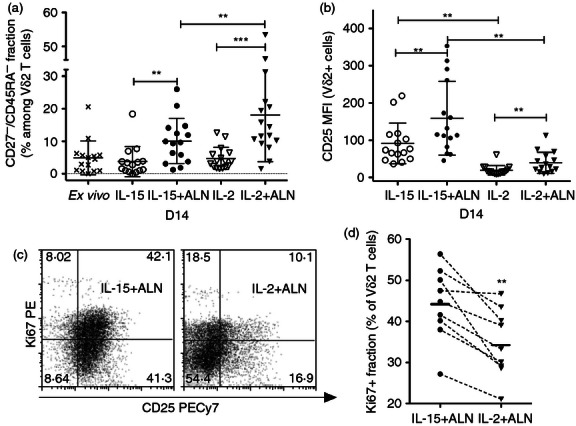
Treatment with interleukin-15 (IL-15) is associated with prolonged activation of cord blood Vγ2Vδ2 T cells. (a) The proportion of CD27− CD45RA− effector memory Vγ2Vδ2 T cells was determined 14 days after stimulation. Scatter plots show individual values, means and standard deviations for 16 specimens. (b) IL-2 Rα chain expression (CD25 mean fluorescence intensity) on Vγ2Vδ2 lymphocytes was determined 14 days after stimulation for 16 specimens, and represented in scatter plots with means and standard deviations for each culture condition. (c) The activation marker Ki67 was analysed on day 14 for nine specimens after stimulation with IL-15 + Alendronate (ALN) or IL-2 + ALN. Dot plots show the results for a representative sample. (d) The fractions of Ki67+ Vγ2Vδ2 T cells for IL-15 + ALN or IL-2 + ALN cultures are shown. Results for each specimen are connected by dashed lines, and horizontal bars indicate mean values for each group. Differences between means were analysed by paired Student's t-test. **P < 0·01; ***P < 0·001
CD25 on day 14 was expressed on a larger fraction of Vδ2 cells and at significantly higher density after IL-15 treatment, with or without ALN (Fig. 3b). This is not likely to be the result of down-modulation of CD25 following IL-2 binding. Exogenous IL-2 was added to cultures 4 days before staining and down-modulation of CD25 following IL-2 binding is usually only a transient phenomenon. Moreover on day 8, < 24 hr after adding IL-2 to cell cultures, a large fraction of IL-2-cultured Vδ2 cells still expressed CD25 at high levels (not shown).
Higher expression of CD25 and a greater proportion of Ki67+ Vδ2 cells were associated with IL-15 + ALN stimulation (Fig. 3c,d). These data suggest that IL-15 prolongs the activation of Vδ2 T cells compared with IL-2.
ALN expanded cord blood Vδ2 lymphocytes produce cytotoxic mediators
Larger proportions of granzyme+ and perforin+ Vδ2 cells were obtained with IL-15 + ALN compared with IL-2 + ALN on days 16 and 28 of culture (Fig. 4a,b). By day 28, cells treated with IL-15 alone also had a significantly larger fraction of granzyme B+ and perforin+ Vδ2 cells compared with IL-2-treated CBMC, and were comparable to IL-15 + ALN (data not shown). The fraction of Vδ2 T cells producing granzyme or perforin depended on the cytokine, but it was similar for CBMC treated with IL-15 whether or not ALN was added. The fraction of perforin+ Vδ2 cells decreased significantly between days 16 and 28, whereas the granzyme+ Vδ2 cells remained stable (Fig. 4a,b). By day 28, Vδ2 cells were mostly double positive for perforin and granzyme B (data not shown).
Figure 4.
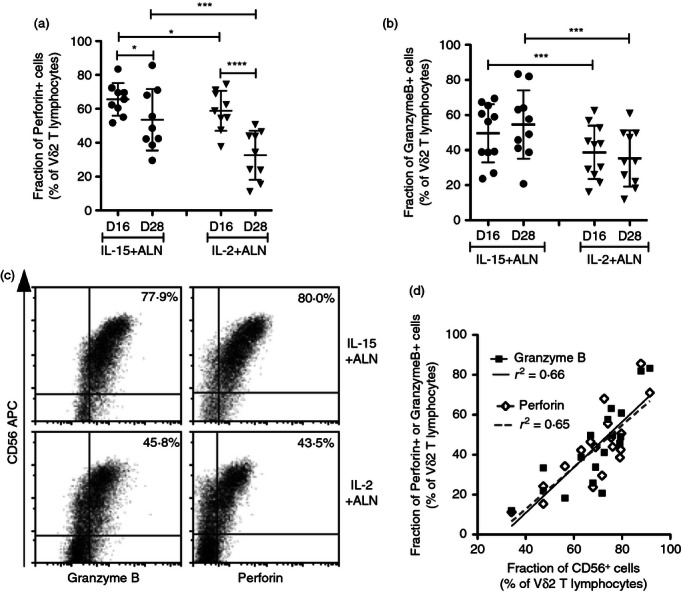
Treatment with interleukin-15 (IL-15) increases cytotoxic mediators in CD56+ Vγ2Vδ2 T cells. The fractions of Vγ2Vδ2 cells expressing (a) perforin (n = 9) or (b) granzyme B (n = 11) were measured on days 16 and 28 after stimulation. Scatter plots show individual values, means and standard deviations for IL-15 + Alendronate (ALN) or IL-2 + ALN cultures. Differences between mean values were analysed by paired Student's t-test. *P < 0·05; ***P < 0·001; ****P < 0·0001. (c) Concomitant expression of CD56 and perforin (right panels) or granzyme B (left panels) was evaluated 28 days after stimulation. The percentages refer to cells positive for CD56/granzyme B or CD56/perforin for a representative sample. (d) The correlation between CD56 and perforin or granzyme B expression in all IL-15 + ALN or IL-2 + ALN cultures (n = 9 for each stimulation) was analyzed 28 days after stimulation (Pearson correlation test). A linear regression plot shows the curve for best-fit values and R2 values.
For all lymphocyte populations (Vδ2+, CD3+ and CD3− subsets), the production of perforin and granzyme B was associated with CD56 expression. CD56− cells were mostly negative for perforin/granzyme B, whereas CD56low cells were negative or expressed intermediate amounts of perforin/granzyme B and CD56bright cells produced the most perforin/granzyme B (Fig. 4c). The fraction of granzyme B+ or perforin+ Vδ2 cells correlated with the fraction of CD56+ Vδ2 cells (Fig. 4d).
Short duration TCR re-stimulation triggers granule release and TNF-α production by cord blood Vδ2 T cells
We tested whether perforin and granzyme B+ cells mobilized cytotoxic granules or produced TNF-α in response to TCR re-stimulation with anti-γδ TCR monoclonal antibody. The fraction of TNF-α+ Vδ2 T cells that released granules and became CD107a+ was typically < 50% (Fig. 5). Most cells releasing granules also produced TNF-α; in some specimens we noted a population that produced TNF-α without releasing granules (Fig. 5a). Vδ2 T cells expanded with IL-15 + ALN had a stronger response in terms of CD107 mobilization and/or TNF-α production (Fig. 5b) but the differences between mean values for these groups were small (for CD107a+ Vδ2 cells: mean = 25·3% versus 18·8%, P = 0·001; for TNF-α+ Vδ2 cells: mean = 34·4% versus 27·3%, P = 0·004).
Figure 5.
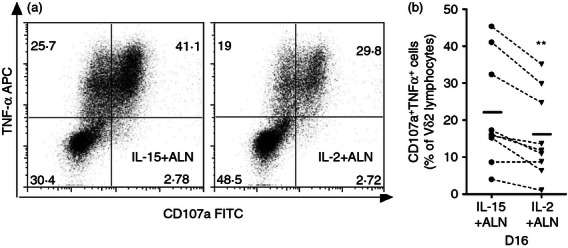
Interleukin -15 (IL-15) + ALN treatment elicits Vγ2Vδ2 T cells to produce tumour necrosis factor-α (TNFα) and mobilize granules in response to T-cell receptor (TCR) re-stimulation. Sixteen days after IL-15 + ALN or IL-2 + ALN stimulation, cord blood mononuclear cells (CBMC) were re-stimulated by transferring then to 96-well plates pre-coated with anti γδ TCR (clone B1.1), then adding CD107a FITC (5 μl/well) and GolgiPlug (1 μg/ml) for 5 hr. TNFα production and expression of CD107a (marker for granule mobilization) were analysed in permeabilized cells by flow cytometry. (a) Results for a representative sample show the proportion of double-positive cells for each culture condition. (b) The fraction of CD107a+ TNFα+ Vγ2Vδ2 T cells is plotted for nine specimens cultured after IL-15 + ALN or IL-2 + ALN, then re-stimulated with anti-γδ TCR; mean values are indicated by horizontal bars. Differences between mean values were analysed by paired Student's t-test. **P < 0·01
ALN stimulation selects a Vγ2 repertoire similar to adults
We compared the Vγ2 repertoire before and after in vitro expansion. Both the Vγ2 and the Vδ2 chains are required for antigen responses;8,52 changes in the Vγ2 repertoire are specifically associated with antigen-driven proliferation.9 We know that Vγ2 chain length is correlated with J segment usage; most (> 75%) of the Vγ2 chains with lengths between 990 and 996 nucleotides carry the Jγ1.2 segment.9 We performed spectratyping on 15 CBMC specimens before and after culture, to compare Vγ2 chain length distributions. The proportions of Vγ2 chains with lengths between 990 and 996 nucleotides (% 990–996) for fresh CBMC were lower than for fresh adult peripheral blood (37·5 ± 13% versus 56 ± 25% for a group of healthy African American individuals or 70 ± 22% for healthy Caucasian individuals, P < 0·05, see Supplementary material, Fig. S2), but comparable to other CBMC specimens.14 In the absence of ALN, both IL-15 and IL-2 increased the frequency of short chains with lengths < 990, and decreased the % 990–996 (from 37·5 ± 13% ex vivo to 26·8 ± 7·7% and 22·8 ± 6·2%, respectively, for IL-15 and IL-2, P = 0·0028 and P = 0·0001, Fig. S2). In particular, the frequency of 993 nucleotide chains was significantly lower in cultures with IL-2 or IL-15 (Fig. 6a). The absolute number of Vδ2 T cells increased 25-fold after IL-15 and 19-fold after IL-2 treatment (Fig. S1), so the relative increase in chains below 990 nucleotides (mostly using Jγ1.3/2.3) is explained by preferential proliferation of lymphocytes with shorter Vγ2 chains. At least some of these Vγ2-Jγ1.3/2.3 chains are paired with Vδ1 chains (data not shown) and are not part of the response to PAg.
Figure 6.
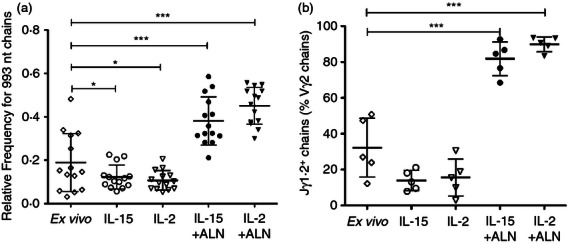
Interleukin-15 (IL)-15 + alendronate (ALN) or IL-2 + ALN select for Jγ1.2+ chains, cytokine alone increases the proportion of Vγ2-Jγ1.2/2.3+ chains. (a) Vγ2 chain length distributions were determined by spectratyping for cord blood mononuclear cells (CBMC) specimens before culture and 14 days after stimulation; the proportions of Vγ2 chains with a length of 993 nucleotides (the single most common length for Jγ1.2+ chains) were compared. The scatter plot shows individual values (n = 14), mean and standard deviation for each condition. (b) The fractions of Jγ1.2+ chains among all Vγ2 were determined by sequence analysis performed before and 14 days after stimulation. At least 200 productively rearranged Vγ2 chains were analysed for each CBMC specimen before culture, and at least 100 productively rearranged Vγ2 chains were analysed for each culture condition after stimulation. The scatter plots show individual values, mean and standard deviation for each condition. Differences between means were analysed by analysis of variance. *P < 0·05; **P < 0·01; ***P < 0·001.
Alendronate (ALN) stimulation (with either IL-15 or IL-2) induced a 200-fold to 300-fold increase in Vδ2 cell numbers by day 14 (Fig. S1), with significant increases in the % 990–996 (from 37·5 ± 13% ex vivo to 62·0 ± 13% and 73 ± 8% respectively for IL-15 + ALN and IL-2 + ALN, P < 0·0001, Fig. S2), due largely to accumulation of 993 nucleotide Vγ2 chains (Fig. 6). After a single round of ALN stimulation, the % 990–996 was comparable to that found in peripheral blood of healthy adults (Fig. S2).
A large fraction of the neonatal Vγ2 repertoire is comprised of public clonotypes with limited junctional diversity
To evaluate changes in the TCR repertoire caused by homeostatic versus antigen-driven proliferation stimulation, we created Vγ2 cDNA libraries from mRNA for CBMC specimens before and after in vitro expansion. For six fresh CBMC specimens, we sequenced 300 Vγ2 cDNA clones, which yielded sequence data for at least 200 productively rearranged chains. For five cultured specimens, we sequenced 150 Vγ2 cDNA clones for each of four different culture conditions. For all CBMC samples before and after culture, Vγ2 chain length distributions were similar whether they were calculated from cDNA sequencing data or measured directly by spectratyping (not shown). This is our standard method to ensure that sufficient cDNA clones were sequenced to represent the entire pool of Vγ2 sequences.
Based on the collection of Vγ2 sequences from six fresh CBMC specimens, we defined a list of public clonotypes. Public Vγ2 clonotypes are amino acid sequences present in two or more donors and identical at all positions in V, N and J regions (Fig. 7). Most public sequences, with both Jγ1.2 and Jγ1.3/2.3 segments, had limited junctional diversity (limited deletion or non-templated nucleotide addition) and a narrow range of lengths (14 out 17 public Jγ1.2 chains were 990–996 nucleotides long, 15 out of 17 public Jγ1.3/2.3 chains were 981–987 nucleotides long).
Figure 7.
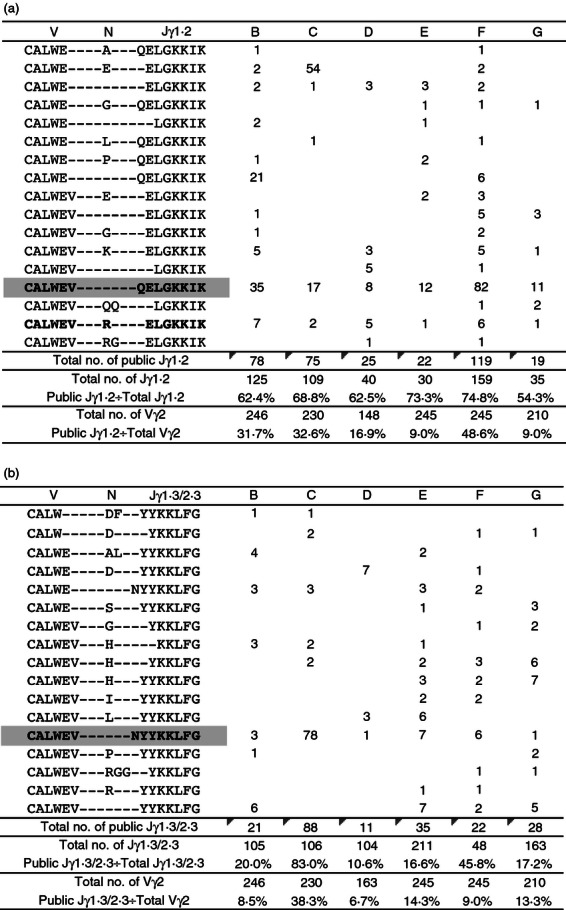
The pool of Vγ2-Jγ1.2+ sequences in cord blood mononculear cells (CBMC) is dominated by public clonotypes. Vγ2 sequences from six fresh CBMC specimens were cross-compared and public clonotypes were identified. Public Vγ2 clonotypes are amino acid sequences present in two or more donors and identical at all positions in V, N and J regions. Abundance of public (a) Jγ1.2+ and (b) Jγ1.3/2.3+ clonotypes, as well as their proportion in the Vγ2 pool or Jγ1.2 or Jγ1.3/2.3 subset are shown for six CBMC specimens. Shaded clonotypes can be encoded by germ-line sequences; bolded clonotypes are present in every specimen.
The fraction of public Jγ1.2 clonotypes in CBMC ranged from 8·6% to 48·6% of all Vγ2 chains and from 51·4% to 74·8% of all Vγ2-Jγ1.2 chains (average 66·8%) (Fig. 7a). The fraction of public Jγ1.3/2.3 clonotypes in CBMC ranged between 6·7% and 38·3% of the Vγ2 chains and between 10·6% and 83% of the Vγ2-Jγ1.3/2.3 chains (average 35·2%) (Fig. 7b). Two of the public Vγ2-Jγ1.2 clonotypes were encoded by up to four different nucleotypes in a single donor (see Supplementary material, Fig. S3a). Public Jγ1.3/2.3 clonotypes were never encoded by more than one nucleotype.
Proliferation after ALN stimulation is due largely to public clonotype responses
We studied the evolution of the Vγ2 repertoire after homeostatic or antigen-driven proliferation to highlight the mechanisms for positive selection. Table 1 shows a summary of J segment usage for each sample before and after culture. Consistent with spectratyping results, the proportion of Jγ1.2 chains tended to be lower after IL-15 or IL-2 culture than in fresh CBMC (even though the difference was not statistically significant due to the low number of samples analysed), whereas it was significantly higher after ALN + IL-15 or ALN + IL-2 stimulation (Fig. 6b).
Table 1.
J segment usage for five specimens sequenced before and after culture
| Ex vivo (%) | IL-15 (%) | IL-2 (%) | IL-15 + ALN (%) | IL-2 + ALN (%) | |
|---|---|---|---|---|---|
| A | |||||
| Jγ1.2 | NA | 10 (7·9) | 3 (2·9) | 110 (84·6) | 128 (93·4) |
| Jγ1.3/2.3 | 113 (89·7) | 101 (97·1) | 19 (14·6) | 9 (6·6) | |
| Jγ1.1 | 3 (2·4) | ND | 1 (0·8) | ND | |
| Jγ2.1 | ND | ND | ND | ND | |
| Total no. | 126 | 104 | 130 | 137 | |
| B | |||||
| Jγ1.2 | 125 (50·8) | 21 (18·1) | 12 (10) | 126 (92·6) | 124 (93·2) |
| Jγ1.3/2.3 | 105 (42·7) | 86 (74·1) | 99 (83·2) | 9 (6·6) | 8 (6) |
| Jγ1.1 | 13 (5·3) | 3 (2·6) | 5 (4·2) | 1 (0·7) | 1 (0·8) |
| Jγ2.1 | 3 (1·2) | 6 (5·2) | 3 (2·5) | ND | ND |
| Total no. | 246 | 116 | 119 | 136 | 133 |
| C | |||||
| Jγ1.2 | 109 (47·4) | 10 (9·6) | 16 (15·5) | 95 (76·6) | 100 (83·3) |
| Jγ1.3/2.3 | 106 (46·1) | 89 (85·6) | 82 (79·6) | 25 (20·2) | 16 (13·3) |
| Jγ1.1 | 16 (6·9) | 3 (2·9) | 4 (3·9) | 4 (3·2) | 4 (3·3) |
| Jγ2.1 | ND | 2 (1·9) | 1 (1) | ND | ND |
| Total no. | 230 | 104 | 103 | 124 | 120 |
| D | |||||
| Jγ1.2 | 40 (27) | 16 (13·2) | 23 (18·9) | 117 (86·7) | 117 (90·7) |
| Jγ1.3/2.3 | 104 (70·3) | 104 (86) | 88 (72·1) | 13 (9·6) | 11 (8·5) |
| Jγ1.1 | 1 (0·7) | ND | 9 (7·4) | 4 (3·0) | 0 (0·0) |
| Jγ2.1 | 3 (2) | 1 (0·8) | 2 (1·6) | 1 (0·7) | 1 (0·8) |
| Total no. | 148 | 121 | 122 | 135 | 129 |
| E | |||||
| Jγ1.2 | 30 (12·2) | 25 (21·2) | 34 (30·6) | 85 (68·5) | 120 (88·9) |
| Jγ1.3/2.3 | 211 (86·1) | 89 (75·4) | 75 (67·6) | 34 (27·4) | 15 (11·1) |
| Jγ1.1 | 4 (1·6) | 2 (1·7) | 2 (1·8) | 3 (2·4) | ND |
| Jγ2.1 | ND | 2 (1·7) | ND | 2 (1·6) | ND |
| Total no. | 245 | 118 | 111 | 124 | 135 |
NA, not available; ND, not detected.
For each specimen, the number (and proportion) of Vγ2 sequences with each J segment are shown for CBMC (ex vivo) and for all culture conditions.
After culture, the fraction of public clonotypes remained around 50% of the Vγ2-Jγ1.2 chains except for IL-15-treated CBMC, where it decreased significantly (Table 2). The proportion of public Jγ1.2 clonotypes among all Vγ2 chains increased significantly for both IL-15 + ALN-treated or IL-2 + ALN-treated CBMC (Fig. 8a), reflecting the expansion of Jγ1.2+ cells. In contrast, in cultures with only IL-2 or IL-15, where the fraction of Jγ1.2+ chains declined, the proportion of public Jγ1.2 clonotypes among all Vγ2 chains also decreased (reaching statistical significance for IL-15, Fig. 8a).
Table 2.
Fraction of Jγ1.2+ or Jγ1.3/2.3+ chains coding public clonotypes
| Ex vivo, % | IL15, % | IL2, % | IL15 + ALN, % | IL2 + ALN, % | |
|---|---|---|---|---|---|
| Jγ1.2 | |||||
| A | NA | 30·0 | 66·7 | 52·7 | 62·5 |
| B | 62·4 | 57·1 | 25·0 | 55·6 | 50·8 |
| C | 68·8 | 30·0 | 43·8 | 48·4 | 44·0 |
| D | 62·5 | 31·3 | 69·6 | 44·4 | 47·0 |
| E | 73·3 | 40·0 | 58·8 | 50·6 | 55·0 |
| Mean | 66·8 | 37·7 | 52·8 | 50·3 | 51·9 |
| Jγ1.3/2.3 | |||||
| A | NA | 8·8 | 3·0 | 0·0 | 22·2 |
| B | 20·0 | 9·3 | 6·1 | 22·2 | 12·5 |
| C | 83·0 | 14·6 | 17·1 | 8·0 | 12·5 |
| D | 10·6 | 13·5 | 11·4 | 30·8 | 9·1 |
| E | 39·8 | 27·0 | 29·3 | 20·6 | 6·7 |
| Mean | 38·4 | 16·1 | 16·0 | 20·4 | 10·2 |
NA, not available.
The fraction of Jγ1.2+ or Jγ1.3/2.3+ chains coding public clonotypes is shown for each specimen before and after culture. Mean values are also calculated for each culture condition.
Figure 8.
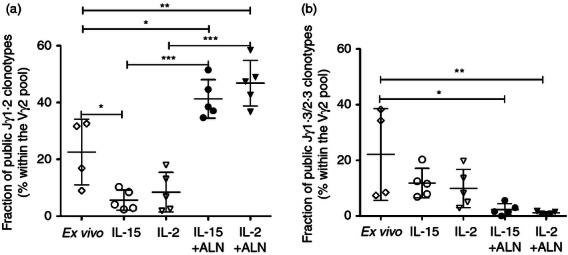
Interleukin-15 (IL-15) + Alendronate (ALN) or IL-2 + ALN preferentially expand cells expressing public Jγ1.2+ clonotypes, and reduce the proportion of public Jγ1.3/2.3+ clonotypes. The proportions of Vγ2 chains coding public clonotypes with (a) Jγ1.2 segment or (b) Jγ1.3/2.3 segments were determined for fresh (n = 6) and cultured (n = 5) CBMC. Scatter plots show individual values, medians and means for each condition. Differences between means were analysed by analysis of variance. *P < 0·05; **P < 0·01; ***P < 0·001
The fraction of Jγ1.3 public clonotypes in the Vγ2 pool (Fig. 8b) or the Jγ1.3/2.3 subset (Table 2) was lower after culture (under all conditions) than ex vivo. Homeostatic proliferation induced by IL-15 or IL-2 caused polyclonal expansion of the Jγ1.3/2.3 subset without selecting for public clonotypes.
Public clonotypes arise by convergent selection
Among Jγ1.2 public sequences from cord blood, the single most abundant clonotype, the canonical CALWEVQELGKKIK, was encoded by six different nucleotypes including the germ-line sequence (Fig. S3b), which was present in all specimens. Two other nucleotypes for CALWEVQELGKKIK were detected in every sample after culture but not in fresh CBMC. Two remaining nucleotypes were present in four of five samples. The second most abundant clonotype, CALWEVRELGKKIK, was encoded by 11 different nucleotypes. Two were present in every cultured sample, one was present in four of five samples, the remaining sequences were less abundant, and present in fewer donors (Fig. S3b).
Frequencies of the Jγ1.2 germ-line nucleotype within all Vγ2 chains decreased after control treatment (IL-15 or IL-2) and increased after ALN stimulation, reflecting changes in the proportion of all chains with Jγ1.2 segments (Fig. 9a). For five samples analysed in this study, the proportion of Vγ2 with the germ-line nucleotype was correlated directly with the stimulation index for ALN (Fig. 9b). This suggests that Vγ2Vδ2 T-cell expansion after ALN stimulation depended to a large extent on the behaviour of cells expressing the canonical Vγ2-Jγ1.2 sequence.
Figure 9.
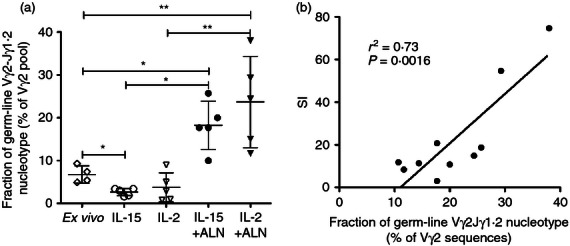
Vγ2Vδ2 T-cell expansion afterAlendronate (ALN) stimulation depends to a large extent on proliferation of cell clones expressing the germ-line Vγ2-Jγ1.2 nucleotype. (a) The fractions of Vγ2 chains with the germ-line Vγ2-Jγ1.2 rearrangement (GAGGTG…CAAGAG) were determined for fresh and cultured cord bloon CBMC. (b) 14 days after stimulation with IL-15 + ALN or IL-2 + ALN, the fraction of germ-line Vγ2-Jγ1.2 sequence was positively correlated with the stimulation index measuring cell proliferation. The correlation was tested with Pearson's correlation test. A linear regression plot shows the curve for best-fit values and R2 value. *P < 0.05; **P < 0.01.
Discussion
A goal of this study was to show whether Vγ2Vδ2 T-cell responses are an important aspect of neonatal immunity. We focused on cord blood Vδ2 cells, their responses to stimulus, and the impact of common γ chain cytokines (IL-2 or IL-15) on repertoire, phenotype and function. Alendronate stimulation of CBMC shifted cord blood Vγ2Vδ2 lymphocytes from naive to mostly central memory cells and produced a small fraction of effector memory with virtually no TEMRA cells, consistent with previous reports.30,53 Vδ2 central memory cells (CD27+ CD45RA−) were more abundant after IL-15 + ALN than IL-2 + ALN stimulation of CBMC, even though IL-15 drives adult Vγ2Vδ2 cells to the effector memory compartment during antigen stimulation.28,29 The central memory Vδ2 population after ALN expansion, responded to TCR re-stimulation with granule mobilization and TNF-α production, hence it also possessed characteristics of effector memory cells (in agreement with a report from Martino et al.28) Sustained expression of CD27, CD25 and Ki67 suggests that central memory Vδ2 T lymphocytes persist as activated cells with low rate of spontaneous apoptosis, well after IL-15 + ALN stimulation.
Surprisingly, cytokine-driven homeostatic proliferation expanded preferentially the cell clones expressing Jγ1.3/2.3+ Vγ2 chains. In contrast, ALN selected the Vγ2-Jγ1.2+ subpopulation, consistent with other studies analysing peripheral blood9 or cord blood54 Vδ2 cell expansion mediated by PAg. Both IL-15 + ALN and IL-2 + ALN increased the proportion of Jγ1.2+ public clonotypes, especially the canonical sequence (CALWEVQELGKKIK). By 2 weeks in culture, the stimulation index for ALN-treated Vγ2Vδ2 T cells was correlated directly with the proportion of cells expressing the Vγ2-Jγ1.2 gem-line nucleotype. This single nucleotype had a significant impact on the response to ALN stimulation. Overall, one round of in vitro CBMC stimulation selected an ‘adult-like’ Vγ2 repertoire, dominated by the Vγ2-Jγ1.2 rearrangement and favoring the accumulation of public clonotypes. These results support the view that neonatal Vγ2Vδ2 cells are capable of strong protective immune responses.
Even though germ-line encoded Vγ2-Jγ1.2 sequences represent a dominant fraction of the neonatal repertoire, Vγ2 diversity is higher in CBMC than during early fetal development,55 and the CBMC repertoire includes several clonotypes that require addition of non-templated (N) nucleotides by terminal deoxynucleotidyl transferase (TdT) activity. Previous repertoire studies on adults, children and fetuses8,55,56 suggested that germ-line encoded residues are crucial for antigen recognition, whereas non-templated residues may be less important because no common N motif was defined for the Vγ2-Jγ1.2 junction. Germ-line encoded sequences may be generated preferentially in the absence of TdT activity,57 explaining why the germ-line Jγ1.2 sequence is highly represented among fetal liver T-cell clones.55 However, the germ-line Jγ1.2 sequence is more abundant than the corresponding Jγ1.3/2.3 germ-line nucleotype, even though Jγ1.3/2.3 chains predominate in cord blood. A mechanism that may explain this difference is convergent recombination, a process by which multiple recombination events involving different splicing of the germ-line V and J, converge to produce the same nucleotide sequence.58,59 This mechanism shapes the clonotypic landscape of the naive CD8 T cells60 and it may similarly model the Vγ2 repertoire, preferentially producing the germ-line nucleotype coding the clonotype CALWEVQELGKKIK, as well as other recurrent public clonotypes. Convergent recombination would also explain why frequently produced nucleotypes (recurrent sequences) are often present in multiple individuals (public).60
In cord blood, a sizable fraction of Vγ2 chains are paired with δ chains other than Vδ2 (especially with Vδ1).12 Conversely, most Vδ2 chains are paired with Vγ2 chains (data not shown). This is also the situation after cytokine treatment (data not shown). We expect that Vγ2 chains paired with non-Vδ2 chains are mostly non-Jγ1.2, so the proportion of Jγ1.2+ clones within the total Vγ2Vδ2 cell population is probably higher than what we report for the Vγ2 pool. Sequencing of ex vivo sorted Vγ2Vδ2 cells would be required to verify this hypothesis.
The use of IL-15 improved the frequency of CD56+ granzyme B+ Perforin+ Vδ2 T lymphocytes compared with IL-2. These effects are independent of selection for specific Vγ2-Jγ1.2 chains; the Vγ2 repertoires after IL-15 + ALN or IL-2 + ALN were very similar and biased equally towards public clonotypes. It was reported that IL-15 sustains IL-2-independent antigen-driven proliferation of adult Vδ2 T cells27 and is required for Vδ2 T-cell responses to mycobacteria.28,29 This cytokine is also critical for NK61–63 and memory CD8 T-cell generation or maintenance.64–68 The ability to use IL-15 and IL-2345 unlinks Vγ2Vδ2 T cells from a dependence on concurrent CD4+ T-cell activation, and may be important for neonatal immunity and neonatal vaccine responses when CD4+ T-cell populations are still immature.
During stimulation, activating and inhibitory NK receptors were up-regulated on Vδ2 cells. Increased expression of CD56, NKG2D and NKG2A was driven by cytokines even in the absence of ALN, and Vγ2 chain sequence is not the determining factor for NK receptor expression. This result supports our previous observation that the Vγ2 sequence does not predict CD56 expression for adult Vγ2Vδ2 T cells.69 The same cells express CD56 and cytotoxic mediators, in agreement with earlier work on CD8 T cells70,71 and studies with adult Vδ2 cells.51 Although not all CD56+ Vδ2 cells contained perforin/granzyme B, > 85% of perforin+ granzyme B+ Vδ2 cells also expressed CD56.
The NKG2A receptor is tightly regulated on neonatal Vδ2 cells, and its expression is limited to a small subset of Vδ2+ NKG2D+ cells. This observation may indicate that cord blood Vδ2 T cells express first NKG2D and later NKG2A, hence NKG2D engagement might be necessary for the expansion and subsequent appearance of NKG2A. The fraction of NKG2A+ Vδ2+ cells after ALN stimulation was comparable to the fraction of CD94+ Vδ2 T cells measured after pamidronate (another bisphosphonate drug) stimulation in our previous study using CBMC samples collected in Rome or Abidjan (19·2 ± 12·2%, 16·6 ± 7·3%, and 19·4 ± 9·7%, respectively).
Our findings, consistent with previous reports for neonatal NK72 or CD8 cells.66 show that cord blood Vδ2 cells have improved survival in response to stimulation and higher expression of CD56, granzyme B and perforin when cultured with IL-15. Differences between the effects of IL-2 and IL-15 are small for in vitro assays; whether those differences are meaningful in vivo is not known. However, the availability of IL-15 during neonatal BCG vaccination, when IL-2 sources may be scarce, would sustain Vγ2Vδ2-cell activation, potentially leading to enhanced responses. Our results are in agreement with the hypothesis proposed in various studies66,73–77 that co-administering IL-15 as molecular adjuvant with BCG might improve immune responses to this vaccine.
Acknowledgments
We thank the ‘Hôpital Central de Yaoundé’, maternity division, for their invaluable assistance with cord blood collection. We are especially grateful to Prof. Pierre Joseph Fouda, at the Centre International de Référence Chantal Biya pour la Recherche sur la Prévention et la Prise en Charge du VIH/SIDA (CIRCB) in Yaoundé, for facilitating the research efforts of Dr Cairo in Cameroon. This work was supported by the Unesco Programme ‘Families First Africa’, the Istituto Superiore di Sanita' (ISS/MAE AID 7999.03.6), the US Public Health Service grant AI068508 (CPD), and the Faculty Development Program, Institute of Human Virology, University of Maryland, School of Medicine (CC).
Glossary
- ALN
Alendronate
- BCG
bacillus Calmette–Guérin
- CBMC
cord blood mononuclear cells
- DC
dendritic cells
- IL-2
interleukin 2
- NK
natural killer
- PAg
phosphoantigen
- TCR
T-cell receptor
Disclosures
The authors declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Homeostatic proliferation induces a modest increase in Vγ2Vδ2 T-cell numbers.
Figure S2. Alendronate stimulation of cord blood mononuclear cells (CBMC) generates a T-cell receptor (TCR- γ2) repertoire with adult-like proportion of Jγ1.2+ TCR-γ2 chains.
Figure S3. Public Vγ2-Jγ1.2 clonetypes in cord blood mononuclear cells (CBMC) are encoded by multiple nucleotypes.
References
- 1.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 2.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human γδ T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 4.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 5.Schild H, Mavaddat N, Litzenberger C, et al. The nature of major histocompatibility complex recognition by γδ T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 6.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 7.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davodeau F, Peyrat MA, Hallet MM, Gaschet J, Houde I, Vivien R, Vie H, Bonneville M. Close correlation between Daudi and mycobacterial antigen recognition by human γδ T cells and expression of V9JPC1 γ/V2DJC δ -encoded T cell receptors. J Immunol. 1993;151:1214–23. [PubMed] [Google Scholar]
- 9.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davodeau F, Peyrat MA, Hallet MM, Houde I, Vie H, Bonneville M. Peripheral selection of antigen receptor junctional features in a major human γδ subset. Eur J Immunol. 1993;23:804–8. doi: 10.1002/eji.1830230405. [DOI] [PubMed] [Google Scholar]
- 11.Casorati G, De Libero G, Lanzavecchia A, Migone N. Molecular analysis of human γδ + clones from thymus and peripheral blood. J Exp Med. 1989;170:1521–35. doi: 10.1084/jem.170.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human γδ T cells at birth. J Immunol. 1994;153:3979–88. [PubMed] [Google Scholar]
- 13.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor γδ repertoire. J Exp Med. 1990;171:1597–612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairo C, Propp N, Auricchio G, et al. Altered cord blood γδ T cell repertoire in Nigeria: possible impacts of environmental factors on neonatal immunity. Mol Immunol. 2008;45:3190–7. doi: 10.1016/j.molimm.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casetti R, Perretta G, Taglioni A, et al. Drug-induced expansion and differentiation of Vγ9Vδ2 T cells in vivo: the role of exogenous IL-2. J Immunol. 2005;175:1593–8. doi: 10.4049/jimmunol.175.3.1593. [DOI] [PubMed] [Google Scholar]
- 16.Pechhold K, Wesch D, Schondelmaier S, Kabelitz D. Primary activation of Vγ9-expressing gamma delta T cells by Mycobacterium tuberculosis. Requirement for Th1-type CD4 T cell help and inhibition by IL-10. J Immunol. 1994;152:4984–92. [PubMed] [Google Scholar]
- 17.Jones SM, Goodier MR, Langhorne J. The response of γδ T cells to Plasmodium falciparum is dependent on activated CD4+ T cells and the recognition of MHC class I molecules. Immunology. 1996;89:405–12. doi: 10.1046/j.1365-2567.1996.d01-762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elloso MM, van der Heyde HC, Troutt A, Manning DD, Weidanz WP. Human γδ T cell subset-proliferative response to malarial antigen in vitro depends on CD4+ T cells or cytokines that signal through components of the IL-2R. J Immunol. 1996;157:2096–102. [PubMed] [Google Scholar]
- 19.Burns J, Lobo S, Bartholomew B. Requirement for CD4+ T cells in the γδ T cell proliferative response to Daudi Burkitt's lymphoma. Cell Immunol. 1996;174:19–24. doi: 10.1006/cimm.1996.0289. [DOI] [PubMed] [Google Scholar]
- 20.Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, Caligiuri MA. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-γ by natural killer cells in vitro. J Clin Investig. 1995;96:2578–82. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–41. [PubMed] [Google Scholar]
- 22.Jonuleit H, Wiedemann K, Muller G, Degwert J, Hoppe U, Knop J, Enk AH. Induction of IL-15 messenger RNA and protein in human blood-derived dendritic cells: a role for IL-15 in attraction of T cells. J Immunol. 1997;158:2610–5. [PubMed] [Google Scholar]
- 23.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–87. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 24.Jullien D, Sieling PA, Uyemura K, Mar ND, Rea TH, Modlin RL. IL-15, an immunomodulator of T cell responses in intracellular infection. J Immunol. 1997;158:800–6. [PubMed] [Google Scholar]
- 25.Frahm M, Goswami ND, Owzar K, et al. Discriminating between latent and active tuberculosis with multiple biomarker responses. Tuberculosis. 2011;91:250–6. doi: 10.1016/j.tube.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeurer M, Seliger B, Trinder P, Gerdes J, Seitzer U. Interleukin-15 in mycobacterial infection of antigen-presenting cells. Scand J Immunol. 1999;50:280–8. doi: 10.1046/j.1365-3083.1999.00593.x. [DOI] [PubMed] [Google Scholar]
- 27.Garcia VE, Jullien D, Song M, Uyemura K, Shuai K, Morita CT, Modlin RL. IL-15 enhances the response of human γδ T cells to nonpeptide (correction of nonpetide) microbial antigens. J Immunol. 1998;160:4322–9. [PubMed] [Google Scholar]
- 28.Martino A, Casetti R, Sacchi A, Poccia F. Central memory Vγ9Vδ2 T lymphocytes primed and expanded by bacillus Calmette–Guérin-infected dendritic cells kill mycobacterial-infected monocytes. J Immunol. 2007;179:3057–64. doi: 10.4049/jimmunol.179.5.3057. [DOI] [PubMed] [Google Scholar]
- 29.Meraviglia S, Caccamo N, Salerno A, Sireci G, Dieli F. Partial and ineffective activation of Vγ9Vδ2 T cells by Mycobacterium tuberculosis-infected dendritic cells. J Immunol. 2010;185:1770–6. doi: 10.4049/jimmunol.1000966. [DOI] [PubMed] [Google Scholar]
- 30.Caccamo N, Meraviglia S, Ferlazzo V, et al. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vγ9Vδ2 naive, memory and effector T cell subsets. Eur J Immunol. 2005;35:1764–72. doi: 10.1002/eji.200525983. [DOI] [PubMed] [Google Scholar]
- 31.Costa G, Loizon S, Guenot M, et al. Control of Plasmodium falciparum erythrocytic cycle: γδ T cells target the red blood cell-invasive merozoites. Blood. 2011;118:6952–62. doi: 10.1182/blood-2011-08-376111. [DOI] [PubMed] [Google Scholar]
- 32.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–94. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 33.Adkins B. T cell function in newborn mice and humans. Immunol Today. 1999;20:330–5. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 34.Adkins B. Development of neonatal Th1/Th2 function. Int Rev Immunol. 2000;19:157–71. doi: 10.3109/08830180009088503. [DOI] [PubMed] [Google Scholar]
- 35.Adkins B, Bu Y, Guevara P. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J Immunol. 2001;166:918–25. doi: 10.4049/jimmunol.166.2.918. [DOI] [PubMed] [Google Scholar]
- 36.Delespesse G, Yang LP, Ohshima Y, Demeure C, Shu U, Byun DG, Sarfati M. Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors. Vaccine. 1998;16:1415–9. doi: 10.1016/s0264-410x(98)00101-7. [DOI] [PubMed] [Google Scholar]
- 37.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–91. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonte D, Willems F, Goldman M. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199:1011–6. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marodi L. IL-12 and IFN-γ deficiencies in human neonates. Pediatr Res. 2001;49:316. doi: 10.1203/00006450-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006;118:137–44. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Naderi N, Pourfathollah AA, Alimoghaddam K, Moazzeni SM. Cord blood dendritic cells prevent the differentiation of naive T-helper cells towards Th1 irrespective of their subtype. Clin Exp Med. 2009;9:29–36. doi: 10.1007/s10238-008-0020-2. [DOI] [PubMed] [Google Scholar]
- 42.Mold JE, Venkatasubrahmanyam S, Burt TD, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–9. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbons DL, Haque SF, Silberzahn T, Hamilton K, Langford C, Ellis P, Carr R, Hayday AC. Neonates harbour highly active γδ T cells with selective impairments in preterm infants. Eur J Immunol. 2009;39:1794–806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- 44.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vγ2Vδ2 T cells. J Immunol. 2010;184:7268–80. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moens E, Brouwer M, Dimova T, Goldman M, Willems F, Vermijlen D. IL-23R and TCR signaling drives the generation of neonatal Vγ9Vδ2 T cells expressing high levels of cytotoxic mediators and producing IFN-γ and IL-17. J Leukoc Biol. 2011;89:743–52. doi: 10.1189/jlb.0910501. [DOI] [PubMed] [Google Scholar]
- 46.Cairo C, Mancino G, Cappelli G, Pauza CD, Galli E, Brunetti E, Colizzi V. Vδ2 T-lymphocyte responses in cord blood samples from Italy and Cote d'Ivoire. Immunology. 2008;124:380–7. doi: 10.1111/j.1365-2567.2007.02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelmann I, Moeller U, Santamaria A, Kremsner PG, Luty AJ. Differing activation status and immune effector molecule expression profiles of neonatal and maternal lymphocytes in an African population. Immunology. 2006;119:515–21. doi: 10.1111/j.1365-2567.2006.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazzola TN, Da Silva MT, Moreno YM, et al. Robust γδ+ T cell expansion in infants immunized at birth with BCG vaccine. Vaccine. 2007;25:6313–20. doi: 10.1016/j.vaccine.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 49.Tastan Y, Arvas A, Demir G, Alikasifoglu M, Gur E, Kiray E. Influence of Bacillus Calmette–Guérin vaccination at birth and 2 months old age on the peripheral blood T cell subpopulations [γδ and αβ T cell] Pediatr Allergy Immunol. 2005;16:624–9. doi: 10.1111/j.1399-3038.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 50.Cairo C, Propp N, Hebbeler AM, Colizzi V, Pauza CD. The Vγ2/Vδ2 T cell repertoire in Macaca fascicularis: functional responses to phosphoantigen stimulation by the Vγ2/Jγ1.2 subset. Immunology. 2005;115:197–205. doi: 10.1111/j.1365-2567.2005.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander AA, Maniar A, Cummings JS, et al. Isopentenyl pyrophosphate-activated CD56+ γδ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232–40. doi: 10.1158/1078-0432.CCR-07-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Libero G, Casorati G, Giachino C, Carbonara C, Migone N, Matzinger P, Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral γδ T cells. J Exp Med. 1991;173:1311–22. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–7. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamashita S, Tanaka Y, Harazaki M, Mikami B, Minato N. Recognition mechanism of non-peptide antigens by human γδ T cells. Int Immunol. 2003;15:1301–7. doi: 10.1093/intimm/dxg129. [DOI] [PubMed] [Google Scholar]
- 55.McVay LD, Carding SR. Extrathymic origin of human γδ T cells during fetal development. J Immunol. 1996;157:2873–82. [PubMed] [Google Scholar]
- 56.Delfau MH, Hance AJ, Lecossier D, Vilmer E, Grandchamp B. Restricted diversity of V γ 9-JP rearrangements in unstimulated human γδ T lymphocytes. Eur J Immunol. 1992;22:2437–43. doi: 10.1002/eji.1830220937. [DOI] [PubMed] [Google Scholar]
- 57.McVay LD, Carding SR. Generation of human γδ T cell repertoires. Crit Rev Immunol. 1999;19:431–60. [PubMed] [Google Scholar]
- 58.Venturi V, Kedzierska K, Price DA, Doherty PC, Douek DC, Turner SJ, Davenport MP. Sharing of T cell receptors in antigen-specific responses is driven by convergent recombination. Proc Natl Acad Sci U S A. 2006;103:18691–6. doi: 10.1073/pnas.0608907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venturi V, Price DA, Douek DC, Davenport MP. The molecular basis for public T cell responses? Nat Rev Immunol. 2008;8:231–8. doi: 10.1038/nri2260. [DOI] [PubMed] [Google Scholar]
- 60.Quigley MF, Greenaway HY, Venturi V, et al. Convergent recombination shapes the clonotypic landscape of the naive T cell repertoire. Proc Natl Acad Sci U S A. 2010;107:19414–9. doi: 10.1073/pnas.1010586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carson WE, Fehniger TA, Haldar S, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Investig. 1997;99:937–43. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warren HS, Kinnear BF, Kastelein RL, Lanier LL. Analysis of the costimulatory role of IL-2 and IL-15 in initiating proliferation of resting (CD56dim) human NK cells. J Immunol. 1996;156:3254–9. [PubMed] [Google Scholar]
- 64.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Investig. 2005;115:1177–87. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–12. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang C, Yamada H, Shibata K, Yoshida S, Wajjwalku W, Yoshikai Y. IL-15 protects antigen-specific CD8+ T cell contraction after Mycobacterium bovis bacillus Calmette–Guérin infection. J Leukoc Biol. 2009;86:187–94. doi: 10.1189/jlb.0608363. [DOI] [PubMed] [Google Scholar]
- 67.Villinger F, Miller R, Mori K, Mayne AE, Bostik P, Sundstrom JB, Sugimoto C, Ansari AA. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22:3510–21. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 69.Urban EM, Li H, Armstrong C, Focaccetti C, Cairo C, Pauza CD. Control of CD56 expression and tumor cell cytotoxicity in human Vγ2Vδ2 T cells. BMC Immunol. 2009;10:50. doi: 10.1186/1471-2172-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cookson S, Reen D. IL-15 drives neonatal T cells to acquire CD56 and become activated effector cells. Blood. 2003;102:2195–7. doi: 10.1182/blood-2003-01-0232. [DOI] [PubMed] [Google Scholar]
- 71.Correia MP, Costa AV, Uhrberg M, Cardoso EM, Arosa FA. IL-15 induces CD8+ T cells to acquire functional NK receptors capable of modulating cytotoxicity and cytokine secretion. Immunobiology. 2011;216:604–12. doi: 10.1016/j.imbio.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 72.Choi SS, Chhabra VS, Nguyen QH, Ank BJ, Stiehm ER, Roberts RL. Interleukin-15 enhances cytotoxicity, receptor expression, and expansion of neonatal natural killer cells in long-term culture. Clin Diagn Lab Immunol. 2004;11:879–88. doi: 10.1128/CDLI.11.5.879-888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kolibab K, Yang A, Derrick SC, Waldmann TA, Perera LP, Morris SL. Highly persistent and effective prime/boost regimens against tuberculosis that use a multivalent modified vaccine virus Ankara-based tuberculosis vaccine with interleukin-15 as a molecular adjuvant. Clin Vaccine Immunol. 2010;17:793–801. doi: 10.1128/CVI.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saito K, Yajima T, Kumabe S, Doi T, Yamada H, Sad S, Shen H, Yoshikai Y. Impaired protection against Mycobacterium bovis bacillus Calmette–Guérin infection in IL-15-deficient mice. J Immunol. 2006;176:2496–504. doi: 10.4049/jimmunol.176.4.2496. [DOI] [PubMed] [Google Scholar]
- 75.Singh V, Gowthaman U, Jain S, Parihar P, Banskar S, Gupta P, Gupta UD, Agrewala JN. Coadministration of interleukins 7 and 15 with bacille Calmette–Gurin mounts enduring T cell memory response against Mycobacterium tuberculosis. J Infect Dis. 2010;202:480–9. doi: 10.1086/653827. [DOI] [PubMed] [Google Scholar]
- 76.Tang C, Yamada H, Shibata K, et al. Efficacy of recombinant bacille Calmette–Guérin vaccine secreting interleukin-15/antigen 85B fusion protein in providing protection against Mycobacterium tuberculosis. J Infect Dis. 2008;197:1263–74. doi: 10.1086/586902. [DOI] [PubMed] [Google Scholar]
- 77.Umemura M, Nishimura H, Saito K, Yajima T, Matsuzaki G, Mizuno S, Sugawara I, Yoshikai Y. Interleukin-15 as an immune adjuvant to increase the efficacy of Mycobacterium bovis bacillus Calmette–Guérin vaccination. Infect Immun. 2003;71:6045–8. doi: 10.1128/IAI.71.10.6045-6048.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


