Abstract
Modulation and suppression of the immune response of the host by nematode parasites have been reported extensively and the cysteine protease inhibitor (CPI or cystatin) is identified as one of the major immunomodulators. In the present study, we cloned and produced recombinant CPI protein from the murine nematode parasite Heligmosomoides polygyrus (rHp-CPI) and investigated its immunomodulatory effects on dendritic cell (DC) function and immune responses in mice. Bone-marrow-derived CD11c+ DC (BMDC) that were exposed to rHp-CPI during the differentiation stage showed reduced MHC-II molecule expression compared with BMDC that were generated in normal culture conditions. The BMDC generated in the presence of rHp-CPI also exhibited reduced expression of CD40, CD86 and MHC-II molecules and reduced interleukin-6 and tumour necrosis factor-α cytokine production when stimulated with Toll-like receptor ligand CpG. Activation of BMDC generated in normal conditions induced by lipopolysaccharide and CpG was also suppressed by rHp-CPI, as shown by reduced co-stimulatory molecule expression and cytokine production. Furthermore, BMDC treated with rHp-CPI before ovalbumin (OVA) antigen pulsing induced a weaker proliferation response and less interferon-γ production of OVA-specific CD4+ T cells compared with BMDC without rHp-CPI pre-treatment. Adoptive transfer of rHp-CPI-treated and OVA-loaded BMDC to mice induced significantly lower levels of antigen-specific antibody response than the BMDC loaded with antigen alone. These results demonstrated that the CPI from nematode parasites is able to modulate differentiation and activation stages of BMDC. It also interferes with antigen and MHC-II molecule processing and Toll-like receptor signalling pathway, resulting in functionally deficient DC that induce a suboptimum immune response.
Keywords: cysteine protease inhibitor, dendritic cell, immunosuppression, nematode
Introduction
Nematode parasite infections are common in many parts of the world and cause significant health problems in humans.1 Infections with this group of pathogens often undergo a chronic and asymptomatic course and induce a T helper type 2-dominated immune response.2,3 In addition, nematode infections often induce immunosuppression, which is believed to be an important strategy for the survival of the parasite in the host.4,5 The immunosuppression associated with nematode infection is also demonstrated as the suppression of immune responses to unrelated antigens and immune protection against concurrent infection with other pathogens.6,7 Epidemiological studies showed that helminth infections in human populations are also associated with decreased prevalence of autoimmune disorders and allergic diseases (hygiene hypothesis).8,9
Although nematode infections are known to elicit T helper type 2-dominant immune responses, which are required for immune protection against the nematode pathogens,10 many studies show that these pathogens also induce a regulatory T-cell response and cytokines that mediate the immunosuppression.11–13 In mice infected with the murine nematode parasite, Heligmosomoides polygyrus, we identified a subset of dendritic cells (DC) that are selectively expanded following H. polygyrus infection and induce interleukin-10 (IL-10) production by T cells and FoxP3+ CD4+ T-cell response.14 Previous studies with H. polygyrus and other nematode species also demonstrated that the crude preparation or excretory–secretory (ES) products from the parasites are able to modulate the phenotypes and functions of immune cells.15–17 It has been reported that the ES products from H. polygyrus can modulate the antigen presentation function of DC and specifically induce an IL-10-producing T-cell response.15 However, the immunoregulatory molecule(s) produced by H. polygyrus have not been fully characterized.
A number of studies in recent years have shown that cysteine proteases inhibitor (CPI; cystatin) is one of the major immune modulators produced by nematode parasites.18,19 Cystatin modulates the activity of cathepsins in the endosome of DC and so interferes with the antigen presentation.20,21 It is also reported that cystatin could induce tumour necrosis factor-α (TNF-α) and IL-10 synthesis, or stimulate production of nitric oxide, which is an inhibitor of parasitic cysteine proteases.22,23 In the present study, we cloned the CPI gene from H. polygyrus, produced the recombinant protein and analysed its immune modulatory activity. We observed that the recombinant CPI from H. polygyrus (rHp-CPI) significantly modulated not only DC differentiation from precursor, but also the phenotype and function of the mature DC in vitro. In vivo study also showed that rHp-CPI can down-regulate the antibody response to antigen stimulation.
Material and methods
Animal and parasite
Six- to 10-week-old female BALB/c mice were obtained from Vital River Laboratory (Beijing, China). DO11.10 ovalbumin (OVA) -specific T-cell receptor (TCR) transgenic mice (on BALB/c background) were purchased from the Nanjing University Model Animal Research Centre (Nanjing, China). Mice were housed in the animal facility of the Guangzhou Institutes of Biomedicine and Health under specific pathogen-free conditions. All animal experiments were carried out in accordance with the national animal protection guidelines and approved by the Institutional Animal Care and Use Committee. The H. polygyrus parasites were kindly provided by Dr M. Scott (McGill University, Montreal, Canada) and maintained in BALB/c mice as previously described.24 To prepare ES products from the parasite, BALB/c mice were infected by oral inoculation with 400 third-stage larvae (L3) and killed 20 days after infection. The H. polygyrus adult worms were collected from the small intestine, washed extensively with sterile endotoxin-free PBS (Ginuo, Hangzhou, China) containing 200 U/ml penicillin and 200 mg/ml streptomycin (HyClone, Beijing, China) and cultured at a density of approximately 1000 worms/ml of RPMI-1640 medium (Invitrogen, Shanghai, China) supplemented with 2% glucose (Sigma-Aldrich, Rockville, MD) and antibiotics for 36 hr at 37°. The supernatant was harvested, centrifuged to remove eggs and worm debris, and stored at −80° until used.
Cloning, expression and purification rHp-CPI
Heligmosomoides polygyrus adult worms were collected from the intestines of mice 3 weeks after H. polygyrus L3 infection. Total RNA was isolated from adult worm homogenate using an RNA isolation kit (Omega Bio-Tek, Guangzhou, China) and reverse transcribed (Promega Corporation, Madison, WI). The cDNA fragment of CPI was amplified with Taq DNA polymerase (TaKaRa, Dalian, China). The sense 5′-TCA TCT CAA GTT GTT GCT GG-3′ and antisense 5′-AAT CTT CCC ATG GCT TCT-3′ primer sequences used for amplification were based on conserved sequences of cystatins previously described for Nippostrongylus brasiliensis, Onchocerca volvulus, Brugia malayi, Haemonchus contortus and Caenorhabditis elegans in GenBank. Based on the nucleotide sequence of cDNA fragments, specific primers were synthesized for 3′- and 5′-rapid amplification of cDNA ends (RACE; TaKaRa Biotechnology, Dalian, China) and used to determine the transcriptional start and terminal sites of CPI transcripts. The full-length cystatin cDNA obtained by RACE was subcloned into expression plasmid vector pET32a and expressed in Escherichia coli (Origami) as a protein fused to a leader sequence of Tobacco Etch virus (TEV) protease and six histidines. The recombinant fusion protein was purified from E. coli lysate by affinity chromatography using chelating Sepharose FF resin (GE Healthcare, Uppsala, Sweden). The His-peptide in the fusion protein was cut off by TEV protease (kindly provided by Dr J. Liu, Guangzhou Institutes of Biomedicine and Health, Guangzhou, China). The purity of the protein obtained was determined by SDS–PAGE and silver staining.
Measurement of protease inhibition activity of rHp-CPI
The activities of cysteine proteases, cathepsin B, C, L and S, was measured following the methods as described by others with some modifications.25 Bovine cathepsin B and C were purchased from Sigma and human cathepsin L and S were purchased from Calbiochem (Shanghai, China) and Enzo (New York, NY), respectively. The fluorogenic substrates for cathepthin B (Z-Arg-Arg-AMC; Sigma–Aldrich), cathepsin C (Gly-PhE-naphthylamide; Sigma-Aldrich), cathepsin S (Z-Phe-Arg-7-amido-4- methylcoumarin; Calbiochem) and cathepsin L (Z-Phe-Arg-7-amido-4-methyl coumarin; Calbiochem) were obtained from individual suppliers. To measure the inhibition activity of rHp-CPI, the protease was incubated with substrate in the absence or presence of serially diluted rHp-CPI in appropriate buffer for 15 min. The amount of product was measured fluorometrically with excitation at 360 nm and emission at 460 nm using a multiwall fluorescence spectrometer (Bio-Tek, Synergy HT, Corning, NY).
Generation of Hp-CPI monoclonal antibody
Monoclonal antibody (mAb) against rHp-CPI was generated following the standard protocol.26 Briefly, female BALB/c mice were immunized subcutaneously with 40 μg rHp-CPI emulsified in complete Freund's adjuvant (Sigma-Aldrich) and boosted twice at 4-week interval with 20 μg rHp-CPI in incomplete Freund's adjuvant. Spleen cells were isolated from the immunized BALB/c mice 1 week after final boosting, and fused with logarithmically growing SP2/0 myeloma cells at a ratio of 1 : 1 in the presence of polyethylene glycol 1500 (Roche, Basle, Switzerland). The treated cells were re-suspended in RPMI-1640 medium supplemented with 20% fetal calf serum, OPI (oxaloacetate, pyruvate, insulin) and HAT (hypoxanthine, aminopterin, thymidine) media supplements (Sigma-Aldrich) and plated into 96-well tissue culture plates at a density of 2·0 × 105 cells per well in a volume of 200 μl. After culturing at 37° with 5·0% CO2 for 7–10 days, the culture wells were screened using indirect ELISA for the presence of anti-rHp-CPI antibody. The cells in positive wells were collected and subjected to cloning by limited dilution. The cloned hybridoma cells were injected into the peritoneal cavity of naive mice. Ascites was collected 10 days after cell implantation and centrifuged at 10 000 g. to remove cells and debris and stored at −20°.
Generation of bone-marrow-derived DC
Bone marrow-derived dendritic cells (BMDC) were generated by culture of bone marrow cells following the method described by Lutz et al.27 Briefly, total bone marrow cells were collected from the femurs and tibias of BALB/c mice, suspended in RPMI-1640 medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (HyClone), 100 U penicillin/ml, 100 mg streptomycin/ml and 50 μm β-mercaptoethanol (Sigma–Aldrich) (complete medium). After lysing red blood cells with ammonium chloride buffer (0·15 m NH4Cl, 10 mm KHCO3 and 0·1 mm Na2 EDTA) and washing with complete medium, bone marrow cells were re-suspended in complete medium that was further supplemented with 10% supernatant from a mouse granulocyte–macrophage colony-stimulating factor (GM-CSF) -transfected cell line (Ag8653, kindly provided by Dr B. Stockinger, National Institute for Medical Research, London, UK) as a source of GM-CSF.28 Cells were cultured at 4 × 106/well in six-well plates (Greiner Bio-one, Frickenhausen, Germany) at 37° for 7–9 days in a humidified CO2 incubator. Cells were fed on days 3, 5 and 7 with complete medium containing GM-CSF supernatant. On day 9, non-adherent cells were collected, washed and used as immature BMDC. Cell viability was determined by trypan blue exclusion test and was 90–94% for the two groups of BMDC. The purity of BMDC was about 70–80% CD11c+ cells as determined by flow cytometry.
To analyse the effects of rHp-CPI on DC differentiation, rHp-CPI (50 μg/ml) were added in appropriate wells beginning at day 3 of culture and the cells were harvested on day 9 and analysed for cell surface molecule expression. In the preliminary experiments, graded doses of rHp-CPI were tested and the dose of 50 μg/ml rHp-CPI was found to be optimum. To investigate the effects of rHp-CPI on DC maturation, the bone marrow cells were cultured in the absence of rHp-CPI as described above for 7 days. The differentiated CD11c+ DC were harvested and activated with 1 μg/ml lipopolysaccharide (LPS; Sigma–Aldrich) or 1 μm CpG oligonucleotide (Invitrogen) with or without rHp-CPI for 18 hr.15,29 Control DC were cultured in complete medium alone. The DC were harvested and analysed for the expression of surface molecules and the cell culture supernatants were collected and stored at −20° for determination of cytokines.
DC and CD4+ T-cell isolation and co-culture
Bone marrow-derived dendritic cells were enriched by positive selection with anti-CD11c magnetic beads (Stemcell Technologies Inc., Vancouver, BC, Canada) according to the manufacturer's instructions. The enriched DC were typically of > 90% purity as determined by flow cytometry. CD4+ T cells in spleen were enriched by magnetic sorting using anti-CD4 magnetic beads (Miltenyi Biotec, Auburn, CA). The enriched CD4+ T cells had > 95% purity. To determine CD4+ T-cell proliferation induced by DC, the enriched BMDC were incubated with rHp-CPI (50 μg/ml) for 2 hr and OVA (1 mg/ml; Calbiochem) was added and incubated for another 2 hr. The DC were then treated with 50 μg/ml mitomycin (Sigma–Aldrich) for 20 min and washed with a sufficient amount of complete medium to remove the mitomycin. Dendritic cells (2 × 104/well) were co-cultured with CD4+ T cells (4 × 104/well) in a 96-well U-bottom plate in the presence of 1 mg/ml OVA for 72 hr. During the last 18 hr, 1 μCi/well of [3H]thymidine was added. Incorporation of [3H]thymidine by the cells was determined by scintillation counting. For determination of cytokine production in DC and CD4+ T-cell co-culture, 2 × 105 CD4+ T cells were co-cultured with 1 × 105 DC in U-bottom plates in the presence of 1 mg/ml OVA for 72 hr. Supernatants were harvested for cytokine analysis by ELISA.
BMDC in vivo transfer
The modulatory effect of rHp-CPI on DC function was analysed by DC transfer experiment. The BMDC were re-suspended at 2 × 106 cells/ml in complete medium and treated with rHp-CPI (50 μg/ml) for 3 hr before pulsing with 1 mg/ml OVA for 4 hr at 37°. After pulsing, cells were harvested, washed extensively with sterile endotoxin-free PBS and re-suspended in RPMI-1640 medium with 5% BALB/c mouse serum. Mice were injected intravenously with 5 × 105 BMDC. Four weeks after DC injection, BALB/c mice were injected intraperitoneally with 10 μg OVA protein emulsified in incomplete Freund's adjuvant (Sigma-Aldrich). Sera were collected 4 weeks after OVA injection and OVA-specific antibody levels were determined by ELISA.
Flow cytometric analysis
For cell surface staining, 106 cells were first incubated with FcR-blocking reagent (BD Biosciences, New York, NY) in sorting buffer (PBS with 1% BSA) on ice for 15 min. The cells were then washed and stained with anti-CD11c-FITC, anti-CD40-phycoerythrin (PE), anti-CD80-PE, anti-CD86-PE and anti-MHC-II-PE fluorescent mAbs (all from eBiosciences, San Diego, CA) following standard protocols. Isotype-matched mAbs were used for control staining. Cells were then washed and re-suspended in sorting buffer and analysed by flow cytometry using FACS Calibur (BD Biosciences). At least 10 000 events were acquired per sample, and the data analysis was performed using Flowjo software (TreeStar, Ashland, OR).
Cytokine and antibody determination by ELISA
Cytokine levels in cell culture supernatants were determined using ELISA kits for IL-12p40, TNF-α, IL-6 and interferon-γ (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Serum levels of OVA-specific antibodies were determined by ELISA. Briefly, ELISA plates were coated with OVA antigen overnight at 4° and subsequently blocked with 1% BSA in PBS for 1·5 hr. After washing, serially diluted serum samples were added and incubated for 1 hr at room temperature. After extensive washing, horseradish peroxidase-conjugated goat anti-mouse total immunoglobulin, IgG1 and IgG2a antibodies (Southern Biotechnology Associates, Birmingham, AL) were added and incubated at room temperature for 1 hr. Reactivity was visualized by addition of substrate and optical density values were read in a microplate reader. Antibody levels in serum are expressed as endpoint titres, the reciprocal of the lowest dilution that yields the background optical density.
Immunoblotting
For immunoblotting, proteins were separated by SDS–PAGE and the gels were electroblotted onto a PVDF membrane (Pall Corporation, East Hills, NY). Anti-rHp-CPI mAb was used as the primary antibody, and horseradish peroxidase-conjugated anti-mouse IgG (Thermo Fisher Scientific, Guangzhou, China) diluted 1 : 50 000 was used as the secondary antibody. Bound antibody was detected using enhanced chemiluminescence reagents (Thermo Fisher Scientific).
Statistical analyses
Statistical analyses were performed with GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA). Significance of differences between groups was analysed using the Student's t-test. Data are presented as mean ± SD. A P-value < 0·05 was considered significant.
Results
Molecular cloning and biological activity analysis of rHp-CPI
Cystatin is known to be conserved in eukaryotes and has been identified in many species of nematode parasite.18 To determine if the H. polygyrus parasite has the CPI gene, we screened the cDNA library of H. polygyrus by RT-PCR using the primers for consensus sequences of cystatin reported in other nematode parasites, and obtained a fragment of cystatin. We then obtained the full length of CPI gene from H. polygyrus (Hp-CPI) using the RACE technique. The open reading frame of Hp-CPI has 432 bp, and the cloned protein (rHp-CPI) consisted of 143 amino acids (Fig. 1a). Comparison of the Hp-CPI amino acid sequence with cystatins from other nematodes showed various levels of homology (Fig. 1a). As observed in cystatin from other nematode species, the CPI protein from H. polygyrus contains a signal peptide of 21 amino acids indicating that the Hp-CPI is a secreted protein. In immunoblotting assay, we confirmed that the mAb generated against the rHp-CPI was able to react with a protein component of 14 000 molecular weight from the excretory and secretory products prepared from adult H. polygyrus (Fig. 1b).
Figure 1.
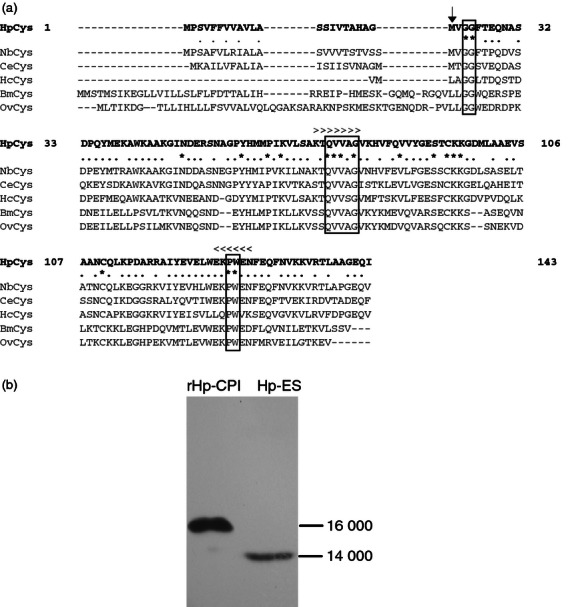
Cysteine protease inhibitor (CPI) of Heligmosomoides polygyrus. (a) Alignment of the amino acid sequences of CPI from H. polygyrus (HpCys), Nippostrongylus brasiliensis (NbCys), Caenorhabditis elegans (CeCys), Haemonchus contortus (HcCys), Brugia malayi (BmCys) and Onchocerca volvulus (OvCys). Three segments of sequences conserved in cystatin superfamily are boxed. Amino acids conserved among the six nematode species are indicated by asterisks and partially conserved amino acids are indicated by dots. Gene-specific PCR primers for cloning Hp-CPI cDNA were designed on the basis of conserved sequences indicated by > for the 5′ primer and by < for the 3′ primer. The first amino acid of mature Hp-CPI is indicated by an arrow. (b) Immunoblotting analysis of recombinant CPI from H. polygyrus (rHp-CPI) and excretory–secretory (ES) products probed with monoclonal antibodies (mAb). The rHp-CPI (signal peptide not removed) and ES products were separated by SDS–PAGE and detected by anti-rHp-CPI mAb.
We then examined the protease inhibitory ability of rHp-CPI to confirm the biological activity of the rHp-CPI protein. The rHp-CPI was produced in E. coli, affinity-purified and analysed for its ability to inhibit the proteolytic activity of cathepsin B, C, L and S, which are known to be important in functions of antigen presentation cells.30,31 We observed that rHp-CPI inhibited the proteolytic activities of cathepsin B, C, L and S in a dose-dependent manner (Fig. 2a), indicating that the recombinant CPI protein from H. polygyrus possesses the biological function of protease inhibition activity. We also analysed the protease inhibitory activity of the ES products from H. polygyrus and observed that H. polygyrus ES products were able to inhibit the proteolytic activities of cathepsin B, C, L and S (Fig. 2b).
Figure 2.
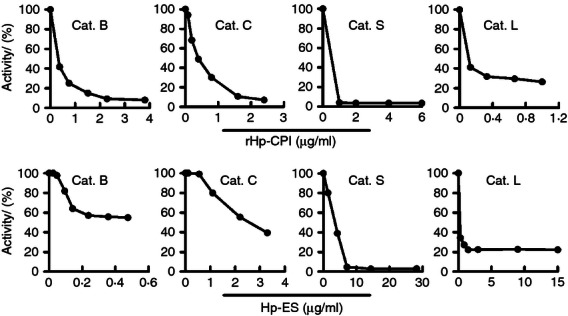
Inhibition of cysteine proteases by recombinant cysteine protease inhibitor from Heligmosomoides polygyrus (rHp-CPI) and excretory–secretory (ES) products of H. polygyrus. Purified cathepsin B (Cat. B), cathepsin C (Cat. C), cathepsin S (Cat. S) and cathepsin L (Cat. L) were incubated with fluorogenic substrates in the presence of different concentrations of rHp-CPI or ES products and the amounts of products were measured using a multiwall fluorescence spectrometer. Products generated in cathepsin and substrate reactions without rHp-CPI or ES are taken as 100% enzyme activity. Results presented are from one of three experiments.
Modulation of BMDC differentiation by rHp-CPI
Although H. polygyrus adult worms dwell in the intestinal lumen of their murine host, the ES products released by the parasites may have the opportunity to enter the bone marrow via the bloodstream or lymphatic system and the immunomodulatory molecules may interfere with the differentiation of DC progenitors.32 To address this question, we examined the modulatory effects of rHp-CPI on the differentiation of DC from BM precursors. Bone marrow cells were cultured in the presence of GM-CSF to induce DC differentiation and, in one group of cultures, rHp-CPI (50 μg/ml) was added on day 3 of culture. The two groups of BMDC were harvested on day 9 and analysed for cell surface co-stimulatory molecule expression by flow cytometry. Addition of rHp-CPI did not show apparent effects on the yield of BMDC (medium control group, 7·8 ± 1·0 × 106 total cells/plate, 79·1 ± 5·1% CD11c+ DC; rHp-CPI-treated group, 6·9 ± 1·2 × 106 total cells/plate, 74·7 ± 8·2% CD11c+ DC). We observed that, although the control and rHp-CPI-treated DC did not show significant differences in frequencies of CD40+, CD80+ and CD86+ cells in total CD11c+ DC and expression level (mean fluorescence intensity, MFI) of these co-stimulatory molecules, the BMDC that were exposed to rHp-CPI on day 3 of culture showed reduced expression of the MHC-II molecule by 48% (Fig. 3). The DC that were exposed to rHp-CPI starting on days 5 and 7 of culture also showed reductions in MHC-II molecules by 37% and 14%, respectively, in comparison with the control DC (data not shown).
Figure 3.
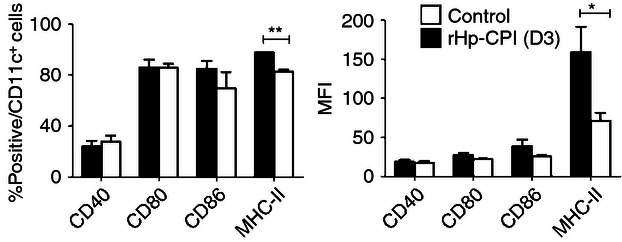
Phenotypes of bone-marrow-derived dendritic cells (BMDC) generated in cultures in the absence or presence of recombinant cysteine protease inhibitor from Heligmosomoides polygyrus (rHp-CPI). Bone marrow cells were cultured in medium alone (medium) or in the presence of rHp-CPI (rHp-CPI) added on day 3 of culture and BMDC were harvested on day 9. Frequencies and mean fluorescence intensity (MFI) of co-stimulatory and MHC-II molecule expression by CD11c+ BMDC were analysed by flow cytometry. (c) Data shown are means ± SD of triplicate samples from one of four experiments. Statistically significant differences, *P < 0·05 and **P < 0·01, are indicated.
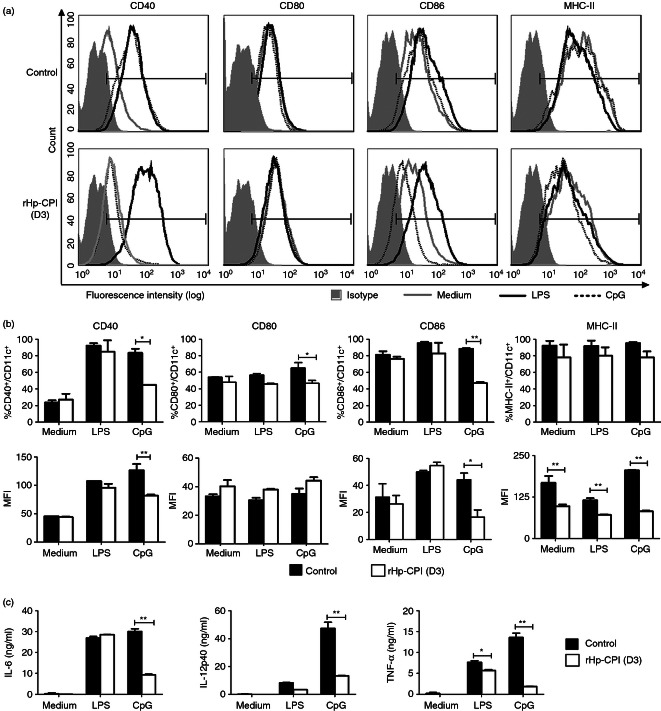
To further analyse the effects of rHp-CPI on DC differentiation, bone marrow cells were cultured for 9 days with or without rHp-CPI, stimulated with the Toll-like receptor (TLR) ligands LPS and CpG and then co-stimulatory molecule expression was examined. Both the control DC (cultured in medium alone) and rHp-CPI-treated DC showed increased expression of CD40 and CD86 in response to stimulation with LPS in comparison with unstimulated DC. Stimulation of control DC with CpG induced increased expression of CD40 whereas this CD40 expression response was absent in BMDC that were treated with rHp-CPI during the differentiation stage. Similarly, LPS stimulation increased the CD86 expression in both groups of BMDC, but the rHp-CPI-treated BMDC showed significantly lower levels of CD86 expression following CpG stimulation than the control BMDC. Furthermore, BMDC that were exposed to rHp-CPI during the differentiation stage exhibited significantly decreased expression of the MHC-II molecule in response to stimulation with LPS and CpG compared with the control DC (Fig. 4a,b). The BMDC exposed to rHp-CPI also produced lower levels of IL-6, IL-12p40 and TNF-α cytokines following CpG stimulation compared with the BMDC generated in normal culture conditions (Fig. 4c). These results demonstrate that exposure of BMDC to rHp-CPI during the differentiation stage modified their ability to respond to the activation signal provided by the TLR9 ligand CpG.
Figure 4.
Co-stimulatory molecules expression and cytokine production by bone-marrow-derived dendritic cells (BMDC) in response to stimulation with lipopolysaccharide (LPS) or CpG. Bone marrow cells were cultured in normal condition (control) or in the presence of recombinant cysteine protease inhibitor from Heligmosomoides polygyrus (rHp-CPI; added on day 3 of culture; rHp-CPI) for 9 days and immature BMDC were harvested and stimulated with LPS (1 μg/ml) or CpG (1 μm) for 18 hr. BMDC were harvested and stained for CD11c and co-stimulatory and MHC-II molecules and analysed by flow cytometry. (a) FACS histograms from one representative experiment. (b) Frequencies and mean fluorescence intensity (MFI) of co-stimulatory and MHC-II molecules expression by CD11c+ cells. Data shown are means ± SD of triplicate samples from one of three experiments. (c) Levels of cytokines in cell culture supernatants were analysed by ELISA. Data presented are means ± SD of triplicate samples from one of three experiments. Statistically significant differences, **P < 0·01, are indicated.
Impaired activation of differentiated DC by rHp-CPI
We next examined the modulatory effects of rHp-CPI on activation of immature BMDC. Bone marrow cells were cultured in the presence of GM-CSF for 7 days and the differentiated immature DC (> 70% CD11c+) were harvested. The harvested BMDC were divided into groups and further cultured for 18 hr in medium alone as control or in the presence of rHp-CPI, LPS, CpG, LPS plus rHp-CPI or CpG plus rHp-CPI. The BMDC were stained and analysed for the expression of co-stimulatory and MHC-II molecules. The results show that treatment of the immature DC with rHp-CPI alone reduced the expression of the MHC-II molecule but did not alter the frequencies of CD11c+ DC that express CD40, CD80 and CD86 and the expression levels of these molecules compared with medium control group (Fig. 5a,b). The immature DC stimulated with LPS showed significantly increased expression of CD40 and CD80 (both the frequencies of positive cells and the MFI) compared with medium control, and rHp-CPI treatment reduced the increased CD80 expression in response to LPS stimulation, but had no effect on CD40 expression (Fig. 5a,b). CpG stimulation of the immature BMDC also induced enhanced expression of CD40 and CD80. The rHp-CPI inhibited the increased expression of CD40 and CD80 induced by CpG (Fig. 5a,b).
Figure 5.
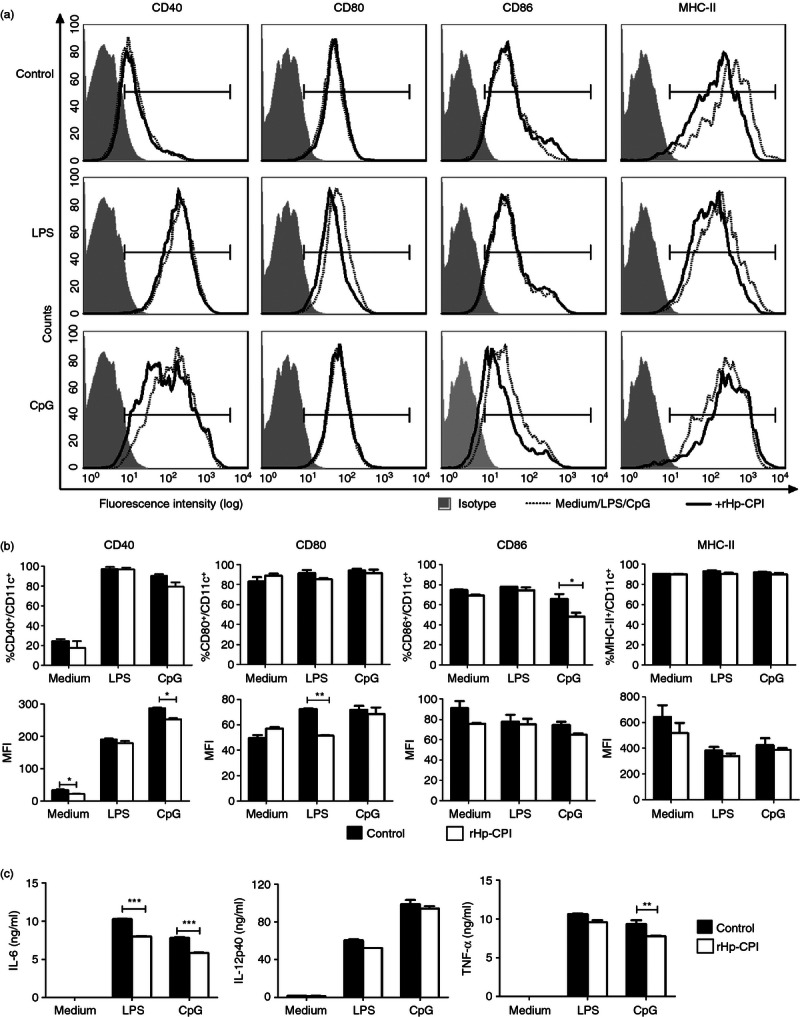
Effects of recombinant cysteine protease inhibitor from Heligmosomoides polygyrus (rHp-CPI) on co-stimulatory molecules expression and cytokine production by immature bone-marrow-derived dendritic cells (BMDC) following stimulation with lipopolysaccharide (LPS) or CpG. BMDC obtained from bone marrow cell culture for 7 days were stimulated with LPS (1 μg/ml) or CpG (1 μm) alone or in combination with rHp-CPI (50 μg/ml) for 18 hr. Cells were harvested and stained for CD11c and co-stimulatory and MHC-II molecule and phenotypic profiles were analysed by flow cytometry. (a) FACS histograms from one representative experiment. (b) Frequencies and mean fluorescence intensity (MFI) of co-stimulatory and MHC-II molecule expression by CD11c+ cells. Data shown are means ± SD of triplicate samples from one of three experiments. (c) Cell culture supernatants were harvested and the levels of cytokines produced by BMDC were analysed by ELISA. Data presented are means ± SD of triplicate samples from one of three experiments. Statistically significant differences, **P < 0·01, ***P < 0·001, are indicated.
We further examined the cytokine production by BMDC and observed that the differentiated immature BMDC with or without rHp-CPI treatment produced minimal levels of IL-6, IL-12p40 and TNF-α. Stimulation of the immature BMDC with LPS and CpG induced increased production of these pro-inflammatory cytokines. The rHp-CPI treatment reduced the IL-6 production induced by both LPS and CpG, and TNF-α production induced by CpG (Fig. 5c). These results show that although treatment of rHp-CPI alone did not alter immature BMDC co-stimulatory molecule expression and cytokine production, it modulates these activation responses of DC induced by LPS and CpG.
Suppressive effects of rHp-CPI on T-cell activation by BMDC
To determine whether the T-cell activation function of DC is altered by rHp-CPI, DC and CD4+ a T-cell co-culture assay was performed. Bone marrow cells were cultured in the medium containing GM-CSF as described above. The immature BMDC were harvested on day 7, re-plated and cultured for 24 hr to obtain matured DC. Mature BMDC were incubated either in medium alone or with rHp-CPI for 2 hr and then pulsed with OVA antigen. The two groups of DC were then co-cultured with OVA-specific CD4+ T at the ratio of 1 : 2. As shown in Fig. 6(a), BMDC treated with rHp-CPI before OVA antigen pulsing induced a lower level CD4+ T-cell proliferation response than the BMDC that were pulsed with OVA only. CD4+ T cells co-cultured with BMDC that were treated with rHp-CPI and pulsed with OVA produced significantly less interferon-γ than the CD4+ T cells co-cultured with BMDC pulsed with OVA only (Fig. 6b). In this DC and CD4 T-cell co-culture, no significant levels of IL-4, IL-10 and IL-13 were detected.
Figure 6.
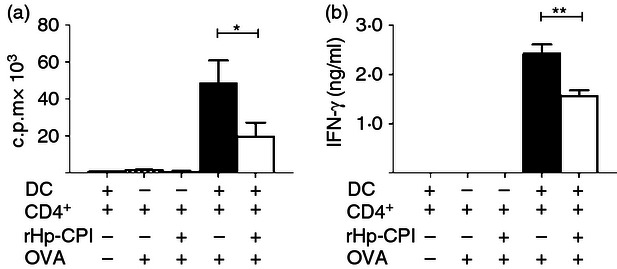
Antigen-specific CD4+ T-cell activation and cytokine production induced by bone-marrow-derived dendritic cells (BMDC) with or without pre-exposure to recombinant cysteine protease inhibitor from Heligmosomoides polygyrus (rHp-CPI). (a) Proliferation response of CD4+ T cells co-cultured with BMDC with our without treatment of rHp-CPI. Enriched CD11c+ BMDC were incubated with rHp-CPIor in medium and treated by mitomycin. After washing, BMDC were co-cultured with ovalbumin (OVA) -specific CD4+ T cells for 72 hr. Cell proliferation was determined by [3H]thymidine incorporation. (b) Cytokine secretion by CD4+ T cells co-cultured with BMDC. Enriched CD11c+ BMDC were treated and co-cultured with CD4+ T cells as described above and cytokine levels in supernatants were determined by ELISA. Results are shown as means ± SD of triplicate samples from one of two experiments. Statistically significant differences, *P < 0·05 and **P < 0·01, are indicated.
BMDC adoptive transfer and antibody response
Adoptive transfer of BMDC was performed to further assess the effect of rHp-CPI on the function of DC. Mice were transferred with enriched BMDC that were pulsed with OVA with or without pre-treatment of rHp-CPI and boosted 4 weeks later with OVA antigen. OVA-specific antibody levels were determined before and after OVA boosting. The two groups of recipient mice produced low levels of antibody in serum 4 weeks after transfer of BMDC and no significant difference in antibody response was observed between the two groups (Fig. 7a). However, OVA antigen boosting 4 weeks after BMDC transfer enhanced the antibody responses. Mice receiving BMDC that were treated with rHp-CPI and pulsed with OVA produced significantly less OVA-specific total immunoglobulin and IgG1 than the mice that received BMDC pulsed with OVA antigen only (Fig. 7b). No significant levels of IgG2a antibody were detected in the BMDC recipient mice and the mice injected with OVA antigen only (Fig. 7b). These data show that rHp-CPI is able to modify the DC phenotype and function resulting in impaired antibody response.
Figure 7.
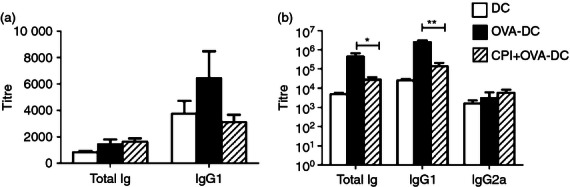
Serum levels of ovalbumin (OVA) -specific antibody in mice adoptively transferred OVA-loaded bone-marrow-derived dendritic cells (BMDC) with or without pre-exposure to recombinant cysteine protease inhibitor from Heligmosomoides polygyrus (rHp-CPI). Normal BMDC or BMDC pre-treated with rHp-CPI for 2 hr were incubated with OVA for 4 hr. The cells were washed and adoptively transferred intravenously (5 × 105/mouse) to recipient mice. The mice were boosted with OVA antigen 4 weeks later. Levels of OVA-specific antibodies in serum samples collected immediately before boosting (a) and 4 weeks after boosting (b) were determined by ELISA. Antibody levels are expressed as endpoint titres. Data shown are means ± SD of three or four mice per group from one of two independent experiments. Statistically significant differences, *P < 0·05 and **P < 0·01, are indicated.
Discussion
Immunosuppression that occurs following infection with murine nematode H. polygyrus has been documented extensively.33–35 The H. polygyrus-derived ES products have been shown to induce immunosuppression in hosts by impairing DC function.15 However, the parasite molecule(s) responsible for induction of immunosuppression are unknown. In this study, we cloned the CPI gene from H. polygyrus, produced recombinant protein rHp-CPI and examined its immunomodulatory effects. Our results demonstrated that the recombinant rHp-CPI protein is biologically functional as shown by its ability to inhibit the protease activity of a panel of cathepsins. Immunoblotting assays revealed that the mAb raised against the rHp-CPI protein was able to recognize a protein component in H. polygyrus ES products, indicating that H. polygyrus produces and secretes the CPI protein. Indeed, the ES products prepared from H. polygyrus adult worms showed inhibitory activity against cathepsins (Fig. 2).
There are several reports to show that nematode parasites that dwell in the gastrointestinal tract of their hosts are able to modulate the immune response systemically.21,36 In a previous study, we have shown that concurrent H. polygyrus infection impairs protective immunity against systemic malarial infection.24 A study by Goodridge et al.32 showed that the immunomodulatory glycoprotein ES-62 of a filarial nematode released by an osmotic pump implanted in the neck of mice is able to induce hyporesponsive DC derived ex vivo from the bone marrow cells of mice. These observations suggest that the immunomodulatory molecules released by adult H. polygyrus may modulate the functions of immune cells locally as well as in other organs of the immune system, including bone marrow where the DC progenitors differentiate and develop into immature DC. To verify this possible mechanism, bone marrow cells were cultured in the presence of rHp-CPI and the phenotypes of the differentiated CD11c+ DC were analysed. We observed that although rHp-CPI did not alter the number of differentiated DC harvested at the end of culture and the expression of co-stimulatory molecules CD40, CD80 and CD86, the rHp-CPI-treated BMDC showed significantly less MHC-II molecule expression in comparison with the DC that were cultured in the absence of rHp-CPI. The responses to stimulation with TLR ligands further revealed the difference between the two groups of differentiated BMDC. The BMDC exposed to rHp-CPI during its differentiation showed significantly lower percentages of CD40+, CD86+ and MHC-II+ cells and IL-6, IL-12p40 and TNF-α cytokine production when stimulated with TLR9 ligand CpG compared with the BMDC that were not exposed to rHp-CPI. Interestingly, the two groups of BMDC generated with or without exposure to rHp-CPI respond in similar manners to stimulation with TLR4 ligand LPS. It is known that a number of cysteine proteases are involved in signalling pathways associated with some TLRs. Proteolytic cleavage of TLR9 by cathepsins is required for TLR9 signalling. The BMDC from cathepsin L-deficient and S-deficient mice showed impaired responses to stimulation with CpG, but the response to LPS stimulation remained unchanged compared with the BMDC from normal wild-type mice.37 Our results that BMDC generated in the presence of rHp-CPI exhibit impaired responses to CpG stimulation, but showed unchanged responses to LPS stimulation, are consistent with the observations made on BMDC from cathepsin-deficient mice.
We then further analysed the modulatory effects of rHp-CPI on differentiated immature BMDC and observed that rHp-CPI treatment alone had no significant effect on DC activation, as shown by the expression of CD40, CD80 and CD86 that was comparable with those detected on control BMDC. In addition, rHp-CPI treatment alone failed to induce production of IL-16, IL-12p40 and TNF-α. These results indicate that the rHp-CPI protein of parasite origin has a negligible effect on differentiated immature BMDC. However, it was observed that rHp-CPI modulates the responses of immature BMDC to stimulation with LPS and CpG. Treatment of immature BMDC with rHp-CPI reduced the CD40 and CD86 expression and IL-6 and TNF-α cytokine production by immature BMDC induced by stimulation with CpG. Treatment with rHp-CPI also suppressed the expression of CD80 and MHC-II molecules and IL-6 production of BMDC induced by LPS stimulation. These results suggest that rHp-CPI modulates the TLR-associated signalling pathways differently at the different stages of BMDC development.
In addition to the modulation effects on responses to stimulation with TLR-associated signalling pathways, rHp-CPI treatment also resulted in impaired antigen-presenting function of BMDC. Cysteine proteases in endosomes and lysosomes of antigen-presenting cells are known to be involved in the processing of protein antigens and MHC-II molecule maturation. Cathepsin S plays an important role in stepwise proteolytic degradation of the invariant chain (Ii) that regulates MHC-II molecule intracellular trafficking and protects the MHC-II molecule from premature binding of antigen peptide.38 Cathepsin B and C are required for processing of antigen peptides and facilitate their binding to the MHC-II peptide-binding groove.39 It is reported that cystatin from Nippostrongylus brasiliensis inhibited the processing of OVA protein by lysosomal cysteine proteases from spleen cells of mice. We also observed in a related study that BMDC exposed to rHp-CPI showed a reduced rate of OVA antigen processing (unpublished observation). Inhibition of the activity of these cathepsins by CPI from H. polygyrus may result in reduced expression of MHC-II–antigen complex on the surface of antigen-presenting cells that are unable to competently activate CD4+ T cells and induce immune responses. We have demonstrated in this study that in the DC and CD4+ T-cell co-culture, the BMDC pre-treated with rHp-CPI exhibited a reduced ability to activate CD4+ T cells and to induce cytokine production. The recipient mice transferred with the BMDC treated with rHp-CPI before OVA antigen loading produced significantly lower levels of OVA-specific total immunoglobulin and IgG1 antibody compared with the mice receiving the BMDC that were loaded with OVA antigen alone, indicating that the antigen-presenting function of BMDC was impaired.
In summary, the results presented in this study demonstrate that the CPI from H. polygyrus exerts its immunomodulatory effects on multiple stages of BMDC development and molecular events that are important for the function of antigen-presenting cells. The observations made in this study may represent one of the important mechanisms by which the nematode parasites induce immunosuppression in the hosts.
Acknowledgments
This work was supported by a Grant to Z.S. from the National Natural Science Foundation of China (No. 30872370).
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Chan MS. The global burden of intestinal nematode infections – fifty years on. Parasitol Today. 1997;13:438–43. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- 2.Gause WC, Urban JF, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–77. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 3.Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–87. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites–masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 5.Else KJ. Have gastrointestinal nematodes outwitted the immune system? Parasite Immunol. 2005;27:407–15. doi: 10.1111/j.1365-3024.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 6.Buendia AJ, Fallon PG, Del Rio L, Ortega N, Caro MR, Gallego MC, Salinas J. Previous infection with the nematode Nippostrongylus brasiliensis alters the immune specific response against Chlamydophila abortus infection. Microb Pathog. 2002;33:7–15. doi: 10.1006/mpat.2002.0507. [DOI] [PubMed] [Google Scholar]
- 7.Chen CC, Louie S, McCormick BA, Walker WA, Shi HN. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J Immunol. 2006;176:472–83. doi: 10.4049/jimmunol.176.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massicot JG, Cohen SG. Epidemiologic and socioeconomic aspects of allergic diseases. J Allergy Clin Immunol. 1986;78:954–8. doi: 10.1016/0091-6749(86)90284-8. [DOI] [PubMed] [Google Scholar]
- 9.Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: implications for the hygiene hypothesis. J Allergy Clin Immunol. 2011;127:479–86. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urban JF, Jr, Madden KB, Svetic A, Cheever A, Trotta PP, Gause WC, Katona IM, Finkelman FD. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992;127:205–20. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 11.Diaz A, Allen JE. Mapping immune response profiles: the emerging scenario from helminth immunology. Eur J Immunol. 2007;37:3319–26. doi: 10.1002/eji.200737765. [DOI] [PubMed] [Google Scholar]
- 12.Grainger JR, Smith KA, Hewitson JP, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–41. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blankenhaus B, Klemm U, Eschbach ML, et al. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J Immunol. 2011;186:4295–305. doi: 10.4049/jimmunol.1001920. [DOI] [PubMed] [Google Scholar]
- 14.Li ZT, Liu GY, Chen Y, Liu YF, Liu BY, Su Z. The phenotype and function of naturally existing regulatory dendritic cells in nematode-infected mice. Int J Parasitol. 2011;41:1129–37. doi: 10.1016/j.ijpara.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37:1887–904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 16.Langelaar M, Aranzamendi C, Franssen F, Van der Giessen J, Rutten V, Van der Ley P, Pinelli E. Suppression of dendritic cell maturation by Trichinella spiralis excretory/secretory products. Parasite Immunol. 2009;31:641–5. doi: 10.1111/j.1365-3024.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- 17.Falcon C, Carranza F, Martinez FF, Knubel CP, Masih DT, Motran CC, Cervi L. Excretory-secretory products (ESP) from Fasciola hepatica induce tolerogenic properties in myeloid dendritic cells. Vet Immunol Immunopathol. 2010;137:36–46. doi: 10.1016/j.vetimm.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Vray B, Hartmann S, Hoebeke J. Immunomodulatory properties of cystatins. Cell Mol Life Sci. 2002;59:1503–12. doi: 10.1007/s00018-002-8525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knox DP. Proteinase inhibitors and helminth parasite infection. Parasite Immunol. 2007;29:57–71. doi: 10.1111/j.1365-3024.2006.00913.x. [DOI] [PubMed] [Google Scholar]
- 20.Manoury B, Gregory WF, Maizels RM, Watts C. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr Biol. 2001;11:447–51. doi: 10.1016/s0960-9822(01)00118-x. [DOI] [PubMed] [Google Scholar]
- 21.Dainichi T, Maekawa Y, Ishii K, Zhang T, Nashed BF, Sakai T, Takashima M, Himeno K. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect Immun. 2001;69:7380–6. doi: 10.1128/IAI.69.12.7380-7386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann S, Kyewski B, Sonnenburg B, Lucius R. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur J Immunol. 1997;27:2253–60. doi: 10.1002/eji.1830270920. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann S, Schonemeyer A, Sonnenburg B, Vray B, Lucius R. Cystatins of filarial nematodes up-regulate the nitric oxide production of interferon-γ-activated murine macrophages. Parasite Immunol. 2002;24:253–62. doi: 10.1046/j.1365-3024.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 24.Su Z, Segura M, Morgan K, Loredo-Osti JC, Stevenson MM. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect Immun. 2005;73:3531–9. doi: 10.1128/IAI.73.6.3531-3539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolenc I, Turk B, Pungercic G, Ritonja A, Turk V. Oligomeric structure and substrate induced inhibition of human cathepsin C. J Biol Chem. 1995;270:21626–31. doi: 10.1074/jbc.270.37.21626. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama WM, Christensen M, Dos Santos G, Miller D. Production of Monoclonal Antibodies. In: Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current Protocols in Immunology. Hoboken: John Wiley & Sons, Inc; 2006. pp. 2.5.1–25. [Google Scholar]
- 27.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 28.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J Exp Med. 1996;183:891–9. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavassani KA, Aliberti JC, Dias ARV, Silva JS, Ferreira BR. Tick saliva inhibits differentiation, maturation and function of murine bone-marrow-derived dendritic cells. Immunology. 2005;114:235–45. doi: 10.1111/j.1365-2567.2004.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa TY, Rudensky AY. The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunol Rev. 1999;172:121–9. doi: 10.1111/j.1600-065x.1999.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 31.Medd PG, Chain BM. Protein degradation in MHC class II antigen presentation: opportunities for immunomodulation. Semin Cell Dev Biol. 2000;11:203–10. doi: 10.1006/scdb.2000.0162. [DOI] [PubMed] [Google Scholar]
- 32.Goodridge HS, Marshall FA, Wilson EH, Houston KM, Liew FY, Harnett MM, Harnett W. In vivo exposure of murine dendritic cell and macrophage bone marrow progenitors to the phosphorylcholine-containing filarial nematode glycoprotein ES-62 polarizes their differentiation to an anti-inflammatory phenotype. Immunology. 2004;113:491–8. doi: 10.1111/j.1365-2567.2004.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finney CA, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4+ CD25+ regulatory T cells in Heligmosomoides polygyrus infection. Eur J Immunol. 2007;37:1874–86. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tetsutani K, Ishiwata K, Ishida H, Tu L, Torii M, Hamano S, Himeno K, Hisaeda H. Concurrent infection with Heligmosomoides polygyrus suppresses anti-Plasmodium yoelii protection partially by induction of CD4+CD25+Foxp3+ Treg in mice. Eur J Immunol. 2009;39:2822–30. doi: 10.1002/eji.200939433. [DOI] [PubMed] [Google Scholar]
- 35.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–66. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–14. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman HA. Endosomal proteases in antigen presentation. Curr Opin Immunol. 2006;18:78–84. doi: 10.1016/j.coi.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Riese RJ, Chapman HA. Cathepsins and compartmentalization in antigen presentation. Curr Opin Immunol. 2000;12:107–13. doi: 10.1016/s0952-7915(99)00058-8. [DOI] [PubMed] [Google Scholar]