Abstract
Ragweed pollen extract (RWE) possesses intrinsic NADPH oxidase activity that induces oxidative stress by initiating the production of intracellular reactive oxygen species (ROS). The ROS are important contributors to the manifestation of allergic inflammation; furthermore, concomitant exposure to an allergen and an endotoxin trigger a stronger inflammatory response. One of the main pro-inflammatory cytokines produced in inflammatory responses is interleukin-1β (IL-1β), and its production is associated with caspase-1-containing inflammasome complexes. Intracellular ROS have been implicated in NLRP3 inflammasome-mediated IL-1β production, therefore, we aimed to study whether RWE influences the function of NLRP3 inflammasome. Here we describe that, in the presence of NADPH, RWE significantly elevates lipopolysaccharide-induced IL-1β production of THP-1 cells as well as human primary macrophages and dendritic cells. We also demonstrate that increased IL-1β production is mediated through NLRP3 inflammasome in THP-1 macrophages. We provide evidence that RWE elevates cytosolic ROS level in these cells, and ROS inhibitors abolish IL-1β production. Furthermore, we show that RWE enhances lipopolysaccharide-induced gene transcription/expression of pro-IL-1β and key components of the inflammasome via a ROS-dependent mechanism.
Keywords: interleukin-1β, NADPH oxidases, NLRP3 inflammasome, ragweed pollen extract, reactive oxygen species
Introduction
Ragweed (Ambrosia artemisiifolia) pollen is one of the most abundant aeroallergens that cause severe allergic symptoms. After hydration in rainwater, or in conditions with high humidity or moisture, ragweed pollen grains release sub-pollen particles of respirable size.1 These particles can easily penetrate the lower airways and trigger or exacerbate asthma symptoms. Sub-pollen particles and pollen extracts have been reported to contain intrinsic NADPH oxidases2 that produce reactive oxygen species (ROS) and therefore induce oxidative stress in the airway epithelium,3 causing allergic airway inflammation.4
It has been demonstrated that allergens in the presence of endotoxins trigger a substantially stronger allergic inflammation, compared with that evoked in the absence of endotoxins.5–7 After inhalation, endotoxins, such as lipopolysaccharide (LPS), encounter and activate alveolar macrophages, leading to the production and release of pro-inflammatory cytokines, chemokines, adhesion molecules and other mediators.8 Nasal and lung lavage samples of allergic subjects show increased levels of interleukin-1β (IL-1β),9 primarily produced by activated macrophages.10 Production of mature IL-1β requires distinct signals, some of which induce gene expression in the so called ‘priming step’, whereas other signals trigger the maturation of pro-IL-1β to IL-1β by a multiprotein complex called inflammasome.
The NLRP3 inflammasome complex consists of NLRP3 (NOD-like receptor family pyrin domain-containing 3) sensor, caspase-1 and ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) adaptor.11,12 NLRP3 inflammasomes play a crucial role in the detection and sensing of exogenous danger signals like pathogen-associated molecular patterns and toxins of microbes, asbestos or silica, as well as endogenous danger signals like monosodium urate and amyloid.13,14 Most NLRP3 activators have been shown to induce ROS generation,15 and inhibitors of ROS production or ROS scavengers attenuate NLRP3 inflammasome activation16 implying an essential role for ROS in NLRP3 function. As pollen NADPH oxidases are able to generate ROS, and ROS have been implicated in the NLRP3 inflammasome-mediated IL-1β production, we hypothesized that exposure to pollen extract may influence inflammatory responses and IL-1β production of macrophages via NLRP3 inflammasome. Here we report for the first time that ragweed pollen extract (RWE), typically used as a model for pollen action,3 significantly elevates LPS-induced IL-1β production of THP-1 or primary macrophages and dendritic cells in an NADPH-dependent manner. We also demonstrate that a caspase-1 inhibitor or NLRP3 silencing abolish this enhancing effect together with the original LPS-triggered inductions. We also show that RWE in the presence of NADPH enhances LPS-induced p38 and Jun N-terminal kinase (JNK) signalling pathways resulting in the activation of AP-1 transcription factors and the subsequent gene transcription/expression of pro-IL-1β and key components of the inflammasome. This effect is mediated by a ROS-dependent mechanism.
Methods
Cell culture of THP-1 macrophages
The THP-1 cell line (ATCC TIB-202) was a generous gift from Professor Laszlo Nagy. THP-1 monocytes were cultured in RPMI-1640 (Gibco BRL Inc., Grand Island, NY) containing 10% heat-inactivated fetal calf serum, penicillin-streptomycin and glutamine, and maintained at 37° under 5% CO2. Cells were differentiated into macrophages in tissue culture dishes with 0·5 μm PMA (InvivoGen, San Diego, CA) for 3 hr, then washed three times with PBS and plated at 5 × 105 cells/ml for ELISA or 106 cells/ml for real-time PCR and Western blot methods. Cells were left to adhere overnight, then they were treated with 100 ng/ml LPS (InvivoGen), 10 μg/ml RWE (Greer Laboratories, Lenoir, NC), 100 μm NADPH (Sigma-Aldrich, St. Louis, MO) or 0·3 mm H2O2 (Sigma-Aldrich). The endotoxin content of pollen extract was 16·31 pg/μg protein, negligible compared with the LPS concentration used. Differences from these treatments are indicated in the corresponding figure legends. N-Acetyl-cysteine (30 mm; NAC, Sigma-ldrich), MitoTEMPO {[2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl]triphenylphosphonium chloride monohydrate} (300 μm; Santa Cruz Biotechnology, Santa Cruz, CA), diphenyleneiodonium chloride (DPI, 10 μm, Sigma-Aldrich) or caspase-1 inhibitor (Z-YVAD-fmk, 20 μm, BioVison, Mountain View, CA) were added to the cells 1 hr before treatments.
Macrophage and dendritic cell generation
For monocyte separation local Ethics Committee approval was received for the studies and the informed consent of all participating subjects was obtained. CD14+ monocytes were separated with anti-CD14-conjugated microbeads (VarioMACS Separation System; Miltenyi Biotec, Bergish Gladbach, Germany) from leucocyte-enriched buffy coats and plated in RPMI-1640. Cells were plated in 12-well culture dishes at a density of 1·5 × 106 cells/ml in RPMI-1640 supplemented with 10% fetal bovine serum, 500 U/ml penicillin-streptomycin (Invitrogen, Carlsbad, CA), and 2 mm l-glutamine (Invitrogen). For macrophage and dendritic cell differentiation cells were treated with 80 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF; Leucomax; Gentaur Molecular Products, Brussels, Belgium) or 80 ng/ml GM-CSF and 100 ng/ml IL-4 (PeproTech EC, London, UK), respectively. IL-4 and GM-CSF were replenished on day 3. The macrophages and dendritic cells were challenged at day 5 of culturing for 24 hr with 500 ng/ml LPS, 100 μg/ml RWE and 100 μm NADPH.
Reactive oxygen species measurement
About 106 cells were loaded with 50 μm 2′-7′-dihydro-dichlorofluorescein diacetate (H2DCFDA, Invitrogen) at 37° for 20 min and treated with the indicated compounds. At the indicated times, cells were resuspended and analysed by flow cytometry using FACSCalibur (BD Biosciences Immunocytometry Systems, Franklin Lakes, NJ). flowjo software was used for analysis. Relative ROS levels are given in arbitrary units of mean intensity of fluorescence with respect to untreated controls.
Small interfering RNA transfection
Differentiated THP-1 cells were electroporated with 2·5 μm NLRP3-specific or scrambled small interfering RNA (siRNA; Silencer Select Pre-Designed and Validated; Ambion Inc., Austin, TX), then plated. After 48 hr, cells were treated with the indicated compounds and 24 hr later the supernatants were collected for ELISA, while cells were used for real-time PCR and/or Western blot.
RNA preparation and RT-PCR
Total RNA was extracted with TriReagent (Molecular Research Center Inc., Cincinnati, OH) and isolated according to the manufacturer's instructions as described previously.17 The concentration and homogeneity of RNA preparations were determined by a spectrophotometer (NanoDrop ND1000; Promega Biosciences, Madison, WI). Standardized amounts of RNA were then digested with DNase (Ambion), and subjected to reverse transcription using Super Script II RNase H – Reverse Transcriptase and Random Primers (Invitrogen).
Real-time quantitative PCR
Real-time analyses were performed in 384-well optical reaction plates in ABI Prism 7900HT Sequence Detector System (Applied Biosystems, Foster City, CA). For real-time PCR, all oligo mixes were purchased from Applied Biosystems. Taq DNA Polymerase (Fermentas, St. Leon-Rot, Germany) was used for amplification, and Rox Reference Dye (Invitrogen) was used for normalization of the fluorescent reporter signal, as described previously.18 Amplification was conducted in a 25 μl reaction mixture containing 125 ng cDNA. Real-time PCR data were analysed by using sequence detector system version 2.1 software (Applied Biosystems). The expression levels were calculated by the ΔCt method using cyclophilin as control.
Western blot analysis
Cells were washed with ice-cold PBS and suspended in a lysis buffer containing 30 mm Tris (pH 7·6), 140 mm NaCl, 5 mm EDTA, 50 mm NaF, 2 mm sodium pyrphosphate, 50 μm phenylasine-oxide, 1% Triton-X and 1 mm Na3VO4 with freshly added protease inhibitors (1 μg/ml aprotinin, 0·5 μg/ml pepstatin, 1·25 μg/ml leupeptin, 1 mm PMSF). The protein concentration of the samples was determined using a bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL); 30 μg of total proteins were heated with SDS sample buffer (0·5 m Tris–HCl, pH 6·8, glycerol, 10% SDS, 0·025% bromophenol blue). Lysates were separated on SDS–PAGE gels, and transferred onto nitrocellulose membranes using wet electro-blotting. Membranes were blocked in Tween-TBS containing 5% non-fat milk and stained with antibodies recognizing NLRP3 (mouse monoclonal; Alexis Biochemicals, San Diego, CA), cleaved IL-1β and caspase-1 (rabbit polyclonal, Cell Signaling Technology, Danvers, MA), procaspase-1 (rabbit polyclonal; Santa Cruz Biotechnology), phospho-p38 mitogen-activated protein kinase (MAPK), phospho-stress-activated protein kinase (SAPK)/JNK (rabbit polyclonal; Cell Signaling Technology), phospho-p38 and p38, phospho-SAPK/JNK and SAPK/JNK, phospho-c-Jun (Ser63 and Ser73) and c-Jun, phospho-c-Fos and c-Fos overnight at 4°. Primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (anti-mouse or anti-rabbit; Amersham Biosciences, Piscataway, NJ) for 1 hr at room temperature. Proteins were visualized by Supersignal West-Pico peroxide/luminol enhancer solution (Pierce). An equal amount of protein sample loading was verified by detecting β-actin (rabbit polyclonal; Sigma-Aldrich) protein expression.
Measurement of caspase-1 activity
Caspase-1 activity in cell lysates was determined using the acetylated and AMC-conjugated fluorometric peptide substrate Acetyl-Tyr-Glu-Val-Asp-7-amino-4-methyl-coumarin (Anaspec, San Jose, CA). Lysis of the cells was performed on ice for 30 min in 50 mm Tris–HCl, pH 7·5, containing 150 mm NaCl, 0·5 mm EDTA, 0·5% Nonidet P-40, 1 mm PMSF, 1 μg/ml aprotinin, 0·5 μg/ml pepstatin, 1·25 μg/ml leupeptin and 1 mm dithiothreitol. After centrifugation (10 000 g, 10 min at 4°), 30 μg protein lysate supernatants were incubated in 100 μl lysis buffer with 40 μm substrate (final concentration) in microtitre plate wells at room temperature, and the increase of fluorescence due to the release of AMC was detected at 460 nm, using a 355-nm excitation wavelength in a Wallac 1420 Victor2 fluorimeter-luminometer (Wallac Oy, Turku, Finland).
Quantification of IL-1β cytokine
The concentrations of secreted IL-1β in the cell culture supernatants after the indicated times of treatments were measured by ELISA (BD Biosciences, San Diego, CA) according to the manufacturer's instructions. Detection limit of the assay was 10 pg/ml.
Statistical analysis
Significance of the differences between mean values was evaluated using a Student's t-test. Data presented as mean ± SD values.
Results
RWE enhances LPS-induced IL-1β secretion of human macrophages
To determine the effect of RWE on IL-1β production, THP-1 macrophages were treated with different combinations of RWE, NADPH and LPS. Although in good agreement with previous findings,19 LPS treatment resulted in a substantial increase of the secreted IL-1β, the treatment with RWE in the absence or presence of NADPH did not trigger the secretion of this cytokine, nor did NADPH alone (Fig. 1a). However, RWE in the presence of NADPH strongly enhanced the LPS-induced IL-1β production in a dose-dependent manner at the lowest saturating LPS concentration (100 ng/ml) (Fig. 1a). A similar induction was observed at an even 10-fold higher LPS concentration and the substantial dose-dependent elevation required 24 hr after treatment (data not shown). Treatment of human monocyte-derived macrophages and dendritic cells with LPS alone or in combination with RWE led to results similar to those found with the THP-1 cell line (Fig. 1b).
Figure 1.
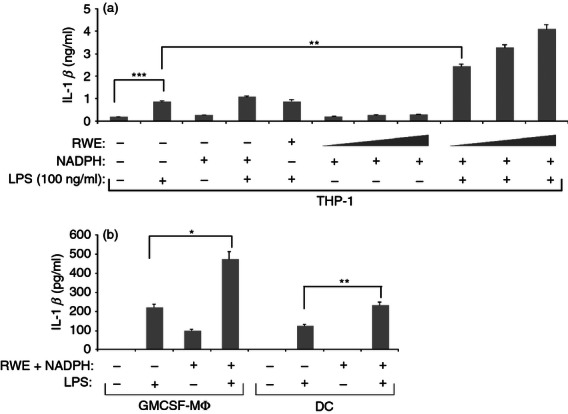
Ragweed pollen extract (RWE) enhances lipopolysaccharide (LPS) -induced interleukin-1β (IL-1β) production by macrophages and dendritic cells in the presence of NADPH. (a) THP-1 macrophages were stimulated for 24 hr with an increasing amount (10, 30 and 100 μg/ml) of RWE in the absence or presence of LPS and 100 μm NADPH, as indicated. The supernatants were collected and their IL-1β content was measured using an ELISA method in triplicates. The experiment was repeated five times and results of a representative set are provided. (b) Human monocyte-derived macrophages and dendritic cells were treated with 500 ng/ml LPS in the absence or presence of 100 ug/ml RWE and 100 μm NADPH. Twenty-four hours after treatment supernatants were collected and their IL-1β content was determined using an ELISA method in triplicates. Experiments were repeated four times and results were combined. Mean ± SD values are provided. *P < 0·05, **P < 0·005.
RWE induces ROS production and ROS inhibitors abolish RWE-enhanced IL-1β production in LPS-treated THP-1 macrophages
Pollen extract has been reported to stimulate ROS production in epithelial cells, for this reason we aimed to see if pollen extract could induce ROS production in THP-1 macrophages. H2O2, used as a positive control, induced a fast increase in intracellular ROS (Fig. 2a). Whereas RWE but not NADPH alone induced some ROS production, their combined effect yielded a continuously increasing ROS level (Fig. 2a). Lipopolysaccharide alone did not produce detectable ROS by this method, in good agreement with previous findings,20 nor did it enhance the ROS produced by RWE treatment in the presence of NADPH (Fig. 2a). To determine whether the RWE-dependent enhancement of LPS-induced IL-1β production is mediated by ROS, THP-1 macrophages were pre-treated with the ROS-scavenger NAC. NAC completely inhibited IL-1β secretion, indicating that ROS play an indispensable role in LPS-induced as well as in RWE-enhanced IL-1β production (Fig. 2b). To verify the source of ROS involved in the IL-1β secretion, cells were treated with MitoTEMPO, which inhibits ROS production by the mitochondria, or with DPI, which inhibits ROS production by NADPH oxidases and mitochondria. In good agreement with previously published results, we found that LPS-induced mitochondrial ROS was substantially contributing to the IL-1β production, as shown by the significant (about two-third) inhibition caused by MitoTempo, However, the RWE-mediated enhancement of the IL-1β production does not appear to be as strongly dependent on mitochondrial ROS because MitoTempo treatment resulted in less than 40% inhibition of IL-1β production. Nevertheless, DPI treatment completely abolished IL-1β production, independently of the stimulating agents (Fig. 2b). This inhibition pattern suggests that while the majority of the ROS involved in the LPS-induced IL-1β production is mitochondrial, the ROS involved in the RWE-dependent enhancement is cytosolic, generated by pollen-derived NADPH oxidases.
Figure 2.
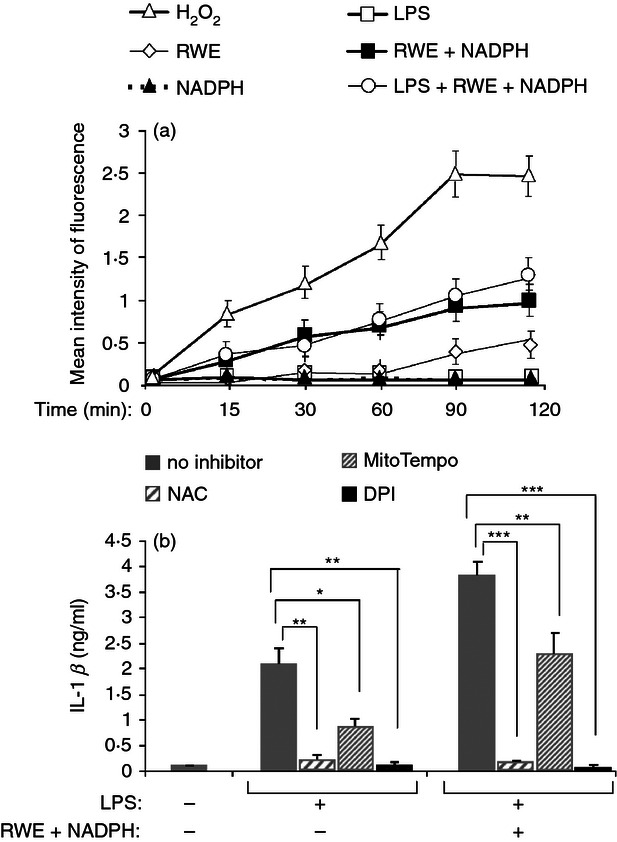
Ragweed pollen extract (RWE) leads to intracellular reactive oxygen species (ROS) production and ROS inhibitors abolish interleukin-1β (IL-1β) production. (a) THP-1 cells were loaded with H2DCFDA, treated with various combinations of 100 μg/ml RWE, 100 μm NADPH and 1000 ng/ml lipopolysaccharide (LPS), and changes in the intracellular ROS level were measured using flow cytometry for the indicated time interval; 1 mm H2O2 was used as a positive control. Mean intensity of fluorescence was calculated from the positive area defined by the stained cells. (b) THP-1 cells were pre-treated with 30 mm NAC, 300 μm MitoTempo or 10 μm DPI for 1 hr then treated with 100 ng/ml LPS in the presence or absence of 10 μg/ml RWE and 100 μm NADPH. Twenty-four hours after treatment the secreted IL-1β was measured from the collected supernatants in triplicates by an ELISA method. Results were obtained in three independent experiments, and a representative result set is shown. *P < 0·1, **P < 0·01, ***P < 0·001.
Caspase-1 inhibition and NLRP3 silencing abolish RWE-enhanced LPS-induced IL-1β production
To find out whether RWE-enhanced IL-1β production is mediated by NLRP3 inflammasome, we treated THP-1 cells with a specific caspase-1 inhibitor. Z-YVAD-FMK significantly reduced the LPS plus RWE-induced IL-1β production, suggesting the involvement of caspase-1 in RWE-enhanced IL-1β production (Fig. 3a). We have also silenced NLRP3 expression using siRNA in THP-1 cells (Fig. 3b,c). Silencing of NLRP3 completely inhibited IL-1β secretion of stimulated THP-1 macrophages (Fig. 3d), indicating that not only the LPS-induced IL-1β production but also its enhancement by RWE are dependent on NLRP3 inflammasome.
Figure 3.
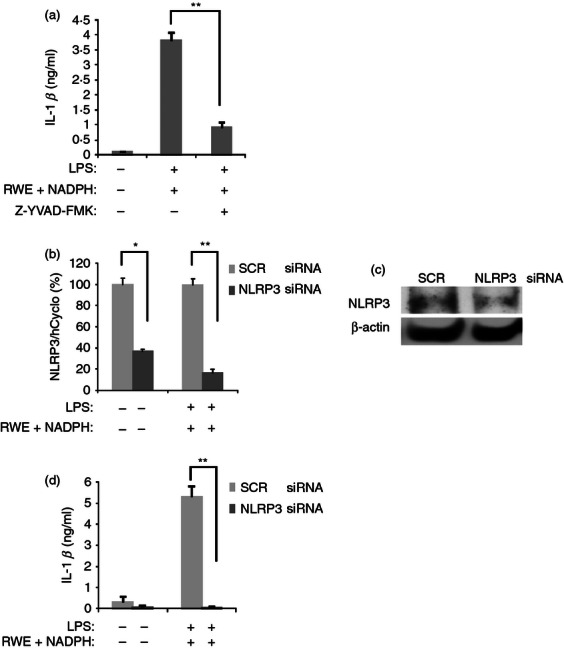
Caspase-1 inhibition and NLRP3 silencing abolish ragweed pollen extract (RWE) -enhanced lipopolysaccharide (LPS) -induced interleukin-1β (IL-1β) production. (a) THP-1 macrophages were treated with 100 ng/ml LPS and 10 μg/ml RWE in the absence or presence of the caspase-1-specific inhibitor Z-YVAD-FMK (20 μm), for 24 hr. IL-1β content of the supernatants was determined using an ELISA method. (b–d) THP-1 cells were electroporated with scrambled (SCR) small interfering (si) RNA, as negative control or siRNA specific for NLRP3. Two days after electroporation, cells were stimulated with LPS (100 ng/ml), RWE (10 μg/ml) and NADPH (100 μm) for 24 hr. After harvesting the cells, RNA was purified and NLRP3 transcription was measured by quantitative real-time PCR (b), while NLRP3 protein expression of NLRP3 was detected by Western blot technique (Alexis Biochemicals) (c). Gene expression is shown as the ratio of the studied transcripts relative to human cyclophilin expression (± SD) measured in triplicates. Equal amount of protein sample loading was verified by detecting β-actin protein expression (rabbit polyclonal antibody, Sigma-Aldrich). (d) The concentration of secreted IL-1β was measured from the supernatant of the cells in triplicates using an ELISA method. Results were obtained in three independent experiments, and a representative result set is shown. * P < 0·05, ** P < 0·005.
RWE enhances the LPS-induced NLRP3 inflammasome function
Priming step of NLRP3 inflammasome function involves the elevated expression of inflammasome components and pro-IL-1β. We sought to determine how RWE and NADPH treatment affect the expression of NLRP3 inflammasome components. We have found that LPS treatment in THP-1 macrophages significantly induced the expression of NLRP3 (Fig. 4a,b) and procaspase-1 (Fig. 4c,d) at both mRNA and protein levels. Whereas RWE in the presence of NADPH did not affect the expression of these molecules, it further enhanced the LPS-induced procaspase-1 expression at both the mRNA and protein levels (Fig. 4c,d). Though an increased transcription of NLRP3 was also observed, this did not result in significant elevation of the protein amount (Fig. 4b). To see whether the elevated level of procaspase-1 is accompanied by increased caspase-1 activity, we detected the processed forms of caspase-1 using immunoblot techniques, furthermore, we also measured the activity of the enzyme in THP-1 cell lysates using a fluorescent substrate. Our results show that LPS treatment significantly induced caspase-1 processing, moreover, in the LPS-primed cells RWE treatment resulted in a further enhancement of the processing of caspase-1 (Fig. 4f). However, we found that while LPS treatment significantly induced caspase-1 enzyme activity (Fig. 4e), treatment of the LPS-primed cells with RWE did not result in further caspase-1 activation. The transcription of pro-IL-1β was also substantially induced by LPS and strongly enhanced by RWE treatment (Fig. 4f) and a substantially stronger production of processed IL-1β protein was detected in the lysate of LPS and RWE plus NADPH-treated cells compared with the LPS-treated ones (Fig. 4g).
Figure 4.
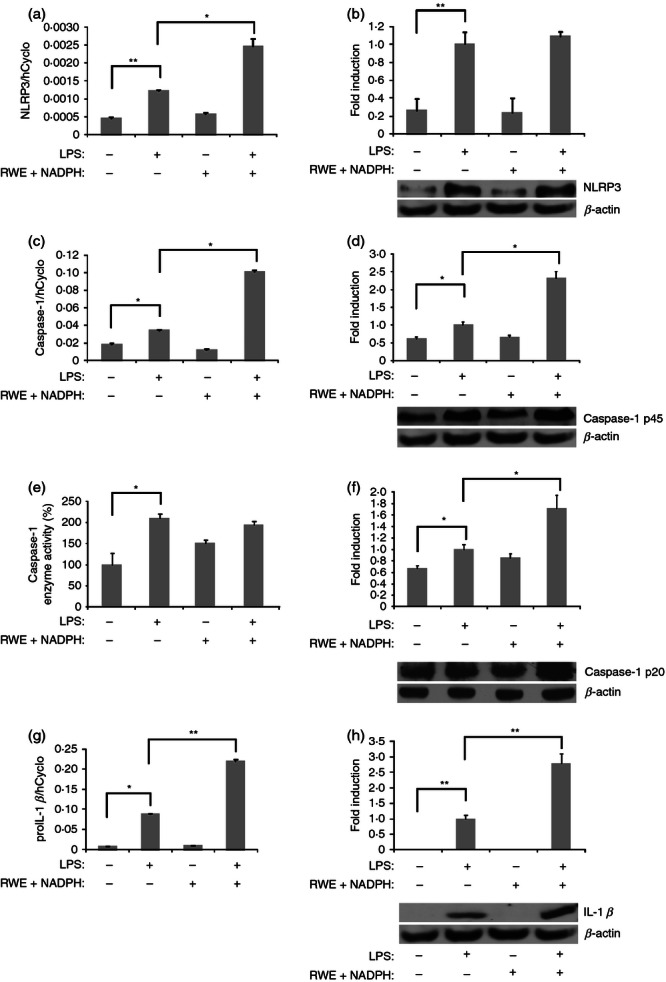
Ragweed pollen extract (RWE) increases NLRP3, caspase-1 and interleukin-1β (IL-1β) gene transcription or expression of lipopolysaccharide (LPS) -treated cells. THP-1 macrophages were treated with LPS (100 ng/ml), RWE and NADPH in the absence or presence of RWE (10 μg/ml) and NADPH (100 μm). Twenty-four hours following treatment cells were collected and gene expression of (a) NLRP3, (c) caspase-1 and (g) pro-IL-1 β was determined using quantitative RT-PCR. Protein expression was determined by Western blot technique using antibodies recognizing (b) NLRP3, (d) procaspase-1, (f) caspase-1 and (h) IL-1β. Primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (anti-mouse and anti-rabbit, Amersham). Gene expression is shown as the ratio of NLRP3, caspase-1 or pro-IL-1β transcripts relative to human cyclophilin expression (± SD) measured in triplicates. For Western blot an equal amount of protein sample loading was verified by detecting β-actin protein expression. Protein levels were estimated by densitometric analysis of the bands using KODAK 1D Image Analysis Software. A representative immunoblot is also shown for NLRP3, caspase-1 and IL-1β. Densitometry values provided are the average of Western blot runs of three independent experiments. (e) From the lysate of THP-1 macrophages the activity of caspase-1 enzyme was determined using fluorometric peptide substrate in triplicates. Mean ± SD values of three independent experiments are shown. *P < 0·1, **P < 0·01
Induction of key inflammasome components and pro-IL-1β by RWE is NADPH-dependent, and is suppressed by an inhibitor of ROS production
To see how NLRP3 and pro-IL-1β expression depends on RWE NADPH oxidase-generated ROS, we studied the RWE-induced transcription of the corresponding genes in the absence or presence of NADPH (Fig. 5). Our results show that all of the studied gene inductions by RWE appeared to be NADPH dependent. Furthermore, we found that ROS-inhibitor DPI substantially inhibited pro-IL-1β and NLRP3 gene expression in the LPS-treated or RWE-treated cells, as well as in those treated with their combination. Interestingly, while the LPS-induced caspase-1 production was not affected by DPI, significant down-regulation was observed in the case of the RWE-treated THP-1 macrophages, regardless of the LPS treatment.
Figure 5.
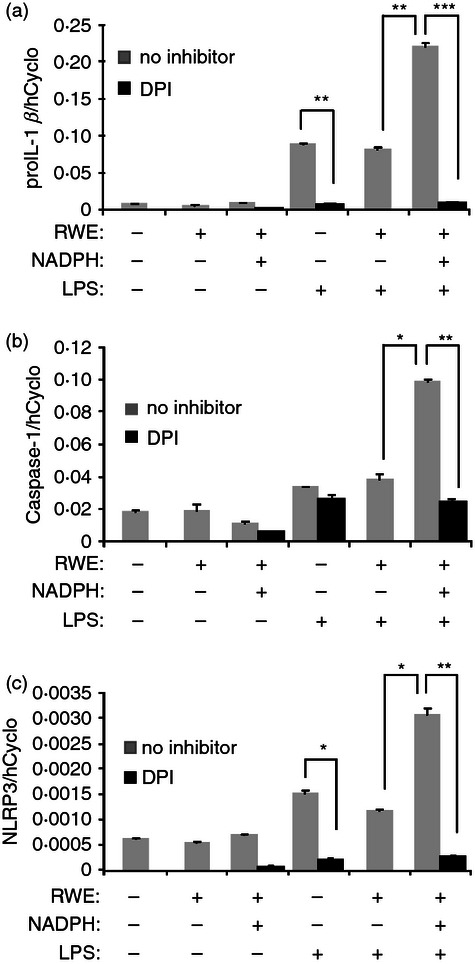
Ragweed pollen extract (RWE)-induced gene expression is NADPH-dependent and is suppressed by a reactive oxygen species (ROS)-inhibitor. THP-1 macrophages were treated for 24 hr with the compounds as indicated, DPI was used in 10 μm. After harvesting the cells, the gene expression of (a) pro-interleukin-1β (pro-IL-1β), (b) caspase-1 and (c) NLRP3 was determined by quantitative real-time PCR. Gene expression is shown as the ratio of the studied transcripts relative to human cyclophilin expression (± SD) measured in triplicates. Results were obtained from four independent experiments, and a representative result set is shown.*P < 0·05, **P < 0·005, ***P < 0·0005.
RWE triggers p38 MAPK and JNK signalling in an NADPH-dependent manner and results in increased p38 MAPK and JNK phosphorylation in LPS-treated cells
To see whether the LPS-activated signal transduction pathways are affected by RWE we studied the phosphorylation of JNK, p38 MAPK and IκBα in response to treatment by various combinations of compounds used in this study. Unlike the phosphorylation of IκBα (data not shown), the RWE-induced p38 MAPK and JNK phosphorylation appeared to be NADPH dependent (Fig. 6a). Furthermore, RWE in the presence of NADPH substantially enhanced the LPS-induced p38 MAPK and JNK phosphorylation (Fig. 6b). p38 and JNK are members of the MAPK family that has been described to activate AP-1 transcription factors.21 To demonstrate the activation of these downstream signalling events we studied the expression and phosphorylation of c-Jun and c-Fos transcription factors. Our results show that the expression of c-Fos and c-Jun was not affected by the NADPH21 (Fig. 6c) or the RWE plus NADPH treatment (Fig. 6d). However, we found that co-treatment with RWE and NADPH significantly increased the phosphorylation of c-Fos and c-Jun compared with that of the RWE-treated cells (Fig. 6c). Similarly, these transcription factors were more phosphorylated in the LPS-activated and RWE plus NADPH-treated cells compared with the only LPS-treated ones (Fig. 6d). These results suggest that the ROS-dependent enhancement of LPS-induced IL-1β production by RWE involves the p38 MAPK and JNK pathways.
Figure 6.
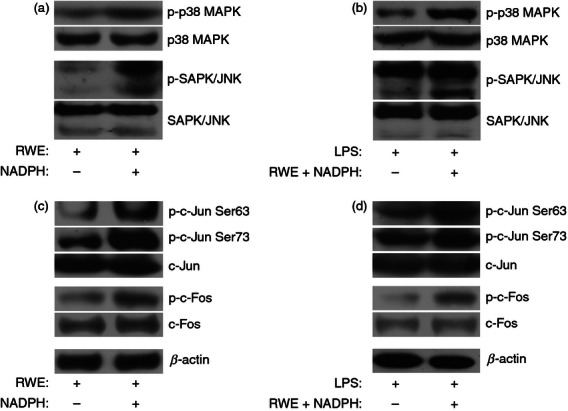
Ragweed pollen extract (RWE) triggers stress-activated protein kinase/Jun N-terminal kinase (SAPK/JNK), p38 mitogen-activated protein kinase (MAPK) and AP-1 signalling. (a, c) THP-1 macrophages were treated with 30 μg/ml RWE in the absence or presence of 100 μm NADPH, or (b, d) with 100 ng/ml lipopolysaccharide (LPS) in the absence or presence of RWE plus NADPH. Five hours after treatment, cells were harvested, protein lysates were prepared and the phosphorylation of (a, b) p38 MAPK, SAPK/JNK, (c, d) c-Jun (phosphorylated at serine 73 or 63) and c-Fos; furthermore total c-Jun and c-Fos were analysed by Western blot method. To verify the loading of equal amounts of protein sample, the β-actin protein expression was detected (c, d) or total p38 and SAPK/JNK were used as loading controls (a, b). Results obtained from three independent experiments, and one representative Western blot are shown.
Discussion
Allergic rhinitis is one of the most common inflammatory disorders accompanied by high levels of IL-1β production. It is hypothesized that combined exposure to endotoxin and an allergen would enhance the influx and activity of macrophages in the lung and increase the symptoms of allergic airway reactions.7,22 Supporting this assumption, here we demonstrate that RWE significantly enhances LPS-induced IL-1β secretion in THP-1 macrophages, as well as in human primary macrophages and dendritic cells.
Both pollen grain and pollen extract have been reported to be able to modify inflammatory responses. However, pollen grains are commonly contaminated with LPS or other microbial products. Furthermore, it has also been described that direct contacts between the antigen-presenting cells and pollen grain particles may strongly influence the outcome of the activation of the cells, which could account for the reported adjuvant activity of intact pollens.23,24 Therefore, to identify the molecular effects of pollen components on antigen-presenting cells, we have used a commercially available pollen extract in our studies that is typically used for skin allergy tests. Furthermore, while pollen grains have been shown to contain endogenous NADPH, the use of pollen extract required exogenous addition of NADPH to study the effect of pollen NADPH oxidase, as this has been established previously.3 Pollen NADPH oxidases are able to induce oxidative stress in various epithelial cells25 and also in dendritic cells.26. Here we show that in THP-1 macrophages RWE causes a steadily increasing level of intracellular ROS and a sustained exposure to ROS, in good agreement with studies that showed long-term intracellular ROS production in pollen-treated A549 alveolar epithelial cells.25 On the other hand, LPS treatment alone neither induced detectable ROS production nor enhanced the RWE-induced one in THP-1 cells, in line with a previous study where, using the same method, no cytoplasmic ROS production was detected in THP-1 cells upon LPS stimulus.20 The primary sources of LPS-generated ROS are the mitochondria,27 into which the de-esterified substrate probe is not expected to penetrate. Our results suggest that agents capable of causing elevated cytoplasmic ROS levels (like H2O2 or RWE with NADPH) can enhance the LPS-induced IL-1β production but cannot alone yield mature IL-1β. In our assay system MitoTempo, a specific mitochondrial ROS production inhibitor, caused a similar degree of inhibition in the LPS and RWE-co-treated THP-1 cells as in the LPS-treated ones, suggesting that the oxidative stress induced by RWE treatment is independent of the mitochondrial ROS generation. The functional involvement of the increased intracellular ROS levels in this enhancing effect was supported by the NADPH-requirement of the RWE and by the strong inhibition of IL-1β production by ROS inhibitors and scavengers.28
Our experiments using a caspase-1 inhibitor as well as silencing of NLRP3 demonstrates that IL-1β production requires NLRP3 inflammasome function. Although various inflammasome complexes have been associated with IL-1β production, such as AIM2 (absent in melanoma 2), IPAF (interleukin-1-converting enzyme protease-activating factor), NLRP1 or NLRP3 inflammasomes,29 only NLRP3 inflammasome-mediated IL-1β production was previously demonstrated to be mediated by intracellular ROS.30,31 It was shown recently that, in the murine system, ROS do not directly affect the activation of the NLRP3 inflammasome but, instead, they are necessary for the priming steps, that is for the induction of the expression of inflammasome components and the pro-IL-1β.31 In good agreement with these findings, the down-regulation of NLRP3 and procaspase-1 gene transcription using ROS-inhibitors suggests that ROS in our experiment is a mediator of the priming of NLRP3 inflammasome. Our results showed that RWE treatment in the presence of NADPH enhanced procaspase-1 and IL-1β protein levels in THP-1 macrophages. This effect was dependent on the presence of exogenously added NADPH, implying the role of pollen NADPH oxidases in these effects.
While using an immunoblotting technique, the RWE treatment in the presence of NADPH further increased caspase-1 processing (Fig. 4f), this did not result in significantly increased caspase-1 activity (see Fig. 4e). These results appear to be contradictory, however, it should be taken into account that the immunoblotting technique detects the processed caspase-1 independent of its activity, and it has been demonstrated that caspase-1 is rapidly inactivated in THP-1 cells (with a half-life of 9 min) leading to the accumulation of processed but inactive caspase-1.32 It should be noted that despite intensive studies on NLRP3 inflammasome and IL-1β production, the molecular and biochemical details of the protein expression, half-life and degradation of NLRP3 and caspase-1 are far from being understood. Post-translational modification, enhanced protein inactivation and degradation may strongly deviate the actual protein levels and activity from those that could be predicted from the gene expression patterns alone.
Various signal pathways have been shown to be involved in LPS-mediated NLRP3 inflammasome component up-regulation.33,34 Based on our studies, RWE induces p38 and JNK phosphorylation in an NADPH-dependent manner; however, this does not lead to elevated pro-IL-1β, NLRP3 and procaspase-1 transcription. Nevertheless, co-treatment of the THP-1 macrophages with LPS and RWE in the presence of NADPH resulted in a substantially more intensive phosphorylation of these proteins, presumably leading to the observed gene expression induction. Unlike LPS, RWE and NADPH did not significantly activate the nuclear factor-κB signalling pathway.
Signals triggering activation of nuclear factor-κB pathways like that of LPS ligating TLR4, induce strong expression of pro-IL-1β because its promoter region contains multiple nuclear factor-κB responsive elements.35 On the other hand, p38 and JNK pathways are typically induced by stress stimuli like ROS. Cross-talk between signalling pathways like phosphorylation of cytosolic elements of the pathway or transcriptional regulators by JNK and p38 kinase may result in the formation of more stable enhancer complexes, as described previously.36
Our results show that LPS-induced p38 and JNK phosphorylation, also the activation of AP-1 (c-Fos and c-Jun) and subsequent gene expressions are enhanced by RWE and NADPH. This suggests that RWE, in the presence of NADPH, essentially participates in the priming step of NLRP3 inflammasome functions.
In summary, our data suggest that RWE-stimulated enhancement of IL-1β production in LPS-treated THP-1 cells is mainly the consequence of the substantially increased pro-IL-1β expression and elevated caspase-1 activation. The induced gene transcription and expression of pro-IL-1β together with key inflammasome components (caspase-1 and NLRP3) is dependent on the ROS production by the RWE-associated NADPH oxidases. Nevertheless, it is important to note that pollen grains and sub-pollen particles are complex biological packages composed of many components that can alter the functions of human cells. However, the observed interplay of RWE and LPS suggests a critical role of bacterial endotoxin in the pollen-induced allergic reactions that should be taken into account in designing treatments for allergic airway inflammations.
Acknowledgments
The work was supported in part by the TÁMOP 4.2.1/B-09/1/KONV-2010-0007 project (to J.T. and A.B.), the TÁMOP-4.2.2.A-11/1/KONV-2012-0023 project (to S.B., J.T. and A.V.) the TÁMOP-4.2.2/B-10/1-2010-0024 project (to A.V.), the UD Faculty of Medicine Research Fund - Bridging Fund (to S.B.) and the Hungarian Science and Research Fund (K-73347 to A.B.). The project is co-financed by the European Union and the European Social Fund. S.B. is a receiver of Lajos Szodoray Post-doctoral Fellowship and Janos Bolyai Post-doctoral Fellowship.
Disclosures
The authors declare no competing interests.
References
- 1.Bacsi A, Choudhury BK, Dharajiya N, Sur S, Boldogh I. Subpollen particles: carriers of allergenic proteins and oxidases. J Allergy Clin Immunol. 2006;118:844–50. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharajiya NG, Bacsi A, Boldogh I, Sur S. Pollen NAD(P)H oxidases and their contribution to allergic inflammation. Immunol Allergy Clin North Am. 2007;27:45–63. doi: 10.1016/j.iac.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Boldogh I, Bacsi A, Choudhury BK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–79. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dharajiya N, Choudhury BK, Bacsi A, Boldogh I, Alam R, Sur S. Inhibiting pollen reduced nicotinamide adenine dinucleotide phosphate oxidase-induced signal by intrapulmonary administration of antioxidants blocks allergic airway inflammation. J Allergy Clin Immunol. 2007;119:646–53. doi: 10.1016/j.jaci.2006.11.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed CE, Milton DK. Endotoxin-stimulated innate immunity: a contributing factor for asthma. J Allergy Clin Immunol. 2001;108:157–66. doi: 10.1067/mai.2001.116862. [DOI] [PubMed] [Google Scholar]
- 6.Kauffman HF. Innate immune responses to environmental allergens. Clin Rev Allergy Immunol. 2006;30:129–40. doi: 10.1385/criai:30:2:129. [DOI] [PubMed] [Google Scholar]
- 7.Jung YW, Schoeb TR, Weaver CT, Chaplin DD. Antigen and lipopolysaccharide play synergistic roles in the effector phase of airway inflammation in mice. Am J Pathol. 2006;168:1425–34. doi: 10.2353/ajpath.2006.050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radon K. The two sides of the “endotoxin coin”. Occup Environ Med. 2006;63:73–8. doi: 10.1136/oem.2004.017616. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linden M, Greiff L, Andersson M, Svensson C, Akerlund A, Bende M, Andersson E, Persson CG. Nasal cytokines in common cold and allergic rhinitis. Clin Exp Allergy. 1995;25:166–72. doi: 10.1111/j.1365-2222.1995.tb01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim TC, Grant JA, Hilsmeier KA, Fukuda Y, Alam R. Proinflammatory cytokines in nasal secretions of allergic subjects after antigen challenge. Am J Respir Crit Care Med. 1994;149:339–44. doi: 10.1164/ajrccm.149.2.8306027. [DOI] [PubMed] [Google Scholar]
- 11.Petrilli V, Papin S, Tschopp J. The inflammasome. Curr Biol. 2005;15:R581. doi: 10.1016/j.cub.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–32. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Benko S, Philpott DJ, Girardin SE. The microbial and danger signals that activate Nod-like receptors. Cytokine. 2008;43:368–73. doi: 10.1016/j.cyto.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–5. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 16.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–9. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 17.Benko S, Magalhaes JG, Philpott DJ, Girardin SE. NLRC5 limits the activation of inflammatory pathways. J Immunol. 2010;185:1681–91. doi: 10.4049/jimmunol.0903900. [DOI] [PubMed] [Google Scholar]
- 18.Benko S, Tozser J, Miklossy G, Varga A, Kadas J, Csutak A, Berta A, Rajnavolgyi E. Constitutive and UV-B modulated transcription of Nod-like receptors and their functional partners in human corneal epithelial cells. Mol Vis. 2008;14:1575–83. [PMC free article] [PubMed] [Google Scholar]
- 19.Netea MG, Nold-Petry CA, Nold MF, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood. 2009;113:2324–35. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carta S, Tassi S, Pettinati I, Delfino L, Dinarello CA, Rubartelli A. The rate of interleukin-1β secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. J Biol Chem. 2011;286:27069–80. doi: 10.1074/jbc.M110.203398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinenov Y, Kerppola TK. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene. 2001;20:2438–52. doi: 10.1038/sj.onc.1204385. [DOI] [PubMed] [Google Scholar]
- 22.Schaumann F, Muller M, Braun A, Luettig B, Peden DB, Hohlfeld JM, Krug N. Endotoxin augments myeloid dendritic cell influx into the airways in patients with allergic asthma. Am J Respir Crit Care Med. 2008;177:1307–13. doi: 10.1164/rccm.200706-870OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currie AJ, Stewart GA, McWilliam AS. Alveolar macrophages bind and phagocytose allergen-containing pollen starch granules via C-type lectin and integrin receptors: implications for airway inflammatory disease. J Immunol. 2000;164:3878–86. doi: 10.4049/jimmunol.164.7.3878. [DOI] [PubMed] [Google Scholar]
- 24.Allakhverdi Z, Bouguermouh S, Rubio M, Delespesse G. Adjuvant activity of pollen grains. Allergy. 2005;60:1157–64. doi: 10.1111/j.1398-9995.2005.00861.x. [DOI] [PubMed] [Google Scholar]
- 25.Bacsi A, Dharajiya N, Choudhury BK, Sur S, Boldogh I. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. J Allergy Clin Immunol. 2005;116:836–43. doi: 10.1016/j.jaci.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csillag A, Boldogh I, Pazmandi K, Magyarics Z, Gogolak P, Sur S, Rajnavolgyi E, Bacsi A. Pollen-induced oxidative stress influences both innate and adaptive immune responses via altering dendritic cell functions. J Immunol. 2010;184:2377–85. doi: 10.4049/jimmunol.0803938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kepp O, Galluzzi L, Kroemer G. Mitochondrial control of the NLRP3 inflammasome. Nat Immunol. 2011;12:199–200. doi: 10.1038/ni0311-199. [DOI] [PubMed] [Google Scholar]
- 28.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 29.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev. 2011;243:136–51. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 31.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–7. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh JG, Logue SE, Luthi AU, Martin SJ. Caspase-1 promiscuity is counterbalanced by rapid inactivation of processed enzyme. J Biol Chem. 2011;286:32513–24. doi: 10.1074/jbc.M111.225862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park MY, Kwon HJ, Sung MK. Dietary aloin, aloesin, or aloe-gel exerts anti-inflammatory activity in a rat colitis model. Life Sci. 2011;88:486–92. doi: 10.1016/j.lfs.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277:22131–9. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 35.Hiscott J, Marois J, Garoufalis J, et al. Characterization of a functional NF-κB site in the human interleukin 1β promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231–40. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escobar J, Pereda J, Lopez-Rodas G, Sastre J. Redox signaling and histone acetylation in acute pancreatitis. Free Radic Biol & Med. 2012;52:819–37. doi: 10.1016/j.freeradbiomed.2011.11.009. [DOI] [PubMed] [Google Scholar]


