Abstract
Background
Noninvasive cortical stimulation could represent an add-on treatment to enhance motor recovery after stroke. However, its clinical value, including anticipated size and duration of the treatment effects, remains largely unknown.
Objective
The authors designed a small semi-randomized clinical trial to explore whether long-lasting clinically important gains can be achieved by adding theta burst stimulation (TBS), a form of repetitive transcranial magnetic stimulation (TMS), to a rehabilitation program for the hand.
Methods
A total of 41 chronic stroke patients received excitatory TBS to the ipsilesional hemisphere or inhibitory TBS to the contralesional hemisphere in 2 centers; each active group was compared with a group receiving sham TBS. TBS was followed by physical therapy for 10 working days. Patients and therapists were blinded to the type of TBS. Primary outcome measures (9-hole Peg Test [9HPT], Jebsen Taylor Test [JTT], and grip and pinch-grip dynamometry) were assessed 4, 30, and 90 days post treatment. The clinically important difference was defined as 10% of the maximum score.
Results
There were no differences between the active treatment and sham groups in any of the outcome measures. All patients achieved small sustainable improvements—9HPT, 5% of maximum (confidence interval [CI] = 3%-7%); JTT, 5.7% (CI = 3%-8%); and grip strength, 6% (CI = 2%-10%)—all below the defined clinically important level.
Conclusions
Cortical stimulation did not augment the gains from a late rehabilitation program. The effect size anticipated by the authors was overestimated. These results can improve the design of future work on therapeutic uses of TMS.
Keywords: transcranial magnetic stimulation, stroke rehabilitation, theta burst stimulation, upper extremity, motor control
Introduction
The prospect of using noninvasive cortical stimulation in the form of repetitive transcranial magnetic stimulation (RTMS) or transcranial direct current stimulation (TDCS) to enhance rehabilitative treatments after stroke has caused excitement among researchers and clinicians. The stimulation can induce long-lasting changes in the excitability of the synapses in motor cortical areas in a manner that is biologically similar to the long-term potentiation/depression (LTP/LTD) phenomena described at the cellular level.1 LTP/LTD are important for learning and memory and are likely to be involved in reacquisition of skill after stroke. It has been hypothesized that priming the correct cortical target areas could improve response to treatment.2,3
The choice of the cortical target has been based on imaging and electrophysiology studies in recovering stroke patients. Most investigators agree that recovery of arm function is often associated with increased activation of ipsilesional motor circuits; abnormal activation of contralesional areas can hamper ipsilesional reorganization via enhanced transcortical inhibition.4-6 Stimulation could therefore be used to excite the ipsilesional or to suppress the contralesional primary motor cortex.
To date, a number of small laboratory-based studies have provided some proof of this principle by showing that a single application of noninvasive cortical stimulation can transiently improve aspects of hand function in chronic stabilized patients with mild residual disability.2,7-13 However, it is still unknown whether these interventions will be useful in a clinical setting. A few clinical studies in acute,14 subacute,15,16 chronic,17-19 or mixed patients20 have shown some positive effects from repeated stimulation, usually in combination with some form of therapy; however, significant variability in effect sizes, short follow-up periods, poorly defined therapy protocols, and/or lack of effect from physical therapy alone make these results difficult to interpret.
We designed a small semi-randomized, placebo-controlled trial to assess whether clinically important long-lasting differences can be achieved by adding theta burst stimulation (TBS) to a standardized physiotherapy protocol for the upper limb in chronic stroke patients. TBS is a robust form of repetitive TMS; its after effects, thought to result from LTP/LTD at NMDA and GABAergic synapses,21-23 can last up to 1 hour, an excellent time window for a therapy session. Previous single-session studies have shown that ipsilesional excitatory TBS produces more consistent behavioral effects than contralesional inhibitory TBS.7,12 However, because a single application may not be enough to reverse chronic interhemispheric inhibition, we decided to test both approaches. We hypothesized that immediate and long-term outcomes from the active treatment would be significantly better when compared with control treatment.
Methods
Participants
Over a period of 50 months, 41 stroke patients were recruited from 4 sites (3 in the UK and 1 in Italy) or from the community via UK-wide advertisement. After initial screening, potentially eligible patients were referred to 1 of the 2 centers undertaking the study procedures (UCL Institute of Neurology, London, UK, and Università Cattolica, Rome, Italy). Inclusion criteria were as follows: (a) first-ever ischemic stroke at least 1 year earlier; (b) mild to moderate residual hand weakness, defined as grasp strength ≥5% of the unaffected hand, preserved extension at the wrist (≥20°), and baseline score in 9 hole Peg Test (9HPT) ≤70% of the unaffected hand; and (c) ability to give informed consent and comprehend instructions. Exclusion criteria included significant spasticity (Ashworth score >2); concomitant neurological conditions, including any history of epilepsy; significant comorbidities; and contraindications to TMS. The study was approved by the main Research Ethics Committee (the joint Institute of Neurology and the National Hospital for Neurology and Neurosurgery) and each local committee.
Intervention
A session of TBS (contralesional TBS [cTBS] or ipsilesional TBS [iTBS] for the active groups and sham for the control group) was followed by physical therapy targeting the arm daily for 10 working days.
TBS consists of short bursts of 3 stimuli at 50 Hz, repeating at 5 Hz.21 The continuous pattern (cTBS; 200 bursts, 600 stimuli, 40 s) suppresses cortical excitability and was delivered to the contralesional hemisphere; the intermittent pattern (iTBS; 20 trains of 10 bursts given with 8-s intervals, 600 stimuli, 200 s) enhances excitability and was delivered to the ipsilesional hemisphere. Similar excitability changes have been produced in chronic7,12 and acute24 stroke patients using the same stimulation parameters.
A 70-mm figure-of-eight coil connected to a Super Rapid Magstim package (Magstim Co, UK) was used to define the motor hotspot for the first dorsal interosseous muscle and the active motor threshold (AMT) as described previously.12 TBS was delivered at 80% of the AMT. Sham stimulation was delivered using a 2-wings 90° positioning at 50% of maximum output.25 A head coordinates system was used to record the position of the hotspot. The hotspot and AMT were confirmed every other day (daily testing in the first 10 patients suggested negligible differences). Patients were instructed not to move their hands for 7 minutes after the end of the stimulation because it has been shown that early contraction can cancel the effects of cTBS.26 All head marks were then removed. The researchers delivering TBS (PT, MvB, GM) were not involved in outcome assessments.
Physical therapy included strength training for the wrist, fingers, and thumb and grasp and repetitive task practice; the latter aimed mainly at hand function, including, however, proximal elements through functional reach to different areas within the work space. It was designed to ensure the same intensity of intervention independent of baseline functional ability, as described in detail in an earlier publication.27 Therapy was given by 3 certified physiotherapists (AW, KB, and CG); KB and CG received 1-to-1 training and continuous support by the lead physiotherapist (AW).
Assessments
The National Institute of Health Stroke Scale and the Barthel Index score were used to assess neurological impairment and disability at enrollment. The Action Research Arm Test (ARAT; score 0-57)28 was used to define the level of arm function. The Rivermead Assessment of Somatosensory Performance29 was used to assess pressure touch (score 0-16) and sensory discrimination (score: fail or pass) in the hand.
Primary Outcome Measures
We assessed key aspects of hand function using measures sensitive to change in patients with mild to moderate hand weakness. These were the 9HPT, the Jebsen Taylor Test (JTT), and maximal grasp and pinch grip dynamometry.
The 9HPT is useful in measuring dexterity in relatively well-recovered patients.30 Participants placed 9 pegs into 9 holes on a board as fast as possible. Scores were computed as pegs/s, averaged over 3 trials and normalized to the average score of the unaffected hand (range 0-1; 0, cannot do).
The JTT has been shown to be valid and reliable in the normal population31 and shows good responsiveness in chronic stroke patients undergoing similar interventions.9,17 The modified version used here consists of 6 sub-sets: turning cards over, putting small objects in a can, mimicking feeding using a spoon, stacking checkers, and moving light and heavy cans. The time to complete each subset was recorded. Performance has been shown to improve with practice9,17; hence items were tested 5 times at each assessment. Performance stabilized after 2 to 3 trials, and the last 2 trials were averaged and used for analysis. Scores were normalized to the performance of the unaffected hand and computed as follows: cannot do or <.05 = 1, 0.05-0.09 = 2, 0.1-0.14 = 3, and so on; thus, the range was 1 to 20, each point reflecting an improvement of 5% of the maximum score—that is, the score of the unaffected hand. The items were then summed to produce a JTT total score (range 6-120, 11.4 points reflecting 10% improvement).
Maximal grip strength represents an important aspect of hand function after stroke; measures, especially when normalized to the unaffected hand, are highly reproducible in chronic patients.32 Grasp and pinch grip dynamometry were performed using a digital dynamometer (Biometrics Ltd). Scores were recorded in kilograms, averaged over 2 trials, and normalized to the unaffected hand (range 0-1, 0 = cannot do).
The clinically important difference was defined as 10% of the maximum score for each test.
Secondary Outcome Measures
Goal attainment scaling was used to evaluate whether any benefits from the intervention were meaningful to the patients. Goals were set by the patients with the therapist’s support and combined in a single T score, using the following formula:
| 1 |
where gi is the score and wi the weight assigned to the ith goal; goals were scored using a 5-point scale (0 = expected outcome; +1 = slightly better; +2 = a lot better than expected; −1 and −2 = slightly worse and a lot worse than expected); at baseline, g was always −1. A change of 10 or more in the baseline T score signifies meaningful change.33
A visual analog scale (VAS)34 was used to assess patients’ rating of the intervention (usefulness, effectiveness, and fatigue) and the stimulation (pain or discomfort). All patients were asked whether they thought they had real or sham stimulation.
Patients, therapists, and researchers involved in outcome assessments were blinded to the type of TBS. All assessors (AW, BC, RO, DH, and MD) received training from the lead physiotherapist (AW); each patient was assessed by a single researcher.
Enrollment and Group Allocation
We aimed to test 2 different types of active TBS: iTBS over the ipsilesional side (iTBS group) and cTBS over the contralesional side (cTBS group). However, in a percentage of clinically eligible patients, there were no adequate responses to TMS on the ipsilesional side—that is, thresholds were too high to identify the hotspot and/or deliver iTBS according to the protocol. We decided to balance clinical realities and scientific accuracy by including these patients but only randomizing them to receive cTBS or sham. To keep our comparisons unbiased. the sham group was split into 2 subgroups: cSham (controlling for the cTBS group, with and without ipsilesional responses) and iSham (controlling for the iTBS group, all patients had ipsilesional responses). In all, 8 patients with responses were included both in the cSham and iSham groups; 4 more patients without responses were recruited into the cSham group, and 4 other patients with responses completed the iSham group. Hence, the total number of patients receiving sham stimulation were 16.
For group allocation, a minimization process35 taking into account the ARAT score and age at baseline was used (Figure 1). This was done centrally for all patients by a single researcher (PT). The allocated intervention was e-mailed directly to the researcher delivering TBS using a secure system, on the first day of the treatment.
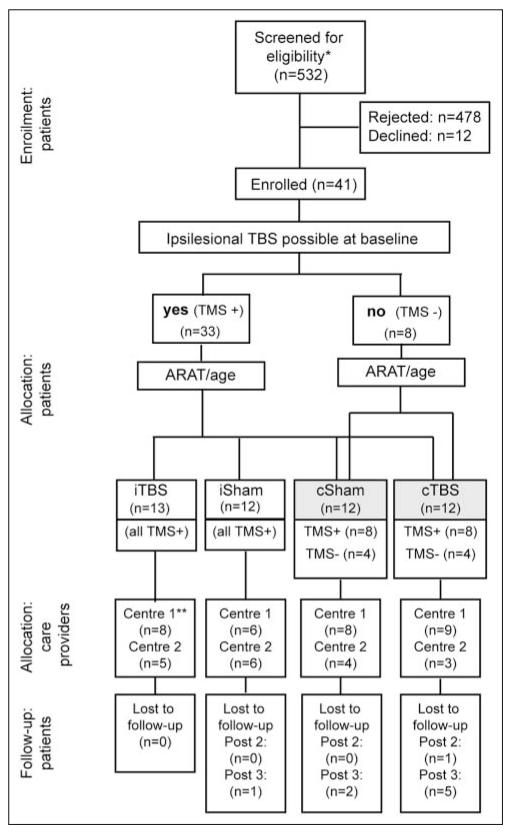
Figure 1.
Participant flow diagram: 8 of the 12 patients (all with preserved ipsilesional responses) are common to the cSham and iSham groups. Abbreviations: ARAT, Action Research Arm Test; TMS, transcranial magnetic stimulation; TBS, theta burst stimulation; cSham, controlling for the cTBS group, with and without ipsilesional responses; iSham, controlling for the iTBS group, and all patients had ipsilesional responses; c, contralesional; i, ipsilesional; post1, post2, post3, outcome measures performed at 4, 30, and 90 days after the end of the treatment period.
*Meeting initial screening criteria (diagnosis of hemiparetic stroke).
**Center 1, UCL Institute of Neurology, UK; Center 2, Institute of Neurology, Università Cattolica, Rome, Italy.
Design
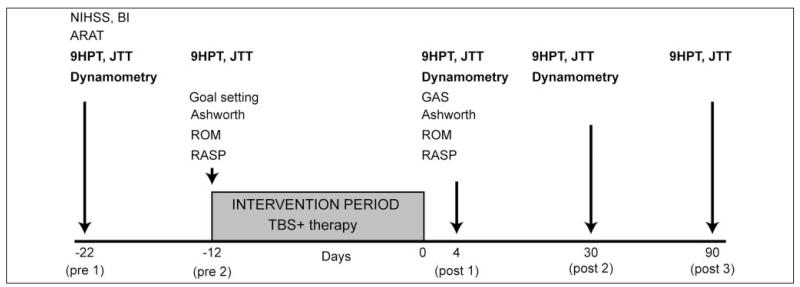
Figure 2 summarizes the study design. Baseline evaluations were performed twice, 10 days apart, to ensure that upper-limb function remained unchanged. The number of tests administered was rationalized to avoid very long sessions. Outcome measures were performed 4 (post1), 30 (post2), and 90 days (post3) after the end of the treatment period.
Figure 2.
Study design; primary outcomes are in bold. Abbreviations: NIHSS, National Institute of Health Stroke Scale; ARAT, Action Research Arm Test; ROM, Range of Movement; RASP, Rivermead Assessment of Somatosensory Performance; 9HPT, 9-Hole Peg Test; JTT, Jebsen Taylor Test; TBS, theta burst stimulation; GAS, goal attainment scaling; post1, post2, post3, outcome measures performed at 4, 30, and 90 days after the end of the treatment period.
This study has been reported in accordance with the CONSORT recommendations for nonpharmaceutical interventions (http://www.consort-statement.org/extensions/interventions).
Statistical Analysis
Power calculations showed that 12 patients per group were needed to detect a 10% difference between the active and sham groups (assuming a standard deviation = 8.5%) with 80% power; details were inferred from previous studies delivering interventions for the upper limb in chronic stroke patients.28,36
Square root transformations were performed as necessary to allow the use of parametric tests. Between-group comparisons (cTBS vs cSham and iTBS vs iSham) were performed to ensure that the groups were matched at baseline. Within-groups comparisons were performed to compare the 2 baselines across the whole population. To investigate the effects of the intervention we used 2-way analyses of variance (ANOVAs) with factors Time and Group. We have adopted one of the suggested methods and divided the P value for the main effects of the ANOVAs by 4 to correct for the multiple primary outcome measures37; thus, the level of significance was set at .0125; for post hoc comparisons, Bonferroni corrections were applied. Please note that we did not perform direct comparisons between the 2 active TBS groups because of differences in the presence of ipsilesional TMS responses.
Changes to the Original Protocol
We originally defined the range of residual disabilities we were targeting as follows: residual hand weakness defined as grasp strength ≥5% of the unaffected side and ARAT score at baseline of 51 or less. We were also looking for a recordable response to TMS on the ipsilesional side. However, the rate of recruitment was very low because many patients within this clinical range often had significant spasticity, could not perform many exercises, and/or had no response to TMS. We therefore modified the clinical range as described earlier in the methods. As a result, we did not use the ARAT as a primary outcome measure because a small percentage of patients had scores that would not allow a measurable 10% change. We did include patients who fulfilled the modified clinical criteria but had no response to TMS and adapted the group allocation process as described above. The power calculation therefore refers to 2 independent group comparisons.
The study protocol in its final form has been published on the UK Stroke Research Network Web site, where it is available to the public: URL, http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=3279; study ID, 03884521.
Results
One patient was unable to attend at 30 days and another 6 at 90 days (Figure 1). There were no adverse events. Table 1 shows that study groups were balanced at baseline apart from more dominant hemisphere infarcts in the cSham group.
Table 1.
Demographics, Stroke Characteristics, and Clinical Severity at Baseline
| cTBS (n = 12) | cSham (n = 12) | P | iTBS (n = 13) | iSham (n = 12) | P | |
|---|---|---|---|---|---|---|
| Age, ya | 55.8 ± 12.4 | 59.4 ± 12.4 | .52 | 54.4 ± 15.8 | 58.5 ± 12.0 | .47b |
| Sex, % female | 41.7 | 50 | .5c | 46.2 | 25 | .41c |
| DOS, moa | 29.8 ± 19.7 | 49.6 ± 76.9 | .9d | 17.5 ± 5.1 | 38.5 ± 57.2 | .38d |
| Lesion location | ||||||
| Subcortical | 2/12 | 7/12 | .09c | 9/13 | 5/12 | .24c |
| Cortical involvemente | 10/12 | 5/12 | 4/13 | 7/12 | ||
| Lesion side, % dominant | 2/12 | 8/12 | .04c | 6/13 | 9/12 | .23c |
| Barthel indexa | 18.3 ± 2 | 18.3 ± 2 | .9d | 18.8 ± 2.1 | 18.8 ± 1.6 | .92d |
| NIHSSa | 3.1 ± 1.9 | 4.2 ± 1.7 | .16b | 3.5 ± 1.4 | 4.2 ± 1.3 | .25b |
| ARATa | 36.5 ± 11.9 | 36.7 ± 13.4 | 0.98b | 45.1 ± 11.4 | 42 ± 10.6 | 0.49b |
| RASP | ||||||
| Light touch, pass, % | 58.3 | 75 | 0.67b | 84.6 | 66.7 | 0.38b |
| Discrimination | 13.2 ± 4.5 | 13.7 ± 4.1 | 0.88c | 15.1 ± 2.5 | 14.9 ± 1.9 | 0.65c |
| 9HPT, range 0-1a | 0.14 ± 0.16 | 0.23 ± 0.26 | 0.54b | 0.32 ± 0.22 | 0.27 ± 0.29 | 0.34b |
| JTT, range 6-114a | 43 ± 17.6 | 47.2 ± 30.6 | 0.34b | 63.8 ± 20.7 | 55.1 ± 24.3 | 0.34b |
| Graspa | 0.47 ± 0.24 | 0.49 ± 0.27 | 0.86b | 0.55 ± 0.22 | 0.55 ± 0.21 | 0.99b |
| Pinch gripa | 0.6 ± 0.3 | 0.55 ± 0.24 | 0.91b | 0.62 ± 0.26 | 0.57 ± 0.19 | 0.75b |
Abbreviations: TBS, theta burst stimulation; DOS, date of stroke; NIHSS, National Institute of Health Stroke Scale; ARAT, Action Research Arm Test; RASP, Rivermead Assessment of Somatosensory Performance; 9HPT, 9-Hole Peg Test; JTT, Jebsen Taylor Test.
Mean ± standard deviation.
t Test.
x2.
Mann-Whitney.
Sparing the primary motor cortex.
Primary Outcome Measures
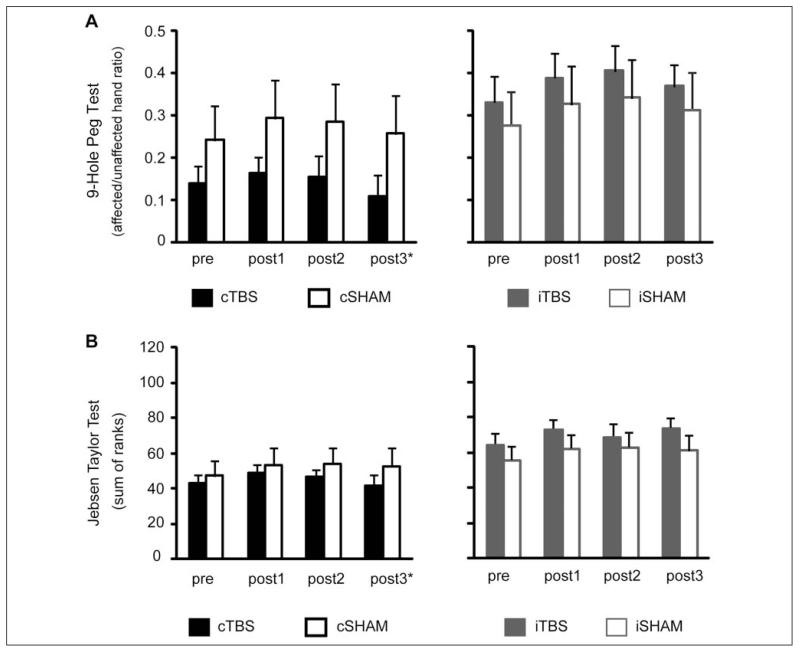
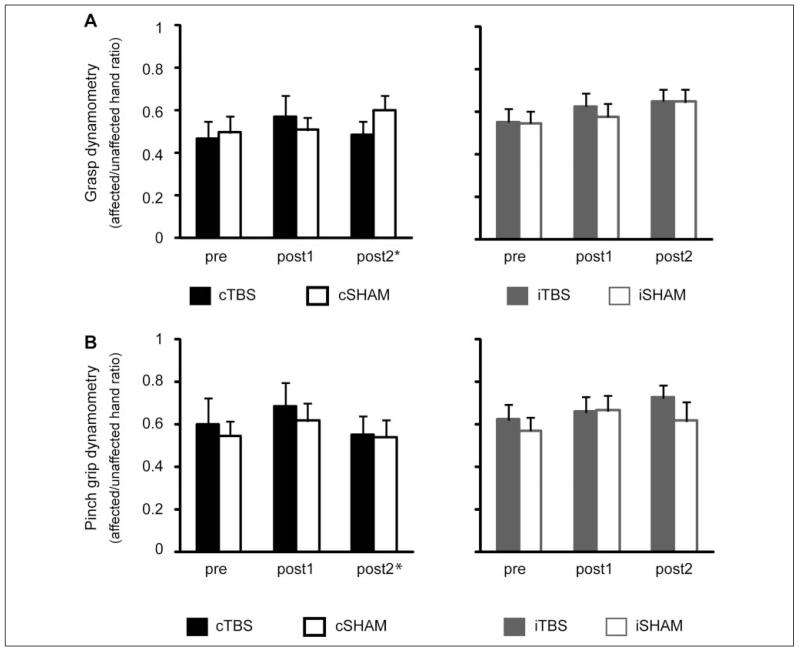
Figures 3 and 4 illustrate the mean scores before and after the intervention. Table 2 summarizes the absolute change seen with the intervention.
Figure 3.
Mean scores in upper-limb function tests before and after the intervention. Error bars represent standard errors. Abbreviations: TBS, theta burst stimulation; cSham, controlling for the cTBS group, with and without ipsilesional responses; iSham, controlling for the iTBS group, and all patients had ipsilesional responses; c, contralesional; i, ipsilesional; post1, post2, post3, outcome measures performed at 4, 30, and 90 days after the end of the treatment period. *The number of patients in the cTBS group was considerably smaller at post3 (n = 6).
Figure 4.
Mean strength measures before and after the intervention. Error bars represent standard errors. Abbreviations: TBS, theta burst stimulation; cSham, controlling for the cTBS group, with and without ipsilesional responses; iSham, controlling for the iTBS group, and all patients had ipsilesional responses; c, contralesional; i, ipsilesional; post1, post2, post3, outcome measures performed at 4, 30, and 90 days after the end of the treatment period. *The number of patients in the cTBS group was smaller at post2 (n = 10 for grip and n = 9 for grasp dynamometry).
Table 2.
Absolute Change in Primary Outcome Measures Stratified by Study Groupsa
| All Patients | cTBS | cSham | iTBS | iSham | ||
|---|---|---|---|---|---|---|
| 9HPT | Post 1 | 0.05 (0.03-0.07), n = 41 |
0.02 (−0.002-0.05), n = 12 |
0.05 (0.02-0.09), n = 12 |
0.06 (0.005-0.11), n = 13 |
0.05 (0.02-0.08), n = 12 |
| Post 2 | 0.06 (0.04-0.08), n = 40 |
0.04 (0.009-0.07), n = 11 |
0.04 (0.008-0.08), n = 12 |
0.07 (0.02-0.13), n = 13 |
0.07 (0.02-0.11), n = 12 |
|
| Post 3 | 0.05 (0.02-0.08), n = 33 |
0.05 (−0.02- 0.12), n = 6 |
0.03 (0.008-0.05), n = 12 |
0.04 (−0.03-0.1), n = 13 |
0.06 (0.02-0.1), n = 12 |
|
| JTT | Post 1 | 6.9 (3.9-9.9), n = 41 |
5.6 (2.9-8.4), n = 12 |
6.3 (−0.1-12.7), n = 12 |
9.2 (3.7-14.7), n = 13 |
6.5 (−0.8-14), n = 12 |
| Post 2 | 5.6 (2.6- 8.6), n = 40 |
6.4 (3.5-9.3), n = 11 |
6.4 (−1.1-13.9), n = 12 |
4.6 (−0.5-9.9), n = 13 |
7.5 (−0.9-16), n = 12 |
|
| Post 3 | 7.6 (4.9-10.2), n = 33 |
5.2 (1.9-8.3), n = 6 |
6.4 (0.9-11.8), n = 12 |
9.5 (4.7-14.3), n = 13 |
7.4 (1.3-13.5), n = 12 |
|
| Grasp dynamometry |
Post 1 | 0.06 (0.02-0.1), n = 39 |
0.01 (−0.001-0.2), n = 11 |
0.01 (−0.08-0.1), n = 12 |
0.07 (−0.006-0.15), n = 13 |
0.03 (−0.03-0.09), n = 11 |
| Post 2 | 0.09 (0.05-0.13), n = 38 |
0.08 (−0.002-0.15), n = 10 |
0.11 (0.01-0.2), n = 12 |
0.09 (0.01-0.18), n = 13 |
0.1 (−0.003- 0.21), n = 11 |
|
| Pinch-grip dynamometry |
Post 1 | 0.06 (0.01-0.1), n = 39 |
0.08 (−0.4-0.21), n = 10 |
0.07 (−0.8-0.21), n = 12 |
0.04 (−0.05-0.12), n = 13 |
0.09 (−0.06-0.24), n = 12 |
| Post 2 | 0.05 (−0.01-0.12), n = 38 |
0.02 (−0.08-0.11), n = 9 |
−0.007 (−0.15- 0.14), n = 12 |
0.1 (−0.02-0.23), n = 13 |
0.04 (−0.13- 0.22), n = 12 |
Abbreviations: TBS, theta burst stimulation; cSham, controlling for the cTBS group, with and without ipsilesional responses; iSham, controlling for the iTBS group, and all patients had ipsilesional responses; c, contralesional; i, ipsilesional; 9HPT: 9-Hole Peg Test; JTT: Jebsen Taylor Test.
Minimal clinically important differences (10% of the maximum score, by approximation the score of the unaffected hand): 9HPT and dynamometry = 0.1, JTT = 11.4.
Nine-Hole Peg Test
Performance was not different between the 2 baseline evaluations—t(40) = −1.049; P = .3; scores were therefore averaged and the mean (pre) used for further analysis. Two-way mixed design ANOVAs with factors Group (levels: cTBS vs cSham or iTBS vs iSham) and Time (levels: pre, post1, and post2) were used.
cTBS versus cSham
There was a significant main effect of Time [F(2, 42) = 10.9; P < .001] with no main effect of Group or Time × Group interaction; post hoc comparisons for Time showed that all patients improved at post1 (P = .001) and maintained their improvements at post2 (P = .006); there were no differences between post1 and post2 (P = .88). We did not look for differences at 3 months because of inadequate numbers in the cTBS group (n = 6).
iTBS versus iSham
there was only a significant main effect of Time [F(2, 46) = 11.7; P < .001]; post hoc comparisons confirmed that all patients improved at post1 (P = .002) and maintained this gain at post2 (P = .002). When post3 was included in the analysis, the results were similar; however, the difference between baseline and post3 was borderline significant (P = .051).
Jebsen Taylor Test
Small improvements in the total JTT score were seen between the 2 baseline assessments [49.2 ± 24.9 vs 51.8 ± 23.7; t(40) = −2.85; P = .007], which could reflect a learning effect. The second baseline was therefore used for further analysis. The ANOVA design was otherwise as above.
We found a significant main effect of Time [F(2, 42) = 10.27; P = .001) for cTBS versus cSham and for iTBS vs iSham [F(2, 46) = 9.154; P = .001], with no effect of Group nor a Time × Group interaction. Post hoc tests revealed that all patients improved at post1—P = .004 and P = .003, respectively; at post2, the respective results were P = .009 and P = .03. When post3 was included in the analysis, results for the iTBS versus iSham were virtually unchanged.
Grasp and Pinch-Grip Dynamometry
The ANOVA design was as above. No effects were found for pinch grip. For grasp, there was a significant main effect of Time [(F(2, 40) = 6.2, P = .005 for cTBS vs cSham and F(2, 44) = 8.1, P = .001 for iTBS vs iSham] without a main effect of Group or an interaction. In all groups and unlike the other tests, significant improvements from baseline could only be demonstrated at post2 (P = .01 and P = .008, respectively).
Comparisons between cTBS and cSham were virtually unchanged for all outcomes when patients with and without responses to TMS were analyzed separately.
Secondary Outcome Measures
The goal attainment scaling is shown in Table 3. Significant improvements were seen in all patients irrespective of the type of TBS they received. VAS scores revealed that all patients found the intervention to be highly effective and well tolerated without any clear differences between the groups (Table 3). Also, 50% of the patients could not tell if they had real or sham stimulation; 15.4% believed that they had had sham and 34.5% that they had had real stimulation. There was no association between the patient’s opinion and the type of stimulation they actually received (x2, P = .3). The former was not correlated with VAS scores.
Table 3.
Visual Analog and Goal Attainment Scaling
| cTBS (n = 12) | cSham (n = 12) | P a | iTBS (n = 13) | iSham (n = 12) | P a | |
|---|---|---|---|---|---|---|
| Usefulnessb | 16.9 ± 3.5 | 16.4 ± 4.2 | .74 | 14.3 ± 4.5 | 15.3 ± 5.5 | .43 |
| Effectivenessb | 14.7 ± 3.9 | 11.7 ± 4.9 | .09 | 13.9 ± 4.8 | 11.6 ± 5.4 | .40 |
| Fatigueb | 8.8 ± 6.2 | 8.1 ± 5.1 | .85 | 8.8 ± 4.9 | 7.6 ± 5.1 | .89 |
| Stimulation associated pain/discomfortb | 2.3 ± 2.5 | 2.5 ± 3.3 | .69 | 1.5 ± 1.4 | 2 ± 2.9 | .69 |
| GAS T score changec (median/IQR) | 20 (10) | 15.5 (10) | .59 | 18.6 (13) | 18.6 (20) | .47 |
Abbreviations: TBS, theta burst stimulation; cSham, controlling for the cTBS group, with and without ipsilesional responses; iSham, controlling for the iTBS group, and all patients had ipsilesional responses; c, contralesional; i, ipsilesional; GAS, goal attainment scaling; IQR, interquartile range.
Mann-Whitney.
Range, 0 to 20, ascending,
Scores ≥10 show significant change.
Discussion
In this population of chronic stroke patients with mild to moderate deficits of upper-limb function, TBS did not augment the gains from a retraining protocol for the upper limb. All patients demonstrated improvements, mainly in measures of dexterity, which were maintained for up to 3 months. The average effect size was 5% of the maximum score, which is comparable to what has been achieved previously with even more intensive late interventions.36 However, addition of TBS did not produce the benefits we were expecting. We aimed for a difference of 10% because this is the minimum effect that has been previously considered to be of clinical significance. Such effects have been previously achieved with intensive late therapy interventions.36 Besides, at the time this study was designed, there was no other source to base our power calculation on. However, this anticipation was clearly overestimated. In fact, our best result was a difference of 2.7 points (2.4% of the maximum, corresponding to a small effect size of 0.25, NS) in the total JTT score change between iTBS and iSham, which would require more than 300 patients to be detected as statistically significant (Table 2).
The idea for this study was based on pilot, single-session studies showing that cortical stimulation alone can transiently improve physiological aspects of hand function, such as reaction times or tapping speed in chronic stroke patients.8-13 There is also some evidence that repeated applications (over a few days) may lead to cumulating longer-lasting effects.17 However, improved performance in a task that is being practiced repeatedly does not necessarily translate into changes in the way the arm is used in everyday life. We have thus designed this small clinical trial to explore whether such clinically significant and long-lasting gains could be achieved. TBS was combined with physical therapy aiming to improve the strength and dexterity of the hand. Our hypothesis was that TBS, as a plasticity-modifying intervention, would enhance and/or accelerate the training process by inducing a critical level of preactivation in the ipsilesional motor circuits. We opted for 2 weeks of therapy because adequate training is essential for the formation of new, stable motor memories leading to skill acquisition.3 To avoid bias associated with the poorly defined “standard physiotherapy” used in some previous studies, we devised a standardized intensity-controlled protocol.27 The standardized protocol along with the training and support provided also allowed us to control the therapy between the 2 centers. We have also provided training and supervision to all our raters and therapists. Despite these efforts, we cannot exclude variability between the 2 treatment centers, and because of the small sample size, we were not able to perform statistical tests for this. We included moderately affected patients because a degree of motor recovery is necessary to perform the exercises and standardize the intensity of the therapy. Besides, it would be unrealistic to attempt to promote ipsilesional reorganization in patients with severe brain damage. Moreover, the more severe the hand weakness, the less likely patients are to have recordable ipsilesional responses to TMS. In fact, in a number of clinically eligible patients, the ipsilesional response was inadequate to deliver iTBS according to the protocol. To keep our study pragmatic, we decided to include these patients. However, instead of arbitrarily using parameters calculated on the healthy side, as done previously, we only randomized them to the cTBS group. Contralesional stimulation, if proven effective, could be the intervention of choice for these patients. As a subgroup, they were slightly more affected but were equally divided between the cTBS and cSham group, and they had a similar response to the intervention. When excluded from the analysis, there was still no difference between the groups. Therefore, although unlikely, we cannot confidently exclude some bias associated with their inclusion. Future work on whether or how to treat these patients would be very useful. The cSham group included slightly more patients with dominant strokes; such imbalances often occur with small group sizes. However, none of our patients had significant side-related problems, such as aphasia or neglect, that could introduce bias. We followed our patients for up to 3 months in order to test the longevity of the effects. Unfortunately, some patients from the cTBS group were lost to follow-up after the 1 month assessment.
Results from previous studies combining rTMS and therapy in chronic stroke patients have been conflicting. In the study by Malcolm et al,18 ipsilesional high-frequency rTMS (5 Hz) did not augment the gains from 2 weeks of constraint-induced movement therapy in chronic stroke patients with disabilities similar to the ones in our study. Together with our results, this may raise the possibility of a ceiling effect. In other words, the amount of improvement that can be achieved or demonstrated in the chronic phase in patients with mild to moderate problems may be limited. However, it is interesting that TBS did not make any difference with outcomes that did not respond to therapy alone, such as pinch grip. Alternatively, longer periods of treatment may be needed at this late stage for a plasticity-modifying intervention to produce a measurable effect. In contrast, it was recently shown that bihemispheric TDCS combined with therapy induced a 9% (of the maximum score) improvement after only 5 days of treatment.19 However, minimal changes were seen in the control group, and the study patients may have been slightly more affected than ours, although different assessment tools make direct comparisons difficult. This result may suggest that either bihemispheric stimulation or TDCS may be a more effective stimulation protocol, so that less therapy is needed. Unfortunately, the follow-up period was only 1 week, and thus, the longevity of these effects and their clinical significance remains unclear.
Positive effects have been reported by adding cortical stimulation to standard rehabilitation protocols delivered earlier during recovery,14-16,20 which appear to outlast the intervention period by 3 months or more. It is possible that during this period, more can be achieved by optimizing brain plasticity. However, careful inspection of the results again reveals significant variability in effect sizes. On some occasions, patients receiving therapy alone show little15 or no20 improvement—an unexpected finding for subacute patients. In fact, the effect size often attributed to the stimulation is similar to the one achieved in our study of chronic patients without any stimulation. Moreover, the use of different outcome measures makes comparisons very difficult.
This is the first treatment study using TBS for cortical stimulation. The stimulation was delivered using standard parameters because it was previously shown that “standard” TBS can change cortical excitability in stroke patients in the same way it does in healthy individuals.7,12,24 Therapeutic TDCS is also applied using standard parameters.9,19 In this study, we have not measured daily excitability changes, mainly to keep the duration of the sessions short and not lose valuable treatment time. It is therefore possible that not all patients responded to TBS, or the response could have been even better if other parameters had been used. Future research is needed to test whether the stimulation protocol itself and/or the stimulation parameters are important factors. So far, a variety of protocols have been used, and the stimulation parameters, for example, intensity and duration, are variable and often arbitrary. Genetic factors deserve further exploration in patient populations and may lead to additional inclusion/exclusion criteria in the future.38
Negative trials,39,40 just like explanatory41-43 and positive44 trials in the field of brain stimulation, have value in bringing clinical research forward.45 They provide data for accurate power calculations, dose–response curves, and stratification of patients to target individuals who have the greatest likelihood to benefit from a given intervention.46 For example, recent work demonstrated an opposite response to stimulation after stroke, depending on whether the location of the primary lesion was cortical or subcortical.47 This finding could be further explored by looking into how the effects of the stroke on brain activation and/or cortical excitability and connectivity interact with response to brain stimulation therapies.46
Conclusions
Our study suggests that the concept of rTMS making rehabilitative treatments for stroke patients even better is perhaps simplistic and that expectations regarding effect sizes should be reconsidered. Instead of adding treatments together hoping to achieve better results, attention should be focused on identifying the patients most likely to respond to a particular intervention. Electrophysiological and functional imaging data that were collected as part of this study, and that will be reported separately, may contribute to that end. Technical difficulties associated with certain interventions, such as ipsilesional rTMS, should also be tackled. Consensus among researchers on clinically relevant outcome measures and the length of follow-up would facilitate comparisons with and interpretation of future studies.
Acknowledgments
Funding The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical Research Council, UK, Rosetrees Trust, European Community (PLASTICISE: FP7 Collaborative Project (223524)
Footnotes
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hess G, Donoghue JP. Long-term potentiation and long-term depression of horizontal connections in rat motor cortex. Acta Neurobiol Exp (Wars) 1996;56:397–405. doi: 10.55782/ane-1996-1143. [DOI] [PubMed] [Google Scholar]
- 2.Talelli P, Rothwell J. Does brain stimulation after stroke have a future? Curr Opin Neurol. 2006;19:543–550. doi: 10.1097/WCO.0b013e32801080d1. [DOI] [PubMed] [Google Scholar]
- 3.Reis J, Robertson EM, Krakauer JW, et al. Consensus: can transcranial direct current stimulation and transcranial magnetic stimulation enhance motor learning and memory formation? Brain Stimul. 2008;1:363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 5.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126(pt 11):2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerloff C, Bushara K, Sailer A, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129(pt 3):791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- 7.Ackerley SJ, Stinear CM, Barber PA, Byblow WD. Combining theta burst stimulation with training after subcortical stroke. Stroke. 2010;41:1568–1572. doi: 10.1161/STROKEAHA.110.583278. [DOI] [PubMed] [Google Scholar]
- 8.Fregni F, Boggio PS, Mansur CG, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- 9.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(pt 3):490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 10.Mansur CG, Fregni F, Boggio PS, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- 12.Talelli P, Greenwood RJ, Rothwell JC. Exploring theta burst stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 2007;118:333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- 14.Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- 15.Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PK. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J Rehabil Med. 2010;42:758–764. doi: 10.2340/16501977-0590. [DOI] [PubMed] [Google Scholar]
- 16.Kim DY, Lim JY, Kang EK, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. 2010;89:879–886. doi: 10.1097/PHM.0b013e3181f70aa7. [DOI] [PubMed] [Google Scholar]
- 17.Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- 18.Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: an exploratory randomized controlled trial. Am J Phys Med Rehabil. 2007;86:707–715. doi: 10.1097/PHM.0b013e31813e0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emara TH, Moustafa RR, Elnahas NM, et al. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. 2010;17:1203–1209. doi: 10.1111/j.1468-1331.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 22.Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Stagg CJ, Wylezinska M, Matthews PM, et al. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Lazzaro V, Pilato F, Dileone M, et al. Modulating cortical excitability in acute stroke: a repetitive TMS study. Clin Neurophysiol. 2008;119:715–723. doi: 10.1016/j.clinph.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 25.Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry. 2001;49:460–463. doi: 10.1016/s0006-3223(00)01110-0. [DOI] [PubMed] [Google Scholar]
- 26.Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- 27.Wallace AC, Talelli P, Dileone M, et al. Standardizing the intensity of upper limb treatment in rehabilitation medicine. Clin Rehabil. 2010;24:471–478. doi: 10.1177/0269215509358944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the Action Research Arm Test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82:14–19. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- 29.Winward CE, Halligan PW, Wade DT. The Rivermead Assessment of Somatosensory Performance (RASP): standardization and reliability data. Clin Rehabil. 2002;16:523–533. doi: 10.1191/0269215502cr522oa. [DOI] [PubMed] [Google Scholar]
- 30.Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. J Neurol Neurosurg Psychiatry. 1987;50:714–719. doi: 10.1136/jnnp.50.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 32.Boissy P, Bourbonnais D, Carlotti MM, Gravel D, Arsenault BA. Maximal grip force in chronic stroke subjects and its relationship to global upper extremity function. Clin Rehabil. 1999;13:354–362. doi: 10.1191/026921599676433080. [DOI] [PubMed] [Google Scholar]
- 33.Turner-Stokes L, Williams H. Goal attainment scaling: a direct comparison of alternative rating methods. Clin Rehabil. 2010;24:66–73. doi: 10.1177/0269215509343846. [DOI] [PubMed] [Google Scholar]
- 34.Price CI, Curless RH, Rodgers H. Can stroke patients use visual analogue scales? Stroke. 1999;30:1357–1361. doi: 10.1161/01.str.30.7.1357. [DOI] [PubMed] [Google Scholar]
- 35.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15:443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 36.van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 37.Pocock SJ, Geller NL, Tsiatis AA. The analysis of multiple endpoints in clinical trials. Biometrics. 1987;43:487–498. [PubMed] [Google Scholar]
- 38.Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586(pt 23):5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothkegel H, Sommer M, Rammsayer T, Trenkwalder C, Paulus W. Training effects outweigh effects of single-session conventional rTMS and theta burst stimulation in PD patients. Neurorehabil Neural Repair. 2009;23:373–381. doi: 10.1177/1545968308322842. [DOI] [PubMed] [Google Scholar]
- 40.Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabil Neural Repair. 2011;25:838–846. doi: 10.1177/1545968311413906. [DOI] [PubMed] [Google Scholar]
- 41.Buetefisch C, Heger R, Schicks W, Seitz R, Netz J. Hebbian-type stimulation during robot-assisted training in patients with stroke. Neurorehabil Neural Repair. 2011;25:645–655. doi: 10.1177/1545968311402507. [DOI] [PubMed] [Google Scholar]
- 42.Castel-Lacanal E, Marque P, Tardy J, et al. Induction of cortical plastic changes in wrist muscles by paired associative stimulation in the recovery phase of stroke patients. Neurorehabil Neural Repair. 2009;23:366–372. doi: 10.1177/1545968308322841. [DOI] [PubMed] [Google Scholar]
- 43.Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabil Neural Repair. 2010;24:125–135. doi: 10.1177/1545968309345270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, Yang YR. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial [published online ahead of print October 5, 2011] Neurorehabil Neural Repair. doi: 10.1177/1545968311423265. doi:10.1177/1545968311423265. [DOI] [PubMed] [Google Scholar]
- 45.Dobkin BH. Progressive staging of pilot studies to improve phase III trials for motor interventions. Neurorehabil Neural Repair. 2009;23:197–206. doi: 10.1177/1545968309331863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward NS. Getting lost in translation. Curr Opin Neurol. 2008;21:625–627. doi: 10.1097/WCO.0b013e32831997af. [DOI] [PubMed] [Google Scholar]
- 47.Ameli M, Grefkes C, Kemper F, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66:298–309. doi: 10.1002/ana.21725. [DOI] [PubMed] [Google Scholar]