Abstract
Background
Despite recommendations in the U.S. for routine HPV vaccination of adolescent girls since 2006, rates of vaccination continue to be low.
Purpose
This study reports vaccination uptake, factors associated with vaccine uptake and reasons for non-vaccination within a national sample of adolescent females during 2010.
Methods
Using a computer administered survey of a national sample of 501 mothers of daughters 14-17 years old we assessed maternal reports of HPV vaccination as well as socio-demographical factors, maternal HPV exposures and reasons chosen for non-vaccination.
Results
Reported HPV vaccination rates were slightly over 50% (51.1%), with 38.3% reporting completion of all 3 doses. Socioeconomic and demographic factors were not associated with vaccination initiation; however, Blacks and Hispanics were less likely to complete vaccination. The most common reasons for non-vaccination were concerns about vaccine safety, danger to daughter, and provider non-recommendation.
Conclusions
Relatively poor HPV vaccine initiation and only modest 3-dose completion continues to be a major public health concern that requires continued efforts to address identified predictors and reasons for non-vaccination.
Keywords: HPV, Vaccination Rates, Adolescent Health Behaviors, Sexually Transmitted Infections
Introduction
Human papillomavirus (HPV) vaccination can lead to substantial reductions in the incidence of HPV infection and HPV-related diseases, including anogenital cancers and genital warts [1]. The introduction and licensing of the quadrivalent HPV vaccine in 2006 and the subsequent licensing of the bivalent vaccine in 2008 created opportunities to counter the existing HPV disease burden [1,2]. Given the high rates of HPV infection that occur shortly after the initiation of sex and the vaccine's ability to prevent infection only prior to exposure, HPV vaccination has been particularly targeted to young adolescents [3,4]. Routine vaccination with either the bivalent or quadrivalent vaccine has been recommended by the Centers for Disease Control and Prevention's (CDC's) Advisory Committee for Immunization Practices (ACIP) for adolescent females ages 11-12, with catch-up vaccination for young women 13-26 years of age. However, four years post vaccination licensure, at least half of the target population of adolescent females had not initiated HPV vaccination [5,6].
Various studies have assessed predictors of HPV vaccination initiation and completion. The CDC reported most recently that poverty was not a factor in HPV vaccination initiation. On the other hand, poverty status and minority group membership were found to be associated with lower HPV vaccine completion rates [5,6]. Other studies have shown conflicting results for the significance of socio-demographic factors in HPV vaccination initiation (e.g., parental education level, income level, insurance status) [7-11]. Other factors that have been evaluated as predictors of vaccine acceptance, include maternal attitudes, such as: knowledge of HPV, perception of daughter's risk for HPV acquisition, belief in vaccination benefits, degree of concern about vaccine side effects, child's age, social influences, concern regarding post-vaccination sexual disinhibition, physician's recommendation, and parent's personal exposure to HPV infection [7,9-14].
In light of the morbidity and mortality associated with HPV disease as well as the disproportionately high HPV disease burden in minority and uninsured women within the United States, it is pivotal that we continue to evaluate factors related to HPV vaccine initiation and completion. Understanding the current trends of vaccination and continuing to re-evaluate predictors of vaccination and non-vaccination allows for the development of more effective strategies designed to increase rates of HPV vaccination and reduce future health disparities.
Objective
In 2010 we conducted a survey with a national U.S. sample of mother-daughter pairs to assess the following aims: (1) number of HPV vaccination doses received (0, 1, 2, or 3) by each daughter in middle to late high school, age of 14-17 years, (2) to evaluate potential predictors of vaccination initiation and completion, and (3) to survey mothers whose daughters had not initiated vaccination to identify reasons for non-initiation of HPV vaccination.
Research Methods
Sample Population
For this cross-sectional study, 501 mother-daughter pairs were recruited from across the U.S. from a pre-formed research panel developed by Knowledge Networks (KN) [Menlo, CA.] This nationally representative panel was acquired using random digit dialing methodology as well as address-based sampling to include those households without landline phones. To ensure adequate representation of African American and Hispanic American households, an oversampling of recruitment was performed in certain Census-defined areas. For each survey administered, a random sample was chosen from the existing panel. Surveys were distributed via link-containing emails with reminder emails and phone calls for non-responders to recruit well-balanced, non-biased samples. All surveys through KN were administered on-line. Those families without a home computer or internet service were provided with a laptop computer as well as free monthly internet access as compensation for participating in the KN. For those families with existing internet access, compensation points, redeemable for cash, were given for each survey in which they participated.
Inclusion criteria for this study required the presence in the home of a mother and daughter (between the ages of 14-17). Only one daughter per home, randomly selected, was allowed to participate in the study; other siblings and those mothers whose daughters did not consent to participate in the study were excluded from participation.
On initial recruitment of the study sample, 857 mothers from the existing KN panel confirmed a 14-17 year old daughter living in their household. Panel recipients were given a brief description of the study, its purpose, and the voluntary nature of participation in the study. Potential participants were asked if they would be willing to participate in a study seeking to understand why some adolescents receive HPV vaccination and others do not. Initial consent was obtained from 637 of these mothers (74.3%). If mothers agreed to participate, their daughters were then asked if they were willing to consent to participate in the study. Six hundred and twenty daughters consented (97.3%) representing 47 of the 50 states. Once consent had been obtained from both mothers and daughters, the daughters were allowed access to the online survey, which was completed by all 620 adolescents (100%). Once the daughters had completed and submitted their surveys, the mothers were then allowed access to complete the surveys through their separate email account access. Five hundred and one of the mothers (80.8%) completed and submitted the survey and were included in this study [Figure 1]. The surveys were distributed in this order to minimize the mother's influence on the daughters' survey responses. Institutional Review Board (IRB) approval was obtained from the Indiana University prior to the beginning of the study.
Figure 1.
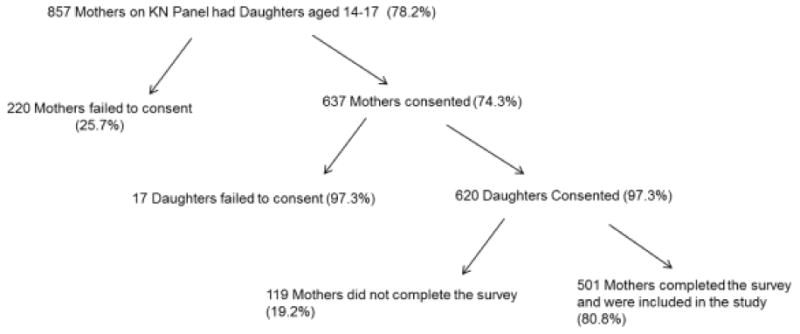
Sample Recruitment Process
Data Collection
Each participant was assigned a separate email account to which all communication was sent. Survey questionnaires were completed in either English or Spanish. A total of nine standard questions were utilized in this study, to assess daughter and maternal socio-demographic factors (daughter's age, daughter's insurance status, daughter's insurance plan, maternal age, maternal racial identification, maternal marital status, maternal education, maternal working status, and maternal geographic location). Additional questions were asked to assess maternal HPV-related exposures, seeking whether the mother had any history of an abnormal Pap test, history of a colposcopy, knowledge of a friend or family member being diagnosed with cervical cancer, or a personal history of cervical cancer.
The number of HPV vaccination doses received (0, 1, 2, or 3) was obtained via maternal report. The mothers of daughters who had initiated vaccination, were asked whether or not their healthcare provider discussed with them that their daughter could receive HPV vaccination. They were also asked to report the strength with which their health care provider had recommended that their daughter receive the vaccine (strongly recommended the vaccine, recommended the vaccine, provided no recommendation, recommended against the vaccine, or strongly recommended against the vaccine).
Mothers of daughters who had not initiated vaccination were asked to select one or more reasons for why their daughters had not received the HPV vaccination; the choices given to select from included: (1) We have not seen a health care provider in a long time, (2) My healthcare provider has not recommended the vaccine, (3) I think the HPV vaccine could be dangerous to my daughter, (4) I don't have insurance or money to pay for the vaccine, (5) I don't believe the vaccine works, (6) I believe the vaccine will have side effects, (7) I believe the vaccine will make it easier for my daughter to have sex.
Data Analysis
Oversampling of African-Americans and Hispanics, which was inclusive of Spanish-speaking, Latino panel members was performed. Data was analyzed using census-based post-stratification probability sampling weights provided by KN to ensure nationally representative data estimates to adjust for sample (including oversampled populations), non-sample error, study design and survey non-response. Bivariate analyses were conducted using SPSS Statistics 17.0 to examine factors associated with HPV vaccination status. Chi-square analyses were performed for categorical variables and analysis of variance tests were performed to assess differences across vaccination status for household income and age. Associations with a p value < 0.05 were considered significant.
Results
A total of 501 mother-daughter dyads were enrolled in the study from 47 states in the U.S. Given the high congruence of daughters' and mothers' reported rates of vaccination (Kappa = .96), only maternal surveys were analyzed in this study and mothers' reports of vaccination status were used as the outcome for vaccination status. Maternal mean age was 45 years. The mothers identified as White (59%), Hispanic (22%), Black (14%), and Other (5%). Almost all surveys were completed in English, except for 14 (2.8%), which were completed in Spanish. Most mothers had some college education, were working, and were married [Table 1]. In regards to maternal experience with HPV related diseases, greater than one-third of the mothers reported a history of an abnormal Pap smear, 26% had undergone a colposcopy, and 16% either had cervical cancer or had a friend or family member with cervical cancer.
Table 1.
Sample Demographics Reported by Mothers
| Socio-economic/demographic Factors | Frequency (N) | Percent (%) |
|---|---|---|
| Daughter Age | - | - |
| 14 | 125 | 25 |
| 15 | 125 | 25 |
| 16 | 122 | 24 |
| 17 | 129 | 26 |
| Maternal Age | - | - |
| 18-39 | 106 | 21.2 |
| 40-44 | 133 | 26.5 |
| 45-49 | 140 | 27.9 |
| 50+ | 122 | 24.4 |
| Maternal Racial Background | - | - |
| White, Non-Hispanic | 297 | 59 |
| Black, Non-Hispanic | 69 | 14 |
| Other or 2+ Races, Non-Hispanic | 26 | 5 |
| Hispanic | 109 | 22 |
| Maternal Marital Status | - | - |
| Married/Partnered | 422 | 84 |
| Single | 79 | 16 |
| Maternal Education | - | - |
| High school or less | 93 | 18.6 |
| Some College | 198 | 39.5 |
| Bachelor's or higher | 210 | 41.9 |
| Maternal Working Status | - | - |
| Working | 355 | 70.9 |
| Non-working | 146 | 29.1 |
| Maternal Geographic Region | - | - |
| Northeast | 71 | 14 |
| Midwest | 156 | 31 |
| South | 136 | 27 |
| West | 138 | 28 |
| Daughter's Insurance Status | - | - |
| Insured | 473 | 94.4 |
| Uninsured | 27 | 5.4 |
| Daughter's Insurance Plan | - | - |
| Private | 286 | 61 |
| Medicaid | 51 | 10.9 |
| Self-pay | 12 | 2.6 |
| Other | 56 | 11.9 |
| Unknown | 63 | 13.4 |
| Refused | 1 | 0.2 |
Half of the daughters had not received HPV vaccination (49.9%), 28 had received 1 dose (5.6%), 28 had received 2 doses (5.6%), and 192 had received all 3 doses of vaccine (38.3%). Three mothers were unsure of how many doses their daughters had received. Of those daughters that had received 1 dose of HPV vaccination, 67.9% were greater than 5 months out from initial vaccination and for those who had received 2 doses, 57.1% were greater than 6 months out from their second dose administration.
Neither socio-economic/demographic factors (including daughter's insurance status, mother's education level, marital status, geographic location, or working status) nor maternal HPV-related exposures were significantly correlated with vaccine initiation. Although daughter's race/ethnicity was not found to be associated with initiation of vaccination, Black and Hispanic girls were both significantly less likely to complete the 3-dose series than non-Hispanic White girls (p<0.001) [Table 2].
Table 2.
Number of HPV Vaccine Doses Received by Racial/Ethnic Groups
| Number of Vaccination Doses Received | White (Non-Hispanic) (n=297) N (%) | Hispanic (n=109) N (%) | Black (Non-Hispanic) (n=69) N (%) | Other (Non-Hispanic) (n=26) N (%)a |
|---|---|---|---|---|
| 0b | 147 (49.8) | 59 (54.1) | 40 (61.5) | 10 (38.5) |
| 1 or 2 | 29 (9.8) | 23 (21.1) | 8 (12.3) | 1 (3.8) |
| 3 | 119 (40.4) | 27 (24.8)* | 17 (26.2)* | 15 (57.7) |
Due to small number and heterogeneity, this group was not included in analysis,
No statistical difference in HPV vaccination initiation rates,
Hispanics and Blacks found to have a statistically significant lower rate of HPV vaccination completion compared to Whites (p < 0.05).
For daughters who had initiated vaccination and had received one or more vaccine doses (n=248), 90% of their mothers (n=223) reported that their healthcare provider had discussed HPV vaccine with them, while only 10% (n=25) indicated that the health care provider had not discussed the vaccine. Looking more closely at strength of provider recommendation, 88.9% of those whose doctor had “strongly recommended” the vaccine completed the vaccination series compared with 71.3% completion in those whose doctors had only “recommended” the vaccine (p=0.013).
Mothers whose daughters had not initiated vaccination were asked to select one or more reasons for non-vaccination. The top three reasons chosen were: concerns about vaccine side effects, fear that the vaccine could be dangerous to their daughter, and provider non-recommendation [Table 3]. There was only moderate overlap of mothers who indicated concern about vaccine side-effect with those who indicated concern that the vaccination could be dangerous (Kappa = .34). Of the 90 non-vaccinating parents reporting concern about side-effects, only 52 also reported concern about the vaccine being dangerous (57.8%). Race and ethnicity was not associated with any of the reasons for non-vaccination.
Table 3.
Maternal Chosen Reasons for Vaccination Non-Initiation*
| Reason for Non-vaccination | Frequency Chosen (N) | Percent of Total Sample (%) |
|---|---|---|
| Concern for vaccine side effect | 90 | 36.0 |
| Concern for danger to daughter | 90 | 36.0 |
| Provider non-recommendation | 86 | 34.4 |
| Long lapse in doctor's visit | 29 | 11.6 |
| Concern for increased ease for daughter to have sex | 19 | 7.6 |
| Doubt of vaccine efficacy | 33 | 6.6 |
| Lack of insurance or finance | 28 | 5.6 |
| No response chosen | 15 | 6.0 |
Multiple reasons could be selected by each mother
Discussion
Results of this study are largely consistent with those of the CDC's most recent adolescent vaccination surveillance report, despite the difference between our methodology and the National Immunization Survey [6]. The strength of our study compared to that of the CDC is that they utilized random-digit-dialing for sample recruitment, while our study utilized both random digit dialing as well as addressed-based sampling to ascertain those individuals without home phones. While our study used internet surveying as compared to the CDC who used mailed surveys, our methodology required that individuals be able and willing to use the internet (which was placed in homes by KN). In our study, we found that 51.1% of our target population initiated vaccination compared to 48.7% reported by the CDC. Additionally, we found that 38.3% completed the vaccine series compared to 32% reported by the CDC [6]. It is concerning that half of this age group continues either delaying or not initiating vaccination.
Our data showed no evidence that socioeconomic/demographic factors (maternal age, education, partner status, working status, geographic location, race/ethnicity, or daughter's insurance status) were associated predictors for the rates of HPV vaccination initiation. This set of findings differs from other studies where insurance status, income and parental education where found to be associated with vaccination initiation [11,15].
Race/ethnicity was the only socioeconomic/demographic factor associated with 3- dose completion showing consistent results with the CDC that Black and Hispanic adolescents were significantly less likely to complete vaccination [6]. This finding has been consistent throughout several other studies that found that adolescents who racially identified as Black were less likely than those who identified as White to complete the vaccine series [16-20]. Some studies have found that even when Blacks had higher rates of initiation compared to Whites, they maintained lower rates of 3-dose completion [11]. A recent study by Chou et al found that practice location was a significant association with vaccination completion and that the combination of being a young woman, 11-17 years old, in an urban practice was a significant predictor of failure to complete vaccination [19]. The etiology of this racial discrepancy for vaccination is not clear, but may be related to variability in access to care, cultural influences, or patient-provider communication. Given the disproportionately higher HPV disease burden and mortality rates amongst Black and other minority populations it will be important to better understand and address these lower rates of vaccination completion [11,17,21-23].
Maternal HPV exposure was not found to be a statistically significant predictor of vaccination, a finding similar to a study by Caskey et al that found no association between history of an abnormal pap smear and vaccination [24]. This lack of association could signify that experience with HPV related conditions does not ensure increased knowledge and understanding of HPV infection, and therefore may not motivate vaccination against HPV. Additionally, there is also no assurance that individuals with friends or family members with cervical cancer are aware of the causal connection between HPV infection and cervical cancer. We note that our findings differ from a previous study that found daughters who received HPV vaccination were fifty percent more likely to have mothers who had received Pap tests, which they interpreted as being due to mothers' attitudes toward preventive measures. However, when this same study looked at mothers' STI exposure history, which included HPV, they found that there was only a slight association with daughters' HPV vaccination status and that the nature of this association was inconsistent across racial/ethnic groups [25].
Provider recommendation shows a strong correlation to vaccination decisions. Previous studies have identified the importance of provider recommendation in HPV vaccination [26,27]. However, studies looking at strength of physician recommendation have shown some physicians continue to alter the strength of their recommendation based on the age of the female patient [28]. One study found that for girls 11-12 years of age, only 56% of physicians and 50% of family physicians strongly recommended vaccination [28]. Another study found 60% of individuals who had not intended to vaccinate reported that they had not received a provider recommendation [27]. In our study, strength of provider recommendation was significantly correlated with higher rates of vaccination completion. Individuals whose providers “strongly recommended” vaccination as compared to those whose providers “recommended” vaccination had higher rates of completion. This could in part indicate that those providers, who “strongly recommended” vaccination, were more proactive about ensuring series completion. Additionally, it may also represent a transfer of vaccine importance from provider to patient, providing further motivation for parents to ensure vaccination completion. Although other studies have shown HPV vaccine recommendations to be influenced by patient age, we found no relationship of provider recommendation with age of the adolescent girls (data not shown), which may be a function of the narrow age range of our study sample [19]. These findings regarding strength of provider recommendation and HPV vaccination highlight the importance of educating physicians to provide “strong recommendations” for all individuals within the vaccine age-targeted population [28].
Mothers, of daughters who had not initiated vaccination, reported the most common reasons for non-vaccination were provider non-recommendation, concern for vaccine side effect, as well as concern that the vaccine could be dangerous to their daughter. Each of these reasons further underscores the importance of the provider's role in vaccine uptake, particularly with almost 50% of the target population having not initiated vaccination [5]. Other studies have found a similar significance of provider influence being crucial to vaccine acceptance [21]. Continued provider support throughout the vaccination process is essential, particularly in recognizing that those who postpone vaccination (or never receive the vaccine) lose potential for protection once exposure to vaccine-related HPV types has occurred.
A limitation of this study is that vaccination rates were based solely on maternal reports and could not include confirmation via medical records. However, congruence between mother and daughter reports of vaccination was found to be high, suggesting good reliability of the self-report measure. Also, the consistency between our findings and the results of the National Immunization Survey suggests that self-reports were fairly accurate. A second limitation is that our assessment of provider non-recommendation as a reason for non-vaccination, does not distinguish between a provider recommendations against vaccinations compared to a complete omission of any recommendation in either direction.
Conclusion
HPV vaccination provides a strong potential to improve public health by decreasing HPV–related anogenital cancers and genital warts. Despite increases in vaccination rates, half of the target population continues to remain unvaccinated and completion rates lag in those populations that are at highest risk for HPV disease morbidity. In light of the recommendation for routine vaccination of young women and the coverage of HPV vaccination by the Vaccines for Children program, there is the potential to greatly decrease HPV-related disease burden. However, reduction in HPV-related diseases will require more uniform vaccination initiation and completion rates across HPV naïve populations and must occur prior to HPV exposure. For individuals who have initiated vaccination, further research is needed to better understand barriers to vaccine completion, especially among minority adolescents. Additionally, improved understanding of the reasons chosen for non-vaccination, including vaccine safety and provider non-recommendation may help improve efforts to increase rates of vaccination. Recognizing that timing of HPV vaccination is important and it is recommended prior to sexual exposure, continued educational interventions with physicians, patients and their parents regarding vaccine safety and vaccine efficacy are clearly needed.
Acknowledgments
Funding support for this research was provided by NIH R56 A1079090-01A1
Footnotes
Disclosures:
Kester, Laura: Has no financial disclosures
Zimet, Gregory: Investigator on grants funded through Merck's Investigator Initiated Studies Program. In the past year served as a consultant to Sanofi Pasteur regarding attitudes about herpes vaccination.
Shew, Marcia: Investigator for Merck and Co. related HPV vaccine trials.
Kahn, Jessica: Co-PI of two clinical trials of HPV vaccine in HIV-infected individuals; vaccine and immunogenicity testing were provided by Merck.
Fortenberry, J Dennis: Receives compensation from American Social Health Association for continuing lectures related to HPV vaccines.
References
- 1.Morbidity and Mortality Weekly Report(MMWR) Quadrivalent Human Papillomavirus Vaccine. RR02. Vol. 56. CDC; 2007. pp. 1–24. 2007. www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm. [PubMed] [Google Scholar]
- 2.Morbidity and Mortality Weekly Report(MMWR) FDA Licensure of Bivalent Human Papillomavirus Vaccine (HPV2, Cervarix) for Use in Females and Updated HPV Vaccination Recommendations from the Advisory Committee on Immunization Practices (ACIP) 2010. 20. Vol. 59. CDC; 2010. pp. 626–629. www.cdc.gov/mmwr/preview/mmwrhtml/mm5920a4.htm. [PubMed] [Google Scholar]
- 3.Morbidity and Mortality Weekly Report(MMWR) National Health and Nutrition Examination Survey, United States2003-2004. CDC; 2007. QuickStats: Prevalence of HPV Infection Among Sexually Active Females aged 14-59 Years by Age Group. www.cdc.gov/mmwr/preview/mmwrhtml/mm5633a5.htm. [Google Scholar]
- 4.Weaver B, Shew M, Qadadri B, et al. Natural History of Multiple Human Papillomavirus Infections in Female Adolescents With Prolonged Follow-up. Journal of Adolescent Health. 2011;48(5):473–480. doi: 10.1016/j.jadohealth.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Morbidity and Mortality Weekly Report(MMWR) National, State, and Local Area Vaccination Coverage among Adolescents Aged 13-17 Years --United States 2009. 32. Vol. 59. CDC; 2010. pp. 1018–1023. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6033a1.htm. [PubMed] [Google Scholar]
- 6.Morbidity and Mortality Weekly Report(MMWR) National and State Vaccination Coverage Among Adolescents Aged 13 Through 17 Years - United States, 2010. 33. Vol. 60. CDC; 2011. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6033a1.htm. [PubMed] [Google Scholar]
- 7.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Preventive Medicine. 2007;45(2-3):107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Guerry SL, De Rosa CJ, Markowitz LE, et al. Human papillomavirus vaccine initiation among adolescent girls in high-risk communities. Vaccine. 2011;29(12):2235–2241. doi: 10.1016/j.vaccine.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 9.Smith L, Brassard P, Kwong J, Deeks S, Ellis A, Levesque L. Factors associated with initiation and completion of the quadrivalent human papillomavirus vaccine series in an Ontario cohort of Grade 8 girls. BMC Public Health. 2011;11(1):645. doi: 10.1186/1471-2458-11-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández ME, Allen JD, Mistry R, Kahn JA. Integrating Clinical, Community, and Policy Perspectives on HPV Vaccination. Annual review of public health. 2010;31:235–252. doi: 10.1146/annurev.publhealth.012809.103609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human Papillomavirus Vaccination Series Initiation and Completion, 2008–2009. Pediatrics. 2011;128(5):830–839. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- 12.Barnack JL, Reddy DM, Swain C. Predictors of Parents' Willingness to Vaccinate for Human Papillomavirus and Physicians' Intentions to Recommend the Vaccine. Women's Health Issues. 2010;20(1):28–34. doi: 10.1016/j.whi.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Allen JD, Othus MKD, Shelton RC, et al. Parental Decision Making about the HPV Vaccine. Cancer Epidemiology Biomarkers & Prevention. 2010;19(9):2187–2198. doi: 10.1158/1055-9965.EPI-10-0217. [DOI] [PubMed] [Google Scholar]
- 14.Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sexually transmitted diseases. 2011;38(3):197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. American journal of preventive medicine. 2010;38(5):525–533. doi: 10.1016/j.amepre.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widdice LE, Bernstein DI, Leonard AC, Marsolo KA, Kahn JA. Adherence to the HPV Vaccine Dosing Intervals and Factors Associated With Completion of 3 Doses. Pediatrics. 2011;127(1):77–84. doi: 10.1542/peds.2010-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schluterman N, Terplan MS, Lydecker AD, Tracy JK. Human papillomavirus (HPV) vaccine uptake and completion at an urban hospital. Vaccine. 2011;29(21):3767–3772. doi: 10.1016/j.vaccine.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Gold R, Naleway AL, Jenkins LL, et al. Completion and timing of the three-dose human papillomavirus vaccine series among adolescents attending school-based health centers in oregon. Preventive Medicine. 2011;52(6):456–458. doi: 10.1016/j.ypmed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Chou B, Krill LS, Horton BB, Barat CE, Trimble CL. Disparities in Human Papillomavirus Vaccine Completion Among Vaccine Initiators. Obstetrics & Gynecology. 2011;118(1):14–20. doi: 10.1097/AOG.0b013e318220ebf3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niccolai LM, Mehta NR, Hadler JL. Racial/Ethnic and Poverty Disparities in Human Papillomavirus Vaccination Completion. American journal of preventive medicine. 2011;41(4):428–433. doi: 10.1016/j.amepre.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 21.Society AC. Cancer Facts and Figures 2010. Alanta: American Cancer Society. 2010 [Google Scholar]
- 22.Bingham AP, Drake JKMPH, LaMontagne SDPMPHF. Sociocultural Issues in the Introduction of Human Papillomavirus Vaccine in Low-Resource Settings. Archives of Pediatrics & Adolescent Medicine. 2009;163(5):455–461. doi: 10.1001/archpediatrics.2009.50. [DOI] [PubMed] [Google Scholar]
- 23.Kahn JA, Lan D, Kahn RS. Sociodemographic factors associated with high-risk human papillomavirus infection. Obstetrics & Gynecology. 2007;110(1):87–95. doi: 10.1097/01.AOG.0000266984.23445.9c. [DOI] [PubMed] [Google Scholar]
- 24.Caskey R, Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and young women: results of a national survey. Journal of Adolescent Health. 2009;45(5):453–462. doi: 10.1016/j.jadohealth.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Chao C, Slezak JM, Coleman KJ, Jacobsen SJ. Papanicolaou screening behavior in mothers and human papillomavirus vaccine uptake in adolescent girls. American journal of public health. 2009;99(6):1137–1142. doi: 10.2105/AJPH.2008.147876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerend MA, Weibley E, Bland H. Parental response to human papillomavirus vaccine availability: uptake and intentions. Journal of Adolescent Health. 2009;45(5):528–531. doi: 10.1016/j.jadohealth.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Dorell C, Yankey D, Strasser S. Parent-Reported Reasons for Nonreceipt of Recommended Adolescent Vaccinations, National Immunization Survey—Teen, 2009. Clinical Pediatrics. 2011;50(12):1116–24. doi: 10.1177/0009922811415104. [DOI] [PubMed] [Google Scholar]
- 28.Daley MF, Crane LA, Markowitz LE, et al. Human papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics. 2010;126(3):425–433. doi: 10.1542/peds.2009-3500. [DOI] [PubMed] [Google Scholar]


