Abstract
The current therapeutic strategies are not efficient in treating disorders related to the central nervous system (CNS) and have only shown partial alleviation of symptoms, as opposed to, disease modifying effects. With change in population demographics, the incidence of CNS disorders, especially neurodegenerative diseases, is expected to rise dramatically. Current treatment regimens are associated with severe side-effects, especially given that most of these are chronic therapies and involve elderly population. In this review, we highlight the challenges and opportunities in delivering newer and more effective bio-therapeutic agents for the treatment of CNS disorders. Bio-therapeutics like proteins, peptides, monoclonal antibodies, growth factors, and nucleic acids are thought to have a profound effect on halting the progression of neurodegenerative disorders and also provide a unique function of restoring damaged cells. We provide a review of the nano-sized formulation-based drug delivery systems and alternate modes of delivery, like the intranasal route, to carry bio-therapeutics effectively to the brain.
Keywords: Central nervous system, blood-brain barrier, nano-sized formulations, intranasal delivery, systemic delivery, bio-therapeutics, drug delivery, olfactory receptor neurons
1. Opportunities and Challenges in CNS Delivery
CNS disorders are one of the largest areas in pharmaceutical world, where there is an unmet medical need and hence, requires adequate treatment. CNS drugs have lower success rate coupled with longer development times, which makes this area quite challenging, and thereby, has recently gained quite some interest among academia, government institutions and private industry. Several biotherapeutics including monoclonal antibodies, peptides, proteins, nucleic acids, etc. are being explored as potential to treat CNS diseases namely, neurodegeneration, pain, psychiatric disorders, gliomas, etc. These have particular advantages over the classical small molecules, because bio-therapeutics are very specific, potent and have reduced side- effects [1]. However, there aren't as many biologics that have been successfully developed in clinic for treating CNS disorders [2]. The lack of drug-like properties coupled with poor solubility, in vivo instability, poor penetration across the CNS and cost of manufacture of biologics have limited their entry into market [1].
1.1. Major Barriers to CNS Delivery
1.1.1. The blood-brain barrier (BBB)
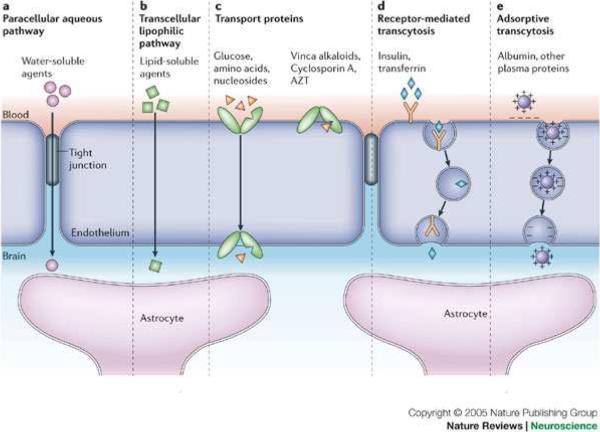
Macromolecules are unable to cross the capillary brain endothelial cells which form tight junctions at the BBB, which prevents 98% of potential drugs from reaching their CNS targets. Figure 1-1 shows some of the common transport pathways across the BBB [3]. Due to the presence of tight junctions at the cerebral microvasculature, the predominant mechanisms for transport of therapeutics across the BBB are either by lipid-mediated free diffusion (which is restricted to lipophilic small molecules), or by use of endogenous uptake transporters present at the luminal side of the BBB [4]. Among the endogenous transporters; transport proteins mainly allow selective uptake of water soluble nutrients like glucose, amino acids or nucleosides; whereas, receptor-mediated transporters are involved in transport of large molecules like insulin, transferrin, etc. across the BBB [4]. Along with the influx transport proteins; there are also efflux transporters like the MDR1, BCRP, MRP1, present at the BBB which pump out the substrates from the cells back into the bloodstream. A recent paper highlights the role of P-glycoprotein in predominantly causing efflux of antiepileptic drugs from the brain[5].
Figure 1-1.
Transport pathways across the blood–brain barrier [112]
A schematic of the blood–brain barrier (BBB) highlighting the proximity of the endothelial cells with the astrocyte foot processes. The major known pathways for transport across the BBB are: a | Paracellular- tight junctions restrict penetration of water-soluble agents. b | Transcellular- lipid bilayer membrane of the endothelium provides diffusive route for lipid-soluble agents. c | Transport proteins- The endothelium contains transport carriers for glucose, amino acids, and other substances. Also, are present the energy-dependent efflux transporters. d | Receptor- mediated-Certain proteins, such as insulin and transferrin, are taken up by specific receptor-mediated endocytosis and transcytosis. e | Adsorptive transcytosis- Uptake of native plasma proteins such as albumin. Pathways b–e are prominent in drug delivery across the BBB; most CNS drugs enter via route b.
1.1.2. The blood- CSF barrier
Unlike the BBB, the blood-CSF barrier has a lot less surface area and hence, likely to pose less obstacle in drug delivery to CNS. However, it does possess tight junctions between the epithelial cells of the choroid plexus, which prevents drugs from entering the CSF.
1.1.3. Systemic distribution and clearance [6]
There is a significant component of dilution and metabolism of drugs due to systemic distribution in peripheral tissues, sepcifically for lipophilic compounds that are synthesized in order to increase CNS permeability. These lipophilic compounds more readily penetrate into peripheral tissues in the body when administered systemically, hence requiring a larger dose to achieve the required therapeutic levels in the brain. This leads to non-specific systemic side-effects. The discovery of antennapedia (Antp) - mediated transduction of heterologous proteins into cells, and of other “Trojan horse peptides”, raised hopes for effective delivery of biological therapeutics across the BBB and cellular membranes [7].
1.2. Bio-therapeutic Delivery for CNS Diseases
Passage of bio-therapeutics into the brain hence needs an effective delivery mechanism that can cross the BBB. Current means to deliver these agents to the CNS can be broadly classified into invasive and non-invasive strategies; some of these are highlighted in Table 1-1. Owing to the complexity of neuro-surgical procedures like the intracerebroventricular and intrathecal injection or intracerebral implantation and the risks involved with invasive strategies; the non-invasive strategies are gaining more attention.
Table 1-1.
Invasive and non-invasive strategies for delivery to the CNS
| Strategies | Description and examples | References |
|---|---|---|
| Invasive strategies | ||
| Intracerebroventricular injection | Intracerebral infusion or implant localized to a small area in the brain, e.g. tumor site, particular failing neuron. Clinical data reported with GDNF, CTNF neurotropic factors. | [85]-88] |
| Intra-arterial administration and transient disruption of the BBB | Intra-arterial administration increases drugs' systemic concentration by eliminating first-pass metabolism. Hyperosmolar sugar solutions (20% mannitol) or immune adjuvants (Freund's) administration in carotid artery or application of ultrasound: shrinkage of endothelial cells, thereby causing transient opening of the tight junction (~20 min). | [86;87] |
| Intraventricular/Intracerebral implants | Direct injection into the CSF; Subcutaneous implant in the scalp connected to the ventricles by a catheter (Convection enhanced drug delivery (CED). Intracerebral polymeric implants for local delivery. | [88-90] |
| Focused Ultra sound (FUS) | Utilizes microbubbles to create an acoustic cavity and a pressure shock wave that temporarily punctures the endothelial wall to cause a transient influx of otherwise impermeable drugs. Biotherapeutics like Rituximab, an anti-CD20 mAb, have been delivered across BBB using this methodology. | [2], [91]-[92] |
| Non- invasive strategies | ||
| Altering the chemistry | Increasing the lipophilicity of molecules (e.g. pro-drug approach), coupling nucleic acids to cell penetrating peptides, `molecular trojan-horse' approach are some of the techniques explored to improve BBB penetration. | [76], [10] |
| Intranasal Delivery | Likely that intranasally delivered therapeutics reach the CNS via the olfactory region or trigeminal pathway, bypassing the BBB. E.g. evidence of delivery of insulin or nerve growth factor (NGF) via intranasal drug delivery. | [93;94] |
| Colloidal drug carriers | Liposomes (e.g. NGF or immunoliposomes), emulsion (e.g. nanoemulsions), solid-lipid nanoparticles (e.g. thiamine-coated nanoparticles) and polymeric nanoparticles (e.g. nerve growth factor using poly(butyl cyanoacrylate) nanoparticles) for delivery to the brain. | [95] |
| Co-administration with inhibitors of efflux | Combination of anticancer drugs with specific P-gp inhibitors, like valspodar, zosuquidar, etc. lead to stronger P-gp inhibition and hence, better CNS penetration. | [87] |
2. Nanotechnology Solutions for CNS Therapy
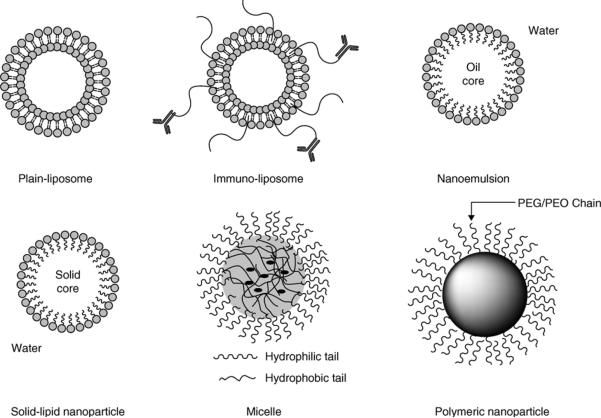
Since majority of CNS compounds have limited brain uptake, due to low permeability across the BBB or high efflux rate or high plasma protein binding; there have been several attempts to use nanotechnology-based drug delivery systems to overcome these challenges. Following intravenous administration, the colloidal systems can extravasate into compromised BBB e.g. the brain tumor, which leads to a more selective drug delivery into brain tumors [8]. Particles that have been reported to cross the BBB include liposomes, solid lipid nanoparticles, nanoemulsion, albumin nanoparticles, and polymeric nanoparticles [9]. Additionally, these delivery technologies can be surface modified with PEG (for longer circulation times) or specific antibody targeting receptors of the brain (immunoliposomes) or chimeric proteins [10] to form multifunctional nanoparticulates. Figure 2-1 illustrates some of the drug-delivery systems reported for systemic delivery of therapeutics to the CNS.
Figure 2-1.
Examples highlighting the span of nano-sized drug carriers for enhanced delivery of biotherapeutics to the brain [6].
2.1 Nanoparticle Delivery Systems
2.1.1. Liposomes
Liposomes are artificial phospholipid vesicles that can be designed for effective encapsulation and systemic delivery of the therapy. Unmodified liposomes are rapidly eliminated from systemic circulation by the cells of reticulo-endothelial system (RES) and hence, long-circulating liposomes and targeted liposomes are being explored [11]. To facilitate targeted delivery of PEG modified liposomes to the brain, they can be further modified with various ligands like monoclonal antibodies against glial fibrillary acidic proteins, transferrin receptors, or human insulin receptors [12] [13]. Transferrin-conjugated liposomes are shown to preferentially deliver the payload e.g. 5-Flurouracil to the rat brain, and likely taken up by receptor-mediated endocytosis [14]. In a rat model of Parkinsonism, OX26 immunoliposomes resulted in a clear pharmacological effect causing reversible normalization of striatal tyrosine hydroxylase expression, thereby demonstrating the transcytosis-mediated delivery across the BBB [15]. Another advantage to surface modified liposome-based drug delivery systems is their property to prolong the half-life of the payload. A recent work highlights glutathione PEG modified (GSH-PEG) liposomes to enhance and prolong blood to- brain drug delivery of the opioid peptide DAMGO (H-Tyr-D-Ala-Gly-MePhe-Gly-ol) [16].
2.1.2 Solid-lipid nanoparticles (SLNs)
Solid–lipid nanoparticles are surfactant-stabilized aqueous colloidal dispersions of lipid nanoparticles that solidify upon cooling. They contain a lipid phase dispersed in an aqueous environment [6]. Poly(ethylene glycol)-modified SLNs have been shown to penetrate the BBB and allow for greater delivery of drug to the CNS [17]. The potential use of SLNs and PEG modified SLNs for antitumor drugs like camptothecin, doxorubicin for brain drug delivering has been widely explored recently [18], and a review article on this topic has been published [19]. SLNs are advantageous over polymeric nanoparticles due to their low intrinsic cytotoxicity, physical stability, protection of labile drugs from degradation, controlled release and ease of preparation, which makes them very attractive candidates for brain delivery and particularly for the treatment of brain tumors [12]. SLNs are less efficient for encapsulation of hydrophilic compounds, and hence may require heating for preparation or a strategy like including an amphiphilic polymer in the lipids to form complexes with the charged, water-soluble drug molecules, thus making polymer-lipid hybrid nanoparticles [20].
2.1.3 Polymeric nanoparticles
Polymeric nanoparticles are solid colloidal particles created from polymeric systems. These nanoparticles are made from biocompatible polymers that encapsulate or adsorb drugs for prolonged release [6]. Polysorbate 80-coated poly(n-butylcyanoacrilate) nanoparticles have been formulated by emulsion polymerization method to target selectively rivastigmine or tacrine to the CNS for Alzheimers dissease [21]. The coating with 1% polysorbate 80 of the nanoparticles increased the concentrations of the drug in the brain when compared with the free drug, indicating potential selective targeting to the CNS. Another advantage to this targeted delivery is its potential to reduce or overcome the hepatic or gastrointestinal side-effects associated with conventional therapy.
2.1.4 Oil-in-water nanoemulsions
Nanoemulsions are oil-in-water (O/W) or water-in-oil (W/O) formulations made with edible oils, surface-active agents (surfactants), and water, where the diameter of inner phase is reduced to nanometer length scale [22]. The versatility of nanoemulsions is based on the different types of oils and surface modifiers that can be used [23]. For instance, we have found that oils that are rich in omega-3 polyunsaturated fatty acids (PUFA) can play a very important role in overcoming biological barriers, including the BBB. Lastly, hydrophobic payloads and imaging agents can be readily incorporated in the oil phase of the nanoemulsions [24]– [25].
2.2. Strategies to Improve CNS Delivery
Multifunctional nanosystems with different types of payloads and targeting capabilities in a single platform are gaining more attention and focus recently, mainly due to their wider capabilities in being amenable to therapeutic, diagnostic and imaging applications. These systems can be designed to achieve active targeting, efflux transporter inhibition or their delivery using alternate routes/ techniques to overcome the BBB.
2.2.1. Active targeting
The nanocarriers can be surface modified to include `active targeting moeities' like monoclonal antibodies, cell penetrating peptides or receptor substrates to improve the specificity of uptake and transcytosis [26]. For example, anti-transferrin receptor antibody (OX26) linked to liposomes lead to enhanced delivery of daunomycin and plasmid to the brain [27]. Cell penetrating peptide, TAT-modified-micelles improved delivery of ciprofloxacin across human brain endothelial cells and coumarin to rat brain [28]. Solid -lipid nanocarriers directly linked or indirectly adsorbed to apolipoproteins (ApoA or ApoE) were able to cross BBB and delivered its payload to brain [29]. Transferrin or folate receptor-coated liposomes significantly improved the uptake of the drugs by the brain.
2.2.2. Enhancing BBB permeability
In addition to the transcellular uptake, receptor-mediated endocytosis and active targeting nanotechnologies; various other strategies have been reported to improve drug delievry to the CNS. One of them is improving paracellular permeability across the tight junctions of the BBB by use of vasoactive agents, momentarily permeabilizes the blood vessel. However, these are associated with significant side effects and dose limitation [18]. Another approach is use of hypertonic soution of mannitol, which transiently opens the tight junctions due to shrinkage of endothelial cells, thereby improving the penetration of therapeutics administered by intraarterial infusion [30]. Another approach that has recently gained much attention is intranasal delivery of nanocarriers. This route allows to bypass BBB by crossing the olfactory epithelium to achieve direct nose-to-brain delivery via the olfactory or trigeminal nerve system [26].
2.2.3. Overcoming efflux transporter inhibition
While the carrier-mediated transport proteins across the BBB specifically carry amino acids, glucose, nutrients, etc. from the blood to the brain; there also exists the ATP-binding cassette (ABC) transporters at the BBB, which are responsible for pumping its substrates out of the brain into the blood. ABC family of active transporters are P-Glycoprotein (P-gp/MDR1), breast cancer resistance protein (BCRP/ABCG2) and the multi-drug resistance-associated protein (MRP) family. This active transport process is one of reasons for CNS anticancer drug resistance. P-gp was found in resistant glioblastomas [31], suggesting a role of P-gp in limiting anticancer drug penetration into brain tumors despite of the leaky nature of glioma vasculature. There have been various preclinical and clinical studies to explore P-gp inhibitors to improve CNS penetration; however, first-generation P-gp inhibitors (e.g., vincristine, virapamil) carried toxicity issues. Several new P-gp inhibitors (e.g., valspodar, elacridar, zosuquidar) may improve the clinical outcome for this strategy [32]. However, there will always be a risk of systemic side effects and pharmacokinetic interactions, and to overcome this risk, one approach is to deliver these efflux transporter inhibitors with nanocarriers [33].
Nanotechnology in neurology has the potential to dramatically affect the ability to specifically target drugs to and across the BBB, to develop potential regenerative therapies and engineer new advanced diagnostic tools for early diagnosis of the disease [21].
3. Intranasal Delivery to the CNS
3.1 Anatomical Structures for Nose-to-Brain Transport
In the last few years lot of research has been carried out both in animals and humans showing the delivery of the exogenous substances from nose to brain, which bypasses the BBB. This route involves the olfactory or trigeminal nerve systems which are initiating from brain parts and are terminating in the nasal cavity at the olfactory epithelium or respiratory epithelium. This section briefly considers the anatomy and details of the structure involved in the transfer of molecules from nose to brain. In humans and most other mammals, nose serve as an organ for olfaction and its primary function includes humidity and temperature regulations of inhaled air and removal of the microorganism from inhaled air. The nasal cavity is divided by the nasal spectrum into two halves. Each half of the nose has three functionally unique regions within the nasal cavity and these are the vestibular, the respiratory and the olfactory regions. The nasal vestibule present just at the opening of the nose is covered with stratified squamous epithelium with long hairs which helps in filtering of airborne particles. Nasal cavity unlike other biological membranes is highly porous and has a thin endothelial basal membrane. It also has a rich supply of blood flow due to highly vascularized epithelial layer where blood is drained directly into systemic circulation.
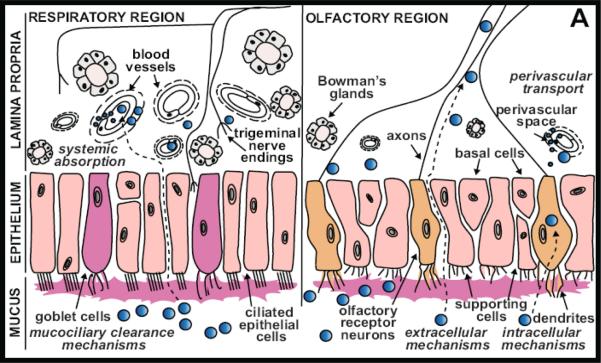
3.1.1. The respiratory epithelium
The largest of the three regions in humans is the respiratory region which lies next to the vestibule region. It is a highly vascularized respiratory epithelium which is made of pseudostratified columnar epithelium, consisting of four main cell types: ciliated and non-ciliated columnar cells, goblet cells, and basal cells (Figure 3-1)./B> These cells facilitate active transport of water and ions between cells. The epithelial cell layer is covered with mucus, which is produced by the goblet cells and cleared by the beating of the cilia. The respiratory epithelial cells are surrounded by microvilli, which provide a high absorptive capacity. In humans the respiratory mucosa covers most of the total surface area and is a major site of systemic drug absorption due to increased surface area provided by the microvilli and also due to the rich vascular capillary bed that lies directly beneath the surface [34].
Figure 3-1.
Pathways of drug distribution in the nasal cavity through the respiratory and olfactory Epithelium Following intranasal administration, drugs (blue circles) come into contact with the nasal mucosa, which is innervated by olfactory and trigeminal nerves.
3.1.2. The olfactory epithelium
The olfactory region is located at the most dorsal and caudal region of the nasal cavity. The olfactory epithelium is located on the top of the nasal cavity and under the cribriform plate of the ethmoid bone which divides the cranial cavity from nasal cavities [34;35]. The cribriform plate is known to have pores that allow passage of the neuronal axons from the olfactory epithelium cells to the CNS. It consists of modified columnar, pseudo-stratified epithelium similar to respiratory epithelium and consists of following main cell types, the ORNs (olfactory receptor neurons), sustentacular (supporting) epithelial cells, horizontal basal cells and globose basal cells [36;37]. The olfactory receptor neurons or the axons are unmyelinated and interspaced between the supporting cells. Tight junctions exist between the supporting cells and olfactory nerve cells.
3.1.3 Olfactory receptors neurons
The first order of neurons that transport odorants and macromolecules are the ORNs. Single dendrites of the olfactory neurons cells terminate in the olfactory knobs and have 10–25 immobile cilia. Cilia express receptor for binding to odorant molecules and once activated causes depolarization of the olfactory axon by ion-gated channels or cAMP operated ion-channels. In the basal regions of the receptor cell bodies, ORNs taper into unbranched and unmyelinated axons eventually forming small bundles with other ORN axons. The olfactory receptor neurons are bipolar neurons that connect the olfactory bulb of the brain with the nasal cavity. The axon of olfactory receptor neurons extend from cell bodies through the lamina propria into cribriform plate and enter perineural space as bundles which are surrounded by Schwann's cells and perineural epithelial cells sleeve. Eventually the olfactory axons enter the olfactory bulb and terminate in spherical neuropils called glomeruli, synapsing with second order mitral and tufted neuronal cells. Olfactory neurons are distinct from other neurons in the CNS and peripheral nervous system (PNS) due to these unique characteristics: 1) ORNs are in direct contact with the external environment due to their peripheral location, which makes them vulnerable compared to internal sensory neurons. 2) With their peripherally located dendrites and direct projection of axons centrally to the forebrain, ORNs form a direct pathway for entry of exogenous substances into the brain 3) ORNs are able to regenerate themselves through continued postnatal neurogenesis which involves neurotrophic, growth and adhesive factors [35;38]
3.2. Nose-to-Brain Transport Pathways
Delivery of large number of small molecules to CNS using the intranasal route has been very successful and now considerable evidence has started to emerge for the success in the delivery of various biologics which are hard to deliver to brain via other routes due to low bioavailability. Attainment of clear understanding of the nose to brain transport pathways for these macromolecules have been extremely complicated but there has been lot of studies showing the transport to the CNS by different pathways. Macromolecules entering the CNS via the olfactory nerve pathway have been found to be distributed within rostral brain regions, such as olfactory bulb, anterior olfactory nucleus, forebrain and hippocampus. Additionally, macromolecules utilizing extracellular transport pathways, such as along the trigeminal nerve complex, are able to distribute within caudal brain regions including the brainstem, cerebellum and hypothalamus [39]. Most studies performed to understand the pathways involved in intranasal delivery to CNS include initial removal of blood from the cerebrovasculature and fixation by cardiac perfusion before measurement of tissue concentration. Based on the various studies the different pathways involved are listed below.
3.2.1. The olfactory nerve pathway
Olfactory nerve pathways are the major transport pathways for the biologics to access the CNS after intranasal dosing evidenced by high concentration obtained in the olfactory bulbs. Also good correlation has been obtained between the concentration in the olfactory epithelium and olfactory bulbs for various compounds. Intranasal administration of [125I]-IGF- I(Insulin growth factor-1) resulted in significantly high concentrations in nearly all CNS regions (olfactory bulb, anterior olfactory nucleus, frontal pole and motor cortex), the trigeminal nerve and areas just below where the trigeminal nerve enters the brainstem (medulla and cervical spinal cord). Levels of [125I]-IGF-I in most CNS areas were either similar to or significantly greater than the final blood sample concentration following i.n. administration [39]. Radiolabeled glucagon like peptide-1 antagonist, when delivered through the intranasal route showed maximum distribution in the olfactory blubs when compared to blood, cervical lymph nodes and other regions of the brain [40].
3.2.2 Intracellular axonal pathway
Intranasal administered substances, including macromolecules are taken up by dendrites of ORNs by either passive diffusion or by receptor mediated endocytosis followed by intracellular transport to the olfactory bulb and further distribution to other CNS parts. Studies comparing the intracellular transport of conjugated WGA-HRP and HRP within the ORN have found that different endocytosis pathways are involved. Unconjugated HRP, enters ORNs by a fluid-phase endocytosis as the protein lacks the binding sites on the ORN membrane. On the other hand, WGA-HRP, a 62kda lectin conjugate binds the cell surface glycoproteins and are taken up by receptor mediated endocytosis followed by trans-neuronal transport after processing in the trans glogi saccule [41]. These and many other studies have shown that this pathway is slow in transport and might take few hours to days to have detectable levels of drugs in the olfactory blubs. There are only a handful of molecules known to be transported through this route as compared to the extracellular pathway, as one would expect that receptor based pathway (as the intracellular axonal pathway) should be saturable and also should be specific. However, evidence has been found for various macromolecules transported through the nasal cavity.
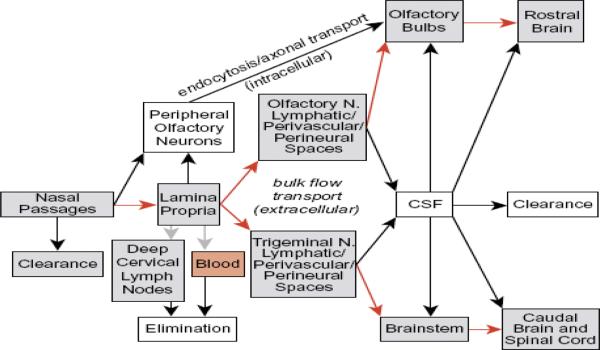
3.2.3. Olfactory epithelium pathway or extracellular pathway
This is an alternative transport pathway which provides fast and direct transportation of macromolecules from nasal cavity to the olfactory bulbs and other regions in the CNS. Transport involves rapid movement of molecules between cells in the nasal epithelium through either receptor mediated endocytosis, fluid-phase endocytosis or by passive diffusion. Molecules may also cross the tight junctions between the supporting cells by paracellular mechanisms (Figure 4-1). Once the olfactory membrane is crossed, molecules enter the lamina propria to gain access the perineural spaces surrounding the olfactory nerve fascicles. Perineural spaces are the extension of the subarachnoid space which allows CSF in the subarachnoid space to be in continuation with perineural fluid. Hence, the intranasal delivered bio-therapeutics could rapidly travel, requiring only several minutes to 30min, through perineural spaces reaching the CSF and CNS. Intranasal delivery of [125I]-IGF-I showed maximum concentration in Olfactory bulbs within 30min, 70 g [125I]-radiolabeled BDNF, CNTF, NT-4, or erythropoietin (EPO) resulted in peak neurotrophin concentrations (0.1–1.0 nM) within 25 min in brain parenchyma [42;43].
Figure 4-1.
Schematic diagram demonstrating the proposed pathways for drugs entering the nasal cavity and passage to brain tissue or CSF. [113]
3.2.4. Transport through the trigeminal nerve pathway
Thorne et. al. [42] recently identified another important pathway connecting the nasal passages to the CNS involving trigeminal nerve which innervates in the respiratory and olfactory epithelium of the nasal cavity and enters the CNS in the pons[42]. Trigeminal nerve conveys sensory information via the ophthalmic division (V1), maxillary division (V2) or mandibular division (V3) to CNS [44;45]. These three branches of the nerve meet at the trigeminal ganglion from where they extend as one to the brain at the pons, terminating in the spinal trigeminal nuclei in the brainstem. Intranasal drug delivery through this pathway was first demonstrated by the evidence of high levels of radioactivity in the trigeminal nerve branches, trigeminal ganglion, pons and olfactory bulbs after the delivery of radiolabeled IGF-1 neuropepitde.There is still controversy regarding which pathway is involved – olfactory or trigeminal nerves, as one portion of the trigeminal nerve enters the brain from cribriform plate alongside the olfactory pathway[42]. Intranasal delivery of various other large molecular weight proteins or peptides- Interferon-B1b, hypocretin, BDNF, CNTF, NT-4, or erythropoietin (EPO) found similar results of high level of radioactivity in the trigeminal nerves.
3.2.5. Vascular and lymphatic pathways
Intranasal route has been utilized traditionally to deliver drugs to the systemic circulation via absorption into the capillary blood vessels which are present in the respiratory and olfactory mucosa. Only molecules which are able to cross the BBB from blood can access the CNS. Mostly small molecules with lipophilicity show 100% bioavailability however hydrophilic and large molecular weight drugs show low nasal mucosa permeability and hence have less than 10% bioavailability for small molecular biologics and less than 1% for the high molecular weight drugs [35;46]
Number of studies have been done that confirms the connection of olfactory subarachnoid space CSF with lymphatics of the nasal mucosa and this connection is proved to be the main pathway through which CSF material flows into the lymphatic system [47]. It is possible that these sites can provide access for the intranasally administered substances, which are able to cross the olfactory epithelium and enter the lamina propria, to the CSF and other regions in CNS. Ultimately the substance would appear in systemic circulation after traveling through the lymphatic flow and has to cross BBB to reach the CNS. Numerous intranasally applied molecules rapidly enter the CSF depending on the lipophilicity, molecular weight, and degree of ionization of the molecules. [48–53]. Large molecular weight compounds such as Evans blue-albumin or [125I]-albumin are concentrated in the perivascular spaces of the middle cerebral arteries and in the deep cervical lymph nodes after injection into CSF or brain [47].
The intracellular and extracellular olfactory pathways constitute direct transport pathways of substance delivery into the brain. However, the systemic and lymphatic pathways are indirect transport pathways to the brain and substances are still required to cross the BBB or blood-CSF barriers. One of the ways to get information regarding the intranasal delivery mechanism involved is by evaluating distribution into the CSF. Due to the various interconnections and overlaps in the different pathways it is difficult to experimentally separate contributions of different pathways into the CNS after intranasal administration.
4. Nanotechnology for Intranasal Delivery to CNS
Even though there has been several biological molecules delivered through the intranasal route (Table 2 and Table 3), evidence of intranasal delivery is still sparse as far as broader therapeutic applications and in higher animal species, including primates, despite the advantages offered for CNS therapy. Most of these biological drugs show less than 1% bioavailability due to various limiting factors involved. The nasal mucosa acts a major obstacle for the passage of large molecules, particularly for those above 1,000 Da in size [54], and these are transported through transcellular pathway by either carrier mediated endocytosis or transporters. Paracellular route is known to be involved with small polar drugs transport and it takes place between adjacent epithelial cells through hydrophilic pores and tight junctions. The tight junctions have a size cutoff in the range of between 3.9–8.4 Å which hinders the large molecules transport [55].
Table 2.
Proteins/Peptides delivered to CNS through the nasal route
| Peptides/Proteins (M.W) | Detected By | Pharmacodynamic data | References |
|---|---|---|---|
| NAP(825da) | Detected in brain, Functional assays | Improved memory; reduced AD-like pathology; decreased hyperactivity; reduced hypoxia-induced oxidative stress | [96] |
| Hypocretin-1(2.6Kda) | Detected highest amounts in trigeminal nerve and olfactory bulbs, small amount in CSF | Improved task performance following sleep deprivation | [97] |
| Pituitary adenylate cyclase-activating peptide(PACAP)(4.5kda | Detected in brain within 5 to 30min, radioactivity | Stimulated non-amyloidogenic processing and improved cognitive function in an AD model | [98] |
| Insulin(5.8kda) | Detected in brain, CSF levels not determined, radioactivity | Slowed cognitive decline, improved mortality, and reduced neuropathic pain in a model of type I diabetes; improved memory recognition, anxiolytic behavior | [99] |
| Leptin(16kda) | Detected in brain, small amount in CSF, Radioactivity count | Inhibited appetite; decreased weight gain | [100] |
| Interferon β1b(18.5kda) | Detected in brain, Radioactivity count, Autoradiography | Phosphorylated INF receptor | [101] |
| Ciliary neurotropic factor(CNTF)(22.7kda) | Detected in brain within 25min, CSF levels not determined, functional assay | pAkt activated in occipital cortex | [102] |
| Transforming growth factor β1(TGF-β1)(25kda) | Detected in brain, no CSF detection, highest levels in 60min | Reduced infarct volume, improved functional recovery, and increased neurogenesis in stroke models | [103] |
| Brain derived neurotropic factor(BNDF)(27kda) | Detected in brain within 25min, functional assay | pAkt activated in frontal cortex | [102] |
| Erythropoietin(EPO)(30-34kda) | Detected in brain | Reduced infarct volume and improved neurologic function in stroke models | [104] |
| Vascular endothelial growth factor(VEGF)(38.2kda) | Detected in brain, no CSF detection | Reduced infarct volume, improved behavioral recovery, and enhanced neurogenesis in stroke models | [105] |
| Ovalbumin(45 kda) | Detected in brain qualitatively by fluorescence microscopy | Not determined | [68] |
Table 3.
Intranasal delivery of other macromolecules
| Molecules | Class | Function/Use | Reference |
|---|---|---|---|
| ADRSVBgal | adenoviral vectors | Detected by histochemical, Intracellular axonal Pathway β-galactosidase activity was detected in the olfactory bulb, locus ceruleus, area postrema, brainstem, and hippocampus 12 days following IN delivery | [106] |
| Semliki Forest Virus-EGFP | IL-10 vector | IL-10 expression seen in nasal passage and olfactory bulb in the infected mice | [107] |
| Plasmid DNA | Gene vector | DNA plasmid expression confirmed in the brain tissues and the lymph nodes by qPCR | [108] |
| Apha B-crytallin siRNA | siRNA | In 12 hours gene reduction was observed in amygdada,entorhinal cortex, and hypothalamus detected by immunohistochemistry | [109] |
| FITC-siRNA | siRNA | siRNA detected along the olfactory nerve bundles and in the olfactory bulbs | [110] |
| Mesenchymal stem cells | Whole cells | MSC in PD animal model and were detected in olfactory bulbs and other parts of brain and cells survived upto 4.5months. Showed improvement in forepaw motor function after in delivery | [111] |
Another major factor is the mucocilliary clearance which is a self clearing mechanism where external agents once bound to the mucus are transported to the nasophayrnx and eventually to the gastrointestinal tract [56]. This mechanism influences the nasal absorption of the biological drugs. Furthermore, enzymatic degradation of the intranasal administered drugs due to the presence of various proteolytic enzymes like aminopeptidases and proteases limits the amount of drug available for transport to the CNS. These proteolytic enzymes were believed to be the major barrier against the absorption of peptide drugs, such as calcitonin, insulin and desmopressin [57;58].
Different multidrug resistance transporters have also been identified in the human nasal respiratory and olfactory mucosa. These transporters actively efflux out the drug from intracellular compartment to the extracellular compartment and they seem to effect the uptake of these biological drugs in the nasal mucosa. P-gp is one of the efflux transporter that is found in the apical area of ciliated epithelial cells and in the submucosal vessels of the human olfactory region [59].
To overcome these various challenges, one of the preferred approaches has been the use of nanotechnology to enhance nasal absorption of large molecules. Over the last few years, specialized systems such as nano/micro-particles, liposomes, lipid emulsions, microspheres, and films have also been developed to improve nasal drug delivery. Few of the research examples focused on the development of formulation strategies to overcome the barriers present in the nasal mucosa to improve intranasal delivery efficiency and targeting to the CNS are presented in this section.
4.1. Nano- and Micro-particles
Nano/micro-particles are solid matrix of colloidal particles with diameters ranging from 1–1000 nm formed using various polymers like degradable starch, dextran, chitosan, microcrystalline cellulose (MCC), hydroxypropyl cellulose (HPC), hydroxypropyl ethylcellulose (HPMC), carbomer, and wax-like starch, gelatin polymers [60]. In these carrier systems, the drug could be loaded via either incorporation with the system or its adsorption on the particulate system. Encapsulated drug can be released from these particles by different mechanisms.
Nanoparticles may offer several advantages in improving nose-to-brain drug delivery, some of which are higlighted here. Due to the small diameter of these particles they can be transported transcellularly through olfactory neurons to the brain via the various endocytic pathways of sustentacular or neuronal cells in the olfactory membrane. Nanoparticles can also be prepared to improve upon the paracellular transport of these molecules. A recent study using estradiol (MW-272) chitosan nanoparticles performed by Wang et al., [61] showed significant increase in the drug concentration in the CSF after intranasal administration in rats. Cyclodextrin complexed chitosan nanoparticles were prepared using the cross-linked chitosan and the resulting diameter was reported to be 265–274 nm. Chitosan is widely used as a permeation enhancer which works by transiently opening the tight junctions and allowing the paracellular transport of drugs through the epithelium barrier. The study suggests that the mucoadhesive nanoparticle formulation can achieve enhanced direct nose-to-brain transport as compared to a simple estradiol solution [62].
As the active drug will be encapsulated inside these particles, macromolecules will be protected from biological and/or chemical degradation and extracellular transport by P-gp efflux proteins. In a study performed by Morimoto et al. [63], sCT (salmon calcitonin MW-3454) was applied nasally to rats in positively and negatively charged gelatin microspheres. The positively charged microspheres showed better ability to adhere to the mucosa, and gelatin microspheres protected the peptide from enzymatic degradation. The hypocalcemic effect obtained with the microspheres was found significantly higher than that of sCT solution. In conclusion, it was demonstrated that gelatin microspheres are a beneficial drug carrier for nasal application of peptide drugs [63]. In another study co-administration of P-gp inhibitor rifampin with P-gp substrate verapamil resulted in significantly greater brain uptake of verapamil as a result of reduced clearance due to P–gp mediated efflux [64].
The mucoadhesive property of these systems is another important parameter that can be explored for improving their retention and action in the nasal mucosa. In one of the study the cationic nanoparticle system having size of 60 nm in diameter made from polysaccharides, dipalmitoyl phosphatidyl choline, and cholesterol, was shown to have an extended mean residence half-life in the nasal cavity of humans to 2.3 h. After 24 h, 33% of the radioactivity was still detectable in the nasal cavity [65]. Due to relatively high surface area offered by these particles, drug release will be faster as compared to larger non encapsulated macromolecules which in turn will be beneficial especially for the acute management of diseases like pain.
4.2. Surface-Modified Polymeric Nanoparticles
The surface of the nanoparticles can be decorated with different targeting molecules. One of the examples of such a surface engineered particles is the wheat germ agglutinin (WGA) lectin coated PEG-PLA nanoparticles, which were used to encapsulate the vasoactive intestinal peptide (VIP; MW 3326). Data reported shows that VIP concentrations were increased in the olfactory bulb, cerebrum and cerebellum for the PEG-PLA particles as compared to non-encapsulated VIP solution and these values further increased by use of the WGA coated–PEG–PLA nanoparticles. The increase in VIP concentrations also correlated to improved memory function, as determined by the water maze behavioral test. This is the first evidence that has shown the ability of nanoparticles to protect a peptide drug from peptidase degradation in the nasal environment, and furthermore, their enhanced pharmacological efficacy compared to control animals. The use of lectins in formulations for coating the surface is advantageous as lectins have selective affinity for biological membranes due to their capacity to recognize the sugar residues [66].
Specific delivery of the drugs to olfactory epithelium is an important factor for the transport of drugs to CNS; otherwise, drugs will be absorbed through the respiratory epithelium into the systemic circulation. One of the strategies that has been adopted for specific targeting to olfactory epithelium for these biologic based drugs is to use ulex europeus aggutinin 1 (UEA 1), which has specific binding affinity to l-fructose residues found on the apical surface of the olfactory epithelium. PEG-PLA nanoparticles have been conjugated to UEA 1 and it was found that the use of these particles increased the fluorescent marker coumarin (loaded in particles) concentration in different regions of brain almost 2 fold as compared to the unmodified particles [67].
4.3. Liposomes
Liposomal drug delivery systems for intranasal delivery to CNS present various advantages as liposomes can be used to encapsulate large molecules with a wide range of hydrophilicity and can also provide higher absorption due to the interaction of the lipids with the lipid bilayer in the epithelium. Liposomes have been found to enhance nasal absorption of peptides such as insulin and calcitonin by increasing their membrane penetration or by providing protection of the entrapped peptides from enzymatic degradation. Cationic liposomes were used to encapsulate a model drug ovalbumin (MW= 45 kDa) and when these liposomes were delivered intranasally they demonstrated high levels in substantia nigra and striatum 6 h and 24 h after intranasal administration [68].
4.4. Cyclodextrin-Inclusion Complexes
Another strategy is to improve upon the drug solubility at the site of delivery in nasal epithelium. Drugs can be encapsulated in carriers, like cyclodextrins inclusion complexes containing a hydrophobic core and a hydrophilic shell which can help improve upon the drug solubility problems and improve brain uptake after intranasal administration. Galanin-like peptide (GALP) mixed with alpha-cyclodextrin resulted in enhanced delivery to all brain regions by two- to threefold, with the greatest uptake in the olfactory bulbs and hypothalamus, while GALP when mixed with beta-cyclodextrin resulted in enhanced uptake of GALP specifically to the olfactory bulbs compared to a simple intranasal solution [65].
4.5. Nano- and Micro-emulsions
These are dispersion consisting naturally occurring lipids or oils, surfactant and an aqueous phase usually with a droplet diameter in the range of 1–1000nm. These formulations have advantages of improving upon the solubility problems and can also provide mucoadhesion due to the use of different types of lipids. Recently, an emulsion-like formulation was patented for use with water-insoluble peptides and proteins by Hanson and Frey's group [69] and it was used to encapsulate the growth differentiation factor (GDF5) protein which may be used to treat Parkinson's disease. It was shown first time that encapsulation of GDF5 in lipid microemulsion increased drug targeting to the midbrain eight fold as compared to intranasal solution [70].
Nanotechnology based particulate systems will be able to facilitate the transport of peptide and protein structured drugs through the nasal mucosa and protect them from enzymatic activity by increasing the retention time of the drug in the nasal cavity, establishing tight contact between the nasal mucosa and the drug, providing localization of the drug at high concentrations, and opening the tight junctions between the epithelial cells. These advantages shows great promise of Nanotechnology based system for improving the intranasal delivery to CNS and need to be further explored for biologics.
5. Formulation Considerations in CNS Therapies
The development of bio-therapeutics for CNS therapy has mainly been hindered owing to their limitations like poor drug-like properties, in vivo instability and short half-life leading to ineffective delivery across the brain. Recent advances in the area of drug delivery mainly via systemic or intranasal route, and discovery of novel devices have enabled over 150 peptide therapeutics already undergoing clinical development [1].
5.1. Formulation Issues in Systemic Delivery
Most peptide therapeutics are susceptible to proteases and have rapid clearance, thereby require higher dose or more frequent administration. Recently, novel approaches in designing synthetic peptides by altering their length and replacing L-amino acids by unnatural D-amino acids, for example, somatostain analog octreotide, have improved the enzymatic stability [71]. PEGylation of nanoformulations has now been well known to improve their circulation time in the blood. The same concept has been applied to peptides, for example, PEGylated interferon for the treatment for hepatitis C has 10-fold increased half-life to native interferon. For CNS delivery, improving the permeability of native therapeutics by increasing the lipophilicity via fatty acid linkers is being explored. A minireview highlights some of the applications of polycyclic cage-like scoffolds of pentacycloundecane and adamantane as moieties to increase the BBB permeability of hydrophobically modified- therapeutics to effectively treat neurodegenerative diseases [72]. Another interesting strategy called as the `lock-in mechanism' has shown drug modifications via the target or peptide conjugate that can be trapped behind the lipoidal BBB for effective therapy [73]– [74]. Hence, there is a need to effectively design molecules that can traverse the BBB and reach the target sites in the brain without altering their potency or safety.
Another problem, especially in the delivery of cancer therapeutics to the brain, is their susceptibility to efflux by transporters like p-glycoprotein and others present at the lumenal side of the BBB. Drugs can be modified so that they are not taken up by these transporters or they can be co-administered with small molecule P-gp inhibitors, like zosquidar, etc. Potent CNS effects of loperamide were seen when administered with P-gp inhibitor quinidine. However, most of the small molecule P-gp inhibitors are also known to cause side-effects due to their low potency and selectivity. We have explored the potential of using safe and well-known natural compound, curcumin to downregulate the efflux transporters and have shown improved uptake of drugs like paclitaxel [75].
Diseases like Alzheimer's, Parkinson's and Huntington's are associated with protein malfunction and hence, can be thought to be effectively treated by downregulating production of offending protein with small interfering RNA (siRNA). It has been shown that by associating siRNA to a brain penetrating peptide, the peptide carries siRNA into the CNS, likely by receptor-mediated transcytosis [76].
Major focus for enabling delivery of therapeutics across the BBB has been using nanoparticulate drug delivery technologies. These formulations, due to their lipophilicity and/ or size are taken up by the cells of the BBB by endocytosis. Nanocariers like liposomes, nanoemulsions, polymeric nanoparticles or solid lipid nanoparticles, can effectively encapsulate the compounds and can be surface modified with specific ligands or antibodies for insulin, transferrin or leptin and Fc fragments for easy identification and uptake by receptor-mediated transport [1]. Conjugation of vasoactive intestinal peptide to an antibody to the transferrin transporter using avidin-biotin technology produced a significant increase in cerebral blood flow [77]. Low density lipoprotein related receptor (LRP-1) family of peptides has been explored to facilitate delivery of therapeutics to the brain [78] via the transcytosis mechanism. ANG1005, is an engineered peptide with angiopeptides (LRP-1) and three molecules of paclitaxel, exhibits greater than 10 times higher uptake into rat brain than paclitaxel alone and is in clinical trials for the treatment of brain cancer [78].
Designing formulations with improved drug loading is the key for enabling nanoparticulate based drug delivery to the CNS via systemic route. Also, adjusting the pH, osmolarity and lipophilicity/targeting potential of these formulations needs to be considered.
5.2. Formulations Issues in Intranasal Delivery
The exact mechanisms by which drugs traverse the olfactory epithelium to reach the brain and CSF are not completely understood. However, it is generally accepted that small lipophilic moieties can be absorbed into the capillaries of olfactory epithelium and subsequently reach the brain by crossing the BBB. In this case, the rate of absorption will largely be influenced by physicochemical properties of the compound itself, including size, ionization state, lipophilicity and its hydrogen-bonding potential with the membrane [79].
Intranasal route of delivery is limited by the volume of formulation that can be administered (<400 μL in humans) and hence, it is important to get adequate solubility and drug loading in the intended formulation. Simple solutions are known to be rapidly cleared along with the mucus in the nasal cavity. Even though this route leads to a faster rate of absortion, it also leads to very short residence time. Also, permeability across the mucus and tissue layers is fairly low. Mucoadhesive excipients like carbopol, starch; polymers like chitosan; and penetration enhancers like surfactants, bile salts, phospholipids can be employed within the formulation to improve the residence time [80]. The formulations need to be adjusted for pH, osmolarity and viscosity to avoid irritation of the mucosal lining.
Olfactory route is gaining more attention mainly because it does not pose restriction similar to that caused by the BBB, however, it should be noted that the epithelial lining of the nasal cavity has other barriers for delivery of large molecular weight drugs due to the tight junctions [81]. Studies published so far with intranasal administration of drugs especially biotherapeutics, has shown very low bioavailability (<1%) and hence, only very potent drugs, genes or siRNA can be attractive candidates for this route of delivery. There is also a need to study their stability in the various metabolic enzymes present in the nasal mucosa and come up with strategies to ensure its protection from enzymatic degradation. Owing to presence of large number of blood vessels surrounding the nasal cavity, drugs can enter the systemic circulation after deposition in the respiratory mucosa. This will in turn can lead to off -target effects. Therefore, targeting the therapeutic to the olfactory mucosa either by use of specific olfactory receptor binding agents or by employing special delivery devices, is an important consideration when delivering molecules to the brain via the intranasal route.
Lastly, pre-clinical to cinical translation of data is debated, given the large differences in anatomy of rodent nose versus human nose. This necessitates the study of therapeutics and intranasal formulations in larger non-human primate models. It may be worthwhile to put some efforts in marrying the drug delivery formulations to devices to effectively introduce therapeutics via the intransal route for brain delivery.
5.3 Material Safety Considerations
High-throughput technologies have become routine in screening candidates during drug discovery. Over recent years, the nature of the disease targets have lead to discovery of molecules with increasing molecular weight, lipophilicity and complexicity [82]. This in turn, has put a significant need to finding novel formulations and excipients to tackle the drug bioavailability issues [83].
The “Guidance for Industry—Drug Product, Chemistry, Manufacturing and Controls Information” issues by FDA classifies excipients into different types:
Compendial, Non-Novel Excipients: generally listed in the formularies and pharmacopeias and thier use is less restricted as the risk is low.
Noncompendial, Non-Novel Excipients: may be listed in US FDA GRAS and may require additional testing.
Novel Excipients: may require additional testing for NDA approval at a level of a drug product.
- In addition, the duration of excipient use is important and can be categorized into:
-
◯short term (<14 days)/ acute
-
◯intermediate term (2 weeks to three months)
-
◯long term (>three months)/ chronic
-
◯
The FDA inactive ingredient (FDA 2006) list includes parenteral formulations containing almost 50% ethanol. Glycerin is also widely available with percentages of greater than 20% in products designed for i.v. or i.m. dosing and greater than 90% for oral administration [83]. Sometimes there is a disconnect between published safety limits and levels that are already being dosed in approved products. Cremophor, polyoxyethylenated castor oil derivative, has become a very common surfactant due to its great solubilizing capacity. However, it is associated with allergic responses in sensitive species, like anaphylatic reaction in dog. In man, there have been varying reports of rash to a shock depending on the product used.
Some excipients are not inert, but can have additional functional properties, like most surfactants can cause P-gp inhibition or CYP3A4 interactions when given orally and hence, alter the in vivo exposure of the active drug. Hence, such excipients need to be screened appropriately. Recently, there has been increased focus to use biodegradable excipients (polymers, oils, etc.) to avoid systemic side- effects. For example, emulsions are gaining popularity not only because of their property to solubilize drugs, but also their ability to provide appropriate kinetics and biodistribution in vivo. Biodegradable nanoparticles of PLGA, etc. have provided improved targeting and hence less systemic toxicity of cancer therapeutics.
Ability of scientists to determine whether improved in vivo performance is due to solubility enhancement alone or in combination with physiological perturbations will allow for a more mechanistic understanding of formulation and excipient function(s)[84].
6. Conclusions and Future Outlook
Bio-therapeutics are attractive as therapeutics owing to their potency, specificity and safety profile. However, their delivery to the CNS has been challenging due to presence of the blood-brain barrier, blood-CSF barrier and the systemic dilution effect and clearance. Various invasive and non-invasive techniques have been explored pre-clinically as well as in clinic to effectively delivery therapies to the CNS. Of these, use of nanoparticulate formulations like liposomes and nanoparticles by systemic administration and via intranasal route has gained recent interest. Furthermore, these systems can be modified to serve as active targeting moieties as well as can be made to incorporate efflux modulators to improve the efficiency of delivery.
Although, many biological molecules have been delivered pre-clinically by intranasal route, much less evidence is present to show enhancement in biodistribution to the brain. Mechanisms involved in delivery of molecules to the CNS via the nasal passages are yet to be completely understood. However, the challenges posed by the intranasal route of administration have been widely studied and are countered by various strategies, like use of absorption enhancers, tight junction openers, permeation enhancers to improve upon the bioavailability of these molecules. Furthermore, novel nanotechnology based approaches have been explored and have shown some pre-clinical promise in terms of providing protection, mucosal retention and improved absorption for these macromolecules.
Detailed evaluation of these different delivery routes and strategies to enhance BBB targeting is warranted for translation to a clinical setting, keeping in mind patient compliance and treatment goal for diseases affecting the CNS.
7. References
- [1].McGonigle P. Peptide therapeutics for CNS indications. Biochem Pharmacol. 2012;83(5):559–566. doi: 10.1016/j.bcp.2011.10.014. [DOI] [PubMed] [Google Scholar]
- [2].Rajadhyaksha M, Boyden T, Liras J, El-Kattan A, Brodfuehrer J. Current advances in delivery of biotherapeutics across the blood-brain barrier. Curr Drug Discov Technol. 2011;8(2):87–101. doi: 10.2174/157016311795563866. [DOI] [PubMed] [Google Scholar]
- [3].Garcel A, Martel S, Carrupt P, Doelker E, Delie F. In vitro blood brain barrier models as a screening tool for colloidal drug delivery systems and other nanosystems. International Journal of Biomedical Nanoscience and Nanotechnology. 2010;1(2):133–163. [Google Scholar]
- [4].Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug Chem. 2008;19(7):1327–1338. doi: 10.1021/bc800148t. [DOI] [PubMed] [Google Scholar]
- [5].Potschka H. Role of CNS efflux drug transporters in antiepileptic drug delivery: overcoming CNS efflux drug transport. Adv Drug Deliv Rev. 2012;64(10):943–952. doi: 10.1016/j.addr.2011.12.007. [DOI] [PubMed] [Google Scholar]
- [6].Barchet TM, Amiji MM. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert Opin Drug Deliv. 2009;6(3):211–225. doi: 10.1517/17425240902758188. [DOI] [PubMed] [Google Scholar]
- [7].Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27(2):85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [8].Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19(3):311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- [9].van R, I, Cakir-Tascioglu S, Hennink WE, Storm G, Schiffelers RM, Mastrobattista E. In vivo methods to study uptake of nanoparticles into the brain. Pharm Res. 2011;28(3):456–471. doi: 10.1007/s11095-010-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Discov Med. 2006;6(34):139–143. [PubMed] [Google Scholar]
- [11].Musacchio T, Torchilin VP. Recent developments in lipid-based pharmaceutical nanocarriers. Front Biosci. 2011;16:1388–1412. doi: 10.2741/3795. [DOI] [PubMed] [Google Scholar]
- [12].Laquintana V, Trapani A, Denora N, Wang F, Gallo JM, Trapani G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin Drug Deliv. 2009;6(10):1017–1032. doi: 10.1517/17425240903167942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pardridge WM. Vector-mediated drug delivery to the brain. Adv Drug Deliv Rev. 1999;36(2–3):299–321. doi: 10.1016/s0169-409x(98)00087-8. [DOI] [PubMed] [Google Scholar]
- [14].Soni V, Kohli DV, Jain SK. Transferrin-conjugated liposomal system for improved delivery of 5-fluorouracil to brain. J Drug Target. 2008;16(1):73–78. doi: 10.1080/10611860701725381. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Y, Calon F, Zhu C, Boado RJ, Pardridge WM. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum Gene Ther. 2003;14(1):1–12. doi: 10.1089/10430340360464660. [DOI] [PubMed] [Google Scholar]
- [16].Lindqvist A, Rip J, Gaillard PJ, Bjorkman S, Hammarlund-Udenaes M. Enhanced Brain Delivery of the Opioid Peptide DAMGO in Glutathione PEGylated Liposomes: A Microdialysis Study. Mol Pharm. 2012 doi: 10.1021/mp300272a. [DOI] [PubMed] [Google Scholar]
- [17].Zara GP, Cavalli R, Bargoni A, Fundaro A, Vighetto D, Gasco MR. Intravenous administration to rabbits of non-stealth and stealth doxorubicin-loaded solid lipid nanoparticles at increasing concentrations of stealth agent: pharmacokinetics and distribution of doxorubicin in brain and other tissues. J Drug Target. 2002;10(4):327–335. doi: 10.1080/10611860290031868. [DOI] [PubMed] [Google Scholar]
- [18].Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- [19].Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–477. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- [20].Wong HL, Bendayan R, Rauth AM, Wu XY. Development of solid lipid nanoparticles containing ionically complexed chemotherapeutic drugs and chemosensitizers. J Pharm Sci. 2004;93(8):1993–2008. doi: 10.1002/jps.20100. [DOI] [PubMed] [Google Scholar]
- [21].Di SA, Iannitelli A, Laserra S, Sozio P. Drug delivery strategies for Alzheimer's disease treatment. Expert Opin Drug Deliv. 2011;8(5):581–603. doi: 10.1517/17425247.2011.561311. [DOI] [PubMed] [Google Scholar]
- [22].Sarker DK. Engineering of nanoemulsions for drug delivery. Curr Drug Deliv. 2005;2(4):297–310. doi: 10.2174/156720105774370267. [DOI] [PubMed] [Google Scholar]
- [23].Ganta S, Deshpande D, Korde A, Amiji M. A review of multifunctional nanoemulsion systems to overcome oral and CNS drug delivery barriers. Mol Membr Biol. 2010;27(7):260–273. doi: 10.3109/09687688.2010.497971. [DOI] [PubMed] [Google Scholar]
- [24].Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm. 2008;347(1–2):93–101. doi: 10.1016/j.ijpharm.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tiwari SB, Amiji MM. A review of nanocarrier-based CNS delivery systems. Curr Drug Deliv. 2006;3(2):219–232. doi: 10.2174/156720106776359230. [DOI] [PubMed] [Google Scholar]
- [26].Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev. 2012;64(7):686–700. doi: 10.1016/j.addr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- [27].Huwyler J, Yang J, Pardridge WM. Receptor mediated delivery of daunomycin using immunoliposomes: pharmacokinetics and tissue distribution in the rat. J Pharmacol Exp Ther. 1997;282(3):1541–1546. [PubMed] [Google Scholar]
- [28].Liu L, Guo K, Lu J, Venkatraman SS, Luo D, Ng KC, et al. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood-brain barrier. Biomaterials. 2008;29(10):1509–1517. doi: 10.1016/j.biomaterials.2007.11.014. [DOI] [PubMed] [Google Scholar]
- [29].Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, et al. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317(3):1246–1253. doi: 10.1124/jpet.105.097139. [DOI] [PubMed] [Google Scholar]
- [30].Fortin D, Gendron C, Boudrias M, Garant MP. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in the treatment of cerebral metastasis. Cancer. 2007;109(4):751–760. doi: 10.1002/cncr.22450. [DOI] [PubMed] [Google Scholar]
- [31].Becker I, Becker KF, Meyermann R, Hollt V. The multidrug-resistance gene MDR1 is expressed in human glial tumors. Acta Neuropathol. 1991;82(6):516–519. doi: 10.1007/BF00293387. [DOI] [PubMed] [Google Scholar]
- [32].Sikic BI, Fisher GA, Lum BL, Halsey J, Beketic-Oreskovic L, Chen G. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 1997;40(Suppl):S13–S19. doi: 10.1007/s002800051055. [DOI] [PubMed] [Google Scholar]
- [33].Lee YJ, Maeda J, Kusuhara H, Okauchi T, Inaji M, Nagai Y, et al. In vivo evaluation of P-glycoprotein function at the blood-brain barrier in nonhuman primates using [11C]verapamil. J Pharmacol Exp Ther. 2006;316(2):647–653. doi: 10.1124/jpet.105.088328. [DOI] [PubMed] [Google Scholar]
- [34].Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379(1):146–157. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- [35].Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- [36].Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- [37].Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- [38].Dhuria SV, Hanson LR, Frey WH., II Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. Journal of Pharmaceutical Sciences. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- [39].Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- [40].Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administration. Journal of Pharmacology & Experimental Therapeutics. 2004;309(2):469–475. doi: 10.1124/jpet.103.063222. [DOI] [PubMed] [Google Scholar]
- [41].Baker H, Spencer RF. Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. 1986;(3):461–473. doi: 10.1007/BF00237470. [DOI] [PubMed] [Google Scholar]
- [42].Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- [43].Alcala-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. Journal of Drug Targeting. 2010;18(3):179–190. doi: 10.3109/10611860903318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Clerico DM, Lanza DC, To WC. Handbook of olfaction and gustation. 2nd edition Marcel Dekker, Inc.; New York: 2003. pp. 1–16. [Google Scholar]
- [45].Gray H. Gray's anatomy. 15th revised edition BountyBooks; New York: 1978. [Google Scholar]
- [46].Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- [47].Bradbury MWB, Cserr HF. Drainage of cerebral interstitial fluid and of cerebrospinal fluid into lymphatics. In: Johnston MG, editor. Experimental Biology of lymphatic circulation. Amsterdam and New York: 1985. pp. 355–391. [Google Scholar]
- [48].Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: A transnasal approach to the human brain. Nature Neuroscience. 2002;5(6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- [49].Dhanda DS, Frey WH, II, Leopold D, Kompella UB. Approaches for drug deposition in the human olfactory epithelium. Drug Delivery Technology. 2005;5:64–72. [Google Scholar]
- [50].Kumar ATC, Umberkoman B, Saini KD, David GFX. Uptake of radioactivity by body fluids and tissues in rhesus monkeys after intravenous injection or intranasal spray of tritium-labelled oestradiol and progesterone. Curr Sci. 1974;43:435–439. [Google Scholar]
- [51].Sakane T, Akizuki M, Yamashita S, Sezaki H, Nadai T. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the dissociation of the drug. J Pharm Pharmacol. 1994;46(5):378–379. doi: 10.1111/j.2042-7158.1994.tb03817.x. [DOI] [PubMed] [Google Scholar]
- [52].Sakane T, Akizuki M, Taki Y, Yamashita S, Sezaki H, Nadai T. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the molecular weight of drugs. J Pharm Pharmacol. 1995;47(5):379–381. doi: 10.1111/j.2042-7158.1995.tb05814.x. [DOI] [PubMed] [Google Scholar]
- [53].Wang Q, Chen G, Zeng S. Pharmacokinetics of Gastrodin in rat plasma and CSF after i.n. and i.v. Int J Pharm. 2007;341(1–2):20–25. doi: 10.1016/j.ijpharm.2007.03.041. [DOI] [PubMed] [Google Scholar]
- [54].Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337(1–2):1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- [55].Illum L. Nanoparticulate systems for nasal delivery of drugs: a real improvement over simple systems? J Pharm Sci. 2007;96(3):473–483. doi: 10.1002/jps.20718. [DOI] [PubMed] [Google Scholar]
- [56].Merkus FW, Verhoef JC, Schipper NG, Marttin E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29(1–2):13–38. doi: 10.1016/s0169-409x(97)00059-8. [DOI] [PubMed] [Google Scholar]
- [57].Lee VHL, Yamamoto A. Penetration and enzymatic barriers to peptide and protein absorption. Advanced Drug Delivery Reviews. 1989;4(2):171–207. [Google Scholar]
- [58].Harris AS, Nilsson IM, Wagner ZG, Alkner U. Intranasal administration of peptides: nasal deposition, biological response, and absorption of desmopressin. J Pharm Sci. 1986;75(11):1085–1088. doi: 10.1002/jps.2600751113. [DOI] [PubMed] [Google Scholar]
- [59].Graff CL, Pollack GM. Functional evidence for P-glycoprotein at the nose-brain barrier. Pharm Res. 2005;22(1):86–93. doi: 10.1007/s11095-004-9013-3. [DOI] [PubMed] [Google Scholar]
- [60].Ozsoy Y. In: Handbook of Particulate Drug Delivery. Kumar MNV, editor. American Scientific Publisher; CA: 2008. p. 143. [Google Scholar]
- [61].Wang X, He H, Leng W, Tang X. Evaluation of brain-targeting for the nasal delivery of estradiol by the microdialysis method. Int J Pharm. 2006;317(1):40–46. doi: 10.1016/j.ijpharm.2006.02.055. [DOI] [PubMed] [Google Scholar]
- [62].Smith J, Wood E, Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharm Res. 2004;21(1):43–49. doi: 10.1023/b:pham.0000012150.60180.e3. [DOI] [PubMed] [Google Scholar]
- [63].Morimoto K, Katsumata H, Yabuta T, Iwanaga K, Kakemi M, Tabata Y, et al. Evaluation of gelatin microspheres for nasal and intramuscular administrations of salmon calcitonin. Eur J Pharm Sci. 2001;13(2):179–185. doi: 10.1016/s0928-0987(01)00094-x. [DOI] [PubMed] [Google Scholar]
- [64].Graff CL, Pollack GM. P-Glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm Res. 2003;20(8):1225–1230. doi: 10.1023/a:1025053115583. [DOI] [PubMed] [Google Scholar]
- [65].Kravtzoff R, Appelqvist T, Haddouk H, Manciaux X, Cholet G, De M, I, et al. Preclinical toxicology of biovectorTM nanoparticles: part II, local tolerance, genetic toxicology and pharmacokinetics. Toxicology Letters 95[1001], 117. 1998 [Google Scholar]
- [66].Gao X, Wu B, Zhang Q, Chen J, Zhu J, Zhang W, et al. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Control Release. 2007;121(3):156–167. doi: 10.1016/j.jconrel.2007.05.026. [DOI] [PubMed] [Google Scholar]
- [67].Gao X, Chen J, Tao W, Zhu J, Zhang Q, Chen H, et al. UEA I-bearing nanoparticles for brain delivery following intranasal administration. Int J Pharm. 2007;340(1–2):207–215. doi: 10.1016/j.ijpharm.2007.03.039. [DOI] [PubMed] [Google Scholar]
- [68].Migliore MM, Vyas TK, Campbell RB, Amiji MM, Waszczak BL. Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. J Pharm Sci. 2010;99(4):1745–1761. doi: 10.1002/jps.21939. [DOI] [PubMed] [Google Scholar]
- [69].Hanson LR, Frey WH, II, Hoekman JD, Pohl J. In: Lipid growth factor formulations. EPO, editor. 2008. inventors. [Google Scholar]
- [70].Hanson LR, Fine JM, Hoekman JD, Nguyen TM, Burns RB, Martinez PM, et al. Intranasal delivery of growth differentiation factor 5 to the central nervous system. Drug Deliv. 2012;19(3):149–154. doi: 10.3109/10717544.2012.657720. [DOI] [PubMed] [Google Scholar]
- [71].Werle M, Bernkop-Schnurch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30(4):351–367. doi: 10.1007/s00726-005-0289-3. [DOI] [PubMed] [Google Scholar]
- [72].Joubert J, Geldenhuys WJ, Van der Schyf CJ, Oliver DW, Kruger HG, Govender T, et al. Polycyclic cage structures as lipophilic scaffolds for neuroactive drugs. ChemMedChem. 2012;7(3):375–384. doi: 10.1002/cmdc.201100559. [DOI] [PubMed] [Google Scholar]
- [73].Bodor N, Prokai L, Wu WM, Farag H, Jonalagadda S, Kawamura M, et al. A strategy for delivering peptides into the central nervous system by sequential metabolism. Science. 1992;257(5077):1698–1700. doi: 10.1126/science.1529356. [DOI] [PubMed] [Google Scholar]
- [74].Wu J, Yoon SH, Wu WM, Bodor N. Synthesis and biological evaluations of brain-targeted chemical delivery systems of [Nva2]-TRH. J Pharm Pharmacol. 2002;54(7):945–950. doi: 10.1211/002235702760089063. [DOI] [PubMed] [Google Scholar]
- [75].Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6(3):928–939. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- [76].Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- [77].Wu D, Pardridge WM. Central nervous system pharmacologic effect in conscious rats after intravenous injection of a biotinylated vasoactive intestinal peptide analog coupled to a blood-brain barrier drug delivery system. J Pharmacol Exp Ther. 1996;279(1):77–83. [PubMed] [Google Scholar]
- [78].Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem. 2008;106(4):1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- [79].Martini Alessandro, Muggetti Lorena, Warchol ark P. Nasal and pulmonary drug delivery systems. Expert Opinion on Therapeutic Patents. 2000;10(3):315–323. [Google Scholar]
- [80].Romeo VD, deMeireles JC, Gries WJ, Xia WJ, Sileno AP, Pimplaskar HK, et al. Optimization of systemic nasal drug delivery with pharmaceutical excipients. Adv Drug Deliv Rev. 1998;29(1–2):117–133. doi: 10.1016/s0169-409x(97)00064-1. [DOI] [PubMed] [Google Scholar]
- [81].Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005;94(6):1187–1195. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- [82].Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012 doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- [83].Mackie C, Brewster M, Noppe M, Loftsson T, Lampo A. Solvent Systems and Their Selection in Pharmaceutics and Biopharmaceutics. Springer; New York: 2007. The Use of Solubilizing Excipients and Approaches to Generate Toxicology Vehicles for Contemporary Drug Pipelines; p. 221. [Google Scholar]
- [84].Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–230. doi: 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- [85].Strasser JF, Fung LK, Eller S, Grossman SA, Saltzman WM. Distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea and tracers in the rabbit brain after interstitial delivery by biodegradable polymer implants. J Pharmacol Exp Ther. 1995;275(3):1647–1655. [PubMed] [Google Scholar]
- [86].Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- [87].Laquintana V, Trapani A, Denora N, Wang F, Gallo JM, Trapani G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin Drug Deliv. 2009;6(10):1017–1032. doi: 10.1517/17425240903167942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dahm P, Nitescu P, Appelgren L, Curelaru I. Efficacy and technical complications of long-term continuous intraspinal infusions of opioid and/or bupivacaine in refractory nonmalignant pain: a comparison between the epidural and the intrathecal approach with externalized or implanted catheters and infusion pumps. Clin J Pain. 1998;14(1):4–16. doi: 10.1097/00002508-199803000-00003. [DOI] [PubMed] [Google Scholar]
- [89].Krewson CE, Klarman ML, Saltzman WM. Distribution of nerve growth factor following direct delivery to brain interstitium. Brain Res. 1995;680(1–2):196–206. doi: 10.1016/0006-8993(95)00261-n. [DOI] [PubMed] [Google Scholar]
- [90].Ilias W, Todoroff B. Optimizing pain control through the use of implantable pumps. Med Devices (Auckl) 2008;1:41–47. doi: 10.2147/mder.s3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Victorov IV, Prass K, Dirnagl U. Improved selective, simple, and contrast staining of acidophilic neurons with vanadium acid fuchsin. Brain Res Brain Res Protoc. 2000;5(2):135–139. doi: 10.1016/s1385-299x(00)00004-0. [DOI] [PubMed] [Google Scholar]
- [92].Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340(4):1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- [93].Liu XF, Fawcett JR, Hanson LR, Frey WH. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis. 2004;13(1):16–23. doi: 10.1016/j.jstrokecerebrovasdis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- [94].Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- [95].Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci U S A. 1996;93(24):14164–14169. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Alcalay RN, Giladi E, Pick CG, Gozes I. Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci Lett. 2004;361(1–3):128–131. doi: 10.1016/j.neulet.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [97].Dhuria SV, Hanson LR, Frey WH. Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system. J Pharm Sci. 2009;98(7):2501–2515. doi: 10.1002/jps.21604. [DOI] [PubMed] [Google Scholar]
- [98].Rat D, Schmitt U, Tippmann F, Dewachter I, Theunis C, Wieczerzak E, et al. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer's disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011;25(9):3208–3218. doi: 10.1096/fj.10-180133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, et al. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain. 2008;131(Pt 12):3311–3334. doi: 10.1093/brain/awn288. [DOI] [PubMed] [Google Scholar]
- [100].Fliedner S, Schulz C, Lehnert H. Brain uptake of intranasally applied radioiodinated leptin in Wistar rats. Endocrinology. 2006;147(5):2088–2094. doi: 10.1210/en.2005-1016. [DOI] [PubMed] [Google Scholar]
- [101].Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH. Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol. 2004;151(1–2):66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- [102].Alcala-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. Journal of Drug Targeting. 2010;18(3):179–190. doi: 10.3109/10611860903318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ma YP, Ma MM, Ge S, Guo RB, Zhang HJ, Frey WH, et al. Intranasally delivered TGF-beta1 enters brain and regulates gene expressions of its receptors in rats. Brain Res Bull. 2007;74(4):271–277. doi: 10.1016/j.brainresbull.2007.06.021. [DOI] [PubMed] [Google Scholar]
- [104].Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 2005;387(1):5–10. doi: 10.1016/j.neulet.2005.07.008. [DOI] [PubMed] [Google Scholar]
- [105].Yang JP, Liu HJ, Cheng SM, Wang ZL, Cheng X, Yu HX, et al. Direct transport of VEGF from the nasal cavity to brain. Neurosci Lett. 2009;449(2):108–111. doi: 10.1016/j.neulet.2008.10.090. [DOI] [PubMed] [Google Scholar]
- [106].Draghia R, Caillaud C, Manicom R, Pavirani A, Kahn A, Poenaru L. Gene delivery into the central nervous system by nasal instillation in rats. Gene Ther. 1995;2(6):418–423. [PubMed] [Google Scholar]
- [107].Jerusalmi A, Morris-Downes MM, Sheahan BJ, Atkins GJ. Effect of intranasal administration of Semliki Forest virus recombinant particles expressing reporter and cytokine genes on the progression of experimental autoimmune encephalomyelitis. Mol Ther. 2003;8(6):886–894. doi: 10.1016/j.ymthe.2003.09.010. [DOI] [PubMed] [Google Scholar]
- [108].Oh YK, Kim JP, Hwang TS, Ko JJ, Kim JM, Yang JS, et al. Nasal absorption and biodistribution of plasmid DNA: an alternative route of DNA vaccine delivery. Vaccine. 2001;19(31):4519–4525. doi: 10.1016/s0264-410x(01)00188-8. [DOI] [PubMed] [Google Scholar]
- [109].Kim ID, Kim SW, Lee JK. Gene knockdown in the olfactory bulb, amygdala, and hypothalamus by intranasal siRNA administration 42 (2009) 285–292. Korean J Anat. 2009;42:285–292. [Google Scholar]
- [110].Renner DB, Frey WH, Hanson LR. Intranasal delivery of siRNA to the olfactory bulbs of mice via the olfactory nerve pathway. Neurosci Lett. 2012;513(2):193–197. doi: 10.1016/j.neulet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- [111].Danielyan L, Schafer R, von Ameln-Mayerhofer A, Bernhard F, Verleysdonk S, Buadze M, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011;14(1):3–16. doi: 10.1089/rej.2010.1130. [DOI] [PubMed] [Google Scholar]
- [112].Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- [113].Dhuria SV, Hanson LR, Frey WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]