Abstract
Recent neuroscience evidence suggests that some higher-order tasks might benefit from a reduction in sensory filtering associated with low levels of cognitive control. Guided by neuroimaging findings, we hypothesized that cathodal (inhibitory) transcranial direct current stimulation (tDCS) will facilitate performance in a flexible use generation task. Participants saw pictures of artifacts and generated aloud either the object’s common use or an uncommon use for it, while receiving cathodal tDCS (1.5 mA) either over left or right PFC, or sham stimulation. A forward digit span task served as a negative control for potential general effects of stimulation. Analysis of voice-onset reaction times and number of responses generated showed significant facilitative effects of left PFC stimulation for the uncommon, but not the common use generation task and no effects of stimulation on the control task. The results support the hypothesis that certain tasks may benefit from a state of diminished cognitive control.
Keywords: semantic memory, creative cognition, transcranial direct current stimulation, prefrontal cortex, cognitive control
Introduction
Cognitive control is the ability to consider multiple types of information from the environment and bias these competing representations toward the optimal alternative to promote the organism’s goals in context (Miller & Cohen, 2001; Shimamura, 2000). Past research has implicated the prefrontal cortex (PFC)— particularly the left ventrolateral regions—in numerous domains requiring such regulatory filtering of bottom-up information, including working memory, language, and attention tasks (Thompson-Schill, Bedny, & Goldberg, 2005). Successful performance in such tasks typically involves representation of explicit abstract rules and inhibition of irrelevant information for the identification of an optimal response (e.g., Barde et al., 2010, Thompson-Schill, et al., 1997). Although the involvement of this region is undeniably critical for several aspects of complex cognition, recent evidence suggests that lack of top-down regulatory filtering, as guided by PFC mechanisms, might—perhaps paradoxically—benefit performance under some circumstances (Thompson-Schill, Ramscar, & Chrysikou, 2009). Diminished PFC functioning (hypofrontality), coupled with increased activity in posterior brain regions, may prove advantageous for tasks that depend on availability of unfiltered information.
Evidence in support of this view comes from different domains. In tasks that require using common objects in ways that deviate from an abstract understanding of their intended function, for example, young children appear less susceptible to functional fixedness than adults, and tend to generate effective solutions inspired by the objects’ low-level, perceptual features (German & Defeyter, 2000). Likewise, patients with frontotemporal dementia, a disease that may selectively affect the left frontotemporal cortex, exhibit spontaneous visual artistic abilities, which they did not possess prior to the onset of their disease (Seeley et al., 2008). Moreover, the abnormal development of PFC in autism, coupled with increased activity in occipital regions, has been hypothesized to underlie exceptional artistic, musical, or visual memory abilities in certain autistic individuals (e.g., Heaton, et al., 2007; Snyder, 2009). Overall, this evidence would suggest that certain tasks might benefit from a temporary disengagement of PFC regulatory mechanisms and a focus on unfiltered information processed in posterior brain regions.
According to this prediction, in past work we hypothesized that under the demands of a flexible thinking task, healthy adults might benefit from a state of lower cognitive control that would likely reflect a lack of regulatory filtering of available information. In a recent fMRI experiment (Chrysikou & Thompson-Schill, 2011), two groups of participants were shown pictures of common objects (e.g., belt) and they were asked to generate either the common use of each (e.g., to keep one’s pants up) or an uncommon use for it (e.g., to use as a tourniquet). The results showed an interaction between task (common vs. uncommon use generation) and brain region (PFC vs. visual association cortex), supporting the hypothesis that generating an uncommon use reduced demands for filtering of low-level object properties (e.g., the shape or materials of the objects) that would support a novel use. This pattern supports the hypothesis that there is tradeoff between unfiltered and PFC-regulated thought, such that tasks that require more of the former are associated with less of the latter, and vice versa.
The aim of this experiment was to amplify these neuroimaging results by using non-invasive brain stimulation to examine the causal relationship between reduced function in this region and performance in tasks involving flexible thought. Specifically, we used cathodal transcranial direct current stimulation (tDCS) to suppress activity in left PFC; tDCS is a noninvasive technique that involves the application of small currents (typically 1–2 mA) to the scalp for a few minutes through two surface electrodes, which can modulate cortical excitability in the underlying brain region. Conventionally, anodal tDCS stimulation increases cortical excitability at the stimulation site through subthreshold neuron soma depolarization, whereas cathodal tDCS stimulation decreases cortical excitability at the stimulation site due to neuron soma hyperpolarization (Nitsche et al., 2008). As such, tDCS has been increasingly used in various domains within cognitive neuroscience to establish relationships between activity in a particular brain region and a specific cognitive function.
A series of studies have employed tDCS to examine the involvement of PFC in tasks that require regulation of thought. For example, increasing PFC activity with anodal tDCS lead to improvements in inhibitory control (Hsu et al., 2011), working memory (Boggio et al., 2006), and increased efficiency in task shifting (Leite et al., 2011; see also Dockery et al., 2009), whereas opposing effects of cathodal versus anodal stimulation over left inferior PFC have recently been reported on a feature categorization task (Lupyan et al. 2012).
Guided by these findings and our earlier fMRI results, we hypothesized that application of cathodal stimulation over left PFC should facilitate performance (compared to those in a sham stimulation condition) for the Uncommon Use (UU) task, which benefits from unfiltered, bottom-up information, but not for the Common Use (CU) task, which benefits from PFC-driven, top-down regulation. To examine the regional specificity of this effect, we also included a group of subjects who received cathodal stimulation over right PFC. To control for the possibility that cathodal stimulation may influence performance regardless of the nature of the tasks (e.g., due to differences in arousal, demand characteristics, etc.) we included as a negative control a Forward Digit Span (FDS) task, which is unrelated to PFC function, and performance on which should not have been affected by our particular electrode size and placement (i.e., electrode montage) and stimulation parameters (Tadini et al., 2011). Although electrical field modeling suggests that tDCS may affect a large area of cortex (Datta et al., 2009) and as such it is not sufficient for the exact specification of structure-function relationships, application of tDCS can be useful to examine the nature of the contribution of a given brain region in a task such as the one employed here, wherein there is no a priori hypothesis that a specific subregion within PFC would be associated with behavior. Notwithstanding the absence of fine-grained brain-behavior predictions, here we used confirmatory electrical field modeling to verify that our particular electrode montage resulted in patterns of current flow that would inhibit lateral PFC.
Method
Participants
Forty-eight right-handed, native English speakers (mean age = 23.38 years, 15 males) participated in the study for monetary compensation, after providing informed consent, as approved by the local Institutional Review Board.
Materials
Sixty gray scale pictures of everyday objects (448 × 336 ppi) presented on a gray background were used as experimental stimuli. The images were a subset of normed stimuli employed in a prior study involving the Uses Task (Chrysikou & Thompson-Schill, 2011). Stimuli were presented for 9000ms each, with a 3000ms interstimulus interval, using E-Prime software (Psychology Software Tools, Inc.) on a PC laptop computer. A brief sound marked the presentation onset of each image. Stimulus onset and participants’ vocal responses were captured through a portable microphone attached to participants’ clothing directly under their mouth and connected to a different laptop computer which recorded stimulus onset and responses using Audacity® software. Stimulus order across participants was randomized.
Design & Procedure
Participants were randomly assigned to one of six conditions (with 8 participants in each) depending on the experimental task they had to perform (either the CU or the UU task) and the type of stimulation that they received (cathodal stimulation either over left-PFC or right-PFC, or sham stimulation). Participants were blind to the stimulation condition. We chose a between-subjects design due to the particular characteristics of the Uses Task, namely, the likelihood that participants would inadvertently think of uncommon uses of the objects in the context of the common uses task, and vice versa; in addition, we wanted to maintain consistency with the design of our previous neuroimaging study (Chrysikou & Thompson-Schill, 2011). For the CU task, participants reported aloud the most typical or commonly-encountered use for each object (e.g., Kleenex tissue: use to wipe one’s nose); for the UU task, participants generated a novel use for the object, one they had not seen or attempted before, that would be plausible, yet, which would deviate significantly from the object’s common uses (e.g., Kleenex tissue: use as stuffing in a box). Participants were informed that the tasks had no right or wrong answers and that they should feel free to produce any response they judged fit. They were instructed to respond as quickly as possible and to remain silent if unable to generate a response. Prior to testing, participants received brief training on either task depending on their condition. Each task lasted 12 minutes. For the FDS control task, participants were read 16 increasingly longer number strings and repeated aloud each string exactly in the order the numbers in each were presented. The task was adapted from the Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV, Pearson Education, Inc.). The duration of the Forward Digit Span task was approximately 5 minutes. Task order was randomized across participants and conditions. The entire experimental session lasted 45 minutes.
tDCS Stimulation Parameters
tDCS was administered via two 5cm × 5cm electrodes covered with saline-soaked sponges. The stimulation site was determined by means of a BraiNet 10/20 Placement cap (bio-medical.com) and was marked with a red marker on the participant’s scalp. Guided by the region of maximal differences in our fMRI study, the cathode was placed either over area F7 or over area F8 on the 10/20 system for stimulation of PFC (Homan et al., 1987) depending on the participant’s condition; the anode was placed over the contralateral mastoid (see Fig. 4 for left cathode/right anode montage). tDCS stimulation was applied using an Eldith DC stimulator (Magstim, Ltd.) at 1.5 mA for a maximum of 20 minutes (including 10 seconds ramp-up and 10 seconds ramp-down time). These parameters are within safety limits established from prior work in humans (e.g., Bikson et al., 2009; Nitsche et al., 2003; Tadini et al., 2011). In the stimulation conditions, stimulation began for 180 seconds prior to the onset of either the experimental or the control task; during this interval participants were looking at a blank fixation screen. This period was introduced based on past results suggesting that cortical excitability changes with tDCS are not observed until after 3–5 minutes of stimulation (Nitsche & Paulus, 2000). Participants in the sham conditions were also presented with a blank fixation screen for 180 seconds prior to the onset of either the experimental or the control task; stimulation began for the first 90 seconds of the 180-second interval and then, unbeknownst to the participants, was automatically turned off. The placement of the cathode for the sham conditions (either over F7 or over F8) was counterbalanced across participants.
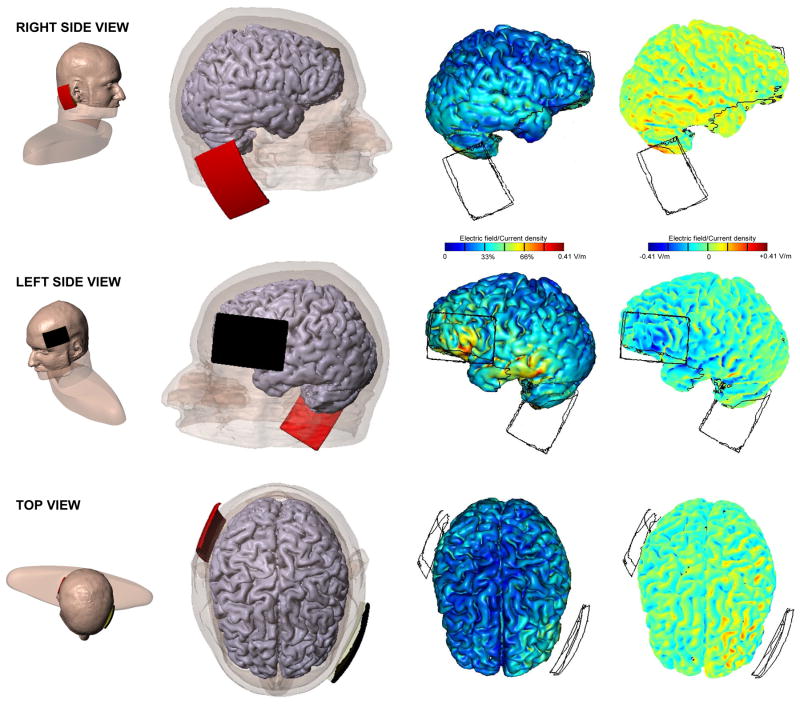
Figure 4.
Modeling of electrical fields induced by tDCS. tDCS stimulation montage and model of current flow for cathodal stimulation over left PFC, with the cathode (depicted in black) over F7 and the anode (depicted in red) over the contralateral mastoid. The ‘mirror,’ reverse configuration was used for cathodal stimulation over right PFC. The third column shows the magnitude of the induced electrical field, which is maximal directly under the electrodes. Points of high current density (peak current flow) are shown in left PFC and left temporal cortex. The fourth column shows the directionality of the current flow, with consistent cathodal (i.e., outward) current flow (depicted in blue) over left PFC (left side view), but inconsistent anodal (i.e., inward) current flow (depicted in red) over right hemisphere (right side view). This pattern of directional current was expected to decrease left PFC excitability. Individual variation in gyral anatomy may result in differences in the precise location of the effect across subjects, but the model serves to confirm the expected effect of this montage on the PFC in general.
This head model was created from a high resolution magnetic resonance image (MRI) of an adult male, and segmented into gray matter, white matter, cerebrospinal fluid (CSF), skull, scalp, eye region, muscle, and air compartments (Custom Segmentation, Soterix Medical, New York, NY). The finite element mesh generated from the segmentation masks was exported to COMSOL Multiphysics 3.5a (Burlington, MA) for computation of electric fields (EF; Datta et al., 2011). To complete the model, a synthetic region was added to replace tissue clipped by the MRI acquisition volume. The following isotropic electrical conductivities (in S/m) were assigned: gray matter: 0.276; white matter: 0.126; CSF: 1.65; skull: 0.01; scalp: 0.465; eye region: 0.4; muscle: 0.334; air: 1e–15; synthetic region: 0.17; sponge: 1.4; electrode: 5.8e7. The Laplace equation was solved and induced cortical EF maps were determined. Cortical EF surface and crosssection magnitude maps for the left cathodal/right anodal electrode montage were determined.
Results
No Effects of Stimulation on Forward Digit Span Performance
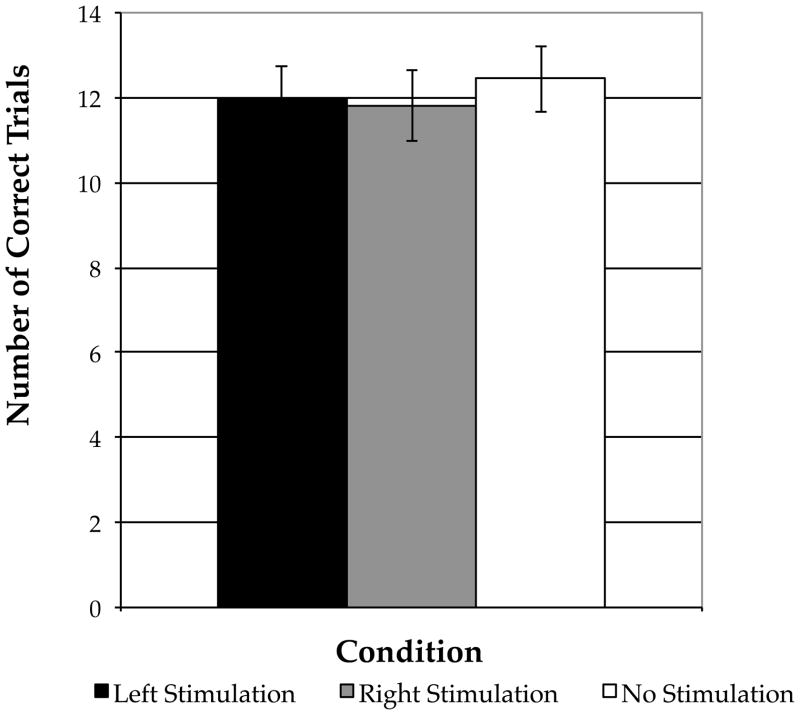
All participants completed the experimental session and no adverse effects were reported. There were no differences in performance on the Forward Digit Span between participants who received the task before and those who received it after the experimental task (p > 0.87), or between participants who received as the experimental task the CU task and those who received as the experimental task the UU task (p > 0.64). Thus, performance on the control task was collapsed across the experimental task conditions. Each participant’s total number of correct responses (out of 16) was entered into one-way Analysis of Variance (ANOVA). There were no effects of stimulation on the Forward Digit Span task across the three stimulation conditions (F[2,42]=0.33, p = 0.72; Fig. 1). Thus, cathodal tDCS stimulation either over left or right PFC did not influence performance on the Forward Digit Span control task.
Figure 1.
Performance on the Forward Digit Span Task by Stimulation Condition. Error bars indicate the standard error of the mean.
Effects of Cathodal Left PFC Stimulation on the Uses Task
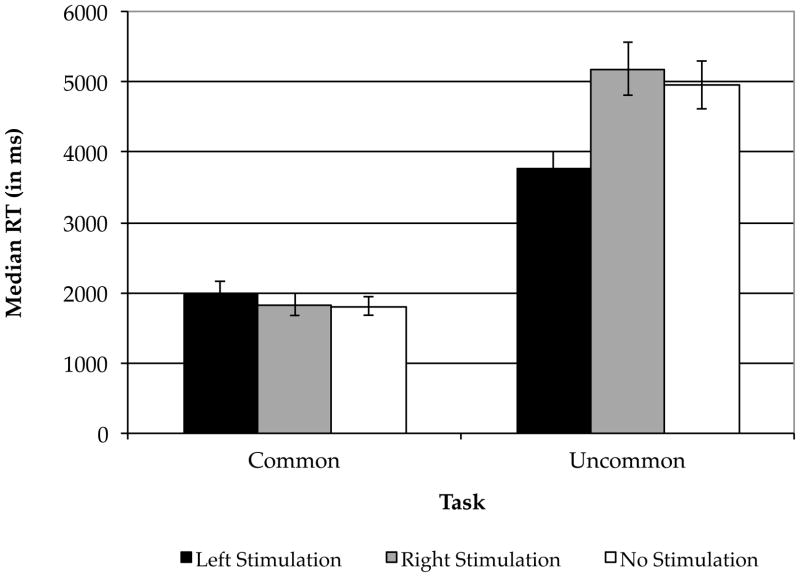
Participants’ responses were transcribed from the audio recordings. Blank responses (see separate analysis below) and answers that did not comply with the experimental instructions (< 1%) were removed from the analysis. Voice-onset reaction times were obtained manually using Audacity® software by an experimenter blind to the participants’ condition. A second experimenter obtained reaction times (RTs) in the same manner from a randomly selected subset of the data (~25%) to verify accuracy (inter-experimenter reliability [Pearson’s r] was over 97%). Following data verification, median RTs were derived for each participant. There were no differences in performance on the Uses Task between participants who received the task before and those who received it after the FDS control task (p > 0.59). Median RTs (Fig. 2) were entered into a 2 (task) × 3 (stimulation type) ANOVA. There was a significant main effect of task (F[1,42]=169.64, p < 0.001) and condition (F[2,42]=3.37, p = 0.04). Critically, the task × condition interaction was significant (F[2,42]=5.55, p = 0.007). For the UU task, two planned orthogonal contrasts revealed that participants who received cathodal tDCS over left PFC generated uncommon uses significantly faster than participants who received cathodal tDCS over right PFC and participants who received sham stimulation (t[22]=3.30, p = 0.003); and, that participants who received cathodal tDCS over right PFC did not generate uncommon uses faster than those who received sham stimulation (t[14]=0.44, p = 0.67). Neither of these contrasts for the CU task was significant (t[22]=0.91, p = 0.37 and t[14]=0.12, p = 0.91, respectively). Overall, these results support the hypothesis that cathodal stimulation over left PFC facilitates performance on a semantic generation task that benefits from unfiltered, bottom-up information.
Figure 2.
Performance on the Uses Task by Stimulation Condition. Error bars indicate the standard error of the mean. ** p < .01
Analysis of Response Omissions
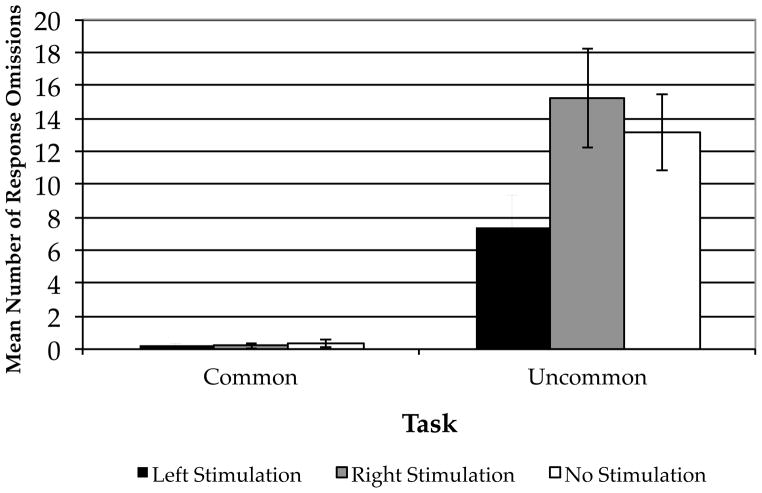
We also assessed whether cathodal stimulation over left PFC would affect the likelihood that participants would generate a response in the time allotted. As with response time, we entered the number of response omissions for each participant in each condition into a 2 (task) × 3 (stimulation type) ANOVA (Fig. 3). There was a significant main effect of task (F[1,42]=65.55, p < 0.001), and there were marginally-significant trends for the main effect of condition (F[2,42]=2.71, p = 0.078) and for the task × condition interaction (F[2,42]=2.66, p = 0.08). Planned orthogonal contrasts revealed that participants who received cathodal tDCS over left PFC omitted fewer responses than did participants who received cathodal tDCS over right PFC and participants who received sham stimulation (t[22]=2.28, p = 0.03); participants who received cathodal tDCS over right PFC did not differ from those who received sham stimulation (t[14]=0.56, p = 0.57). Neither of the planned comparisons for the CU task was significant (t[22]=0.26, p = 0.80 and t[14]=0.40, p = 0.69, respectively). These results suggest that, beyond increasing the speed of generation of uncommon uses, cathodal tDCS over left PFC also reduced the number of response omissions in the UU condition.
Figure 3.
Mean Number of Response Omissions by Stimulation Condition. Error bars indicate the standard error of the mean. * p < .05.
Model of Current Flow
In studies using tDCS, the electrode montage in conjunction with the anatomy of the underlying brain regions, determines the resulting current flow in the brain and, as such, any possible neurophysiogical effects on task performance. Although a growing body of literature has reported significant effects of tDCS in behavior as discussed above, the majority of studies have not incorporated current flow analyses to guide or confirm that a particular montage is associated with the predicted patterns of anodal or cathodal current flow. Computational models using finite element methods (FEM) are standard tools for predicting current flow through the brain during tDCS (Datta et al., 2011). Accordingly, in the present study induced electrical fields were modeled in a single individual (Fig. 4) to verify the mode of polarization under the cathode leading to somatic hyperpolarization/decreased excitability in PFC (Radman et al., 2007).
Consistent with findings using other bipolar montages, tDCS generated a complex pattern of current flow across the brain with clusters of electrical field peaks reflecting the idiosyncratic cortical gyrations. Consistent with our objective, peak current was under the posterior portion of the cathode electrode (PFC), which was the most homogeneous region of consistently unidirectional, soma hyperpolarizing current flow. A second current peak in the temporal cortex was more heterogeneous and bidirectional, and therefore less likely to drive consistent resting membrane potential hyperpolarization in that region. The use of a mastoid anode resulted in diffused inward current flow across the right hemisphere and also superior to the cathode electrode, but there was no uniformly inward region comparable to that observed under the cathode (Fig. 4). The model thus confirmed that the selected montage produces dominantly outward current flow in PFC, with diffuse bi-directional current flow in other regions between the electrodes.
Discussion
We used tDCS to examine the hypothesis that certain tasks involving flexible thought may benefit from reduced PFC regulation, and a focus, instead, on unfiltered bottom-up information. As predicted, cathodal tDCS over left PFC, relative to cathodal tDCS over right PFC and sham stimulation, lead to significant improvements in the total number and the speed in which participants generated uncommon uses, but not typical uses, for everyday objects. Furthermore, tDCS did not alter performance on the FDS control task. Our results offer support for the hypothesis that a hypofrontal cognitive state—and consequent lack of top-down regulatory filtering—may benefit performance in tasks that require availability of unfiltered, low-level information.
Our computational model confirmed that our particular electrode montage lead to a pattern of current flow concentrated in the stimulated hemisphere. Although the exact manner in which tDCS influences cortical excitability is currently unknown, based on the model’s output we hypothesize that cathodal stimulation may have led to PFC hypofunction, which selectively facilitated performance on the UU task for the left PFC stimulation condition. This interpretation is in line with past findings suggesting benefits to creative cognition under hypofrontal cognitive states (e.g., Mölle et al., 1999; Limb & Braun, 2008).
Our findings do not indicate that all aspects of behavior that could be characterized as creative benefit from a state of reduced cognitive control. Flexible idea generation can benefit from availability of unfiltered information (as we have just shown), but creative thought may also depend on access to remote associations that have been associated with right-hemisphere functions (Kounios & Beeman, 2009) or the evaluation and selection of an optimal idea when implementing a particular goal. Recent evidence in the neural stimulation literature supports this point. For example, cathodal tDCS over the left anterior temporal lobe but with concurrent anodal tDCS over the right anterior temporal lobe lead to performance benefits in a matchstick arithmetic task that involves violations of rule-based thought (Chi & Snyder, 2011). Similarly, anodal stimulation over left PFC, but not cathodal or sham stimulation, lead to improvements in performance in a remote associates task (RAT) that incorporates a creative component and, critically, convergence to a single correct response unlike our open-ended task (Cerruti & Schlaug, 2008)1. Studies such as these, and the present one, illustrate the need to better characterize the component processes that contribute to behaviors that are conveniently (but perhaps not usefully) lumped together as examples of “creativity.”
The application of cathodal tDCS over PFC that was employed in this study was guided by past research on the possible benefits of hypofrontality in a variety of tasks, as well as our own fMRI findings on the Uses Task. Whether or not anodal tDCS over left PFC would lead to the reverse effects, namely performance deficits in the Uses Task and any other tasks that depend on availability of unfiltered data (Ambrus et al., 2011), remains an open question. Furthermore, in this experiment we chose to increase the availability of object information (e.g., shape, material) by using pictorial stimuli, which may have strengthened our observed effect; thus, it is possible that stimulus modality (i.e., verbal or pictorial) can moderate the outcome of tDCS. Finally, in this paradigm the application of tDCS was concurrent with the experimental tasks, and it is possible that there are differences in performance with different timing parameters, including application of tDCS prior to testing (Stagg et al., 2011; Zheng et al., 2011). Notwithstanding these remaining questions, the present experiment is the first to demonstrate that alterations in PFC function by means of cathodal tDCS over left PFC can be associated with the availability of low-level, bottom-up information in a task involving flexible object use.
Acknowledgments
This research was supported by NIH grant R21-MH083029 to S.T.S, a grant through the Robert Wood Johnson Foundation Amos Medical Faculty Development Program to R.H.H. The City University of New York has patents on brain stimulation with M.B. and A.D. as inventors. M.A. and A.D. have equity in Soterix Medical Inc. A.D. is CTRO of Soterix Medical.
Footnotes
The current flow model revealed an unpredicted, secondary current peak, albeit not uniform, in lateral temporal cortex that may, in part, account for the magnitude of the observed effect on RTs under left PFC stimulation. Specifically, cathodal tDCS over left PFC may have inhibited PFC-guided tendencies to organize knowledge according to abstract, categorical rules. On the other hand, the effects of cathodal tDCS in lateral temporal cortex may have inhibited access to stored categorical knowledge per se, which has been associated with activity in this region in past research. For example, in an fMRI adaptation paradigm it has been shown that activation in posterior lateral temporal cortex is associated with sematic retrieval, whereas activation in left vetrolateral PFC is associated with semantic ambiguity (Bedny et al., 2008). Limited access to stored categorical knowledge through left cathodal tDCS may have lead to accumulated benefits for the UU task, performance in which depends on the temporary distancing from exactly this type of well-established knowledge of object function that is associated with activity in left lateral temporal cortex. Although it is difficult in the present paradigm to isolate the independent contributions of left PFC and temporal cortex to the observed effects, decreased function in these regions is consistent with the observed facilitation in performance in the UU task.
References
- Ambrus GG, Zimmer M, Kincses ZT, Harza I, Kovács G, Paulus W, Antal A. The enhancement of cortical excitability over the DLPFC before and during training impairs categorization in the prototype distortion task. Neuropsychologia. 2011;49:1974–1980. doi: 10.1016/j.neuropsychologia.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Barde LHF, Schwartz MF, Chrysikou EG, Thompson-Schill SL. Reduced short-term memory span in aphasia and susceptibility to interference: Contribution of material-specific maintenance deficits. Neuropsychologia. 2010;48:909–920. doi: 10.1016/j.neuropsychologia.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, McGill M, Thompson-Schill SL. Semantic adaptation and competition during word comprehension. Cerebral Cortex. 2008;18:2574–2585. doi: 10.1093/cercor/bhn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, Fregni F. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249:31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiology. 2009;120:1033–1034. doi: 10.1016/j.clinph.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerruti C, Schlaug G. Anodal transcranial direct current stimulation of the prefronal cortex enhances complex associative thought. Journal of Cognitive Neuroscience. 2008;21:1980–1987. doi: 10.1162/jocn.2008.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi RP, Snyder AW. Facilitate insight by non-invasive brain stimulation. PLoS One. 2011;2:e16655. doi: 10.1371/journal.pone.0016655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Thompson-Schill SL. Dissociable brains states linked to common and creative object use. Human Brain Mapping. 2011;32:665–675. doi: 10.1002/hbm.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–207. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Baker JM, Bikson M, Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimulation. 2011;4:169–194. doi: 10.1016/j.brs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery CA, Hueckel-Weng R, Birbaumer N, Plewnia C. Enhancement of planning ability by transcranial direct current stimulation. J Neurosci. 2009;29:7271–7277. doi: 10.1523/JNEUROSCI.0065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German TP, Defeyter MA. Immunity to functional fixedness in young children. Psychonomic Bulletin & Review. 2000;7:707–712. doi: 10.3758/bf03213010. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett HB. Hemispheric mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain & Language. 2011;118:40–50. doi: 10.1016/j.bandl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton P, Ludlow A, Roberson D. When less is more: poor discrimination but good colour memory in Autism. Research in Autism Spectrum Disorders. 2008;2:147–156. [Google Scholar]
- Homan RW, Herman J, Purdy P. Cerebral location of international 10–20 system electrode placement. Electroencephalography and Clinical Neurophysiology. 1987;66:376–382. doi: 10.1016/0013-4694(87)90206-9. [DOI] [PubMed] [Google Scholar]
- Hsu TY, Tseng LY, Yu JX, Kuo WJ, Hung DL, Tzeng OJL, Javadi AH, Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimulation 2011 [Google Scholar]
- Kounios J, Jung-Beeman M. Aha! The cognitive neuroscience of insight. Current Directions in Psychological Science. 2009;18:210–216. [Google Scholar]
- Leite J, Carvalho S, Fregni F, Gonçalves ÓF. Task-specific effects of tDCS-induced cortical excitability changes on cognitive and motor sequence set shifting performance. PLoS ONE. 2011;6:e24140. doi: 10.1371/journal.pone.0024140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb CJ, Braun AR. Neural substrates of spontaneous musical performance: An fMRI study of jazz improvisation. PLoS ONE. 2008;32:e1679. doi: 10.1371/journal.pone.0001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupyan G, Mirman D, Hamilton R, Thompson-Schill SL. Categorization is modulated by transcranial direct current stimulation over left prefrontal cortex. Cognition. 2012;124:36–49. doi: 10.1016/j.cognition.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Wolf B, Fehm HL, Born J. EEG complexity and performance measures of creative thinking. Psychophysiology. 1999;36:95–104. doi: 10.1017/s0048577299961619. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Cohen L, Wassermann E, Priori A, Lang N, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiology. 2003;114:2220–2222. doi: 10.1016/s1388-2457(03)00235-9. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman T, Su Y, An JH, Parra LC, Bikson M. Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J Neurosci. 2007;27:3030–3036. doi: 10.1523/JNEUROSCI.0095-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini ML, Foti D, Mackenzie IR, Miller BL. Unravelling Boléro: Progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131:39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. The role of the prefrontal cortex in dynamic filtering. Psychobiology. 2000;28:207–218. [Google Scholar]
- Snyder A. Explaining and inducing savant skills: Privileged access to lower level, less-processed information. Philosophical Transactions of the Royal Society B. 2009;364:1399–1405. doi: 10.1098/rstb.2008.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. The Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011;49:800–804. doi: 10.1016/j.neuropsychologia.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadini L, El-Nazer R, Brunoni AR, et al. Cognitive, mood, and electroencephalographic effects of noninvasive cortical stimulation with weak electrical currents. J ECT. 2011;27:134–140. doi: 10.1097/YCT.0b013e3181e631a8. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left prefrontal cortex in retrieval of semantic knowledge: A re-evaluation. Proceedings of the National Academy of Sciences USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Ramscar M, Chrysikou EG. Cognition without control: When a little frontal lobe goes a long way. Current Directions in Psychological Science. 2009;18:259–263. doi: 10.1111/j.1467-8721.2009.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V, Muggleton NG, Juan CH. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage. 2011;56:2249– 2257. doi: 10.1016/j.neuroimage.2011.03.059. [DOI] [PubMed] [Google Scholar]
- Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58:26–33. doi: 10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]