Abstract
OBJECTIVE:
To characterize and identify determinants of HIV disease progression among a predominantly injection drug use (IDU) HIV population in the highly active antiretroviral therapy era.
METHODS:
The present retrospective study was based on 343 HIV patients diagnosed from 2005 to 2010 from two clinics in Saskatoon, Saskatchewan. Disease progression was defined as the time from diagnosis to immunological AIDS (CD4 count <200 cells/μL) and death. Uni- and multivariable Cox proportional hazards models were used.
RESULTS:
Of the 343 patients, 79% had a history of IDU, 77% were hepatitis C virus (HCV) coinfected and 67% were of Aboriginal descent. The one-year and three-year immunological AIDS-free probabilities were 78% and 53%, respectively. The one-year and three-year survival probabilities were 97% and 88%, respectively. Multicollinearity among IDU, HCV and ethnicity was observed and, thus, separate models were built. HCV coinfection (HR 2.9 [95% CI 1.2 to 6.9]) was a significant predictor of progression to immunological AIDS when controlling for baseline CD4 counts, treatment, age at diagnosis and year of diagnosis. For survival, only treatment use was a significant predictor (HR 0.34 [95% CI 0.1 to 0.8]). HCV coinfection was marginally significant (P=0.067).
CONCLUSION:
Baseline CD4 count, HCV coinfection, year of diagnosis and treatment use were significant predictors of disease progression. This highlights the importance of early treatment and the need for targeted interventions for these particularly vulnerable populations to slow disease progression.
Keywords: Aboriginal ethnicity, Disease progression, Hepatitis C coinfection, HIV/AIDS, Injection drug use, Survival
Abstract
OBJECTIF :
Caractériser et établir les déterminants de la progression du VIH dans une population atteinte du VIH surtout composée d’utilisateurs de drogues injectables (UDI) à une époque d’antirétrovirothérapie très active.
MÉTHODOLOGIE :
La présente étude rétrospective s’est fondée sur 343 patients atteints du VIH diagnostiqués entre 2005 et 2010 dans deux cliniques de Saskatoon, en Saskatchewan. La progression de la maladie était définie comme le moment du diagnostic jusqu’à l’apparition du sida immunologique (numération de CD4 inférieure à 200 cellules/μL), puis jusqu’au décès. Les chercheurs ont utilisé le modèle univariable et multivariable de risques proportionnels de Cox.
RÉSULTATS :
Sur les 343 patients, 79 % avaient déjà été UDI, 77 % étaient co-infectés par le virus de l’hépatite C (VHC) et 67 % étaient d’origine autochtone. La probabilité de non-apparition de sida immunologique au bout d’un an et de trois ans correspondait à 78 % et à 53 %, respectivement. La probabilité de survie au bout d’un an et de trois ans s’élevait à 97 % et à 88 %, respectivement. Les chercheurs ont observé une multicolinéarité entre les UDI, le VHC et l’ethnie et ont donc préparé des modèles différents. La co-infection par le VHC (RC 2,9 [95 % IC 1,2 à 6,9]) était un prédicteur important de progression en sida immunologique lorsqu’on contrôlait l’effet de la numération de CD4 de référence, du traitement, de l’âge au diagnostic et de l’année de diagnostic. Seule l’utilisation du traitement était une prédicteur important de la survie (RC 0,34 [95 % IC 0,1 à 0,8]). La co-infection par le VHC avait peu d’importance (P=0,067).
CONCLUSION:
La numération de CD4 de référence, la co-infection par le VHC, l’année de diagnostic et l’utilisation d’un traitement étaient des prédicteurs importants de progression de la maladie. Ces constatations font ressortir l’importance d’un traitement rapide et la nécessité de procéder à des interventions ciblées pour ces populations particulièrement vulnérables afin de ralentir la progression de la maladie.
HIV disease progression is the result of a complex interplay among viral, host and environmental factors. HIV disease progression is a continuum of progressive damage to the immune system that advances to severe immunological damage defined as AIDS, which, if left untreated, leads to death. Highly active antiretroviral therapy (HAART) has significantly altered HIV disease progression by reducing the incidence of AIDS and death (1–3). However, while HAART is now widely available in most developed countries, the benefits of HAART have not been uniformly distributed.
Disparities in disease progression have been noted among ethnic groups in numerous countries. For instance, an American study found that among AIDS-diagnosed individuals, the HR for survival increased from 1.18 to 1.51, a 33% increase, after the introduction of HAART (1993 to 1995 versus 1996 to 2001) for non-Hispanic black individuals compared with white (4). The authors highlighted that the increased risk was a result of the decreased progression rate in white individuals due to HAART. Similarly, a study from British Columbia found that, following HAART initiation, Aboriginal persons had all-cause mortality rates 3.12 times higher than that of non-Aboriginals, even after controlling for adherence (5).
These health disparities have also been evident among injection drug users (IDUs), when compared with men who have sex with men (MSM). In a Spanish seroconversion cohort, it was noted that in 1998 to 1999 the relative risk (RR) of AIDS did not significantly differ from 1992 to 1995 for IDU (RR 0.72 [95% CI 0.44 to 1.16]), while an 89% reduction (RR 0.11 [95% CI 0.02 to 0.49]) was noted among MSM (6). A composition of 22 cohorts from Australia, Europe and Canada similarly showed that compared with pre-1997 data, IDUs had significantly higher mortality in 1999 to 2001 compared with MSM (HR 4.28 [95% CI 2.86 to 6.41]) (7).
Thus, acknowledging the important difference between efficacy and effectiveness, the present study aimed to characterize HIV disease progression following HIV diagnosis in the HAART era among an HIV population that is comprised predominantly of IDU and those of Aboriginal ethnicity. Complementing efficacy studies, the present study provides an indirect measure of the accessibility and utilization of services and adherence with medication (8). Moreover, knowledge on the rate of disease progression following HIV diagnosis enables informed decision making on resource allocation, prevention interventions and treatment efforts. Finally, the identification of clinical features and characteristics associated with a more rapid progression can help identify patients who may benefit from a closer and more frequent clinical follow-up.
METHODS
Setting and population
The present study was based on a Canadian province (Saskatchewan) that has experienced a consistent rise in the incidence of HIV, from 3.3 per 100,000 population in 2002 to 20.8 per 100,000 population in 2008; this is the highest in Canada and more than twice the national average (9). The Positive Living Program and the Westside Community Clinic are the only two sites in Saskatoon that provide specialized HIV/AIDS care. The Positive Living Program, located at the Royal University Hospital (Saskatoon, Saskatchewan), serves adults and children with HIV and/or hepatitis C virus (HCV) who reside in Central and Northern Saskatchewan. The Westside Community Clinic serves the core residents of Saskatoon who are primarily marginalized populations. This community clinic has gained skills and expertise in HIV/AIDS care due to the growing demand from its clients.
Data collection
Data were extracted from medical charts of patients who made ≥1 clinic visits at either one of two sites specializing in HIV/AIDS care in Saskatoon. HIV-positive patients ≥18 years of age, diagnosed between January 1, 2005, and December 31, 2010, were eligible for inclusion in the study. Data included demographics and clinical information such as age, sex, ethnicity, date of HIV diagnosis, HIV exposure categories (IDU, MSM, etc), medical history, comorbidities, HIV treatment, CD4 cell counts, CD4 percentages and viral load data.
The independent variables of interest were categorized as follows: IDU was categorized as a history of injection drug use or no injection drug use; HCV coinfection was based on an HCV antibody-positive test; ethnicity was categorized as self-identified Aboriginal ethnicity and non-Aboriginal ethnicity; baseline CD4 count was defined as the first CD4 count measure within six months of diagnosis; and treatment use was categorized as ever undergoing antiretroviral therapy (ART) versus never undergoing ART during follow-up.
Disease progression was defined as the time from HIV diagnosis to immunological AIDS (CD4 count <200 cells/μL) and death. For time to immunological AIDS, patients were censored at their last CD4 count measure. Patients with a concurrent immunological AIDS diagnosis (CD4 count <200 cells/μL within one month of HIV diagnosis) were excluded from the immunological AIDS analysis. For survival analysis, patients were censored at their final clinic visit before December 31, 2010.
Analysis
Descriptive statistics were performed to summarize the data. Pearson correlation analysis was performed. The Kaplan-Meier method (10) was used to estimate the survival probability of time to immunological AIDS and death. Univariate and multivariable Cox proportional hazards models (11) were used to determine independent predictors of disease progression. Only fixed covariates were included in the model. The fixed covariates were baseline demographics, history of IDU, HCV coinfection, ever undergoing ART and baseline CD4 counts. Based on the literature, potential confounders assessed in the multivariable models included age, sex, year of diagnosis and treatment use. Collinearity and interaction among covariates was verified. Models’ goodness of fit was also checked. A significance level of 0.05 was used. All data analyses were performed using SAS version 9.2 (SAS Institute Inc, USA).
The study was approved by the University of Saskatchewan Ethics Review Board.
RESULTS
There were a total of 343 adult (≥18 years of age) HIV-positive patients who met the inclusion criteria. One hundred eighty-seven patients (55%) were from the Positive Living Program, 84 (25%) patients were from the Westside Community Clinic and an additional 72 (21%) attended both clinics. Of these, 177 were male (52%). The mean (± SE) age of the population was 35±0.6 years at diagnosis.
Self-reported Aboriginal ethnicity represented 230 (67%) of all patients. These individuals represented First Nations (89%) and Métis (11%). The remaining non-Aboriginals were comprised of 79 (23%) Caucasians and 12 (4%) other ethnicities; 22 (6.4%) were of unknown ethnicity.
A history of IDU was reported by 272 (79%) patients. Of the 343 patients with an antibody test, HCV antibodies were present in 264 (77%) patients. Nine patients (2.6%) had no laboratory evidence of HCV antibody test. Table 1 summarizes the study population characteristics.
TABLE 1.
Study population characteristics (n=343)
| Characteristic | |
|---|---|
| Sex | |
| Male | 177 (51.6) |
| Female | 166 (48.4) |
| Ethnicity | |
| Aboriginal | 230 (67.1) |
| Caucasian | 79 (23.0) |
| Other | 12 (3.5) |
| Unknown | 22 (6.4) |
| Age at diagnosis, years | |
| <20 | 15 (4.4) |
| 20–29 | 108 (31.5) |
| 30–39 | 108 (31.5) |
| 40–49 | 80 (23.3) |
| ≥50 | 32 (9.3) |
| Year of diagnosis | |
| 2005 | 54 (15.7) |
| 2006 | 41 (12.0) |
| 2007 | 55 (16.0) |
| 2008 | 74 (21.6) |
| 2009 | 88 (25.7) |
| 2010 | 31 (9.0) |
| Site of care | |
| Positive Living Program | 187 (54.5) |
| Westside Community Clinic | 84 (24.5) |
| Both | 72 (21.0) |
| History of injection drug use | |
| Yes | 272 (79.3) |
| No | 64 (18.7) |
| Unknown | 7 (2.0) |
| Hepatitis C virus antibodies | |
| Present | 264 (77.0) |
| Absent | 70 (20.4) |
| Unknown | 9 (2.6) |
| Ever on antiretroviral therapy | |
| Yes | 167 (48.7) |
| No | 123 (35.9) |
| Unknown | 53 (15.5) |
| Baseline CD4 counts*, cells/μL | |
| <200 | 53 (15.5) |
| 200–349 | 74 (21.6) |
| ≥350 | 126 (36.7) |
| Unknown | 90 (26.2) |
| Immunological AIDS | |
| Yes | 132 (38.5) |
| No | 192 (56.0) |
| Unknown | 19 (5.5) |
| Deaths | 23 (6.7) |
| Age at diagnosis, years, mean ± SE | 35.1±0.6 |
| Baseline CD4 count*, cells/μL, mean ± SE | 382.1±14.4 |
| Baseline viral load log10, mean ± SE | 4.38±0.1 |
Data presented as n (%) unless otherwise specified.
Baseline defined as the first measure within six months of HIV diagnosis
The mean (± SE) baseline CD4 count was 382±14 cells/μL. The mean log viral load was 4.38±0.1. During follow-up, 58% of cases were undergoing ART. Among patients with a CD4 count <350 cells/μL at any point during follow-up (ie, eligible for treatment), 71% were on ART (data not shown).
There was high correlation between IDU and HCV (Pearson’s χ2=226.96; P<0.001), IDU and Aboriginal ethnicity (Pearson’s χ2=91.18; P<0.001) and HCV and Aboriginal ethnicity (Pearson’s χ2=66.02; P<0.001). To further illustrate this point, among those that reported a history of IDU, 83% were of Aboriginal descent and 95% were HCV coinfected. Among those HCV coinfected, 98% reported a history of IDU.
HIV diagnosis to immunological AIDS
Nineteen patients had no CD4 count measures, and an additional 45 patients had a CD4 count <200 cells/μL within one month of HIV diagnosis and were thus excluded from the analysis. Of the remaining 279, during the study time, 101 (36%) developed immunological AIDS.
The median follow-up time to immunological AIDS event was 1.3 years. Following HIV diagnosis, the one-year and three-year immunological AIDS-free probability was 77.8% (95% CI 72.1 to 82.5) and 53% (95% CI 44.8% to 60.9%), respectively (Figure 1).
Figure 1).
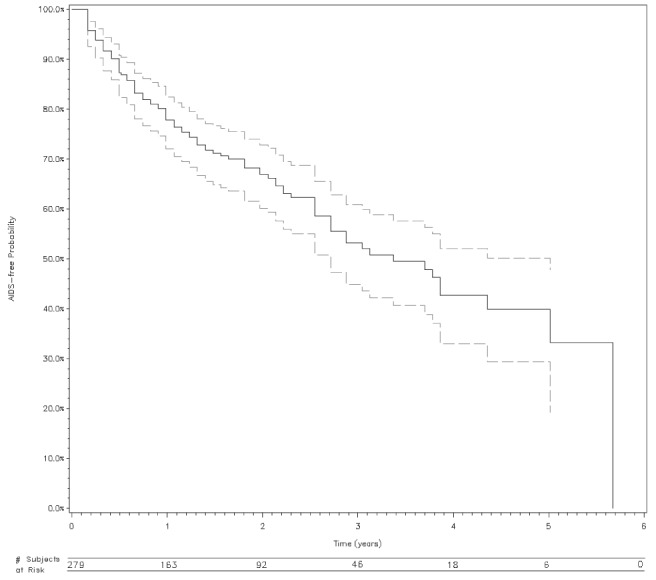
Survival probability (with 95% CI and number of subjects at risk) of immunological AIDS from HIV diagnosis
In the univariable analysis for time to immunological AIDS, year of diagnosis, site of care, ever recipient of ART, CD4 count and viral load at baseline were significant predictors, while HCV coinfection, history of IDU and ethnicity were not (Table 2). The two latter years were found to be significantly associated with progression, while the earlier years were not and, thus, this variable was dichotomized into 2005 to 2008 and 2009 to 2010.
TABLE 2.
Univariable Cox regression analysis for time to immunological AIDS and death
| Immunological AIDS, HR (95%CI) | Death, HR (95%CI) | |
|---|---|---|
| Sex | ||
| Female | 1 | 1 |
| Male | 1.30 (0.87–1.91) | 0.99 (0.43–2.29) |
| Ethnicity | ||
| Non-Aboriginal | 1 | 1 |
| Aboriginal | 1.41 (0.88–2.26) | 0.90 (0.36–2.24) |
| Age at diagnosis | 1.02 (1.00–1.04) | 1.03 (0.99–1.07) |
| Year of diagnosis | ||
| 2005 | 1 | 1 |
| 2006 | 1.48 (0.76– 2.88) | 2.09 (0.65– 6.72) |
| 2007 | 1.28 (0.63– 2.62) | 2.38 (0.58– 9.81) |
| 2008 | 1.77 (0.87– 3.59) | 3.02 (0.54– 16.88) |
| 2009 | 5.77 (2.83–11.78) | 4.28 (0.58– 31.84) |
| 2010 | 14.97 (4.93– 45.45) | 48.65 (4.88– 484.63) |
| Year of diagnosis | ||
| 2005–2008 | 1 | 1 |
| 2009–2010 | 4.48 (2.67–7.52) | 3.26 (0.83–12.80) |
| Site of care | ||
| Positive Living Program | 1.00 | 1.00 |
| Westside Community Clinic | 1.81 (1.09–3.03) | 0.68 (0.15–3.04) |
| Both | 1.02 (0.61–1.70) | 0.33 (0.08–1.41) |
| History of injection drug use | ||
| No | 1 | 1 |
| Yes | 1.25 (0.74–2.11) | 5.37 (0.76–42.24) |
| Hepatitis C virus antibodies | ||
| No | 1 | 1 |
| Yes | 1.52 (0.90–2.58) | 6.51 (0.88–48.39) |
| Ever on antiretroviral therapy | ||
| No | 1 | 1 |
| Yes | 2.25 (1.37–3.69) | 0.34 (0.14–0.82) |
| Baseline viral load log10, mean ± SE | 1.71 (1.31–2.25) | 1.82 (1.01– 3.29) |
| Baseline CD4 counts* | 0.91 (0.89–0.92) | 1.01 (0.98–1.03) |
Within six months of HIV diagnosis, per every 10-unit increase for CD4 count
In the multivariable analysis, separate models were built for the three variables of interest due to the high collinearity among Aboriginal ethnicity, IDU and HCV infection. In model 1, Aboriginal ethnicity was not found to be a significantly associated with progression to immunological AIDS, when controlling for age at diagnosis, treatment use, year of diagnosis and baseline CD4 count. In model 2, HCV coinfection was found to be associated with an increased risk of progression to immunological AIDS (HR 2.1 [95% CI 1.1 to 4.4]) when controlling for the same variables as mentioned above. In model 3, history of IDU approached significance (P=0.071). In all three models, treatment use, year of diagnosis and baseline CD4 count were observed to be commonly significant. There was no interaction observed in each model (Table 3).
TABLE 3.
Three separate multivariable Cox regression analysis for time to immunological AIDS HR (95% CI)
|
HR (95% CI)
|
|||
|---|---|---|---|
| Ethnicity (model 1) | HCV coinfection (model 2) | History of IDU (model 3) | |
| Age at diagnosis | 1.01 (0.98–1.03) | 1.01 (0.98–1.03) | 1.01 (0.98–1.03) |
| Ever on ART | |||
| No | 1 | 1 | 1 |
| Yes | 1.95 (0.94–4.06) | 2.24 (1.07–4.67) | 2.23 (1.06–4.66) |
| Year of diagnosis | |||
| 2005–2008 | 1 | 1 | 1 |
| 2009–2010 | 2.46 (1.20–5.07) | 2.91 (1.43–5.91) | 2.75 (1.36–5.57) |
| Site of care | |||
| PLP | 1 | 1 | 1 |
| WSC | 1.29 (0.51–3.25) | 0.94 (0.38–2.33) | 0.92 (0.36–2.35) |
| Both | 1.31 (0.63–2.76) | 1.22 (0.62–2.42) | 1.25 (0.63–2.48) |
| Baseline CD4 count* | 0.92 (0.90–0.94) | 0.92 (0.90–0.94) | 0.92 (0.90–0.94) |
| Ethnicity | |||
| Non-Aboriginal | 1 | – | – |
| Aboriginal | 1.29 (0.62–2.66) | – | – |
| Hepatitis C virus antibodies | |||
| Absent | – | 1 | – |
| Present | – | 2.13 (1.04–4.38) | – |
| History of IDU | |||
| No | – | – | 1 |
| Yes | – | – | 1.99 (0.93–3.92) |
Within six months of HIV diagnosis, per every 10-unit increase in CD4 count. ART Antiretroviral therapy; HCV Hepatitis C virus; IDU Injection drug use; PLP Positive Living Program; WSC Westside Community Clinic
Further analysis of year of diagnosis showed a few significant differences. A higher percentage of cases came from Westside Community Clinic in the latter years (49% in 2009 to 2010 versus 12% in 2005 to 2008) and ever being on ART was lower in the latter years (43% versus 64%, χ2 P=0.001). Moreover, among those eligible for ART (CD4 count <350 cells/μL), a declining proportion of cases was recorded as being on ART (2005, 87.5%; 2006, 76.8%; 2007, 70.0%; 2008, 70.2%; 2009, 64.6%; 2010, 33.3%). The mean log viral load was also significantly higher in 2009 to 2010 compared with 2005 to 2008 (4.3 versus 4.6, P=0.02), while CD4 count approached significance. No differences were noted in age at diagnosis, IDU or HCV coinfection. The addition of viral load into the multivariate model did not alter the significance of year of diagnosis and was itself not significant; therefore, it was not included in the final models.
HIV diagnosis to death
Of the 343 patients, 23 (7%) died during follow-up. Cause of death was non-HIV-related for nine (39%) patients, HIV-related for six (26%) patients and unknown for eight (35%) patients.
The median follow-up time for survival was 1.7 years. The one-year and three-year survival probability was 98% (95% CI 95% to 99%) and 88% (95% CI 82% to 93%), respectively (Figure 2).
Figure 2).

Survival probability (with 95% CI and number of subjects at risk) for all-cause mortality from HIV diagnosis
Univariate Cox regression analysis for survival time is summarized in Table 2. These results show that treatment was the only significant predictor of survival (HR 0.34 [95% CI 0.1 to 0.8]). In the multivariable analysis, after controlling for treatment, HCV coinfection was marginally significant (P=0.067), while a history of IDU or ethnicity were not found to be significant (data not shown).
DISCUSSION
The present study was based on a retrospective chart review of 343 HIV-positive patients receiving HIV/AIDS care in Saskatoon, Saskatchewan. It characterized HIV disease progression among this study population and identified factors associated with disease progression to immunological AIDS and death.
Our findings show continued and unacceptable progression of HIV disease. There was a 25% probability of reaching immunological AIDS (ie, CD4 count <200 cells/μL) within one year of diagnosis, which rose to 50% by three years. The findings are similar to that reported among HIV-positive IDUs in the United States (49% probability of progression to AIDS, defined as an AIDS-defining illness or CD4 count <200 cells/μL, within three years of HIV diagnosis [12]). Progression to death was also very similar (three-year survival probability of 86% compared with 89% in our study) (12). In contrast, the MSM population had a 20% higher AIDS-free probability in the same three-year time frame (13).
Progression of HIV disease is concerning and likely attributed to issues with access, engagement and retention into care, and subsequently treatment initiation and adherence. A survey administered among IDUs in Saskatoon found 46% of participants reported not accessing a health care centre even when they believed they should; discrimination was the most commonly reported reason for not seeking care (14). Addiction, highly prevalent in this population, further compounds the problem by introducing issues with readiness and adherence to treatment (15). Issues such as criminal sanctions, low self-efficacy, addiction-related instability and provider reluctance to prescribe treatment have been previously identified among IDUs (15). Underlying these issues are social determinants of health such as low education, homelessness and poverty, which were common among this study population and IDUs in the province (16). The authors would also argue that Aboriginal-specific factors, such as intergenerational trauma, abuse and potentially high mobility, play a role in and interacts with these other factors, resulting in these poor outcomes.
While the various determinants of progression to immunological AIDS identified in the present study were previously established, a few were unexpected. As expected, baseline CD4 count was the most important predictor of progression to immunological AIDS, a finding consistent with other studies (17,18). Similar to other studies (19,20), our analysis showed HCV coinfection was associated with faster progression, independent of baseline CD4 count. HIV treatment is complex, particularly in HCV-infected patients, and it is possible that delays in and difficulties with HAART may explain these differences. Ninety-eight per cent of HCV-coinfected patients reported a history of IDU, further suggesting disparities in receipt of, and/or adherence to, treatment (16,21). Individuals in the present study reported multiple highly correlated risk behaviours, making it difficult to distinguish between direct and indirect effects. While it may appear to be contradictory, a positive association between treatment and disease progression was observed in our study, as in other studies (22,23), due to the preferential prescription of ART for persons with advanced disease (24). To draw conclusions regarding the association of ART use and immunological parameters, the nonrandom allocation of treatment would need to be accounted for. The association with year of diagnosis was not clearly understood. Lower CD4 counts at diagnosis and reduced treatment use for cases diagnosed were observed in the latter years compared with 2005 to 2008. Moreover, a higher proportion of cases came from the Westside Community Clinic, which serves a more marginalized population. Year of diagnosis could be a surrogate marker for these other variables.
ART use was the only significant predictor of survival, highlighting the large benefits of HIV treatment. The present study did not have access to vital statistics and, therefore, mortality data were only representative of the information held within the patients’ medical charts, which likely resulted in an under-representation of deaths.
There were several limitations to our study. First, this was a retrospective cohort, limiting the availability and quality of data collected. Second, the use of HIV diagnosis time may introduce a substantial amount of noise, resulting in the attenuation of coefficients toward the null. If the time to diagnosis depends on factors such as sex or socioeconomic status, there is also the potential for systemic bias. Advanced statistical analysis and the use of a cohort with known dates of HIV infection and time-dependent covariates are needed to confirm the determinants of disease progression identified in the present study. Third, the present study only included HIV-positive individuals in care. This has likely biased toward a more stable study population and could have biased against rapid progressors. Moreover, rapid progressors may have been excluded from the first analysis, time from HIV diagnosis to immunological AIDS, because they were more likely to be diagnosed with a CD4 count <200 cells/μL.
Our study population corresponds to a representation of 47% of all HIV diagnoses in Saskatchewan from 2005 to 2010. While the results of the present study cannot be generalized to the entire province, it does represent much of the HIV-positive population in care in Saskatoon.
CONCLUSION
HIV disease progression among this predominantly IDU HIV population is concerning. HCV coinfection, when controlling for other variables, was associated with disease progression. Treatment was the only predictive variable for survival. The present study highlights the need for targeted interventions for these particularly vulnerable populations to slow disease progression and maximize the benefits of HAART. For instance, avenues of increasing treatment uptake and adherence, including evidence-based strategies coupling treatment with opioid substitution therapies and direct administered treatment programs (16,21), need to be further explored in this setting. Addiction management, along with outreach and case management, are essential to improve and sustain engagement. The need to address the underlying social determinants of health (eg, housing, poverty) are important to reduce disease progression and prevent the spread of HIV. Finally, further research is required to support these findings and continue to investigate other relevant clinical and public health issues.
Acknowledgments
The authors acknowledge the support and contributions from the Positive Living Program, Westside Community Clinic, Saskatoon Health Region and the thesis committee members. This work was written in partial fulfillment of the requirements for a Master’s degree from the University of Saskatchewan.
REFERENCES
- 1.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Interm Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 2.Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med. 2005;6:99–106. doi: 10.1111/j.1468-1293.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 3.Ormaasen V, Sandvik L, Dudman SG, Bruun JN. HIV related and non-HIV related mortality before and after the introduction of highly active antiretroviral therapy (HAART) in Norway compared to the general population. Scand J Infect Dis. 2007;39:51–7. doi: 10.1080/00365540600904779. [DOI] [PubMed] [Google Scholar]
- 4.Woldemichael G, Christiansen D, Thomas S, Benbow N. Demographic characteristics and survival with AIDS: Health disparities in Chicago, 1993–2001. Am J Public Health. 2009;99:S118–23. doi: 10.2105/AJPH.2007.124750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lima VD, Kretz P, Palepu A, et al. Aboriginal status is a prognostic factor for mortality among antiretroviral naive HIV-positive individuals first initiating HAART. AIDS Res Ther. 2006;3:14. doi: 10.1186/1742-6405-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Hoyos S, Del Amo J, Muga R, et al. Effectiveness of highly active antiretroviral therapy in Spanish cohorts of HIV seroconverters: Differences by transmission category. AIDS. 2003;17:353–9. doi: 10.1097/00002030-200302140-00009. [DOI] [PubMed] [Google Scholar]
- 7.Porter K, Babiker A, Bhaskaran K, et al. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362:1267–74. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 8.Munoz A, Gange SJ, Jacobson LP. Distinguishing efficacy, individual effectiveness, and population effectiveness of therapies. AIDS. 2000;14:754–6. doi: 10.1097/00002030-200004140-00020. [DOI] [PubMed] [Google Scholar]
- 9.Surveillance Report to December 31, 2008. Ottawa, Ontario: Surveillance and Risk Assessment Division, Centre for Communicable Diseases and Infection Control, Public Health Agency of Canada; 2009. HIV and AIDS in Canada. [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–81. [Google Scholar]
- 11.Cox D. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 12.Grigoryan A, Hall HI, Durant T, Wei X. Late HIV diagnosis and determinants of progression to AIDS or death after HIV diagnosis among injection drug users, 33 US States, 1996–2004. PLoS ONE. 2009;4:e4445. doi: 10.1371/journal.pone.0004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall HI, Byers RH, Ling Q, Espinoza L. Racial/ethnic and age disparities in HIV prevalence and disease progression among men who have sex with men in the United States. Am J Public Health. 2007;97:1060–6. doi: 10.2105/AJPH.2006.087551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plamondon K, de Bruin P. Saskatoon Health Region. Mount Royal College; 2009. Bridging services with community voices around injection drug use; p. 6. [Google Scholar]
- 15.Wood E, Kerr T, Tyndall MW, Montaner JS. Review of barriers and facilitators of HIV treatment among injection drug users. AIDS. 2008;22:1247–56. doi: 10.1097/QAD.0b013e3282fbd1ed. [DOI] [PubMed] [Google Scholar]
- 16.Public Health Agency of Canada . I-track: Enhanced Surveillance of Risk Behaviours among Injecting Drug Users in Canada. Ottawa: Public Health Agency of Canada; Aug, 2006. [Google Scholar]
- 17.Diamond C, Davidson A, Sorvillo F, Buskin S. HIV-infected American Indians/Alaska Natives in the Western United States. Ethn Dis. 2001;11:633–44. [PubMed] [Google Scholar]
- 18.Chaisson RE, Keruly JC, Moore RD. Race, sex, drug use, and progression of human immunodeficiency virus disease. New Engl J Med. 1995;333:751–6. doi: 10.1056/NEJM199509213331202. [DOI] [PubMed] [Google Scholar]
- 19.Carlos Martin J, Castilla J, Lopez M, Arranz R, Gonzalez-Lahoz J, Soriano V. Impact of chronic hepatitis C on HIV-1 disease progression. HIV Clin Trials. 2004;5:125–31. doi: 10.1310/YFV8-FE5K-5LN9-DQ4C. [DOI] [PubMed] [Google Scholar]
- 20.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus co-infection. The Swiss HIV Cohort Study. Lancet. 2000;356:1800–5. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 21.Spire B, Lucas GM, Carrieri MP. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST) Int J Drug Policy. 2007;18:262–70. doi: 10.1016/j.drugpo.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Thorpe LE, Frederick M, Pitt J, et al. Effect of hard-drug use on CD4 cell percentage, HIV RNA level and progression to AIDS-defining class C events among HIV-infected women. J Acquir Immune Defic Syndr. 2004;37:1423–30. doi: 10.1097/01.qai.0000127354.78706.5d. [DOI] [PubMed] [Google Scholar]
- 23.Chaisson RE, Keruly JC, Moore RD. Association of initial CD4 cell count and viral load with response to highly active antiretroviral therapy. JAMA. 2000;284:3128–9. doi: 10.1001/jama.284.24.3128. [DOI] [PubMed] [Google Scholar]
- 24.Ahdieh I, Gange SJ, Greenblatt RM, et al. Selection by indication of potent antiretroviral therapy use in a large cohort of women infected with human immunodeficiency virus. Am J Epidemiol. 2000;152:923–33. doi: 10.1093/aje/152.10.923. [DOI] [PubMed] [Google Scholar]


