CASE PRESENTATION
An 80-year-old man living in Eastern Ontario was assessed at a local emergency department for a syncopal episode that occurred after awakening from sleep and ambulating to the bathroom. He had experienced malaise, anorexia and episodes of presyncope for four months. His medical history was significant for rheumatic fever in childhood without known cardiac sequelae, diverticulitis, hypertension and gastroesophageal reflux disease. A review of systems was notable for the absence of fever, weight loss, arthralgias, gastrointestinal (GI) and neurological complaints. The patient’s medications were hydrochlorothiazide and rabeprazole.
A transthoracic echocardiogram revealed a mobile mass on the aortic valve. There was no history of antimicrobial exposure within the preceding 12 months, and the patient denied illicit drug use, travel or exposure to animals. A physical examination revealed a systolic ejection murmur at the left upper sternal border. No stigmata of infective endocarditis (IE) were present. The remainder of the general examination was normal.
A transesophageal echocardiogram revealed a 1.5 cm × 0.5 cm mobile mass on the right coronary cusp of the aortic valve and moderate-severe aortic insufficiency. Laboratory investigations were significant for normocytic anemia, with a hemoglobin level of 109 g/L and a serum albumin level of 39 g/L.
The patient underwent mechanical aortic valve replacement for severe aortic insufficiency. Intraoperatively, the lesion appeared to be a vegetation of infectious origin, and valve specimens were sent for Gram stain and culture. Empirical antimicrobial therapy was initiated with ceftriaxone and vancomycin for presumed IE.
Gross pathological examination revealed a thrombotic vegetation attached to the aortic valve. On high-power light microscopy, numerous small Gram-negative coccobacilli were observed along the margin of the vegetation, with no inflammatory cells present. Cultures from the valve and blood were negative after 21 days of incubation. Serologies for Bartonella henselae and Coxiella burnetii were negative. Broad-based 16S rDNA polymerase chain reaction (PCR)-based amplification and sequencing of the valvular tissue was requested through the Ontario Public Health Laboratory (Toronto, Ontario).
DIAGNOSIS
PCR molecular diagnostic testing performed on tissue from the resected valve identified Tropheryma whipplei. Periodic acid-Schiff (PAS) staining was subsequently performed on sections of the resected valve and demonstrated abundant PAS-positive material within macrophages (Figure 1). On identification of T whipplei, vancomycin was discontinued and ceftriaxone was continued for a total of six weeks. The patient was subsequently treated with oral trimethoprim-sulfamethoxazole, which resulted in significant GI intolerance. Doxycycline combined with hydroxychloroquine was continued for a total of 12 months.
Figure 1).
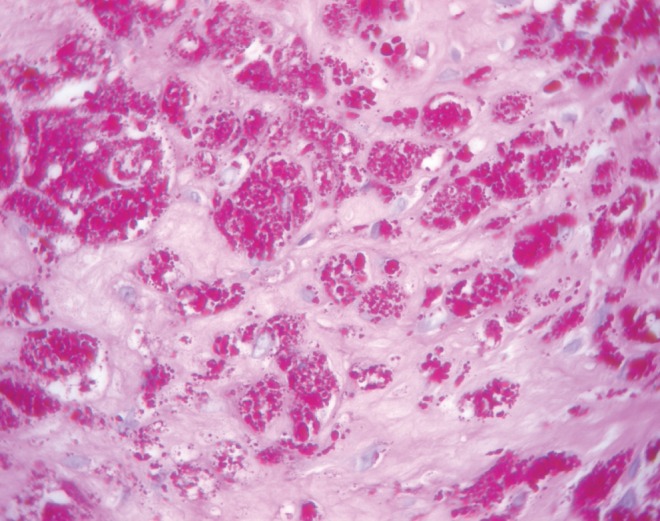
Aortic valve vegetation with numerous macrophages containing periodic acid-Schiff-positive material (original magnification ×60)
DISCUSSION
In as many as 40% of IE cases, no organism is identified (1). Causes of blood culture-negative endocarditis include recent antimicrobial use, fastidious bacterial organisms, fungi and noninfective causes of endocarditis. In the largest diagnostic series of IE reported to date, Coxiella burnetti, Bartonella species and T whipplei were the most commonly isolated fastidious organisms (Table 1) (1). Of these, only serology for C burnetii has been included in the modified Duke criteria (2).
Table 1.
Organisms and their relative frequencies identified in cases of blood culture-negative endocarditis
| Microorganism | Percentage of isolates (n=740) |
|---|---|
| Coxiella burnetii | 37.0 |
| Bartonella species | 12.4 |
| Streptococcus species | 4.4 |
| Tropheryma whipplei | 2.6 |
| Fungi | 1.0 |
| Other bacteria | 6.5 |
| No etiology | 36.5 |
Adapted from reference 1
T whipplei is a ubiquitous bacterium that is classically implicated in Whipple’s disease. Although the disease was first described in 1907, the bacterium was not successfully cultured until 1999 (3,4). T whipplei is phylogenetically described as a Gram-positive bacillus, although it may appear as Gram-negative and gram-indeterminate. PAS staining frequently reveals PAS-positive organisms within macrophages in infected tissues (4). Whipple’s disease most commonly occurs in Caucasian men of European descent, with a mean age at diagnosis of 50 years (3). The incidence is unknown, with approximately 1000 cases reported.
T whipplei manifests as two distinct clinical presentations: classical Whipple’s disease, characterized by a prodrome of polyarthritis, fever and weight loss followed by GI involvement with diarrhea and abdominal pain; and as isolated organ involvement without features of systemic infection. Isolated organ involvement can occur in the heart, central nervous system (CNS) and joints. Cardiac involvement is most common and occurs in 20% to 55% of cases of T whipplei infection. The valvular system, myocardium or pericardium may be involved, and IE most commonly involves the aortic valve (1). Patients with isolated T whipplei endocarditis generally lack symptoms of classical Whipple’s disease and present with an indolent, chronic disease course, similar to that experienced by the patient highlighted in the present case (5). In a recent prospective study (6), only one case of 16 fulfilled the Duke criteria.
16S rDNA PCR diagnostic testing has been performed on EDTA-anticoagulated blood or valvular tissue since the early 1990s and demonstrates higher sensitivity and specificity than culture for detecting organisms in IE, with a reported sensitivity of 72% and specificity of 100% (7). This has led to the suggestion of including a molecular diagnostic criterion in the Duke criteria (8). However, sensitivity of PCR-based molecular diagnostic testing is lower when sampling is performed on blood compared with valvular tissue (1). PCR-based molecular diagnostic testing detects both viable and nonviable organisms and may, therefore, remain positive following effective antimicrobial therapy. Although PCR-based molecular diagnostic testing identifies pathological organisms, determination of antimicrobial susceptibility is not possible (7).
Recommended therapy for Whipple’s endocarditis consists of intravenous ceftriaxone for two weeks followed by oral trimethoprim-sulfamethoxazole for 12 months (9). Alternative regimens include meropenem for initial intravenous therapy, and a combination of doxycycline and hydroxychloroquine as oral therapy. Symptoms typically resolve within two to four weeks. In cases with GI involvement, duodenal biopsies with PCR testing have been recommended to document resolution of infection (10).
In the present case, the patient was not initially evaluated for CNS involvement because clinical suspicion for T whipplei was low. Once the diagnosis was confirmed, the patient had been empirically treated with ceftriaxone for two weeks. The likelihood of a positive cerebro-spinal fluid PCR result would have been low; therefore, cerobrospinal fluid investigations were not performed. Similarly, duodenal biopsies were considered unlikely to affect subsequent therapeutic decision making in this setting.
T whipplei is increasingly recognized as a common fastidious organism identified in blood culture-negative endocarditis. When valvular tissue is available, 16S rDNA PCR-based amplification and sequencing can be a valuable tool for diagnosing T whipplei IE. In confirmed cases, prolonged antimicrobial therapy is required, and early CNS and GI evaluation should be considered.
REFERENCES
- 1.Fournier PE, Thuny F, Richet H, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: A prospective study of 819 new cases. Clin Infect Dis. 2010;51:131–40. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]
- 2.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 3.Whipple G. A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Johns Hopkins Hospital Bulletin. 1907;18:382–91. [Google Scholar]
- 4.Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med. 2000;342:620–25. doi: 10.1056/NEJM200003023420903. [DOI] [PubMed] [Google Scholar]
- 5.Richardson DC, Burrows LL, Korithoski B, et al. Tropheryma whippelii as a cause of afebrile culture-negative endocarditis: The evolving spectrum of Whipple’s disease. J Infect. 2003;47:170–3. doi: 10.1016/s0163-4453(03)00015-x. [DOI] [PubMed] [Google Scholar]
- 6.Geissdorfer W, Moos V, Moter A, et al. High frequency of Tropheryma whipplei in culture-negative endocarditis. J Clin Microbiol. 2012;50:216–22. doi: 10.1128/JCM.05531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voldstedlund M, Pedersen LN, Baandrup U, Klaaborg KE, Fuursted K. Broad-range PCR and sequencing in routine diagnosis of infective endocarditis. APMIS. 2008;116:190–8. doi: 10.1111/j.1600-0463.2008.00942.x. [DOI] [PubMed] [Google Scholar]
- 8.Millar B, Moore J, Mallon P, et al. Molecular diagnosis of infective endocarditis – a new Duke’s criterion. Scand J Infect Dis. 2001;33:673–80. doi: 10.1080/00365540110026764. [DOI] [PubMed] [Google Scholar]
- 9.Fenollar F, Puechal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356:55–66. doi: 10.1056/NEJMra062477. [DOI] [PubMed] [Google Scholar]
- 10.Schneider T, Moos V, Loddenkemper C, Marth T, Fenollar F, Raoult D. Whipple’s disease: New aspects of pathogenesis and treatment. Lancet Infect Dis. 2008;8:179–90. doi: 10.1016/S1473-3099(08)70042-2. [DOI] [PubMed] [Google Scholar]


