Abstract
BACKGROUND:
Nocardia species are rare, opportunistic organisms that cause disease in both immunocompetent and immunocompromised individuals.
OBJECTIVE:
To investigate the clinical presentations of various Nocardia infections based on the 16S ribosomal RNA gene of the isolate, as well as related risk factors and susceptibility patterns to antimicrobial agents
METHODS:
Thirteen patients with a diagnosis of nocardiosis were included in the present study. Seven Nocardia species were identified by 16S ribosomal RNA. Susceptibility testing was performed using six antimicrobial agents.
RESULTS:
Five patients were immunocompromised, and eight were immunocompetent with predisposing factors including cystic fibrosis, tuberculosis and ophthalmic infections. Nocardia caused pulmonary infections in eight patients (61.5%), invasive systemic infections in three patients (23%) and local (ophthalmic) infections in two patients (15.4%). In the patients with pulmonary disease, nocardiosis was caused by six species (Nocardia cyriacigeorgica, Nocardia otitidiscaviarum, Nocardia farcinica, Nocardia carnea, Nocardia testacea and Nocardia asiatica). The seventh species identified in the present study was Nocardia crassostreae.
DISCUSSION:
N crassostreae is a multidrug-resistant organism that was reported to be an emerging human pathogen causing invasive nocardiosis in a patient with non-Hodgkin’s lymphoma. N farcinica was isolated from blood in a patient with breast cancer. None of the Nocardia isolates were resistant to linezolid. One N otitidiscaviarum isolate was a multidrug-resistant organism. All patients in the present study were treated with the appropriate antibiotics and their condition resolved without further sequelae.
CONCLUSIONS:
The present study is the first report on N crassostreae as a human pathogen. The detection of multidrug-resistant species necessitate molecular identification and susceptibility testing, and should be performed for all Nocardia infections. Nocardiosis manifests various clinical features depending on the Nocardia species and underlying conditions.
Keywords: Antibiotic susceptibility, Clinical cases, Molecular identification, Nocardia, Nocardia crassostreae
Abstract
HISTORIQUE :
Les espèces de Nocardia sont des organismes opportunistes rares qui sont pathogènes à la fois chez les personnes immunocompétentes et immunodéprimées.
OBJECTIF :
Explorer la présentation clinique de diverses infections à Nocardia d’après le gène d’ARN ribosomique 16S de l’isolat, ainsi que les facteurs de risque connexes et les profils de susceptibilité aux antimicrobiens.
MÉTHODOLOGIE :
Treize patients ayant un diagnostic de nocardiose ont participé à la présente étude. Les chercheurs ont repéré sept espèces de Nocardia au moyen de l’ARN ribosomique 16S. Ils ont effectué les tests de susceptibilité à six antimicrobiens.
RÉSULTATS :
Cinq patients étaient immunodéprimés et huit étaient immunocompétents, mais présentaient des facteurs de prédisposition, y compris la fibrose kystique, la tuberculose et des infections ophtalmiques. La Nocardia a provoqué des infections pulmonaires chez huit patients (61,5 %), des infections systémiques invasives chez trois patients (23 %) et des infections locales (ophtalmiques) chez deux patients (15,4 %). Chez les patients atteints d’une maladie pulmonaire, la nocardiose était attribuable à six espèces (Nocardia cyriacigeorgica, Nocardia otitidiscaviarum, Nocardia farcinica, Nocardia carnea, Nocardia testacea et Nocardia asiatica). La septième espèce observée dans la présente étude était la Nocardia crassostreae.
EXPOSÉ :
La N crassostreae, un organisme multirésistant considéré comme un agent anthropopathogène émergent, était responsable d’une nocardiose invasive chez un patient atteint d’un lymphome non hodgkinien. Les chercheurs ont isolé le N farcinica dans le sang d’un patient atteint d’un cancer du sein. Aucun des isolats de Nocardia n’était résistant à la linézolide. Un isolat de N otitidiscaviarum était multirésistant. Tous les patients participant à la présente étude ont reçu un traitement aux antibiotiques pertinent et se sont rétablis sans autres séquelles.
CONCLUSION :
La première étude est la première à faire état de la N crassostreae comme agent anthropopathogène. Pour déceler les espèces multirésistantes, il faut procéder à une identification moléculaire et à un test de susceptibilité, des mesures qu’il faudrait prendre à l’égard de toutes les infections à Nocardia. La nocardiose s’associe à diverses caractéristiques cliniques, selon l’espèce de Nocardia et les maladies sousjacentes.
Nocardia are ubiquitous organisms distributed worldwide in the environment as saprophytic components of fresh and salt water, soil, dust, decaying vegetation and decaying fecal deposits from animals (1). Nocardia are rare opportunistic organisms that cause diseases in immunocompetent and immunocompromised individuals, and are reported in all ages and ethnic groups. Immunocompetent patients usually develop localized cutaneous lesions, such as cellulitis, abscesses or sporotrichoid forms (2), and endogenous endophthalmitis (3). However, most cases are reported in immunocompromised patients, and manifest as deep infections or disseminated diseases (4–6). Risk factors for infection with Nocardia include solid organ transplant (5,7–9) or bone marrow transplant recipients (10), patients with hematological diseases (11,12) and HIV infection (13–16). Pulmonary nocardiosis can be a cause of disease in populations with risk factors such as immunosuppression, malignancies and severe lung disease (17), patients with cystic fibrosis (18), and in patients with chronic obstructive pulmonary disease and bronchiectasis (19,20). Lung infections are frequent and, in many cases, disease can spread to the central nervous system, including the brain (4,21), with poor prognosis in some cases, irrespective of antimicrobial therapy (22).
Identification of clinical isolates beyond the genus level is important because Nocardia species differ in clinical spectrum and their susceptibility to antibiotics. The classic laboratory methods of identification, including direct examination and culture, are insufficient; therefore, sequence analysis of the 16S ribosomal (r)RNA gene is mainly used for the identification of Nocardia isolates to species level (23,24).
Trimethoprim-sulfamethoxazole has traditionally been the agent of choice for the treatment of nocardiosis, with alternative drugs including amikacin and imipenem (1,25). Resistance and therapeutic failure may occur, which necessitates a search for alternative agents. The aim of the present study was to investigate the clinical presentations of various Nocardia infections based on the 16S rRNA gene of the isolate, related risk factors and susceptibility patterns to various antimicrobial agents.
METHODS
Patients
The present laboratory-based study was approved by the Scientific Council and Ethics Committee of Hamad Medical Research Center, Doha, Qatar (proposal number 10174/10).
Thirteen patients of different nationalities with various clinical symptoms and risk factors admitted to Hamad Hospital, Doha, Qatar, were diagnosed with nocardial infections by the main Microbiology Laboratory of Hamad Medical Corporation from January 2006 to June 2010. Patients’ clinical records, demographic data and treatment outcomes were included in the present study. Information regarding sex, age, underlying conditions including history of immunosuppressant drug use, tuberculosis and malignancy were analyzed (Table 1). Disseminated nocardiosis was considered for infection of two organs or more, such as lungs, lymph nodes, brain or blood. The respiratory samples included sputum, endotracheal aspiration and bronchoalveolar lavage. A diagnosis of pulmonary nocardiosis required at least one positive culture from respiratory samples, and the presence of clinical symptoms and an abnormal chest radiograph.
TABLE 1.
Clinical characteristics and outcome of 13 patients with nocardiosis
| Case | Age, years/sex | Patient origin | Clinical diagnosis | Clinical specimen | 16S rRNA identification (genus Nocardia) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 42/F | Qatar | Non-Hodgkin’s lymphoma, abscess on back at L1 level | Pus aspirate | N cyriacigeorgica | Ceftriaxone + SXT | Recovered |
| 2 | 33/M | India | Chest pain, pleural effusion, lingular infiltrates | Sputum | N cyriacigeorgica | Azithromycin | Recovered |
| 3 | 36/M | India | Pulmonary infection mimicking tuberculosis | Sputum | N cyriacigeorgica | NA | NA |
| 4 | 45/M | India | Corneal abscess | Corneal scraping | N otitidiscaviarum | Local gentamicin and levofloxacin eye drops | Recovered |
| 5 | 17/F | Qatar | Cystic fibrosis with pneumonia | BAL | N otitidiscaviarum | Clarithromycin + SXT, moxifloxacin | Recovered |
| 6 | 50/F | Qatar | Conjunctivitis | Conjunctival swab | N farcinica | Fusidic acid + lomefloxacin | Recovered |
| 7 | 68/M | Qatar | Renal transplant | Sputum | N farcinica | Meropenem + SXT | Recovered |
| 8 | 50/F | India | Breast cancer on chemotherapy with central line-related sepsis | Blood | N farcinica | SXT | Recovered |
| 9 | 30/M | India | Pulmonary tuberculosis, bronchiectasis | Sputum | N carnea | Anti-TB | Recovered |
| 10 | 36/M | Egypt | Pulmonary infection mimicking tuberculosis | Sputum | N carnea | NA | NA |
| 11 | 73/F | Qatar | Non-Hodgkin’s lymphoma, paravertebral abscess at L3–L5, on chemotherapy | CT-guided aspirated fluid | N crassostreae | Meropenem + SXT | Improved; died from progressive disease |
| 12 | 34/M | India | Previous case of pulmonary tuberculosis | Sputum | N testacea | None | NA |
| 13 | 44/F | Syria | Breast cancer with pulmonary infection | Sputum | N asiatica | SXT | Recovered |
Anti-TB Antituberculosis treatment; BAL Bronchoalveolar lavage; CT Computed tomography; F Female; M Male; NA Not available; rRNA Ribosomal RNA; SXT Trimethoprim-sulfamethoxazole
Isolation and identification of Nocardia species
A total of 13 clinical specimens positive for Nocardia species were recorded over a four-year period. Nocardia species were isolated and identified according to standard laboratory procedures. Identification of Nocardia species was based on Gram-positive branching, beaded and filamentous bacilli, and positive modified acid-fast stain results. The clinical specimens were generally cultured on chocolate agar and blood agar media, and incubated in both aerobic and anaerobic conditions at 37°C. Characteristic dry, chalk-like Nocardia colonies appeared on aerobic cultures after three to seven days of incubation, depending on the species. Blood cultures were performed using the Bactec automated culturing system (BD Diagnostic Systems, USA).
Molecular analysis
The identities of the clinical isolates were further confirmed by 16S rRNA gene analysis (26). The DNA was isolated from freshly grown colonies using a MagNA Pure LC instrument in combination with MagNA Pure LC DNA isolation kit III according to the instructions of the manufacturer (Roche Diagnostics, The Netherlands). An approximately 500 bp fragment from the 5′ end of the 16S rRNA gene was amplified using polymerase chain reaction containing 1 U of FastStart Taq DNA polymerase (Roche Diagnostics), 0.2 mM dNTPs, 1.5 mM MgCl2 and 0.5 μM of both amplification primers (forward: 5′-CCT AAC ACA TGC AAG TCG ARC G-3′; reverse: 5′-CGT ATT ACC GCG GCT GCT-3′) in 1× polymerase chain reaction reaction buffer. Cycling conditions were as follows: 30 s at 94°C, 30 s at 56°C and 1 min at 72°C repeated 30 times, preceded by a 10 min activation step at 94°C and followed by an additional 10 min elongation step at 72°C. The amplified product was purified using SPRI chemistry (AMPure, Beckman Coulter, The Netherlands) and subjected to DNA sequence analysis with the reverse amplification primer using the DYEnamic ET dye terminator kit (GE Healthcare, Belgium) as recommended. Sequence reaction products were purified using SPRI chemistry (CleanSeq Beckman Coulter) and analyzed on a MegaBACE 500 automated DNA analysis platform (GE Healthcare) using standard electrophoretic conditions. The obtained sequences were verified and manually corrected when necessary using MegaBACE Sequence Analyzer v3.0 (GE Healthcare). Sequences were then compared with the public DNA databases using the BLAST interface (www.ncbi.nlm.nih.gov/BLAST/).
Susceptibility testing
Antimicrobial susceptibility testing was performed using Etest (AB biodisk, Sweden). A suspension of the microorganism, with turbidity equivalent to 1.0 McFarland standard, was inoculated (150 μL/plate) by confluent swabbing on Mueller-Hinton agar plates. A maximum of two Etest strips were applied to each plate. Etest plates were incubated at 35°C and results were recorded after 48 h (or after 72 h if growth was insufficient after 48 h). The following antimicrobial agents were tested (concentration ranges): amikacin (0.016 μg/mL to 256 μg/mL), moxifloxacin (0.002 μg/mL to 32 μg/mL), cefotaxime (0.016 μg/mL to 256 μg/mL), cotrimoxazole (0.002 μg/mL to 32 μg/mL), linezolid (0.016 μg/mL to 256 μg/mL) and imipenem (0.002 μg/mL to 32 μg/mL). Agents were determined by Etest for 13 Nocardia isolates from clinical specimens. Minimum inhibitory concentrations were determined according to manufacturer’s guidelines. Results were interpreted as susceptible, intermediate or resistant according the breakpoints recommended by the Clinical and Laboratory Standards Institute for Nocardia and other aerobic actinomycetes (27).
Beta-lactamase activity:
All Nocardia clinical isolates were tested for beta-lactamase activity using the nitrocefin disk test
RESULTS
Microbiological investigation
A total of 13 Nocardia isolates were identified from 13 patients during the period January 2006 to June 2010. Molecular identification yielded Nocardia cyriacigeorgica (n=3; Genbank accession number JN041560), Nocardia otitidiscaviarum (n=2; Genbank accession number JN041512), Nocardia farcinica (n=3; Genbank accession number JN041682), Nocardia carnea (n=2; Genbank accession number JN041599) and one each of Nocardia asiatica, Nocardia crassostreae and Nocardia testacea (Genbank accession numbers JN041487, AY756548 and AB192415, respectively).
Clinical features
Demographic data and information pertaining to the source of isolation, as well as the clinical symptoms of the patients yielding these isolates, are provided in Table 1. Thirteen patients (seven male and six female, 17 to 73 years of age) were diagnosed as having Nocardia infections. Five patients were immunocompromised, and eight were apparently immunocompetent with predisposing factors such as cystic fibrosis, tuberculosis and ophthalmic infections with no apparent risk factor. Nocardia caused pulmonary infection in eight patients (61.5%), invasive infections in three patients (23%) and local (ophthalmic) infections in two patients (15.4%). Pulmonary nocardiosis was found to be caused by N cyriacigeorgica, N otitidiscaviarum, N farcinica, N carnea, N testacea or N asiatica. Two Nocardia species were involved in ophthalmic infections (N otitidiscaviarum and N farcinica) and three species caused invasive infections (N cyriacigeorgica, N farcinica and N crassostreae). Of the three patients with predisposing factors for invasive infections, two had non-Hodgkin’s lymphoma and were currently undergoing chemotherapy, and one had breast cancer and was also undergoing chemotherapy.
Invasive infections
Patients with non-Hodgkin’s lymphomas (cases 1 and 11):
Case 1 was a female patient with a Nocardia cavitary lesion in the left upper lung lobe who developed an abscess approximately 3.5 cm in diameter with thick wall present at the left lumborum muscle at the level of L1 vertebral body, as shown in magnetic resonance imaging (Figure 1). Under computed tomography (CT) guidance, a large-gauge needle was inserted into the abscess and a small amount of pus was aspirated and sent to the microbiology laboratory for microscopy and culture; the culture grew N cyriacigeorgica. The patient recovered after treatment with ceftriaxone and sulfamethoxazole-trimethoprim (cotrimoxazole) and is still doing well. In the other woman (case 11), a CT scan revealed a large, paravertebral abscess at the L3–L5 level, present in the left psoas muscle and compressing and displacing the left kidney. A catheter was inserted percutaneously into the abscess; more than 50 mL of pus was aspirated and the catheter was left in situ for further drainage. The abscess extended outward and produced another abscess collection in the left lumborum muscle (Figure 2); the abscess was aspirated under CT guidance, and sent to the microbiology laboratory for microscopy and culture (the culture grew N crassostreae). There was no patient history of contact with or ingestion of molluscs. The patient improved after treatment with meropenem and trimethoprim-sulfamethoxazole, but died later from progressive disease.
Figure 1).
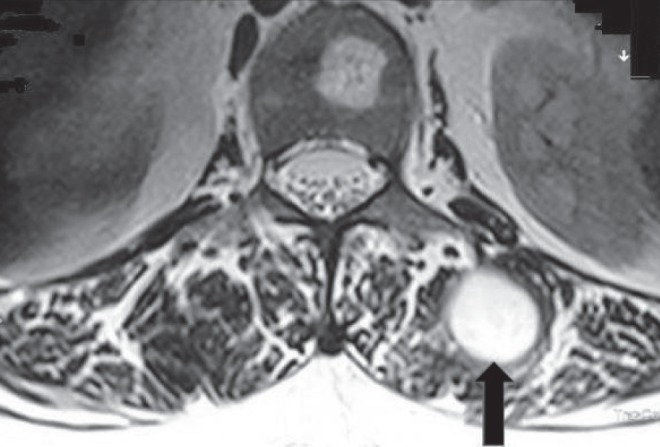
Magnetic resonance image of a patient (case 1) with pulmonary nocardiosis and dissemination to an abscess approximately 3.5 cm in diameter with thick wall (arrow) in the left lumborum muscle at level of L1 vertebral body caused by Nocardia cyriacigeorgica
Figure 2).

Computed tomography scan shows a large abscess caused by Nocardia crassostreae, in the left psoas muscle (solid arrow) extending outward and producing a multiloculated abscess (open arrow) in the left lumborum muscle (case 11)
Blood stream infection:
The third case of invasive nocardiosis was found in an immunocompromised patient with breast cancer undergoing chemotherapy (case 8), who was diagnosed with N farcinica infection. She was successfully treated with trimethoprim-sulfamethoxazole.
Pulmonary nocardiosis
Pulmonary nocardiosis was diagnosed in eight patients in the present study. In case 3, an immunocompetent male patient with chest pain, pleural effusion and lingular infiltrates, a CT scan showed a small area of consolidation present in the inferior segment of lingula of the left lung, along with ill-defined hazy linear shadowing present at the periphery of the lateral segment of the left lower lobe (Figure 3). Sputum culture grew N cyriacigeorgica, and the patient recovered after treatment with azithromycin.
Figure 3).
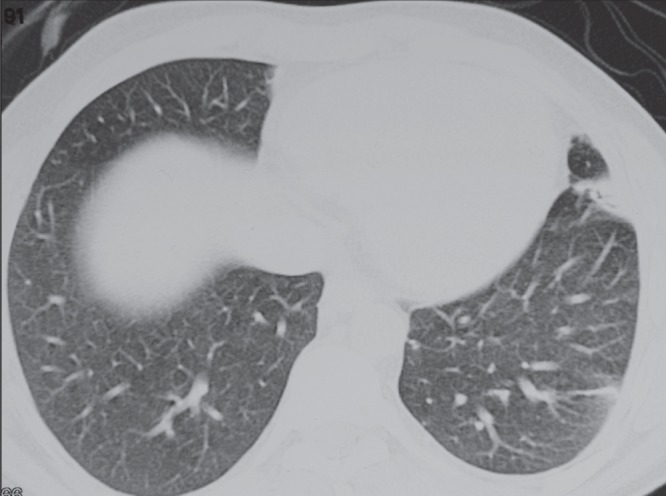
Chest computed tomography scan of an immunocompetent patient (case 3). A small area of consolidation in the inferior segment of lingual (left lung) caused by Nocardia cyriacigeorgica is apparent
Antimicrobial susceptibility
The antimicrobial susceptibility patterns of the 13 Nocardia species for six antibacterial agents are summarized in Table 2. Growth inhibition ellipses were uniform and well delineated, and the points of intersection with the Etest strips were easy to determine, except for N otitidiscaviarum (case 4), in which the mutant colonies appeared with cotrimoxazole Etest strips at a minimum inhibitory concentration of 12 μg/mL. All Nocardia isolates were susceptible to linezolid, but showed various susceptibility patterns to other antimicrobial agents. The N crassostreae isolate (case 11) was resistant to amikacin, cefotaxime and imipenem. The N otitidiscaviarum isolate (case 4) was resistant to imipenem, cotrimoxazole and cefotaxime, and intermediate for moxifloxacin. N otitidiscaviarum isolate (case 5) was resistant to the antimicrobial agents cefotaxime and imipenem. Eight Nocardia isolates showed beta-lactamase activity by nitrocefin disk test. Positive reaction was demonstrated within 5 min. The nitrocefin test was negative for N carnea, N testacea and N asiatica, whereas N cyriacigeorgica isolates showed variability in the results of nitrocifin test, with one of the three strains being negative. Positive beta-lactamase Nocardia included N otitidiscaviarum and N crassostreae, which also exhibited resistance to cefotaxime and imipenem.
TABLE 2.
Susceptibility pattern of Nocardia species to most common antibiotics (μg/mL)
| Case | Organism (genus Nocardia) | AK | MOX | CTX | SXT | LIN | IMI | BL |
|---|---|---|---|---|---|---|---|---|
| 1 | N cyriacigeorgica | 0.5 | 0.19 | 0.38 | 0.016 | 0.25 | 0.19 | + |
| 2 | N cyriacigeorgica | 1.5 | 4 | 0.75 | 0.64 | 0.38 | 0.19 | + |
| 3 | N cyriacigeorgica | 0.75 | 6 | ND | 0.19 | 0.75 | 0.75 | − |
| 4 | N otitidiscaviarum | 1 | 3 | >256 | 12 | 0.75 | >32 | + |
| 5 | N otitidiscaviarum | 1.5 | 1 | >256 | 0.19 | 0.5 | >32 | + |
| 6 | N farcinica | 2 | 0.023 | 1.5 | 0.064 | 0.38 | 0.094 | + |
| 7 | N farcinica | 2 | 0.047 | 6 | 0.5 | 0.75 | 1 | + |
| 8 | N farcinica | 0.75 | 0.064 | 16 | 0.5 | 0.5 | 0.5 | + |
| 9 | N carnea | 0.19 | 0.38 | 2 | 0.5 | 0.25 | 0.38 | − |
| 10 | N carnea | 0.25 | 0.19 | 2 | 0.25 | 0.094 | 0.75 | − |
| 11 | N crassostreae | >256 | 0.38 | >256 | 1.5 | 0.25 | >32 | + |
| 12 | N testacea | 0.38 | 0.094 | 1.5 | 0.38 | 0.19 | 0.25 | − |
| 13 | N asiatica | 0.094 | >32 | 0.094 | 0.08 | 0.032 | 0.19 | − |
AK Amikacin; BL Beta-lactamase; CTX Cefotaxime; IMI Imipenem; LIN Linezolid; MOX Moxifloxacin; SXT Trimethoprim-sulfamethoxazole;
− Negative;
+ Positive
DISCUSSION
Identification of Nocardia to species level using phenotyping is difficult (1,2). Sequencing of the 16S rRNA gene enables more accurate identification, and the application of this technique has resulted in the identification of new and clinically important species of Nocardia over the past 10 years (28–30). The number of reported clinical cases caused by opportunistic nocardiosis infections is constantly rising. Seven species of Nocardia from 13 cases were reported in a relatively short period during the present study (Table 1). N cyriacigeorgica and N farcinica are the most common species associated with clinical specimens in Qatar, each represented by three cases, collectively constituting 46% of the cases. N cyriacigeorgica is frequently isolated from clinical specimens (31,32). The most frequently reported cases of N cyriacigeorgica constitute disseminated infections with various risk factors including bacteremia in a renal transplant (33), brain abscess in HIV (34), endocarditis (35) and pulmonary infections (36). In the present study, N cyriacigeorgica was isolated from pulmonary infections in two cases and one disseminated infection in a patient with non-Hodgkin’s lymphoma. N farcinica has been reported to be an increasing cause of localized and disseminated infections in immunocompromised patients in recent years (37–39), but bacteremia remains a rare finding (40,41). In the present study, we reported a case of bacteremia in a 50-year-old woman with breast cancer undergoing chemotherapy. The second case was a pulmonary infection in a renal transplant patient, treated successfully with meropenem/trimethoprim-sulfamethoxazole, whereas the third case was conjunctivitis in a patient with no apparent immune dysfunction. Although Nocardia infection of any type involving the eye is rare, several species have been diagnosed as a cause of keratitis (42); the isolation of N farcinica in the present study will be added as a possible etiological agent of eye infection. N otitidiscaviarum has been isolated from a fatal brain abscess in a patient with chronic obstructive pulmonary disease (22), in a case of bacteremia (43) and also from pulmonary infection (44); in the present study, this species was found in two cases: corneal abscess in an immunocompetent patient and severe pneumonia in a patient with cystic fibrosis. In a retrospective analysis that included 17 cystic fibrosis patients (18), five Nocardia species (including N otitidiscaviarum) were considered as colonizers and oral antibiotic therapy did not appear to affect the clinical outcome. A patient with cystic fibrosis in the present study (case 5) was hospitalized for severe pneumonia that was treated successfully with clarithromycin, trimethoprim-sulfamethoxazole and moxifloxacin with clinical improvement and negative post-treatment sputum culture.
Several rare pathogens were identified in the present study. N carnea, less frequently encountered as a human pathogen (32), was isolated from two cases of pulmonary infections. In one report, the species was isolated from a pulmonary infection in a patient with tuberculosis (45). To our knowledge, N crassostreae has not been reported as a human pathogen since its isolation in 1998 from Pacific oysters (46). This species was isolated from a paravertebral abscess at L3–L5 from a patient with a non-Hodgkin’s lymphoma undergoing chemotherapy. N testacea, isolated from sputum of a patient with previous pulmonary tuberculosis in the present study, has been rarely isolated from clinical specimens (47). N asiatica, a rare agent of nocardiosis, was first described in 2004, including five strains isolated from Asia (48), and six documented clinical isolates causing pneumonia or cutaneous infections in patients with HIV and a bone marrow recipient (32), and a disseminated infection in HIV patient (31). It was isolated in the present study from a breast cancer patient with pulmonary infection (case 13). This suggests that, although rarely seen in clinical specimens, N asiatica is associated with immune dysfunction. All patients were treated with appropriate antibiotics and the infection resolved without further sequelae.
The organisms are readily aerosolized with dust and the respiratory tract remains the main portal of entry, with the majority of patients presenting with pulmonary involvement (4,49). Due to its nontypical manifestations, nocardiosis is frequently misdiagnosed; the initial diagnosis is often pneumonia, tuberculosis or lung abscesses. Radiographic presentation may reveal bronchiectasis with pneumonia (Figure 3).
Five immunocompromised patients (Table 1; cases 1, 7, 8, 11 and 13) were successfully treated with the appropriate antibiotics, but case 11 died from a progressive hematological disease. Disseminated nocardiosis, particularly in those with central nervous system involvement or bacteremia, has a poor prognosis with a high mortality rate in immunocompromised hosts (6,17,22,50).
Accurate identification of Nocardia species is important because different species may have different antimicrobial susceptibilities. Linezolid had a distinctive activity pattern against all Nocardia species (Table 2); these results are in accordance with previously reported susceptibility patterns for linezolid (51–53). The data support linezolid as an alternative for the treatment of nocardiosis. All but one isolate of N otitidiscaviarum isolates were susceptibile to trimethoprim-sulfamethoxazole. Susceptibility of Nocardia species to trimethoprim-sulfamethoxazole is variable; it was reported that only 2% of Nocardia isolates (total n=138) in Taiwan were resistant (51), compared with a higher resistance rate (42%, total n=765) in the United States (53).
In the present study, various Nocardia species exhibited different drug susceptibility patterns to cefotaxime and imipenem. The two isolates of N otitidiscaviarum showed a typical multidrug resistance pattern, characterized by resistance to cefotaxime and imipenem and positive for beta-lactamase activity by nitrocefin disk test. Similar resistance patterns were documented in other reports (51–53). N crassostreae was resistant to cefotaxime, imipenem and amikacin; however, amikacin was uniformly active against all other Nocardia isolates (Table 2). Amikacin was demonstrated to be highly active against all tested Nocardia species (51,52). Resistance to amikacin is rare and has been reported mainly for N transvalensis (53,54); because this is the first report for N crassostreae as a human pathogen, susceptibility data were not available in the literature for clinically isolated strains. N farcinica, one of the predominant species in the present study, was susceptible to all agents. Despite exhibiting beta-lactamase activity by nitrocefin disk test, all isolates were susceptibile to cefotaxime and imipenem. One strain was intermediately susceptible to cefotaxime. Resistance to cefotaxime and imipenem does not appear to be mediated by beta-lactamase, but rather by decreased affinities of penicillin binding-proteins for these molecules (55).
Among other species, isolates of the N asiatica, N carnea and N testacea have been reported only rarely as human pathogens. N asiatica was resistant to moxifloxacin but susceptibile to all other five tested antimicrobial agents. However, few isolates were tested for susceptibility, which showed a resistance pattern for ciprofloxacin (51,53), suggestive of a quinolone resistance profile. This class of antibiotics may not be considered for the treatment of infections caused by this species.
The present study is the first report of series of Nocardia infection, documenting the species prevalent in Qatar. N crassostreae was reported for the first time as a human pathogen. The detection of multidrug resistance species necessitate molecular identification and susceptibility testing, and should be performed for all Nocardia infections. The disease manifests with different clinical features depending on the Nocardia species and underlying conditions. Most patients recovered with combined antimicrobial agents. Trimethoprim-sulfamethoxazole alone or in combination, or sequential with other agents, was effective in treating the majority of patients.
Acknowledgments
The authors thank Kim Driessen and Antoinette Stevens-Krebbers for the technical assistance, and Dr Ferry Hagen for reviewing the manuscript.
REFERENCES
- 1.Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ., Jr Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259–82. doi: 10.1128/CMR.19.2.259-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeil MM, Brown JM. The medically important aerobic actinomycetes: Epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357–417. doi: 10.1128/cmr.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilman T, Trubnik V, Shah M, McCormick SA, Finger PT. Isolated Nocardia exalbida endogenous endophthalmitis. Ocul Immunol Inflamm. 2011;19:237–9. doi: 10.3109/09273948.2011.563898. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosioni J, Lew D, Garbino J. Nocardiosis: Updated clinical review and experience at a tertiary center. Infection. 2010;38:89–97. doi: 10.1007/s15010-009-9193-9. [DOI] [PubMed] [Google Scholar]
- 5.Parks BS, Parks WJ, Kim YJ, et al. A case of disseminated Nocardia farcinica diagnosed through DNA sequencing in a kidney transplantation patient. Clin Nephrol. 2008;70:542–5. [PubMed] [Google Scholar]
- 6.Tan CK, Lai CC, Lin CH, et al. Clinical and microbiological characteristics of nocardiosis including those caused by emerging Nocardia species in Taiwan, 1998–2008. Clin Microbiol Infect. 2010;16:966–72. doi: 10.1111/j.1469-0691.2009.02950.x. [DOI] [PubMed] [Google Scholar]
- 7.Hussain S, McCurry K, Dauber J, Singh N, Kusne S. Nocardia infection in lung transplant recipients. J Heart Lung Transplant. 2002;21:354–9. doi: 10.1016/s1053-2498(01)00394-1. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez-Galanes Marchan S, Meneu Diaz JC, Caso Maestro O, et al. Disseminated nocardiosis: A rare infectious complication following non-heart-beating donor liver transplantation. Transpl Proc. 2009;41:2495–7. doi: 10.1016/j.transproceed.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Yu X, Han F, Wu J, et al. Nocardia infection in kidney transplant recipients: Case report and analysis of 66 published cases. Transpl Infect Dis. 2011;13:385–91. doi: 10.1111/j.1399-3062.2011.00607.x. [DOI] [PubMed] [Google Scholar]
- 10.Dehghani M, Davarpanah MA. Epididymo-orchitis and central nervous system nocardiosis in a bone marrow transplant recipient for acute lymphoplastic leukemia. Exp Clin Transplant. 2009;7:264–6. [PubMed] [Google Scholar]
- 11.Sharma M, Gilbert BC, Santoro J. Disseminated Nocardia otitidiscaviarum infection in a women with sickle cell anemia and end-stage renal disease. Am J Med Sci. 2007;333:372–5. doi: 10.1097/MAJ.0b013e318065ab26. [DOI] [PubMed] [Google Scholar]
- 12.Mete B, Yemisen M, Demirel AE, et al. A case of nocardiasis complicated with meningitis in a patient with immune thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2010;21:185–7. doi: 10.1097/MBC.0b013e3283338bf5. [DOI] [PubMed] [Google Scholar]
- 13.Jinno S, Jirakulaporn T, Bankowski MJ, Kim W, Wong R. Rare case of Nocardia asteroides pericarditis in a human immunodeficiency virus-infected patient. J Clin Microbiol. 2007;45:2330–3. doi: 10.1128/JCM.00149-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unkle DW, Ricketti AJ, Cleri DJ, Moser RL, Vernaleo JR. An HIV-infected patient with Nocardia asteroides bilateral pneumonia. AIDS Read. 2008;18:566–8. [PubMed] [Google Scholar]
- 15.Liu WL, Lai CC, Hsiao CH, et al. Bacteremic pneumonia caused by Nocardia veterana in an HIV-infected patient. Int J Infect Dis. 2011;15:e430–e2. doi: 10.1016/j.ijid.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Martinaud C, Verdonk C, Bousquet A, et al. Isolation of Nocardia beijingensis from a pulmonary abscess reveals human immunodeficiency virus infection. J Clin Microbiol. 2011;49:2748–50. doi: 10.1128/JCM.00613-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corti M. Nocardiosis: A review. Int J Infect Dis. 2003;7:243–50. doi: 10.1016/s1201-9712(03)90102-0. [DOI] [PubMed] [Google Scholar]
- 18.Thorn ST, Brown MA, Yanes JJ, et al. Pulmonary nocardiosis in cystic fibrosis. J Cyst Fib. 2009;8:316–20. doi: 10.1016/j.jcf.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Aidê MA, Lourenço SS, Marchiori E, Zanetti G, Mondino PJ. Pulmonary nocardiosis in a patient with chronic obstructive pulmonary disease and bronchiectasis. J Bras Pneumol. 2008;34:985–8. doi: 10.1590/s1806-37132008001100016. [DOI] [PubMed] [Google Scholar]
- 20.Cargill JS, Boyd GJ, Weightman NC. Nocardia cyriacigeorgica: A case of endocarditis with disseminated soft-tissue infection. J Med Microbiol. 2010;59:224–30. doi: 10.1099/jmm.0.011593-0. [DOI] [PubMed] [Google Scholar]
- 21.Lin YJ, Yang KY, Ho JT, Lee TC, Wang HC, Su FW. Nocardial brain abscess. J Clin Neurosci. 2010;17:250–3. doi: 10.1016/j.jocn.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Pelaez AI, Garcia-Suarez Mdel M, Manteca A, et al. A fatal case of Nocardia otitidiscaviarum infection and brain abscess: Taxonomic characterization by molecular techniques. Ann Clin Microbiol Antimicrob. 2009;8:11. doi: 10.1186/1476-0711-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conville PS, Brown JM, Steigerwalt AG, et al. Nocardia veterana as a pathogen in North American patients. J Clin Microbiol. 2003;41:2560–8. doi: 10.1128/JCM.41.6.2560-2568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conville PS, Fischer SH, Cartwright CP, Witebsky FG. Identification of Nocardia species by restriction endonuclease analysis of an amplified portion of the 16S rRNA gene. J Clin Microbiol. 2000;38:158–64. doi: 10.1128/jcm.38.1.158-164.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saubolle MA, Sussland D. Nocardiosis: Review of clinical and laboratory experience. J Clin Microbiol. 2003;41:4497–501. doi: 10.1128/JCM.41.10.4497-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerrits GP, Klaassen C, Coenye T, Vandamme P, Meis JF. Burkholderia fungorum septicemia. Emerg Infect Dis. 2005;11:1115–7. doi: 10.3201/eid1107.041290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CLSI . Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic Actinomycetes; Approved Standards. 2nd edn. Wayne: Clinical and Laboratory Standards Institute; 2011. CLSI document M24-A2. [PubMed] [Google Scholar]
- 28.Moser BD, Klenk HP, Schumann P, et al. Nocardia niwae sp. nov., isolated from human pulmonary sources. Int J Syst Evol Microbiol. 2010;60:2272–6. doi: 10.1099/ijs.0.020370-0. [DOI] [PubMed] [Google Scholar]
- 29.Conville PS, Brown JM, Steigerwalt AG, Brown-Elliott BA, Witebsky FG. Nocardia wallacei sp. nov. and Nocardia blacklockiae sp. nov., human pathogens and members of the “Nocardia transvalensis Complex”. J Clin Microbiol. 2008;46:1178–84. doi: 10.1128/JCM.02011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurent F, Rodríguez-Nava V, Noussair L, Couble A, Nicolas-Chanoine MH, Boiron P. Nocardia ninae sp. nov., isolated from a bronchial aspirate. Int J Syst Evol Microbiol. 2007;57:661–5. doi: 10.1099/ijs.0.64476-0. [DOI] [PubMed] [Google Scholar]
- 31.Minero MV, Marin M, Cercenado E, Rabadán PM, Bouza E, Muñoz P. Nocardiosis at the turn of the century. Medicine (Baltimore) 2009;88:250–61. doi: 10.1097/MD.0b013e3181afa1c8. [DOI] [PubMed] [Google Scholar]
- 32.Liu WL, Lai CC, Ko WC, et al. Clinical and microbiological characteristics of infections caused by various Nocardia species in Taiwan: A multicenter study from 1998 to 2010. Eur J Clin Microbiol Infect Dis. 2011;30:1341–7. doi: 10.1007/s10096-011-1227-9. [DOI] [PubMed] [Google Scholar]
- 33.Namnyak S, Uddin M, Ahmod N. Nocardia cyriacigeorgica bacteraemia presenting with cytomegalovirus disease and rapidly fatal pneumonia in a renal transplant patient: A case report. J Med Case Reports. 2011;5:228–31. doi: 10.1186/1752-1947-5-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnaud G, Deschamps C, Manceron V, et al. Brain abscess caused by Nocardia cyriacigeorgica in a patient with human immunodeficiency virus infection. J Clin Microbiol. 2005;43:4895–7. doi: 10.1128/JCM.43.9.4895-4897.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cargill JS, Boyd GJ, Weightman NC. Nocardia cyriacigeorgica: A case of endocarditis with disseminated soft-tissue infection. J Med Microbiol. 2010;59:224–30. doi: 10.1099/jmm.0.011593-0. [DOI] [PubMed] [Google Scholar]
- 36.Chavez TT, Fraser SL, Kassop D, Bowden LP, Skidmore PJ. Disseminated Nocardia cyriacigeorgica presenting as right lung abscess and skin nodule. Mil Med. 2011;176:586–8. doi: 10.7205/milmed-d-10-00346. [DOI] [PubMed] [Google Scholar]
- 37.Sim SH, Park HC, Kim CJ, et al. A case of Nocardia farcinica brain abscess in the patient receiving steroid treatment. Infect Chemother. 2008;40:301–4. [Google Scholar]
- 38.Bruno P, Ricci A, Pezzuto A, Martone L, Gencarelli G, Mariotta S. Severe pneumonia caused by Nocardia farcinica and complicated by Staphylococcus haemoliticus superinfection. Eur Rev Med Pharmacol Sci. 2011;15:401–5. [PubMed] [Google Scholar]
- 39.Parande MV, Shinde RS, Mantur BG, et al. A fatal case of empyema thoracis by Nocardia farcinica in an immunocompromised patient. Indian J Med Microbiol. 2010;28:390–2. doi: 10.4103/0255-0857.71831. [DOI] [PubMed] [Google Scholar]
- 40.Lai CC, Lee LN, Teng LJ, Wu MS, Tsai JC, Hsueh PR. Disseminated Nocardia farcinica infection in a uraemia patient with idiopathic thrombocytopenia purpura receiving steroid therapy. J Med Microbiol. 2005;54:1107–10. doi: 10.1099/jmm.0.46084-0. [DOI] [PubMed] [Google Scholar]
- 41.Heo ST, Ko KS, Kwon KT, et al. The first case of catheter-related bloodstream infection caused by Nocardia farcinica. J Korean Med Sci. 2010;25:1665–8. doi: 10.3346/jkms.2010.25.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalitha P. Nocardia keratitis. Curr Opin Opthalmol. 2009;20:318–23. doi: 10.1097/ICU.0b013e32832c3bcc. [DOI] [PubMed] [Google Scholar]
- 43.Candel FJ, González J, Matesanz M, et al. Bacteremic infection due to Nocardia otitidiscaviarum: Case report and review. An Med Interna. 2005;22:489–92. doi: 10.4321/s0212-71992005001000009. [DOI] [PubMed] [Google Scholar]
- 44.Ramamoorthi K, Pruthvi BC, Rao NR, Belle J, Chawla K. Pulmonary nocariosis due to Nocardia otitidiscaviarum in an immunocompetent host – a rare case report. Asian Pac J Trop Med. 2011;4:414–6. doi: 10.1016/S1995-7645(11)60116-8. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe K, Shinagawa M, Amishima M, et al. First clinical isolates of Nocardia carnea, Nocardia elegans, Nocardia paucivorans, Nocardia puris and Nocardia takedensis in Japan. Nippon Ishinkin Gakkai Zasshi. 2006;47:85–9. doi: 10.3314/jjmm.47.85. [DOI] [PubMed] [Google Scholar]
- 46.Friedman CS, Beaman BL, Chun J, Goodfellow M, Gee A, Hedrick RP. Nocardia crassostreae sp. nov., the causal agent of nocardiosis in Pacific oysters. Int J Syst Bacteriol. 1998;8:237–46. doi: 10.1099/00207713-48-1-237. [DOI] [PubMed] [Google Scholar]
- 47.Maki Y, Uchida Y, Monji N, et al. Microbiological and clinical features of nine cases with nocardial infections. Rinsho Byori. 2011;59:213–8. (Abst). [PubMed] [Google Scholar]
- 48.Kageyama A, Poonwan N, Yazawa K, Mikami Y, Nishimura K. Nocardia asiatica sp. nov., isolated from patients with nocardiosis in Japan and clinical specimens from Thailand. Int J Syst Evol Microbiol. 2004;54:125–30. doi: 10.1099/ijs.0.02676-0. [DOI] [PubMed] [Google Scholar]
- 49.Agterof MJ, Van der Bruggen T, Tersmette M, Ter Borg EJ, Van den Bosch JM, Biesma DH. Nocardiosis: A case series and a mini review of clinical and microbiological features. Neth J Med. 2007;65:199–202. [PubMed] [Google Scholar]
- 50.Lederman ER, Crum NF. A case series and focused review of nocardiosis: Clinical and microbiological aspects. Medicine (Baltimore) 2004;83:300–13. doi: 10.1097/01.md.0000141100.30871.39. [DOI] [PubMed] [Google Scholar]
- 51.Lai CC, Liu WL, Ko WC, et al. Antimicrobial-resistant Nocardia isolates, Taiwan, 1998–2009. Clin Infect Dis. 2011;52:833–5. doi: 10.1093/cid/ciq255. [DOI] [PubMed] [Google Scholar]
- 52.Glupczynski Y, Berhin C, Janssens M, Wauters G. Determination of antimicrobial susceptibility patterns of Nocardia spp.from clinical specimens by Etest. Clin Microbiol Infect Dis. 2006;12:905–12. doi: 10.1111/j.1469-0691.2006.01460.x. [DOI] [PubMed] [Google Scholar]
- 53.Uhde KB, Pathak S, McCullum I, Jr, et al. Antimicrobial-resistant Nocardia isolates, United States, 1995–2004. Clin Infect Dis. 2010;51:1445–8. doi: 10.1086/657399. [DOI] [PubMed] [Google Scholar]
- 54.Cercenado E, Marín M, Sánchez-Martínez M, Cuevas O, Martínez-Alarón J, Bouza E. In vitro activities of tigecycline and eight other antimicrobials against different Nocardia species identified by molecular methods. Antimicrob Agents Chemother. 2007;51:1102–04. doi: 10.1128/AAC.01102-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laurent F, Poirel L, Naas T, et al. Biochemical-genetic analysis and distribution of FAR-1, a class A beta-lactamase from Nocardia farcinica. Antimicrob Agents Chemother. 1999;43:1644–50. doi: 10.1128/aac.43.7.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]


