Abstract
BACKGROUND:
Current literature reports that outpatient parenteral antimicrobial therapy (OPAT) programs improve cure rates, and reduce length of hospitalization and costs. OPAT programs are still relatively new in Canada.
OBJECTIVE:
To evaluate the benefits of an OPAT program initiated at a multispecialty tertiary care facility in Toronto, Ontario, compared with the previous standard of care.
METHODS:
The present retrospective observational study was conducted using data from a group of surgical patients who were treated for active infections. Between February 1, 2010 and November 30, 2010, a total of 108 surgical patients were enrolled in the OPAT program. Patients were matched 1:1 with historical controls discharged between January 1, 2001 and January 1, 2010 according to age, sex, type of surgery, infection and comorbidities (Charlson Comorbidity Index). Cure rate, 30-day rehospitalization and length of stay were evaluated as primary end points.
RESULTS:
Of 108 eligible OPAT patients, 21 were matched to the control group using the prespecified criteria. For this cohort, the OPAT program was associated with improved cure rates (OPAT 61.7% versus control 57.1%; P>0.10), reduction in rehospitalization rate (14.3% versus 28.6%; P>0.10) and reduced length of stay (10.7 versus 13.9 days, P>0.10) compared with the control group.
CONCLUSIONS:
For this cohort of surgery patients, the OPAT program demonstrated a trend toward improved outcomes but did not achieve statistical significance. Due to the lack of statistical power, further evaluation is required to determine the full benefit of OPAT to patients and the health care system.
Keywords: Home parenteral antimicrobial therapy, OPAT, Outpatient parenteral antimicrobial therapy
Abstract
HISTORIQUE :
D’après les rapports bibliographiques actuels, les programmes d’antibiothérapie parentérale ambulatoire (ATPA) améliorent les taux de guérison et réduisent la durée d’hospitalisation et les coûts. Les programmes d’ATPA sont encore relativement nouveaux au Canada.
OBJECTIF :
Évaluer les avantages d’un programme d’ATPA lancé dans un centre de soins tertiaires multidisciplinaire de Toronto, en Ontario, par rapport aux normes de soins antérieures.
MÉTHODOLOGIE :
Les chercheurs ont mené la présente étude d’observation rétrospective à l’aide des données d’un groupe de patients opérés traités en raison d’infections actives. Entre le 1er février et le 30 novembre 2010, 108 patients opérés ont été inscrits au programme d’ATPA. Les patients ont été jumelés 1:1 avec des sujets témoins historiques qui ont obtenu leur congé entre le 1er janvier 2001 et le 1er janvier 2010 d’après leur âge, leur sexe, le type d’opération, l’infection et les comorbidités (indice de comorbidité de Charlson). Les paramètres principaux étaient le taux de guérison, la réhospitalisation au bout de 30 jours et la durée d’hospitalisation.
RÉSULTATS :
Sur les 108 patients du programme d’ATPA admissibles, 21 ont été jumelés au groupe témoin au moyen des critères pré-définis. Dans cette cohorte, le programme d’ATPA s’associait à un meilleur taux de guérison (61,7 % pour l’ATPA par rapport à 57,1 % pour le groupe témoin; P>0,10), à une réduction du taux de réhospitalisation (14,3 % par rapport à 28,6 %; P>0,10) et à diminution de la durée d’hospitalisation (10,7 par rapport à 13,9 jours, P>0,10) que dans le groupe témoin.
CONCLUSIONS :
Auprès de cette cohorte de patients opérés, le programme d’ATPA démontrait une tendance vers de meilleures issues, sans pour autant avoir de signification statistique. En raison de l’absence d’efficacité statistique, il faudra approfondir l’évaluation afin de déterminer les avantages du programme d’ATPA pour les patients et le système de santé.
Outpatient parenteral antimicrobial therapy (OPAT) is officially defined as at least two doses of an intravenous (IV) antibiotic on different days without hospitalization, and was first introduced in the United States in the 1970s (1). OPAT programs usually involve a team comprised of an infectious diseases physician, an infectious diseases or antimicrobial stewardship pharmacist, and a registered nurse (1,2). Other stakeholders may also be involved on an ad hoc basis (1,2). After determining patient eligibility, the OPAT team ensures the appropriate selection and dosing of antimicrobial(s), as well as administration and storage of the drug(s) for the patient on discharge and throughout the duration of therapy (3). The OPAT pharmacist is responsible for helping with the selection of the appropriate antimicrobial(s) and vascular access device (VAD), as well as providing patient education and appropriate monitoring for safety and efficacy of drug therapy (1,4).
Due to evidence of cost savings, reductions in hospitalization, similar or improved efficacy and safety compared with inpatient therapy, and improvements in quality of life, OPAT programs have grown worldwide, including in Canada (1–3,5–10). However, minimal Canadian data on the efficacy and safety of an OPAT program have been published in the current literature.
The OPAT program at the University Health Network (UHN) (Toronto, Ontario) was initiated at two of its hospital sites (Toronto General Hospital and Toronto Western Hospital) in February 2010 as part of the Antimicrobial Stewardship Program following the identification of patients developing significant nephrotoxicity while continuing IV vancomycin after hospital discharge. The purpose of the program was to optimize antimicrobial therapy for patients discharged on parenteral antimicrobials, with an emphasis on patient safety. The program included patients who were medically stable for discharge, and accepted by ambulatory care centres province-wide. The OPAT team monitored the patient’s clinical status, laboratory values and drug levels, and performed regular follow-up as defined by existing guidelines (1). The program had more than 200 patients in its roster with six to eight new patient referrals per week, with the majority from priority surgery programs (cardiovascular, vascular, orthopedic and neurosurgery) as well as general internal medicine. The present study aimed to compare the clinical outcomes of patients discharged on the UHN OPAT program with patients discharged with the previous standard of care.
METHODS
A retrospective chart review was conducted using a pre-post study design from the OPAT perspective for surgery patients who received more than two doses of a parenteral antimicrobial for more than one day, as per the definition. The project focused on surgery patients as the most prevalent users of the OPAT program and also to minimize confounders. The project received approval from the UHN Research Ethics Board. Patients in the standard of care group were monitored, discharged and followed up by their surgical team at approximately six weeks post discharge, while patients in the OPAT group were given one predischarge counselling session plus follow-up of laboratory values and clinical status at approximately weekly to biweekly intervals throughout the duration of therapy.
Patients from the pre-OPAT period were derived from the Radiology Information Service (RIS) database for VAD insertions, because no database stratifying patients discharged on parenteral antimicrobials existed before the OPAT program. The pre-OPAT period evaluated was nine years (January 1, 2001 to January 1, 2010) due to limitations of the data storage system. Patients from the OPAT group were derived from the OPAT database. The OPAT period evaluated was from February 1, 2010 to November 30, 2010.
To minimize confounders, patients were matched within their surgery type according to age, sex, comorbidities as defined by the Charlson Comorbidity Index and type of infection (11). Patients with multiple courses within the OPAT group and patients who were admitted both before and after OPAT program implementation were also eligible for inclusion. Patients with more than one course followed by the OPAT team or in the control group only had the initial course included in the outcome analysis.
Clinical status at the end of therapy (cure versus improved or controlled versus failure) was the major primary end point. Cure was defined as clinical signs and symptoms resolved at the end of therapy, and either no further antimicrobial treatment or a finite period of oral step-down therapy was required after parenteral therapy. Controlled/improved was defined as partial resolution of clinical signs and symptoms, and/or need for additional therapy or change in therapy at the end of the initial course due to the above reasons, or suppression of signs and symptoms with current antimicrobial therapy and indefinite oral step-down antimicrobial therapy was required. Failure was defined as no change in clinical status, or worsening signs and symptoms, or need for additional therapy or change in therapy due to the above reasons. Other primary end points included primary length of stay and all-cause 30-day rehospitalization at one of the two UHN sites included in the present study. Secondary end points included incidence of device-related complications and adverse drug reactions.
Based on a hypothesized difference in cure rate of 5% to 10% compared with the standard-of-care group, with the OPAT cure rate estimated at 90%, a sample size of 37 pairs, with a minimum sample size of 12 pairs, was needed, to achieve 80% power and type I error of 0.05. Due to the matched nature of the study, the paired t test was used to compare the means of continuous variables for baseline characteristics as well as the means of continuous variable end points, and the McNemar’s χ2 test was used for discrete end points. All tests were two-tailed, with P<0.05 considered to be statistically significant.
RESULTS
OPAT group
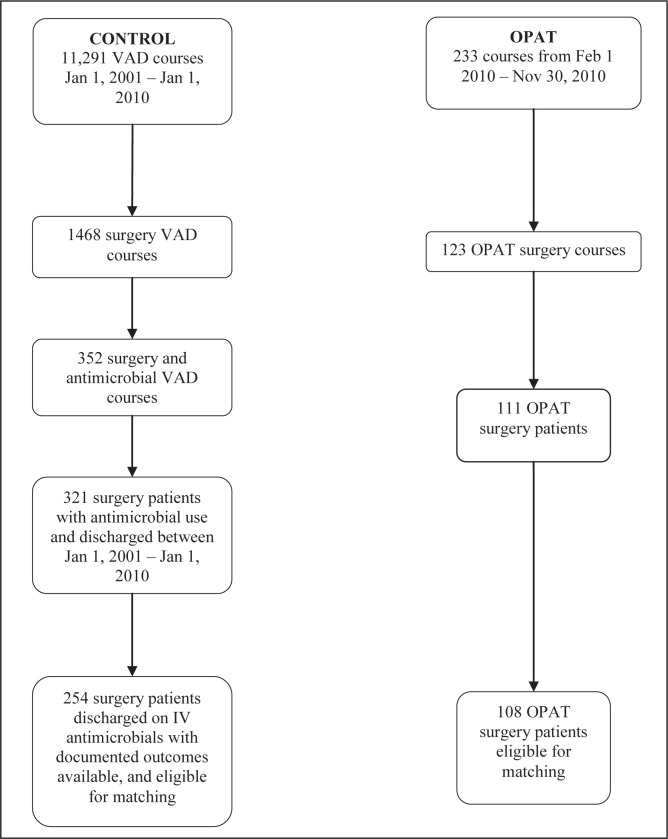
Two hundred thirty-three courses were recorded for OPAT patients discharged between February 1, 2010 and November 30, 2010, with 111 patients from the surgical programs of interest (23 cardiovascular surgery, 29 neurosurgery, 17 vascular surgery and 42 orthopedic surgery). Three patients were excluded from the orthopedic surgery group – one patient initiated OPAT therapy on an outpatient basis, one patient was switched to oral therapy before discharge and one patient was diagnosed with gout. In total, 108 patients were eligible for matching (Figure 1).
Figure 1).
Screening process. Due to the very large initial term of derivation (11,291 vascular access device [VAD] courses) for control patients, Excel (Microsoft Corporation, USA) was used for filtering the initial steps. Patients were determined to be using the VAD for antimicrobial purposes by the reported descriptions in the database. Once antimicrobial courses were determined, the rest of the screening process was performed manually. Feb February; IV Intravenous; Jan January; OPAT Outpatient parenteral antimicrobial therapy
Control group
The RIS database yielded a total of 11,291 VAD procedures from January 1, 2001 to January 1, 2010. From these, 254 patients (76 cardiovascular surgery, 51 neurosurgery, 33 vascular surgery and 94 orthopedic surgery) were discharged on IV antimicrobials with outcome data available (Figure 1).
Matching
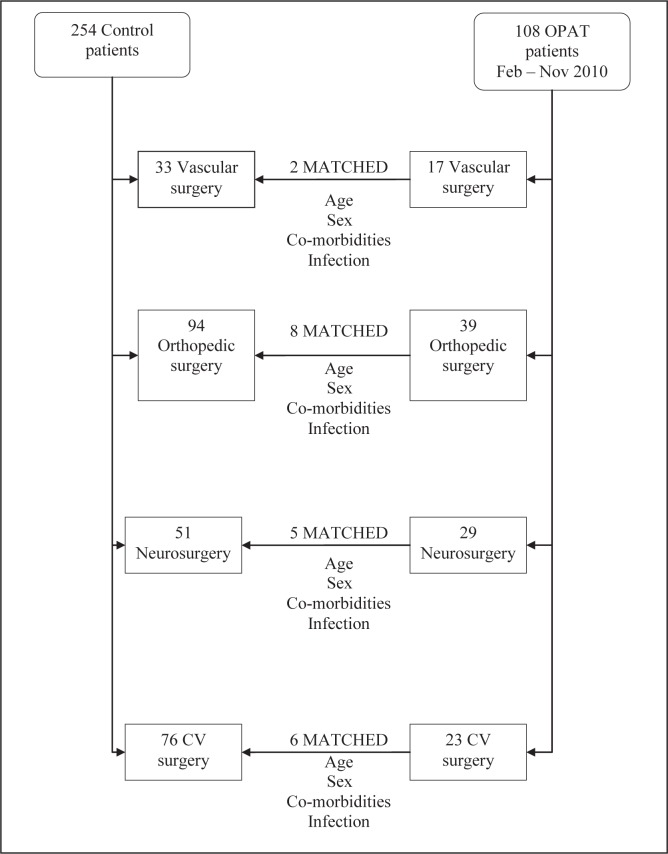
From the 108 OPAT patients eligible for matching, 21 were able to be matched using all four criteria (Figure 2). The distribution of patients according to surgery type was similar to the entire OPAT population. Patients were similar between the two groups, with the majority of infections being osteomyelitis and prosthetic joint infections (Table 1). Despite not being matching parameters, the non-Charlson Comorbidity Index comorbidities and antibiotic use were similar.
Figure 2).
Schematic diagram of the matching process undertaken. Due to the stringent matching criteria, only 21 of the 108 outpatient parenteral antimicrobial therapy (OPAT) patients had a matched control. CV Cardiovascular; Feb February; Nov November
TABLE 1.
Population characteristics
| Factor | Control group | OPAT group |
|---|---|---|
| n | 21 | 21 |
| Age, years, mean ± SD | 59.1±17.3 | 58.8±17.2 |
| Female sex | 9 (42.9) | 9 (42.9) |
| Procedure | ||
| Neurosurgery | 5 (23.8) | 5 (23.8) |
| Vascular surgery | 2 (9.5) | 2 (9.5) |
| Cardiovascular surgery | 6 (28.6) | 6 (28.6) |
| Orthopedic surgery | 8 (38.1) | 8 (38.1) |
| Infection | ||
| Osteomyelitis | 7 (33.3) | 7 (33.3) |
| Joint (endogenous) | 1 (4.8) | 1 (4.8) |
| Joint (prosthetic) | 7 (33.3) | 7 (33.3) |
| Vascular graft | 1 (4.8) | 1 (4.8) |
| Endocarditis/pacemaker | 4 (19.0) | 4 (19.0) |
| Brain abscess | 1 (4.8) | 1 (4.8) |
| Charlson Comorbidity Index, mean | 1.33 | 1.33 |
| Source control achieved | 17 (81.0) | 19 (90.4) |
| Treatment duration, days, mean ± SD | 39.0±6.9 | 42.7±5.4 |
Data presented as n (%) unless otherwise specified. OPAT Outpatient parenteral antimicrobial therapy
Primary end points
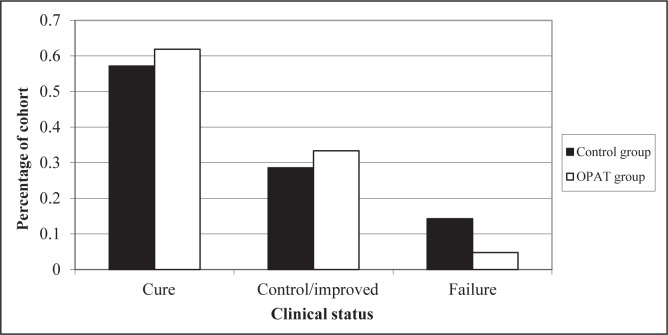
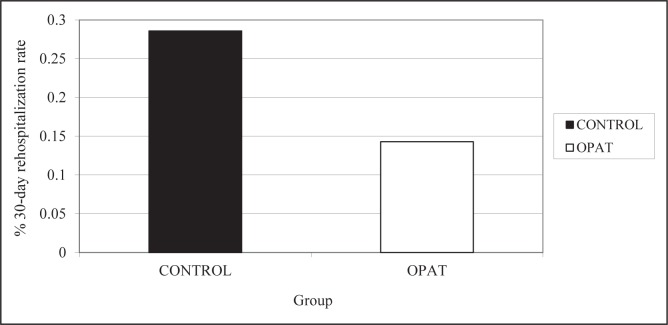
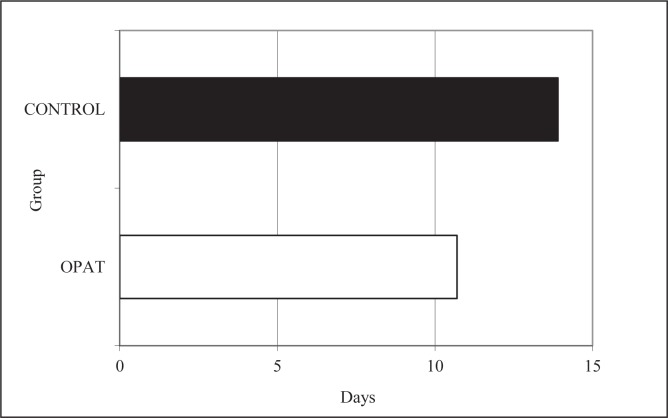
The OPAT group had a better overall cure rate than the control group (61.9% versus 57.1%, P=1.00), as shown in Figure 3, but this did not achieve statistical significance. For a composite primary end point of clinical successes (ie, combined cures and controlled/improved cases), the results were 95% (OPAT) versus 86% (control), P=0.62. For three patients who experienced treatment failure in the control group, the reason was documented to be previous source control measures; for one patient in the OPAT group, treatment failure was due to drug therapy complicated by an occluded peripherally inserted central catheter line. The incidence of 30-day rehospitalization in the OPAT group was also nonsignificantly reduced by 50% compared with the control group (Figure 4, P=0.51). The mean hospital length of stay was shorter in the OPAT group than the control group by 3.2 days (Figure 5, P=0.36).
Figure 3).
Clinical status at the end of therapy for both groups. OPAT Outpatient parenteral antimicrobial therapy
Figure 4).
Rate of 30-day rehospitalization for both groups. OPAT Outpatient parenteral antimicrobial therapy
Figure 5).
Mean hospital length of stay for both groups
Secondary end points
On the basis of similar antimicrobial use, the total incidence of adverse drug reactions and VAD-related complications were similar between the two groups (Table 2). In the control group, 60% of patients experiencing an adverse event required a change or discontinuation of antimicrobial therapy compared with 20% of patients in the OPAT arm (Table 2, P=0.62). There were no rehospitalizations for adverse drug reactions or VAD-related complications in the OPAT group, while there was one case of each in the control group.
TABLE 2.
Secondary end points
| Secondary end point | Control group | OPAT group | P |
|---|---|---|---|
| Patients with reported adverse drug reactions, n (%) | 5 (23.8) | 5 (23.8) | 0.72 |
| Allergic reaction | 2 | 1 | |
| Hematological | 0 | 1 | |
| Renal/liver toxicity | 1 | 1 | |
| Gastrointestinal | 0 | 1 | |
| Neurological | 1 | 0 | |
| Abnormal drug level | 0 | 2 | |
| Other | 1 | 0 | |
| Total reported adverse drug reactions | 5 | 6 | |
| Patients with therapy stopped/switched due to ADR, n (%) | 3 (60.0) | 1 (20.0) | 0.62 |
| VAD-related complications, n (%) | 2 (9.5) | 2 (9.5) | 0.62 |
Data presented as n unless otherwise specified. ADR Adverse drug reaction; OPAT Outpatient parenteral antimicrobial therapy; VAD Vascular access device
DISCUSSION
Our cure rate of 62% in the OPAT program was lower than the 80% to 90% reported in the literature (3,5,12–20). However, the majority of these studies were published more than five years ago, and the intervention arms included all enrolled OPAT patients versus a stringently selected surgical population. The lower cure rate may also be explained by the increasing complexities of patients being managed on an out-patient basis. Our results showed less frequent failures due to source control, possibly due to greater involvement of the OPAT team in decisions involving optimal source control.
Our rehospitalization rate of 14.3% for the OPAT group was within the range of approximately 7% to 18% reported in the literature (3,19,21–23). No patients in the OPAT group were rehospitalized for adverse drug reactions or complications, while there were such cases in the control group (eg, rehospitalization for peripherally inserted central catheter line change for a suspected line infection). The mean length of stay for the OPAT group was similar to the 12 days reported in a Canadian OPAT cost analysis, which evaluated a more general population and compared with inpatient controls (21).
The incidences of adverse drug reactions for the two groups were both within the range of 5% to 35% reported in the literature (3,12,13,19,21–23). For the OPAT group, two patients experienced abnormal serum drug levels while none was observed in the control group. This may reflect the fact that the OPAT program provided more frequent therapeutic drug monitoring.
The present study reflected our OPAT program experience at a single centre and may also represent the OPAT experience within Ontario. To our knowledge, the present study was the first to attempt a comparison with the current ambulatory centre standard of care model in Canada rather than with inpatients or no control groups. This was also one of the few studies that attempted to minimize baseline differences using a matched study design.
Our results were affected by program limitations and study design. While our program attempted to maximize quality and efficiency in its first year, a significant amount of time was devoted to successful coordination with community infrastructure, reducing the time available for direct patient care activities, which could have influenced our results. Given project feasibility, the timeframe of collected data was only 10 months, compared with a minimum of 12 months in previous published reports. The duration may be insufficient in reflecting the true steady state outcomes of the program.
The greatest challenge of the present study involved patient matching, due to the size ratio of the OPAT group to the control group being only 1:2.4. While a size ratio of at least 1:5 would have been ideal, we were hindered by the lack of a standardized database at UHN tracking patients discharged on IV antimicrobials before this program, and attempted to circumvent this challenge by using the RIS database. However, eligible patients may still have been omitted, which resulted in our smaller-than-optimal sample size and our study being underpowered. Other limitations included a heavy reliance on accurate documentation of outcomes, and results were only applicable for surgery patients and rehospitalizations were limited to our institution.
Finally, there were factors we were unable to account for, eg, clinician differences and changes in practice over time. All decisions regarding source control and initial choice of antimicrobials were left to the discretion of referring physicians. It was beyond the scope of the present study to determine whether best practice was being followed. All of the above may have affected the observed clinical outcomes.
CONCLUSION
The results at the 10-month point of the OPAT program in this tertiary teaching hospital in Canada demonstrated that for surgery patients with similar age, sex, comorbidities, types of infection and antimicrobial therapy, there was a trend toward improved cure rate, reduced primary length of hospital stay and reduced 30-day rehospitalization rate in the OPAT group compared with the previous standard of care. The above results may suggest more confidence in discharging patients on the appropriate antimicrobial treatment earlier, given the availability of the OPAT program to provide consistent follow-up and measures to manage complications and adverse drug reactions on an outpatient basis. Future studies focusing on a more general group of patients and on provincial health care system usage as a broader outcome measure would better evaluate applicability and utility.
Acknowledgments
The authors thank the University Health Network/Mount Sinai Hospital joint Antimicrobial Stewardship Program, the University Health Network department of Pharmacy and department of Medicine (division of Infectious Diseases), as well as the University of Toronto for support of the project.
REFERENCES
- 1.Tice A, Rehm SH, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38:1651–65. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 2.Gilchrist M, Franklin BD, Patel JP. An outpatient parenteral antibiotic therapy (OPAT) map to identify risks associated with an OPAT service. J Antimicrob Chemother. 2008;52:177–83. doi: 10.1093/jac/dkn152. [DOI] [PubMed] [Google Scholar]
- 3.Kieran J, O’Reilly A, Parker J, Clarke S, Bergin C. Self-administered outpatient parenteral antimicrobial therapy: A report of three years experience in the Irish healthcare setting. Eur J Clin Microbiol Infect Dis. 2009;28:1369–74. doi: 10.1007/s10096-009-0794-5. [DOI] [PubMed] [Google Scholar]
- 4.American Society of Health-System Pharmacists ASHP guidelines on the pharmacist’s role in home care. Am J Health-Syst Pharm. 2000;57:1252–7. doi: 10.1093/ajhp/57.13.1252. [DOI] [PubMed] [Google Scholar]
- 5.Nathwani D. Developments in outpatient parenteral antimicrobial therapy (OPAT) for Gram-positive infections in Europe, and the potential impact of daptomycin. J Antimicrob Chemother. 2009;64:447–53. doi: 10.1093/jac/dkp245. [DOI] [PubMed] [Google Scholar]
- 6.Choudri S. The Manitoba Community Intravenous Therapy Program: Then and now. Can J Infect Dis. 2000;11(Suppl A):5. [Google Scholar]
- 7.Stiver G, Wai A, Chase L, Frighetto L, Marra CA, Jewesson P. Outpatient intravenous antibiotic therapy: The Vancouver Hospital experience. Can J Infect Dis. 2000;11(Suppl A):11–4. [Google Scholar]
- 8.Martel A. Monitoring guidelines for home and outpatient parenteral antibiotic therapy. Can J Infect Dis. 2000;11(Suppl A):35–44. [Google Scholar]
- 9.Delorme L, Frenette C, LeCorre I, Duchesne J, Delorme C, Plourde P. Ambulatory intravenous antibiotic therapy in Quebec: The Hôpital Charles LeMoyne experience in 1996. Can J Infect Dis. 2000;11(Suppl A):6–10. [Google Scholar]
- 10.Pennie RA, Glavin V, Smaill FM. Experience with a special outpatient antimicrobial program at McMaster University Medical Centre in 1997. Can J Infect Dis. 2000;11(Suppl A):15–6. [Google Scholar]
- 11.Abdullah M, Al-Salamah SM. Impact of comorbidity on outcome among acute non-traumatic surgical patients. Evaluation of Charlson comorbidity index. Saudi Med J. 2009;30:228–33. [PubMed] [Google Scholar]
- 12.Tice A. The use of outpatient parenteral antimicrobial therapy in the management of osteomyelitis: Data from the Outpatient Parenteral Antimicrobial Therapy Outcomes Registries. Chemotherapy. 2001;47(Suppl 1):5–16. doi: 10.1159/000048563. [DOI] [PubMed] [Google Scholar]
- 13.Upton A, Ellis-Pegler R, Woodhouse A. Outpatient parenteral antimicrobial therapy (OPAT): A review of experience at Auckland Hospital. N Z Med J. 2004;117:1–9. [PubMed] [Google Scholar]
- 14.Martone WJ, Lindfield KC, Katz DE. Outpatient parenteral antibiotic therapy with daptomycin: Insights from a patient registry. Int J Clin Pract. 2008;62:1183–7. doi: 10.1111/j.1742-1241.2008.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard L, El-hajj E, Pron B, et al. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: Evaluation of efficacy, tolerance and cost. J Clin Pharm Ther. 2001;26:445–51. doi: 10.1046/j.1365-2710.2001.00380.x. [DOI] [PubMed] [Google Scholar]
- 16.Rehm S. Community-based outpatient parenteral antimicrobial therapy (CoPAT) for Staphylococcus aureus bacteraemia with or without infective endocarditis: Analysis of the randomized trial comparing daptomycin with standard therapy. J Antimicrob Chemother. 2009;63:1034–42. doi: 10.1093/jac/dkp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tice A, Strait K, Ramey R, Hoaglund PA. Outpatient parenteral antimicrobial therapy for central nervous system infections. Clin Inf Dis. 1999;29:1394–9. doi: 10.1086/313503. [DOI] [PubMed] [Google Scholar]
- 18.Wolter JM, Cagney RA, McCormack JG. A randomised trial of home vs hospital intravenous antibiotic therapy in adults with infectious diseases. J Infect. 2004:48263–8. doi: 10.1016/S0163-4453(03)00135-X. [DOI] [PubMed] [Google Scholar]
- 19.Yong C, Fisher DA, Sklar GE, Li S. A cost analysis of outpatient parenteral antibiotic therapy: An Asian perspective. Int J Antimicrob Agents. 2009;33:46–51. doi: 10.1016/j.ijantimicag.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Gesser RM, McCarroll KA, Woods GL. Evaluation of outpatient treatment with ertapenem in a double blind controlled clinical trial of complicated skin/skin structure infections. J Infect. 2004;48:32–8. doi: 10.1016/j.jinf.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Wai A, Frighetto L, Marra C, Chan E, Jewesson PJ. Cost analysis of an adult outpatient parenteral antimicrobial therapy (OPAT) programme: A Canadian teaching hospital and Ministry of Health perspective. Pharmacoeconomics. 2000;18:451–7. doi: 10.2165/00019053-200018050-00004. [DOI] [PubMed] [Google Scholar]
- 22.Matthews PC, Conlon CP, Berendt AR, et al. Outpatient parenteral antimicrobial therapy (OPAT): Is it safe for selected patients to self-administer at home? A retrospective analysis for a large cohort over 13 years. J Antimicrob Chemother. 2007;60:356–62. doi: 10.1093/jac/dkm210. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman-Terry ML, Fraimow HS, Fox TR, Swift BG, Wolf JE. Adverse effects of outpatient parenteral antimicrobial therapy. Am J Med. 1999;106:44–9. doi: 10.1016/s0002-9343(98)00362-3. [DOI] [PubMed] [Google Scholar]