Abstract
Humans have hunted wildlife in Central Africa for millennia. Today, however, many species are being rapidly extirpated and sanctuaries for wildlife are dwindling. Almost all Central Africa's forests are now accessible to hunters. Drastic declines of large mammals have been caused in the past 20 years by the commercial trade for meat or ivory. We review a growing body of empirical data which shows that trophic webs are significantly disrupted in the region, with knock-on effects for other ecological functions, including seed dispersal and forest regeneration. Plausible scenarios for land-use change indicate that increasing extraction pressure on Central African forests is likely to usher in new worker populations and to intensify the hunting impacts and trophic cascade disruption already in progress, unless serious efforts are made for hunting regulation. The profound ecological changes initiated by hunting will not mitigate and may even exacerbate the predicted effects of climate change for the region. We hypothesize that, in the near future, the trophic changes brought about by hunting will have a larger and more rapid impact on Central African rainforest structure and function than the direct impacts of climate change on the vegetation. Immediate hunting regulation is vital for the survival of the Central African rainforest ecosystem.
Keywords: Central Africa, hunting, future, wildlife, land-use change, ecological function
1. Introduction
Hunting is a ubiquitous part of daily life in rural Central Africa. Wild meat is part of the village subsistence economy, and commercial wildlife hunting—practised in Central Africa for at least two millennia—continues today [1,2]. Contemporary illegal wildlife trade, now one of the three most important types of crime on the planet [3], uses village hunters to secure tusks, meat and skins, but an increasing number of commercial hunters, using heavier-calibre weapons than those available to villagers, and particularly targeting ivory-bearing elephants, are also hunting in the region; meat and ivory then pass via highly organized trade chains to their destinations in the cities of the region and overseas [4–7]. The ecological consequences of wildlife hunting in the tropics are far-reaching, with knock-on effects disrupting ecological function over large areas and in key ecosystems [8–10].
Human shaping of the rainforest environment is a significant evolutionary force for the biome [1,11], and African rainforest species, unlike those endemic to other continents, have evolved in the constant presence of hominid hunters and their ancestors [12]. However, modern human hunting pressure, driven largely by increasing commercial trade, has resulted in the local extirpation of many larger African rainforest mammals [13–17] and is changing wildlife assemblages and species interactions [18,19]. Overhunting of wildlife is highly associated with loss of ecological integrity in tropical forests [20] and significant, deleterious trophic cascades in other ecosystems [9,10], and similar changes are almost certainly happening in the rainforests of Central Africa, although empirical studies are few. Modern hunting practices in Central Africa are already modifying rainforest ecosystem function, and in synergy with changes in climate and land use may become increasingly influential in the future.
Within this special issue, the future of African rainforests is discussed in relation to likely climate change and land-use scenarios. In this paper, we
— collate and synthesize existing data on direct hunting impacts in the Central African region;
— review the factors driving human hunting in Central African forests and the empirical evidence for indirect ecological impacts of hunting, and discuss how the African rainforests of the twenty-first century are being shaped by current hunting activity; and
— consider how the future scenarios for land-use change (LUC) and climate change outlined in this special issue are likely to influence, and interact with, the drivers of wildlife hunting in Central Africa, to explore the potential long-term consequences for the region's rainforests.
2. The hunting footprint
Central Africa is one of the ‘most remote’ areas of tropical moist forest in the world, based on human settlement density, infrastructure and road location [21], yet empirical data from village hunting studies and ecological surveys in the region show that much of this remote forest is already accessed by hunters (table 1).
Table 1.
Village and commercial hunting distances.
| (a) evidence of village hunting distances, from village hunting surveys | |||
|---|---|---|---|
| source | site | method | maximum distance of traps and hunting camps from village |
| [22] | Ekom, Cameroon | GPS follow | hunting camps: 18 km |
| [23] | Oleme and Diba, ROC | GPS follow | traplines: 8.0 (Oleme) and 3.9 km (Diba) |
| [24] | Ituri, DRC | estimate | most hunting occurs within approximately 15 km of settlements |
| [25] | Mossapouna, CAR | GPS follow | day hunting: 10 km from village; hunting camps: 21 km from village |
| [26] | Sendje, Equatorial Guinea | GPS follow | hunting camps: 30 km, hunters trap a max of 3.2 km from the village or a hunting camp (1 day trip) |
| [27] | Zoulabot Ancien, Cameroon | GPS follow | hunting camps: 21.5 km from village, snares set in a radius of approximately 3 km from camp; every 2 years hunters stay in a hunting camp less than 50 km from the village, for 2 months or more |
| [28] | Mekas, Cameroon | estimate | village hunting: approximately 5–10 km; hunting camps: approximately 40 km |
| [29] | Nsiete, Gabon | hunter interviews | more than 10 km in the dry season. Less than 2 km in the wet season |
| [30] | Midyobo Anvom, Equatorial Guinea | GPS follow | hunting camps: 13.2 km, hunters travelled 2–3 km from hunting camps to place traps |
| [31]a | Dibouka and Kouagna, Gabon | GPS follow | village traplines: 6.5 km; hunting camps 12.7 km |
| (b) evidence of hunting presence in protected areas, from ecological transect surveys | |||
| source | site | furthest record of hunting sign, from nearest road or river (foot transect surveys; in kilometres) | |
| [32] | Nouabale Ndoki, protected landscape ROC | <20 | |
| [32] | Ntokou Pikounda forest, ROC | <25 | |
| [33] | Mbam Djerem National Park, Cameroon | <27 | |
| [34] | Odzala-Koukoua National Park, ROC | <30 | |
| [35] | Sankuru landscape, DRC | <40 | |
| [36] | Lopé National Park, Gabon | <22 | |
| [37] | Mont de Cristal, Gabon | <10 | |
| [38] | Lac Tele National Park, ROC | <25 | |
| [39] | Waka National Park, Gabon | <10 | |
aMaximum trapping distances calculated for this paper; analysis of trapping distances and methods can be found in Coad et al. [31].
Humans, as central place foragers, gather food resources in a halo around the village [40,41], and village hunters in Central Africa will usually travel less than 10 km from the village during a day trip (table 1). However, most studies also document village hunters' use of hunting camps. These satellite ‘central places’, situated up to 50 km from the village, are used to catch larger bodied species favoured by the commercial trade [41,42], which are often highly depleted closer to the village where hunting pressure is more intense [31,43]. There have been no direct studies of forest use and offtake by purely commercial hunters in Central Africa: salaried hunters tend not to be based at a fixed point, and their activity is predominantly illegal and therefore concealed. However, ecological transect surveys often record hunting signs such as snares, gun cartridges and hunting paths, which suggests that hunters are penetrating up to 40 km into the forest from the nearest access point such as roads and rivers (table 1).
Even in 1998, more than 40% of Central African forest was within 10 km of a road, and more than 90% was within 50 km of a road [44]. Recent improvements in the road networks of many countries, expansion of logging and other industries and availability of mechanized transport in the region have increased the number of access points for hunters, who are likely to now be accessing the majority of the ‘most remote’ lands throughout the Congo Basin [44,45] (see the electronic supplementary material, figure S1).
3. Biological extent and impact of hunting
The biological impacts of hunting comprise both the direct impact on prey species (removal of individuals) and the cascade effects of changing ecological function across the trophic web, as species declining under extreme hunting pressure change their ecological interactions with others [10].
(a). Prey species
An estimated 178 species are currently hunted and used in the wild meat industry in Central Africa [46]. The survival of over half (97) of these species is deemed threatened by this hunting [47]. Site-specific species lists compiled throughout the region typically show that the majority of local species are used in wild meat consumption and trade, with mammals dominating the harvest (see the electronic supplementary material, table S1). On average, over 60% of village hunting offtake comprises small ungulate and rodent species (see the electronic supplementary material, table S1), which are often caught using wire cable or tough plastic snares. However, escalating gun hunting facilitates the hunting of larger bodied animals, such as elephant or buffalo and primates, which can become the most common taxonomic group at market and village level in regions opened to gun hunting [18,48]. Ivory poaching is currently having exceptionally high impacts in Central Africa [49–51]. Forest elephants across the region declined by 62% between 2002 and 2011 and there is no sign of a fall in the rate of poaching [17]. Almost 90% of elephant carcasses found by guard patrols and on surveys within Central African protected areas in 2011 had been illegally killed [52,53]. Ape populations are also dropping very rapidly in the region, declining by 50% between 1984 and 2000 [54], targeted by hunters for commercial opportunity rather than for family meat [55].
(b). Changes in wildlife assemblages
Wildlife species are not equally affected by hunting, although some general ecological rules are clear: large, low-density, slow-reproducing and specialist species, such as elephants, will be more vulnerable to increases in predation pressure than smaller, fast-reproducing and high-density generalist species, such as rodents [56,57].
As forest elephants can represent between 33 and 89% of the animal biomass of intact Central African forests, and diurnal primates up to 30% [58,59], the dramatic declines recorded for these species will radically alter functional relationships in which they play a key role [19,60,61]. However, the detrimental and cascading effects of losing large fauna from an ecosystem are not always visible in tropical forests where forest cover and tree density are often used as proxy indicators of ecosystem health [20,62].
Although loss of the ‘large forest architects’ may cause the most obvious ecosystem changes, other shifts in species composition will also have important impacts on forest structure and function. Small species released from predation pressure and competition as their natural predators and competitors are hunted to low densities, yet themselves unattractive to human hunters, can find conditions of high hunting pressure favourable and densities may even locally increase, with knock-on consequences for the area's ecology [19,40]. In Central Africa, frugivorous medium-bodied duikers and primates make up a high proportion of hunting offtakes (see figure 1 and electronic supplementary material, table S1), and reductions in their abundance can be dramatic: Lahm [63] recorded local extirpation of yellow-backed duiker around a village of northeastern Gabon less than a year from the onset of the unregulated hunting now typical of the entire region. The role of these species in the middle level of the trophic web, as browsers, seed dispersers and producers of food for the higher trophic levels, makes them a key part of the ecosystem: changes in the abundance of the entire guild are certain to have multiple consequences for the ecosystem [13,19,64]. Leopards, the apex predator in Central African forests, have already been lost from heavily hunted areas due to loss of the species which are their prey base, rather than direct persecution [15].
Figure 1.
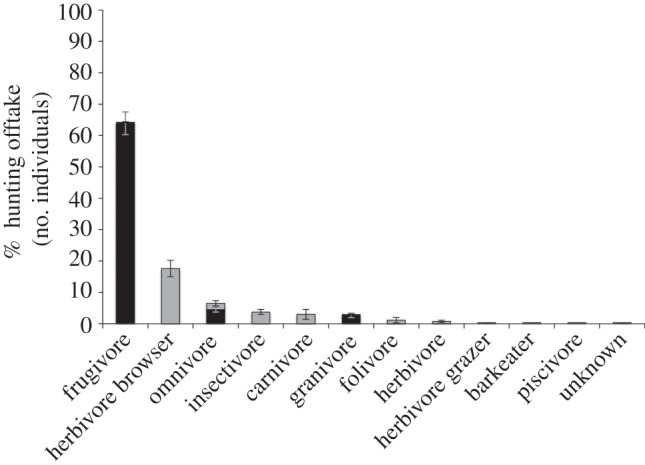
The impact of village hunting on trophic guilds of the Central African forests. Classification of species to these guilds can be found in the electronic supplementary material, table S1. Grey bars represent non-seed disperser, and black bars represent seed disperser.
4. Current drivers of hunting
Globally, the need for both protein and income are well recognized as the ultimate drivers of hunting [31,65–67].
Wild meat is a traditional food for almost all ethnic groups in Central Africa, consumed by rich and poor, in urban and rural communities [68,69]. While hunting is predominantly a poor man's activity [70], eating wild meat is not; hunting traditions are a source of pride in urban populations, who pronounce preferences for wild meat and will pay a premium for it, even when their protein requirement can be met with cheaper sources [67,68,71,72]. Vigorous trading of wild meat to satisfy urban demand is widespread in all major Central African cities [71,73–78], and the purchase of wild meat is common in even relatively small towns [41,68,79,80]. The certainty of demand, ease of entering the market and low risk of penalties have encouraged villagers in subsistence economies across the region to use local wildlife as a cash crop [66,81–83].
Commercial hunting has increased markedly in the last 50 years [18,63,81]. Recent studies at village subsistence level have found that more than 90% of resident village hunters sell at least part of their catch in all countries of the region [45,67,81,82,84,85] and that demand along the commodity chain is biased towards larger and rarer species [83,84,86–88]. In addition to village hunters, who are in part hunting to feed themselves, the purely commercial hunters, who often hunt species to order, are accessing more remote land in production forests or protected areas where village land rights do not apply and resources are de facto open access [62,69]. This type of hunting is particularly deleterious to the largest species, such as elephants or gorillas, which are typically less highly impacted by village subsistence activities [17,55].
(a). Sustainability of hunting offtakes
Considerable effort has been made in the last 20 years to develop indices and methods for evaluating hunting sustainability [89–93]. However, contemporary impacts depend enormously on the history of the local area and the dynamic interactions between hunters and the local wildlife community. Impacts of a given hunting pressure can only be predicted in the light of the area's recent past, which has already shaped the communities of both hunters and their available prey [31,94]. None of the sustainability indicators currently in use have been found to perform well [95], and empirical data on wildlife population trends remain the only valid measure of hunting impacts at the species level. To date, using either proxies or direct measures of population trends, only smaller species, such as blue duiker or large rodents, have shown any indication of being resilient to modern Central African hunting regimes sustained over several years [19,29,31,90,96]. If current hunting offtakes are unsustainable, both direct impacts and cascading ecological impacts will intensify over coming years unless hunting practices change.
5. Ecosystem function
Ecological systems are shaped by ‘top-down’ forces, such as predation, and ‘bottom-up’ forces, such as climate or land use [10]. The top-down force of overhunting seems already to be causing tropic cascade changes in Central Africa. A suite of empirical studies give evidence for relational changes between species and disruption of ecological function in hunted tropical forests. Although much of the evidence available to date is from outside Central Africa, recent studies in the region confirm similar responses to overhunting to those documented in other tropical forests. Functional changes recorded relate to trophic roles, structural changes in forests, changes in species diversity and richness, seed dispersal, pollination and soil nutrient cycling. Table 2 collates existing empirical evidence for ecological change as a result of hunting, in all tropical forests and African rainforests. Central African forests still harbour large populations of extremely large mammalian ‘forest architects’ or ‘ecological engineers’, such as elephant, hippo, buffalo and gorilla. They are likely to experience significant changes in forest structure after their loss [117]. Importantly, significant implications of mesofaunal loss are also emerging from recent empirical studies [15,19,115].
Table 2.
Empirical evidence of the impacts of hunting on ecological function in tropical forest ecosystems.
| disruption to ecological functioning as a result of hunting | examples from literature | empirical studies from African rainforests | empirical studies from other tropical forests | |
|---|---|---|---|---|
| changes in wildlife assemblages | interspecific competition (between predator and prey) | apex and mesopredators inhabiting heavily hunted areas are forced to prey on smaller species through direct competition with hunters for larger bodied species; this reduces predator population sizes | [15] | [8,97] |
| interspecific competition (between prey species) | heavy hunting pressure and selective hunting can change the competitive balance between sympatric species; many species have separate niches due to competition rather than habitat constraints | [96,98] | [40] | |
| ecological and predator release | as large herbivores and top predators are extirpated, an initial increase in secondary game species is witnessed; populations of small, non-target species increase but overall animal biomass decreases | [19,79] | [40] | |
| changes in relative seed dispersal success | seed dispersal failure due to seed predators both increasing and switching to more abundant seeds | in heavily hunted areas, rodents and other small seed predators switch feeding behaviour to animal-dispersed species whose undispersed seeds are now found in clusters around the parent plant preference by small-bodied mammal species for small seeds; low predation on large seeds in heavily hunted area |
[19] | [99,100] |
| reduced seed dispersal, sapling recruitment and plant regeneration | in hunted areas where large, long distance dispersers are absent, rates of dispersal and regeneration of animal-dispersed trees are much lower than where these species are present | [101–103] | [104–107] | |
| increased kin competition between seedlings | seedlings, which remain under parent plant due to reduced dispersal in hunted areas, experience increased competition | [108] | ||
| changes in relative pre-dispersion predation pressure for seeds | pre-dispersal seed predation by larger mammals is higher in protected areas than in hunted areas | [109,110] | ||
| changes in vegetation structure and composition | reduced plant species richness and diversity | tree species richness is lower in regenerating cohorts in hunted areas than in protected sites | [19] | [104,111–113] |
| changes in tree spatial structure | increased clustering and densities of saplings of animal-dispersed plants due to loss of large herbivores | [102] | [104] | |
| increased proportion of lianas (and other wind-dispersed species) due to decrease in animal seed dispersers | wind-dispersed climbing species, which are overwhelmingly woody lianas, are found in higher proportions in the seedling bank at heavily hunted sites than at protected sites | [19] | [106] | |
| changes in vegetation composition | diversity of plants with large- and medium-sized seeds was significantly lower while diversity of plants with small-sized seeds increased significantly with hunting pressure | [114] | [100] | |
| soil quality | altered nutrient cycling and parasite suppression, disturbed soil fertilization and aeration | reduced abundance of Scarabaeinae beetles as a result of reduced availability of mammal dung alters ecosystem functioning | [113,115] | |
| carbon balance | reduced carbon storage | reduced woody plant recruitment due to decreased large seed species will threaten carbon-storage capacity | [105,116] |
Central African hunting systems are biased towards heavy offtakes of seed-dispersing frugivorous mammals; over 70% of animals in an average village hunting offtake have a seed dispersal role (see figure 1 and electronic supplementary material, table S1). Animals disperse the seeds of the majority of tree species in tropical forests [118] and seed dispersal by larger animal guilds particularly affected by hunting plays a key role in both the spatial pattern of tree recruitment and survival [19,61,102,107], and the relationship between tree and liana establishment [104,119,120]. In the absence of specialized dispersing fauna, recruitment and survival of seedlings is reduced for tree species that depend on them [19,121]. Animal-dispersed tropical trees are biased towards species with slower growth, longer life and higher wood density than abiotically dispersed species [105]. A positive (if weak) correlation between seed size and wood density also exists [122]. Animal-dispersed tree species, particularly those with large seeds dispersed by large mammals, therefore contribute a high proportion of the overall carbon-storage capacity of tropical forests. Carbon storage may therefore erode over time if tree regeneration is hampered by changes in faunal guilds, including extinction of large specialized disperser species [61,104,123,124] or increases in seed-predating species enjoying ecological release from their predators [99,125]. Recent data already show significant changes in the seedling layer of hunted West African forests [19,107] with regeneration in defaunated forests favouring faster growing, lower density plants and resulting in overall loss of diversity. This effect has also been recorded in Asian forests [104]. Across the tropics, forests suffering high levels of hunting over the past three decades have also recorded increases in the establishment of fast-growing pioneer species and lianas, and the loss of large hardwood trees [20].
6. Impacts of land-use change and climate change on Central African forest structure and function
Central African rainforests will be influenced by both climate change and LUC as well as by hunting over the coming decades. We summarize the climate change and LUC scenarios outlined in this special issue and the recent literature and go on to discuss their potential interaction with the ecological changes caused by hunting, already in motion.
(a). Land-use change: reduced habitat for forest species, more forests accessed
Central African economies rely on extractive industries, allocating a large part of their territories to formal sector oil, mining, agriculture and extensive timber use [126]. For example, in Gabon, 59% of land is currently allocated to oil and mining production, industrial agriculture and commercial logging, with logging comprising 54% [127]. In 2007, over 30% of all Central African forests were allocated to logging [45]. Despite this allocation, current levels of deforestation in Central Africa are low (0.08% per year from 2000 to 2010, [128]) and driven mainly by increases in population density and subsequent land conversion for small-scale agriculture [128].
Although land conversion and commercial logging are yet to result in large loss of forest canopy cover, the secondary impacts of extractive industries on forests have been substantial. In 2007, logging roads accounted for 38% of the road network in Central Africa, ranging from 13% in DRC to over 60% in Gabon and the Republic of Congo [45], significantly influencing forest disturbance and unregulated human access. Logging infrastructure and industrial roads usher in a domino effect of factors known to intensify hunting pressure, such as population growth from an immigrant workforce [129], increased income and demand for wild meat [130], increased forest access [131–133] and increased extraction to international markets for specialist products like ivory [6,17]. Although logging itself can affect animal densities by modifying habitat at landscape and local scales [134], evidence across the region indicates that secondary impacts of logging activity are currently of far greater ecological importance [135].
Future LUC in Central Africa is difficult to predict beyond the medium term in a region of political and economic instability; however, under current socio-economic conditions, land use may change rapidly, even in the short term [126]. The recent road network expansion in Central Africa, driven by global demand for raw natural resources, is expected to continue to increase access to the remaining remote tracts of forest [45,128], reducing the contiguous forest to smaller blocks both at local scales [136,137] and across the Congo Basin [138]. International demand for agro-industrial products such as oil palm, rubber, sugar cane, coffee and cocoa, is already responsible for forest conversion in the region [128] with governments actively soliciting agro-industrial investment. For example, the government of Gabon recently created a 40/60 joint venture with a large multinational to develop agroindustry as well as other products and services like industrial parks, which ultimately support extractive activities and redistribute worker populations to forest areas [127]. Under scenarios of increasing agro-industrialization and forest access, coupled with intrinsic population growth, unless regulated, hunting offtakes in Central African forests are expected to increase in the short term, and the hunting footprint will be likely to spread into the last remnants of remote forest.
(b). Climate change impacts: resilient forests?
Climate models for Central Africa, presented in this special issue, suggest that direct impacts of climate change on the region's forest cover may be lower than previously thought by the Intergovernmental Panel on Climate Change. These models predict an increase in temperature, and increased dry signals for Central Africa [139], yet importantly also suggest that extreme precipitation or drought events are unlikely to increase [140,141]. Central African rainforests are also suggested to be more resilient to water deficits compared with moist tropical forests elsewhere [142].
Although extreme change linked to temperature and drying, such as forest die back, seems unlikely for the Central African region [142–144] and devastating forest fires are not predicted in the near future by any of the climate models for Central Africa, even the slight drying associated with higher temperatures, rainfall changes and increased human incursion predicted for this region [128,139,140] may make forests more vulnerable to fire in the future [145,146]. Forest resilience has been used to mean the resistance of the vegetation to change [144]; however, ecological function in even a seemingly ‘resilient’ forest may be significantly affected by the relatively small increases in temperature predicted. Tutin & Fernandez [147] record a guild of trees in Gabon relying on temperature triggers for flowering (and thus fruiting) events. In Uganda, increases in annual temperatures over 30 years have been correlated with a decline in the fruiting and flowering of some tropical tree species, while increasing the fecundity of others [148]. How Central African ligneous species will respond to rapidly changing temperature is poorly understood, but significant impacts on forest species composition or tree productivity could potentially change food availability for animals, affecting animal ranging patterns and densities across the region [149] and initiating trophic cascades as prey distributions, seed dispersal functions and nutrient cycling are in turn changed. These changes would be likely to be additive to any trophic change initiated as a direct result of hunting.
7. Discussion
In this paper, we set out to assemble the current empirical knowledge on hunting in Central Africa, its extent, drivers and direct and indirect ecological consequences, and to consider how interactions between hunting and current scenarios for LUC and climate change, outlined in this special issue, might influence the future of the African rainforests.
The data reviewed show that village hunting has been studied in detail at several sites in the six main forested countries of the African rainforest region over the past two decades (table 1). Drivers of hunting are very similar across all countries [65] and direct impacts on species are broadly similar across the Central African forests (see the electronic supplementary material, table S1). Twenty years ago, Barnes et al. [150] first documented the deleterious effect of human access on elephant densities in Gabon. Ten years later, Wilkie et al. [151] postulated that hunting, rather than habitat loss, would be the greatest driver of wildlife declines across Central African forests, due to increasing road access, and Barnes [152] further predicted that the insidious effects of hunting on wildlife populations would not be realized until species were close to collapse. Barnes' and Wilkie's hypotheses have been upheld for large species [15,17,20,50,51,133]. However, the majority of studies on hunting have focused on hunters, using only proxies for impacts on prey species [88,153]. Data for wildlife population responses to hunting are only available for large species for which robust census methods are available [154,155]. Data on the true status of smaller species, which form the bulk of the wildmeat harvest (see the electronic supplementary material, table S1), are almost entirely absent. Thus, although we have now registered the catastrophic decline of megafauna in the Central African rainforests, the immense consequences of the resulting trophic cascades, on mesofauna densities, forest structure and overall ecosystem function, are only now becoming understood.
As the extent and drivers of the ecological changes underway across the world's ecosystems in the twenty-first century become apparent [9,20,121], research paradigms must widen to become more multidisciplinary. Research on individual impacts of climate change, LUC or hunting on Central African forests risks missing the interactions between these factors. Considering one without the others could lead to widely different conclusions on the future health of Central African rainforests, and conservation, management and research priorities. For example, if viewed solely through the climate change lens, the scenarios outlined in this issue predict minimal change for African rainforests and reasonable resilience to ongoing change [156]. However, coupled with more certain likelihood of LUC, the picture becomes increasingly dynamic and threatening to intact forests and wildlife. LUC predictions, though limited to the medium term, broadly indicate loss of contiguous forest blocks [138], increased accessibility of remote areas, overall forest degradation and increased local populations [128] and permanent agricultural lands [126,128]. The drivers of hunting summarized in this paper are likely to intensify under these LUC scenarios, particularly in regard to the large-bodied, commercially valuable species, which are also more vulnerable to forest cover fragmentation and loss [62,157]. Without greatly improved regulation, intensified drivers will result in intensified hunting offtakes and an increase in the rate of ecological change that these offtakes are effecting. Central African forests now look highly threatened.
Assuming current levels of hunting are maintained, evidence from the studies we reviewed might indicate one possible future scenario for African rainforests: Overhunting severely reduces large ‘forest architects’ and mesofauna, releasing small-bodied seed predators. This promotes fast-growing abiotically dispersed tree species over large-seeded, animal-dispersed, slow-growing, shade-bearing species, reducing carbon storage, fruit availability and associated biodiversity. In turn, the forests' ability to support large frugivorous, seed-dispersing mammals declines, spurring a negative feedback loop whereby both large mammals and large-seeded, long-lived, hard-wooded trees further decline, further reducing carbon storage and thus global resilience to climate change. Although this scenario represents dramatic, permanent change for African rainforests, it may be one plausible, logical outcome that puts the risks of not regulating hunting into perspective.
As Central Africa moves into the twenty-first century, the challenges that LUC and climate change bring are sure to be complex. However, they will be unlikely to reduce the demand for hunting in the region, nor to mitigate any of its ecological impacts. As outlined above, anticipated LUC in the region will probably exacerbate the impacts of hunting and the ecological changes resulting from hunting will probably in turn exacerbate the effects of climate change. LUC and hunting effects are likely to combine to reduce overall carbon-storage potential and biodiversity, diminish overall forest area and, through the structural and compositional changes initiated by hunting, lessen the resilience of remaining forests to drought, and possibly fire, over the long term.
Good hunting management practices and planning are clearly vital to maintaining ecological function in the African tropical forests and must be incorporated into research priorities and overall land-use and climate change strategies [158], as well as impact assessments and private sector management practices on the ground. Conservation practitioners in Central Africa have shown that multiple-use landscapes under efficient management can maintain the large wide-ranging species critical for large tree seed dispersal [159], sustain game populations for hunting needs [160] and support threatened species [161], if a few design rules are applied: for example, including sufficient protected areas and maintaining large timber concessions with a mix of logging histories, including unlogged patches, and ensuring sufficient resources are allocated to controlling hunting and enforcing management plans [160,162]. Similar kinds of landscape-level work are also needed to rethink and reinvent how agriculture might be designed and implemented in Central Africa: for example, to intensify local food production without converting new lands, siting large-scale concessions in already degraded areas, and designing and managing them to mitigate landscape-scale impacts.
The studies on trophic cascades reviewed here (table 2) already indicate that limiting the loss of megafauna and apex predators should become a first priority for conservation strategies that seek to sustain intact ecosystem function in tropical forests [9,121]. However, global demand for ivory, human–wildlife conflicts with megafauna and overall forest loss, coupled with wildlife population declines already suffered, may make retaining viable populations of these species impossible outside large protected areas [51]. Research to determine the responses of mesopredators and ecological niche adjustments, in both wildlife assemblages impacted by overhunting and the plant communities they live in, will be critical in determining the true resilience of the ecosystem and thus in designing hunting management plans and multiple land-use scenarios that could mitigate spiralling and irreversible ecological change.
If future Central African human and wildlife communities are to rely on the range of ecosystem services currently provided by their rainforests—and if the value of these global goods is to be maintained—immediate management of hunting and integration of good hunting practices into large-scale land-use planning must be considered an urgent priority for rainforest preservation and thus an integral and important part of planning for climate change management and mitigation.
Acknowledgements
We would like to acknowledge three anonymous referees whose comments improved the manuscript.
Funding statement
L.C. and G.T. would like to acknowledge the support of the Oxford University John Fell Fund and the Oxford Martin School.
References
- 1.Barton H, Denham T, Neumann K, Arroyo Kalin M. 2012. Long-term perspectives on human occupation of tropical rainforests: an introductory overview. Quat. Int. 249, 1–3 (doi:10.1016/j.quaint.2011.07.044) [Google Scholar]
- 2.Nasi R, Taber A, Van Vliet N. 2011. Empty forests, empty stomachs? Bushmeat and livelihoods in the Congo and Amazon Basins. Int. For. Rev. 13, 355–368 (doi:10.1505/146554811798293872) [Google Scholar]
- 3.INTERPOL 2012. Environmental crime. See http://www.interpol.int/Public/EnvironmentalCrime/Default.asp
- 4.Milliken T, Burn RW, Underwood FM, Sangalakula L. 2012. The elephant trade information system (ETIS) and the illicit trade in ivory: a report to the 16th meeting of the Conference of the Parties to CITES (Cop 16). See http://www.cites.org/eng/cop/16/doc/E-CoP16-53-02-02.pdf.TRAFFIC
- 5.Roe D, Milliken T, Milledge S, Mremi J, Mosha S, Grien-Gran M. 2002. Making a killing or making a living? Wildlife trade, trade controls and rural livelihoods. Nairobi, Kenya: IIED, TRAFFIC International [Google Scholar]
- 6.Stiles D. 2011. Elephant meat and ivory trade in Central Africa. Pachyderm 50, 26–36 [Google Scholar]
- 7.UNEP, CITES, IUCN, TRAFFIC 2013. Elephants in the dust—the African elephant crisis: United Nations Environment Programme, GRID-Arendal. See http://www.grida.no/_cms/OpenFile.aspx?s=1&id=1570 (accessed 15 May 2013.) [Google Scholar]
- 8.Brashares J, Pruh L, Stoner CJ, Epps CW. (eds) 2010. Ecological and conservation implications of mesopredator release, 1st edn Washington, DC: Island Press [Google Scholar]
- 9.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306 (doi:10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 10.Terborgh J, Holt RD, Estes JA. 2010. Trophic cascades: what they are, how they work and why they matter. In Trophic cascades (eds Terborgh J, Estes JA.), 1st edn, pp. 1–19. Washington, DC: Island Press [Google Scholar]
- 11.Bayon G, Dennielou B, Etoubleau J, Ponzevera E, Toucanne S, Bermell S. 2012. Intensifying weathering and land use in iron age Central Africa. Science 335, 1219–1222 (doi:10.1126/science.1215400) [DOI] [PubMed] [Google Scholar]
- 12.Brunet M, et al. 2002. A new hominid from the Upper Miocene of Chad, Central Africa. Nature 418, 145–151 (doi:10.1038/nature00879) [DOI] [PubMed] [Google Scholar]
- 13.Jimoh SO, Ikyaagba ET, Alarape AA, Adeyemi AA, Waltert M. 2012. Local depletion of two larger duikers in the Oban hills region, Nigeria. Afr. J. Ecol. 50, 1–7 (doi:10.1111/j.1365-2028.2011.01285.x) [Google Scholar]
- 14.Hearn GW, Morra WA. 2001. The approaching extinction of monkeys and duikers in Bioko Island, Equatorial Guinea, Africa. Glenside, PA: Bioko Biodiversity Protection Program, Arcadia University [Google Scholar]
- 15.Henschel P, Hunter LTB, Coad L, Abernethy KA, Muhlenberg M. 2011. Leopard prey choice in the Congo Basin rainforest suggests exploitative competition with human bushmeat hunters. J. Zool. 285, 11–20 (doi:10.1111/j.1469-7998.2011.00826.x) [Google Scholar]
- 16.Maisels F, Keming E, Menei M, Toh C. 2001. The extirpation of large mammals and implications for montane forest conservation: the case of the Kilum-Ijim Forest, North-west Province, Cameroon. Oryx 35, 322–331 (doi:10.1046/j.1365-3008.2001.00204.x) [Google Scholar]
- 17.Maisels F, et al. 2013. Devastating decline of forest elephants in Central Africa. PLoS ONE 8, e59469 (doi:10.1371/journal.pone.0059469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fa JE, Brown D. 2009. Impacts of hunting on mammals in African tropical moist forests: a review and synthesis. Mamm. Rev. 39, 231–264 (doi:10.1111/j.1365-2907.2009.00149.x) [Google Scholar]
- 19.Effiom OA, Nunez-Iturri G, Smith HG, Olsson O. 2013. Bushmeat hunting changes regeneration of African rainforests. Proc. R. Soc. B 280, 20130246 (doi:10.1098/rspb.2013.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurance WF, et al. 2012. Averting biodiversity collapse in tropical forest protected areas. Nature 489, 290–294 (doi:10.1038/nature11318) [DOI] [PubMed] [Google Scholar]
- 21.WCS, CIESIN, Columbia University 2005. Last of the wild project, Version 2, 2005 (LWP-2): global human footprint dataset (IGHP). Palisades, NY: NASA Socioeconomic Data and Applications Center (SEDAC) See http://sedac.ciesin.columbia.edu/data/set/wildareas-v2-human-footprint-ighp (accessed 24 September 2012). [Google Scholar]
- 22.Dethier 1995. Etude chasse. Brussels, Belgium: ECOFAC, AGRECO-CTFT; See http://www.ecofac.org/Biblio/Download/EtudesChasse/Cameroun/DethierChasseCameroun.pdf [Google Scholar]
- 23.Vanwijnsberghe S. 1996. Etude sur la chasse villegeoise aux environs du Parc d'Odzala. Brussels, Belgium: ECOFAC [Google Scholar]
- 24.Wilkie DS, Curran B, Tshombe R, Morelli GA. 1998. Modeling the sustainability of subsistence farming and hunting in the Ituri forest of Zaire. Conserv. Biol. 12, 137–147 (doi:10.1046/j.1523-1739.1998.96156.x) [Google Scholar]
- 25.Noss AJ. 1998. The impacts of BaAka net hunting on rainforest wildlife. Biol. Conserv. 86, 161–167 (doi:10.1016/S0006-3207(98)00013-5) [Google Scholar]
- 26.Kumpel NF. 2006. Incentives for sustainable hunting of bushmeat in Rio Muni, Equatorial Guinea. PhD thesis, Imperial College, London, UK [Google Scholar]
- 27.Yasuoka H. 2006. The sustainability of duiker (Cephalophus spp.) hunting for the Baka hunter-gatherers in southeastern Cameroon. Afr. Study Monogr. 33, 95–120 [Google Scholar]
- 28.Solly H. 2004. Bushmeat hunters and secondary traders: making the distinction for livelihood improvement. London, UK: ODI [Google Scholar]
- 29.Van Vliet N, Nasi R. 2008. Hunting for livelihood in northeast Gabon: patterns, evolution, and sustainability. Ecol. Soc. 13, 33 See http://www.ecologyandsociety.org/vol13/iss2/art33/ [Google Scholar]
- 30.Rist J. 2008. Bushmeat catch per unit effort in space and time: a monitoring tool for bushmeat hunting. PhD thesis, Imperial College, London, UK [Google Scholar]
- 31.Coad L, et al. 2013. Social and ecological change over a decade in a village hunting system, central Gabon. Conserv. Biol. 27, 270–280 (doi:10.1111/cobi.12012) [DOI] [PubMed] [Google Scholar]
- 32.Maisels F, et al. 2012. Great ape and human impact monitoring training, surveys, and protection in the Ndoki-Likouala Landscape, Republic of Congo. GACF Agreement: 96200–9-G247. Final report. New York, NY: Wildlife Conservation Society.
- 33.Malonga R. 2008. Final report on elephants and great apes survey in the Ngombe FMU and Ntokou-Pikounda forest, Republic of Congo. Contribution to a status survey of Great Apes. New York, NY: Wildlife Conservation Society [Google Scholar]
- 34.Maisels F, Ambahe E, Ambassa R, Nvemgah Yara C, Fosso B.2009. Great ape and human impact monitoring in the Mbam et Djerem National Park, Cameroon. Final report to USFWS-GACF Agreement 98210–7-G290. New York, NY: Wildlife Conservation Society.
- 35.Blake S. 2006. Final report on elephant and ape surveys in the Odzala-Koukoua National Park, 2005. New York, NY: Wildlife Conservation Society [Google Scholar]
- 36.Liengola I, Maisels F, KNkumu P, Bonvenge A.2010. Conserving bonobos in the Lokofa Block of the Salonga National Park, Democratic Republic of Congo. Report to the Beneficia Foundation. New York, NY: Wildlife Conservation Society.
- 37.Maisels F, Bechem M, Mihindou Y. 2006. Lope National Park, Gabon. Large mammals and human impact. Monitoring program 2004–2005. New York, NY: Wildlife Conservation Society [Google Scholar]
- 38.Aba'a R. 2006. Abondance relative des grands mammiferes et des activités humaines au parc National des Monts de Cristal et sa peripherie. Libreville, Gabon: Wildlife Conservation Society [Google Scholar]
- 39.Abitsi G. 2006. Inventaires de reconnaissance des grands mammiferes et de l'impact des activités anthropiques. Parc National de Waka, Gabon. December 2005–Juillet 2006. Libreville, Gabon: Wildlife Conservation Society [Google Scholar]
- 40.Peres CA, Palacios E. 2007. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: implications for animal-mediated seed dispersal. Biotropica 39, 304–315 (doi:10.1111/j.1744-7429.2007.00272.x) [Google Scholar]
- 41.Coad L. 2007. Bushmeat hunting in Gabon: socioeconomics and hunter behaviour. PhD thesis, University of Cambridge, Cambridge, UK [Google Scholar]
- 42.Colom A. 2006. Aspects Socio-economiques de la gestion des ressources naturelles dans le paysage Salonga-Lukenie-Sankuru, DRC. Kinshasa, Democratic Republic of the Congo: WWF CARPO [Google Scholar]
- 43.Kumpel NF, Rowcliffe JM, Cowlishaw G, Milner-Gulland EJ. 2009. Trapper profiles and strategies: insights into sustainability from hunter behaviour. Anim. Conserv. 12, 531–539 (doi:10.1111/j.1469-1795.2009.00279.x) [Google Scholar]
- 44.WRI 2009. Earthtrends. Washington, DC: World Resources Institute [Google Scholar]
- 45.Laporte NT, Stabach JA, Grosch R, Lin TS, Goetz SJ. 2007. Expansion of industrial logging in Central Africa. Science 316, 1451 (doi:10.1126/science.1141057) [DOI] [PubMed] [Google Scholar]
- 46.Taylor G. 2012. A systematic review of the bushmeat trade in West and Central Africa. MSc thesis, University of Oxford, Oxford, UK [Google Scholar]
- 47.IUCN 2012. The red list of threatened species. See http://www.iucnredlist.org/ (accessed 27 September 2012).
- 48.Fa JE, Johnson PJ, Dupain J, Lapuente J, Koster P, Macdonald DW. 2004. Sampling effort and dynamics of bushmeat markets. Anim. Conserv. 7, 409–416 (doi:10.1017/S136794300400160X) [Google Scholar]
- 49.Beyers RL, Hart JA, Sinclair ARE, Grossmann F, Klinkenberg B, Dino S, Somers M. 2011. Resource wars and conflict ivory: the impact of civil conflict on elephants in the Democratic Republic of Congo: the case of the Okapi Reserve. PLoS ONE 6, e27129 (doi:10.1371/journal.pone.0027129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouche P, Douglas-Hamilton I, Wittemyer G, Nianogo AJ, Doucet JL, Lejeune P, Vermeulen C. 2011. Will elephants soon disappear from West African Savannahs? PLoS ONE 6, e20619 (doi:10.1371/journal.pone.0020619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouche P, Mange RNM, Tankalet F, Zowoya F, Lejeune P, Vermeulen C. 2012. Game over! Wildlife collapse in northern Central African Republic. Environ. Monit. Assess. 184, 7001–7011 (doi:10.1007/s10661-011-2475-y) [DOI] [PubMed] [Google Scholar]
- 52.CITES 2012. Monitoring the illegal killing of elephants. CoP16 Doc. 53.1 See http://www.cites.org/eng/cop/16/doc/E-CoP16-53-01.pdf [Google Scholar]
- 53.CITES 2013. Monitoring the illegal killing of elephants—Addendum. CoP16 Doc. 53.1: Addendum See http://www.cites.org/eng/cop/16/doc/E-CoP16-53-01-Addendum.pdf [Google Scholar]
- 54.Walsh PD, et al. 2003. Catastrophic ape decline in western equatorial Africa. Nature 422, 611–614 (doi:10.1038/nature01566) [DOI] [PubMed] [Google Scholar]
- 55.Kuëhl HS, Nzeingui C, Yeno SLD, Huijbregts B, Boesch C, Walsh PD. 2009. Discriminating between village and commercial hunting of apes. Biol. Conserv. 142, 1500–1506 (doi:10.1016/j.biocon.2009.02.032) [Google Scholar]
- 56.Berryman AA. 1992. The origins and evolution of predator prey theory. Ecology 73, 1530–1536 (doi:10.2307/1940005) [Google Scholar]
- 57.Bennett EL, Robinson JG. 1999. Hunting for sustainability in tropical forests. New York, NY: Columbia University Press [Google Scholar]
- 58.White LJT, Tutin CEG, Fernandez M. 1993. Group composition and diet of forest elephants, Loxodonta africana cyclotis Matschie 1900, in the Lopé Reserve, Gabon. Afr. J. Ecol. 31, 181–199 (doi:10.1111/j.1365-2028.1993.tb00532.x) [Google Scholar]
- 59.Prins HHT, Reitsma JM. 1989. Mammalian biomass in an African equatorial rain-forest. J. Anim. Ecol. 58, 851–861 (doi:10.2307/5128) [Google Scholar]
- 60.Tutin CEG, Williamson EA, Rogers ME, Fernandez M. 1991. A case-study of a plant–animal relationship: Cola lizae and lowland gorillas in the Lopé Reserve, Gabon. J. Trop. Ecol. 7, 181–199 (doi:10.1017/S0266467400005320) [Google Scholar]
- 61.Campos-Arceiz A, Blake S. 2011. Megagardeners of the forest: the role of elephants in seed dispersal. Acta Oecol. 37, 542–553 (doi:10.1016/j.actao.2011.01.014) [Google Scholar]
- 62.Wilkie DS, Bennett EL, Peres CA, Cunningham AA. 2011. The empty forest revisited. Ann. NY Acad. Sci. 1223, 120–128 (doi:10.1111/j.1749-6632.2010.05908.x) [DOI] [PubMed] [Google Scholar]
- 63.Lahm S. 2001. Hunting and wildlife in Northeastern Gabon. Why conservation should extend beyond protected areas. In African rain forest ecology and conservation: an interdisciplinary perspective (eds Weber W, White LJT, Vedder A.), pp. 344–354 New York, NY: Yale University Press [Google Scholar]
- 64.Kumpel NF, Milner-Gulland EJ, Rowcliffe JM, Cowlishaw G. 2008. Impact of gun-hunting on diurnal primates in continental Equatorial Guinea. Int. J. Primatol. 29, 1065–1082 (doi:10.1007/s10764-008-9254-9) [Google Scholar]
- 65.Brashares JS, Golden CD, Weinbaum KZ, Barrett CB, Okello GV. 2011. Economic and geographic drivers of wildlife consumption in rural Africa. Proc. Natl Acad. Sci. USA 108, 13 931–13 936 (doi:10.1073/pnas.1011526108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumpel NF, Milner-Gulland EJ, Cowlishaw G, Rowcliffe JM. 2010. Incentives for hunting: the role of bushmeat in the household economy in rural Equatorial Guinea. Hum. Ecol. 38, 251–264 (doi:10.1007/s10745-010-9316-4) [Google Scholar]
- 67.Foerster S, Wilkie DS, Morelli GA, Demmer J, Starkey M, Telfer P, Steil M, Lewbel A. 2012. Correlates of bushmeat hunting among remote rural households in Gabon, Central Africa. Conserv. Biol. 26, 335–344 (doi:10.1111/j.1523-1739.2011.01802.x) [DOI] [PubMed] [Google Scholar]
- 68.Wilkie DS, Starkey M, Abernethy K, Effa EN, Telfer P, Godoy R. 2005. Role of prices and wealth in consumer demand for bushmeat in Gabon, Central Africa. Conserv. Biol. 19, 268–274 (doi:10.1111/j.1523-1739.2005.00372.x) [Google Scholar]
- 69.Nasi R, Brown D, Wilkie D, Bennett EL, Tutin C, van Tol G, Christophersen T. 2008. Conservation and use of wildlife-based resources: the bushmeat crisis. Bogor, Indonesia: Secretariat of the Convention on Biological Diversity, Montreal, and Center for International Forestry Research (CIFOR) [Google Scholar]
- 70.Wilkie DS, Carpenter JF. 1999. Bushmeat hunting in the Congo Basin: an assessment of impacts and options for mitigation. Biodivers. Conserv. 8, 927–955 (doi:10.1023/A:1008877309871) [Google Scholar]
- 71.Schenck M, Effa EN, Starkey M, Wilkie D, Abernethy K, Telfer P, Godoy R, Treves A. 2006. Why people eat bushmeat: results from two-choice, taste tests in Gabon, Central Africa. Hum. Ecol. 34, 433–445 (doi:10.1007/s10745-006-9025-1) [Google Scholar]
- 72.East T, Kumpel NF, Milner-Gulland EJ, Rowcliffe JM. 2005. Determinants of urban bushmeat consumption in Rio Muni, Equatorial Guinea. Biol. Conserv. 126, 206–215 (doi:10.1016/j.biocon.2005.05.012) [Google Scholar]
- 73.Hennessey AB, Rogers JA. 2008. A study of the bushmeat trade in Ouesso, Republic of Congo. Conserv. Soc. 6, 179–184 (doi:10.4103/0972-4923.49211) [Google Scholar]
- 74.Edderal D, Dame M. 2006. A census of the commercial bushmeat in Yaounde, Cameroon. Oryx 40, 472–475 (doi:10.1017/S0030605306001256) [Google Scholar]
- 75.Malonga R. 1996. Dynamique socio-economique du circuit commerical de viande de chasse a Brazzaville. New York, NY: Wildlife Conservation Society [Google Scholar]
- 76.Fa JE, Garcia-Yuste JE. 2001. Commercial bushmeat hunting in the Monte Mitra forests, Equatorial Guinea: extent and impact. Anim. Biodivers. Conserv. 24, 31–52 [Google Scholar]
- 77.Mbayma G. 2009. Bushmeat consumption in Kinshasa, DRC. Analysis at the household level. New York, NY: Wildlife Conservation Society [Google Scholar]
- 78.Mbete RA, et al. 2011. Profile of bushmeat sellers and evaluation of biomass commercialized in the municipal markets of Brazzaville, Congo. Trop. Conserv. Sci. 4, 203–217 [Google Scholar]
- 79.Fa JE, Seymour S, Dupain J, Amin R, Albrechtsen L, Macdonald D. 2006. Getting to grips with the magnitude of exploitation: bushmeat in the Cross–Sanaga rivers region, Nigeria and Cameroon. Biol. Conserv. 129, 497–510 (doi:10.1016/j.biocon.2005.11.031) [Google Scholar]
- 80.Tieguhong JC, Zwolinski J. 2009. Supplies of bushmeat for livelihoods in logging towns in the Congo Basin. J. Horticulture For. 1, 2–9 [Google Scholar]
- 81.Starkey M. 2004. Commerce and subsistence: the hunting, sale and consumption of bushmeat in Gabon. Cambridge, UK: University of Cambridge [Google Scholar]
- 82.de Merode E, Homewood K, Cowlishaw G. 2004. The value of bushmeat and other wild foods to rural households living in extreme poverty in Democratic Republic of Congo. Biol. Conserv. 118, 573–581 (doi:10.1016/j.biocon.2003.10.005) [Google Scholar]
- 83.Coad L, Abernethy K, Balmford A, Manica A, Airey L, Milner-Gulland EJ. 2010. Distribution and use of income from bushmeat in a rural village, central Gabon. Conserv Biol. 24, 1510–1518 (doi:10.1111/j.1523-1739.2010.01525.x) [DOI] [PubMed] [Google Scholar]
- 84.Abernethy K, Coad L, Ilambu O, Makiloutila F, Easton J, Akiak J. 2010. Wildlife hunting, consumption and trade in the Oshwe sector of the Salonga-Lukenie-Sankuru Landscape. Kinshasa, Democratic Republic of the Congo: WWF CARPO [Google Scholar]
- 85.Auzel P, Wilkie D. (eds) 2000. Wildlife use in northern Congo: hunting in a commercial logging concession. New York, NY: Columbia University Press [Google Scholar]
- 86.Carpaneto GM, Fusarii A, Okongo H. 2007. Subsistence hunting and exploitation of mammals in the Haut-Ogooué province, south-eastern Gabon. J. Anthropol. Sci. 85, 183–193 [Google Scholar]
- 87.Lahm S. 1994. Hunting and wildlife in northeastern Gabon: why conservation should extend beyond protected areas. Makoukou, Gabon: Institut de Recherche en Ecologie Tropicale [Google Scholar]
- 88.Allebone-Webb SM, Kumpel NF, Rist J, Cowlishaw G, Rowcliffe JM, Milner-Gulland EJ. 2011. Use of market data to assess bushmeat hunting sustainability in Equatorial Guinea. Conserv. Biol. 25, 597–606 (doi:10.1111/j.1523-1739.2011.01681.x) [DOI] [PubMed] [Google Scholar]
- 89.Robinson JG, Redford KH. (eds) 1991. Sustainable harvest of neotropical forest mammals. Chicago, IL: University of Chicago Press [Google Scholar]
- 90.Cowlishaw G, Mendelson S, Rowcliffe JM. 2005. Evidence for post-depletion sustainability in a mature bushmeat market. J. Appl. Ecol. 42, 460–468 (doi:10.1111/j.1365-2664.2005.01046.x) [Google Scholar]
- 91.Kumpel NF, Milner-Gulland EJ, Cowlishaw G, Rowcliffe JM. 2010. Assessing sustainability at multiple scales in a rotational bushmeat hunting system. Conserv. Biol. 24, 861–871 (doi:10.1111/j.1523-1739.2010.01505.x) [DOI] [PubMed] [Google Scholar]
- 92.Ling S, Milner-Gulland EJ. 2006. Assessment of the sustainability of bushmeat hunting based on dynamic bioeconomic models. Conserv. Biol. 20, 1294–1299 (doi:10.1111/j.1523-1739.2006.00414.x) [DOI] [PubMed] [Google Scholar]
- 93.Robinson JG, Redford KH, Bennett EL. 1999. Conservation—Wildlife harvest in logged tropical forests. Science 284, 595–596 (doi:10.1126/science.284.5414.595) [Google Scholar]
- 94.Papworth SK, Rist J, Coad L, Milner-Gulland EJ. 2009. Evidence for shifting baseline syndrome in conservation. Conserv. Lett. 2, 93–100 (doi:10.1111/j.1755-263X.2009.00049.x) [Google Scholar]
- 95.Weinbaum KZ, Brashares JS, Golden CD, Getz WM. 2013. Searching for sustainability: are assessments of wildlife harvests behind the times? Ecol. Lett. 16, 99–111 (doi:10.1111/ele.12008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newing H. 2001. Bushmeat hunting and management: implications of duiker ecology and interspecific competition. Biodivers. Conserv. 10, 99–118 (doi:10.1023/A:1016671524034) [Google Scholar]
- 97.Treves A. 2009. Hunting for large carnivore conservation. J. Appl. Ecol. 46, 1350–1356 (doi:10.1111/j.1365-2664.2009.01729.x) [Google Scholar]
- 98.Linder JM, Oates JF. 2011. Differential impact of bushmeat hunting on monkey species and implications for primate conservation in Korup National Park, Cameroon. Biol. Conserv. 144, 738–745 (doi:10.1016/j.biocon.2010.10.023) [Google Scholar]
- 99.Dirzo R, Mendoza E, Ortiz P. 2007. Size-related differential seed predation in a heavily defaunated neotropical rain forest. Biotropica 39, 355–362 (doi:10.1111/j.1744-7429.2007.00274.x) [Google Scholar]
- 100.Terborgh J, Nunez-Iturri G, Pitman NCA, Valverde FHC, Alvarez P, Swamy V, Pringle EG, Paine CET. 2008. Tree recruitment in an empty forest. Ecology 89, 1757–1768 (doi:10.1890/07-0479.1) [DOI] [PubMed] [Google Scholar]
- 101.Babweteera F, Savill P, Brown N. 2007. Balanites wilsoniana: regeneration with and without elephants. Biol. Conserv. 134, 40–47 (doi:10.1016/j.biocon.2006.08.002) [Google Scholar]
- 102.Blake S, Deem SL, Mossimbo E, Maisels F, Walsh P. 2009. Forest elephants: tree planters of the Congo. Biotropica 41, 459–468 (doi:10.1111/j.1744-7429.2009.00512.x) [Google Scholar]
- 103.Wang BC, Sork VL, Leong MT, Smith TB. 2007. Hunting of mammals reduces seed removal and dispersal of the afrotropical tree Antrocaryon klaineanum (Anacardiaceae). Biotropica 39, 340–347 (doi:10.1111/j.1744-7429.2007.00275.x) [Google Scholar]
- 104.Harrison RD, Tan S, Ploktin JB, Slik F, Detto M, Brenes T, Itoh A, Davies SJ. 2013. Consequences of defaunation for a tropical tree community. Ecol. Lett. 16, 687–694 (doi:10.1111/ele.12102) [DOI] [PubMed] [Google Scholar]
- 105.Brodie JF, Gibbs HK. 2009. Bushmeat hunting as climate threat. Science 326, 364–365 (doi:10.1126/science.326_364b) [DOI] [PubMed] [Google Scholar]
- 106.Wright SJ, Stoner KE, Beckman N, Corlett RT, Dirzo R, Muller-Landau HC, Nuñez-Iturri G, Peres CA, Wang BC. 2007. The plight of large animals in tropical forests and the consequences for plant regeneration. Biotropica 39, 289–291 (doi:10.1111/j.1744-7429.2007.00293.x) [Google Scholar]
- 107.Nunez-Iturri G, Olsson O, Howe HF. 2008. Hunting reduces recruitment of primate-dispersed trees in Amazonian Peru. Biol. Conserv. 141, 1536–1546 (doi:10.1016/j.biocon.2008.03.020) [Google Scholar]
- 108.Muller-Landau HC. 2007. Predicting the long-term effects of hunting on plant species composition and diversity in tropical forests. Biotropica 39, 372–384 (doi:10.1111/j.1744-7429.2007.00290.x) [Google Scholar]
- 109.Beckman NG, Muller-Landau HC. 2007. Differential effects of hunting on pre-dispersal seed predation and primary and secondary seed removal of two neotropical tree species. Biotropica 39, 328–339 (doi:10.1111/j.1744-7429.2007.00273.x) [Google Scholar]
- 110.Holbrook KM, Loiselle BA. 2009. Dispersal in a Neotropical tree, Virola flexuosa (Myristicaceae): Does hunting of large vertebrates limit seed removal? Ecology 90, 1449–1455 (doi:10.1890/08-1332.1) [DOI] [PubMed] [Google Scholar]
- 111.Nunez-Iturri G, Howe HF. 2007. Bushmeat and the fate of trees with seeds dispersed by large primates in a lowland rain forest in western Amazonia. Biotropica 39, 348–354 (doi:10.1111/j.1744-7429.2007.00276.x) [Google Scholar]
- 112.Stevenson PR. 2011. The abundance of large ateline monkeys is positively associated with the diversity of plants regenerating in neotropical forests. Biotropica 43, 512–519 (doi:10.1111/j.1744-7429.2010.00708.x) [Google Scholar]
- 113.Nichols E, Larsen T, Spector S, Davis AL, Escobar F, Favila M, Vulinec K. 2008. Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol. Conserv. 141, 1932–1939 (doi:10.1016/j.biocon.2008.06.004) [Google Scholar]
- 114.Vanthomme H, Belle B, Forget PM. 2010. Bushmeat hunting alters recruitment of large-seeded plant species in Central Africa. Biotropica 42, 672–679 (doi:10.1111/j.1744-7429.2010.00630.x) [Google Scholar]
- 115.Andresen E, Laurance SGW. 2007. Possible indirect effects of mammal hunting on dung beetle assemblages in Panama. Biotropica 39, 141–146 (doi:10.1111/j.1744-7429.2006.00239.x) [Google Scholar]
- 116.Jansen PA, Muller-Landau HC, Wright SJ. 2010. Bushmeat hunting and climate: an indirect link. Science 327, 330 (doi:10.1126/science.327.5961.30-a) [DOI] [PubMed] [Google Scholar]
- 117.Guimaraes PR, Galetti M, Jordano P. 2008. Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS ONE 3, e1745 (doi:10.1371/journal.pone.0001745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marquis R. 2010. The role of herbivores in terrestrial trophic cascades. In Trophic cascades (eds Terborgh J, Estes JA.), 1st edn, pp. 109–124 Washington, DC: Island Press [Google Scholar]
- 119.Phillips OL, et al. 2002. Increasing dominance of large lianas in Amazonian forests. Nature 418, 770–774 (doi:10.1038/nature00926) [DOI] [PubMed] [Google Scholar]
- 120.van der Heijden GMF, Phillips OL. 2009. Liana infestation impacts tree growth in a lowland tropical moist forest. Biogeosciences 6, 2217–2226 (doi:10.5194/bg-6-2217-2009) [Google Scholar]
- 121.Terborgh J. In press. Using Janzen–Connell to predict the consequences of defaunation and other disturbances of tropical forests. Biol. Conserv. (doi:10.1016/j.biocon.2013.01.015) [Google Scholar]
- 122.Wright IJ, et al. 2007. Relationships among major dimensions of plant trait variation in seven Neotropical forests. Ann. Bot. Lond. 99, 1003–1015 (doi:10.1093/aob/mcl066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Voysey BC, McDonald KE, Rogers ME, Tutin CEG, Parnell RJ. 1999. Gorillas and seed dispersal in the Lopé Reserve, Gabon. II: survival and growth of seedlings. J. Trop. Ecol. 15, 39–60 (doi:10.1017/S0266467499000668) [Google Scholar]
- 124.Cochrane EP. 2003. The need to be eaten: Balanites wilsoniana with and without elephant seed-dispersal. J. Trop. Ecol. 19, 579–589 (doi:10.1017/S0266467403003638) [Google Scholar]
- 125.Stoner KE, Riba-Hernandez P, Vulinec K, Lambert JE. 2007. The role of mammals in creating and modifying seedshadows in tropical forests and some possible consequences of their elimination. Biotropica 39, 316–327 (doi:10.1111/j.1744-7429.2007.00292.x) [Google Scholar]
- 126.Rudel TK. 2013. The national determinants of deforestation in sub-Saharan Africa. Phil. Trans. R. Soc. B 368, 20120405 (doi:10.1098/rstb.2012.0405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oxford Business Group 2011. Gabon: the report. Oxford, UK: Oxford Business Group [Google Scholar]
- 128.Mayaux P, et al. 2013. State and evolution of the African rainforests between 1990 and 2010. Phil. Trans. R. Soc. B 368, 20120300 (doi:10.1098/rstb.2012.0300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Poulsen JR, Clark CJ, Mavah G, Elkan PW. 2009. Bushmeat supply and consumption in a tropical logging concession in Northern Congo. Conserv. Biol. 23, 1597–608 (doi:10.1111/j.1523-1739.2009.01251.x) [DOI] [PubMed] [Google Scholar]
- 130.Thibault M, Blaney S. 2003. The oil industry as an underlying factor in the bushmeat crisis in Central Africa. Conserv. Biol. 17, 1807–1813 (doi:10.1111/j.1523-1739.2003.00159.x) [Google Scholar]
- 131.Blake S, et al. 2008. Roadless wilderness area determines forest elephant movements in the Congo Basin. PLoS ONE 3, e3546 (doi:10.1371/journal.pone.0003546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wilkie D, Shaw E, Rotberg F, Morelli G, Auzel P. 2000. Roads, development, and conservation in the Congo basin. Conserv. Biol. 14, 1614–1622 (doi:10.1046/j.1523-1739.2000.99102.x) [DOI] [PubMed] [Google Scholar]
- 133.Yackulic CB, Strindberg S, Maisels F, Blake S. 2011. The spatial structure of hunter access determines the local abundance of forest elephants (Loxodonta africana cyclotis). Ecol. Appl. 21, 1296–1307 (doi:10.1890/09-1099.1) [DOI] [PubMed] [Google Scholar]
- 134.Poulsen JR, Clark CJ, Bolker BM. 2011. Decoupling the effects of logging and hunting on an Afrotropical animal community. Ecol. Appl. 21, 1819–1836 (doi:10.1890/10-1083.1) [DOI] [PubMed] [Google Scholar]
- 135.Nasi R, Billand A, Vanvliet N. 2012. Managing for timber and biodiversity in the Congo Basin. For. Ecol. Manag. 268, 103–111 (doi:10.1016/j.foreco.2011.04.005) [Google Scholar]
- 136.Mertens B, Lambin EF. 2000. Land-cover-change trajectories in southern Cameroon. Ann. Assoc. Am. Geogr. 90, 467–494 (doi:10.1111/0004-5608.00205) [Google Scholar]
- 137.Galford GL, Soares-Filho B, Cerri CEP. 2013. Prospects for land-use sustainability on the agricultural frontier of the Brazilian Amazon. Phil. Trans. R. Soc. B 368, 20120171 (doi:10.1098/rstb.2012.0171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang QF, Justice CO, Jiang MX, Brunner J, Wilkie DS. 2006. A GIS-based assessment on the vulnerability and future extent of the tropical forests of the Congo Basin. Environ. Monit. Assess. 114, 107–121 (doi:10.1007/s10661-006-2015-3) [DOI] [PubMed] [Google Scholar]
- 139.James R, Washington R, Rowell DP. 2013. Implications of global warming for the climate of African rainforests. Phil. Trans. R. Soc. B 368, 20120298 (doi:10.1098/rstb.2012.0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Otto FEL, Jones RG, Halladay K, Allen MR. 2013. Attribution of changes in precipitation patterns in African rainforests. Phil. Trans. R. Soc. B 368, 20120299 (doi:10.1098/rstb.2012.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fisher JB, et al. 2013. African tropical rainforest net carbon dioxide fluxes in the twentieth century. Phil. Trans. R. Soc. B 368, 20120376 (doi:10.1098/rstb.2012.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Asefi-Najafabady S, Saatchi S. 2013. Response of African humid tropical forests to recent rainfall anomalies. Phil. Trans. R. Soc. B 368, 20120306 (doi:10.1098/rstb.2012.0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Malhi Y, Aragao LEOC, Galbraith D, Huntingford C, Fisher R, Zelazowski P, Sitch S, McSweeney C, Meir P. 2009. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl Acad. Sci. USA 106, 20 610–20 615 (doi:10.1073/pnas.0804619106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huntingford C, et al. 2013. Simulated resilience of tropical rainforests to CO2-induced climate change. Nat. Geosci. 6, 268–273 (doi:10.1038/ngeo1741) [Google Scholar]
- 145.Cochrane MA. 2003. Fire science for rainforests. Nature 421, 913–919 (doi:10.1038/nature01437) [DOI] [PubMed] [Google Scholar]
- 146.Bowman DMJS, et al. 2011. The human dimension of fire regimes on earth. J. Biogeogr. 38, 2223–2236 (doi:10.1111/j.1365-2699.2011.02595.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tutin CEG, Fernandez M. 1993. Relationships between minimum temperature and fruit production in some tropical forest trees in Gabon. J. Trop. Ecol. 9, 241–248 (doi:10.1017/S0266467400007239) [Google Scholar]
- 148.Chapman CA, Chapman LJ, Struhsaker TT, Zanne AE, Clark CJ, Poulsen JR. 2005. A long-term evaluation of fruiting phenology: importance of climate change. J. Trop. Ecol. 21, 31–45 (doi:10.1017/S0266467404001993) [Google Scholar]
- 149.Tutin CEG, White LJ. 1998. Primates, phenology and frugivory: present past and future patterns in the Lopé Reserve, Gabon. In Dynamics of tropical communities (eds Newbury DH, Prins HT, Brown N.), pp. 309–337 Oxford, UK: Blackwell Science [Google Scholar]
- 150.Barnes RFW, Barnes KL, Alers MPT, Blom A. 1991. Man determines the distribution of elephants in the rain-forests of northeastern Gabon. Afr. J. Ecol. 29, 54–63 (doi:10.1111/j.1365-2028.1991.tb00820.x) [Google Scholar]
- 151.Wilkie D, Sidle JG, Boundzanga GC, Blake S, Auzel P. 2001. Defaunation or deforestation: commercial logging and market hunting in northern Congo. In The cutting edge: conserving wildlife in logged tropical forests (eds Fimbel R, Robinson JG, Graial A.), pp. 375–399 New York, NY: Columbia University Press [Google Scholar]
- 152.Barnes RFW. 2002. The bushmeat boom and bust in West and Central Africa. Oryx 36, 236–242 (doi:10.1017/S0030605302000443) [Google Scholar]
- 153.Rist J, Milner-Gulland EJ, Cowlishaw G, Rowcliffe M. 2010. Hunter reporting of catch per unit effort as a monitoring tool in a bushmeat-harvesting system. Conserv. Biol. 24, 489–499 (doi:10.1111/j.1523-1739.2010.01470.x) [DOI] [PubMed] [Google Scholar]
- 154.Hedges S. 2012. Monitoring elephants and assessing threats: a manual for researchers, managers and conservationists. Himayatnagar, India: Hyderabad Universities [Google Scholar]
- 155.Kuëhl H, Maisels F, Ancrenaz M, Williamson EA. (eds) 2008. Best practice guidelines for surveys and monitoring of great ape populations. Gland, Switzerland: IUCN [Google Scholar]
- 156.Lewis SL, et al. 2013. Above-ground biomass and structure of 260 African tropical forests. Phil. Trans. R. Soc. B 368, 20120295 (doi:10.1098/rstb.2012.0295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Prugh LR, Hodges KE, Sinclair ARE, Brashares JS. 2008. Effect of habitat area and isolation on fragmented animal populations. Proc. Natl Acad. Sci. USA 105, 20 770–20 775 (doi:10.1073/pnas.0806080105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chazdon RL, et al. 2009. Beyond reserves: a research agenda for conserving biodiversity in human-modified tropical landscapes. Biotropica 41, 142–153 (doi:10.1111/j.1744-7429.2008.00471.x) [Google Scholar]
- 159.Stokes EJ, et al. 2010. Monitoring great ape and elephant abundance at large spatial scales: measuring effectiveness of a conservation landscape. PLoS ONE 5, e10294 (doi:10.1371/journal.pone.0010294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Clark CJ, Poulsen JR, Malonga R, Elkan PW. 2009. Can wildlife management in logging concession extend the ‘conservation estate’ of tropical forests? Conserv. Biol. 23, 1281–1293 (doi:10.1111/j.1523-1739.2009.01243.x) [DOI] [PubMed] [Google Scholar]
- 161.Tranquilli S, et al. 2011. Lack of conservation effort rapidly increases African great ape extinction risk. Conserv. Lett. 5, 48–55 (doi:10.1111/j.1755-263X.2011.00211.x) [Google Scholar]
- 162.Stokes EJ. 2010. Improving effectiveness of protection efforts in tiger source sites: developing a framework for law enforcement monitoring using MIST. Integr. Zool. 5, 363–377 (doi:10.1111/j.1749-4877.2010.00223.x) [DOI] [PubMed] [Google Scholar]


