Abstract
Rinderpest was a devastating disease of livestock responsible for continent-wide famine and poverty. Centuries of veterinary advances culminated in 2011 with the UN Food and Agriculture Organization and the World Organization for Animal Health declaring global eradication of rinderpest; only the second disease to be eradicated and the greatest veterinary achievement of our time. Conventional control measures, principally mass vaccination combined with zoosanitary procedures, led to substantial declines in the incidence of rinderpest. However, during the past decades, innovative strategies were deployed for the last mile to overcome diagnostic and surveillance challenges, unanticipated variations in virus pathogenicity, circulation of disease in wildlife populations and to service remote and nomadic communities in often-unstable states. This review provides an overview of these challenges, describes how they were overcome and identifies key factors for this success.
Keywords: rinderpest, morbillivirus, eradication
1. Introduction and history
Rinderpest (cattle plague) is caused by a morbillivirus of the family Paramyxoviridae, which cause diseases affecting mammals, including man. Rinderpest virus affects mainly ungulates, both wild and domestic, as does peste des petits ruminants virus to which, with measles virus, it is most closely related. Other related viruses are largely defined by the genera which they were first associated with but this is proving to be a simplification and host specificity is still ill-defined: canine distemper virus, which also affects a number of other carnivore families with epidemics reported in African lions and pinnipeds [1–4]; phocid distemper virus [5]; cetacean morbillivirus [4]; measles in humans [6] and a newly discovered felid morbillivirus [7]. Rinderpest in cattle and buffaloes is marked by fever with ocular and nasal discharges and is capable of causing high morbidity and mortality rates from oral and gastrointestinal tract ulceration, diarrhoea, dysentery, dehydration, protein loss and immunosuppression resulting from lymphocyte depletion. Pathogenesis in wildlife species can be highly variable, for example, a significant proportion of lesser kudu (Tragelaphus imberbis imberbis) develop ocular lesions, including corneal opacity and/or panophthalmitis causing total blindness, often leading to mortality without evident gastrointestinal lesions or diarrhoea.
Rinderpest and measles most probably had their origins in an environment where cattle and humans were living in close proximity; probably the cattle herds of Central or South Asia some 10 000 years ago at the time of domestication of the wild aurochs. However, in contradiction to this understanding, molecular clock analysis indicates that the divergence of rinderpest and the related measles virus might not have occurred until as recently as the eleventh or twelfth centuries [6].
Three human activities were responsible for the expansion of rinderpest from its origins: a rapidly growing human population with increasing dependence on cattle for food; draught power and status; and disease spread associated firstly with waging war and secondly with trading in livestock.
From the fourth to the twentieth centuries, large cattle herds travelled with marauding armies to feed the soldiers and provide draught power for their baggage trains (figure 1); victorious armies amassed large herds as the spoils of war to take home. Rinderpest was rarely far away. The Hun and Mongol invaders, raiding as far as present-day Iraq and Austria, brought rinderpest from their homeland in the east of Asia into Europe. Their Asian Grey Steppe oxen were remarkably resistant to the effects of rinderpest, and large herds could shed the virus for months and provoke epidemics that devastated native cattle and buffalo herds [8]. Repeated barbarian invasions introduced rinderpest into Europe and were responsible for massive human migrations that spread the disease widely. In the far east of Asia, conflict between China, Korea and Japan acted in the same way.
Figure 1.

Cattle-drawn campaign tent used by Chinggis Khaan portrayed on a Mongolian bank note.
Increasingly, in the seventeenth, eighteenth and nineteenth centuries, organized cattle trade, largely from Russia to feed the growing European cities, repeatedly introduced rinderpest into Europe and elsewhere. The ‘Russian disease’ was spread not only by cattle traded for meat but also by the trade in corn transported in massive quantities by ox-drawn carts [9]. The development of steam power in the nineteenth century enabled the shipment of live cattle by rail and sea in numbers previously unthought of. As a result, in the mid-nineteenth century, Europe was denuded of cattle by rinderpest. Massive trade of Baghdadli cattle [10] into Egypt from Iraq during the early twentieth century was also notorious for introducing rinderpest without the imported cattle themselves being seriously affected [11]. War and civil disturbance continued spreading rinderpest until the late-twentieth century: Israeli and Syrian armies, withdrawing from Lebanon in the early 1970s, took rinderpest with looted cattle into their own countries; goats were incriminated in the inadvertent reintroduction of rinderpest to Sri Lanka in 1978 by Indian peacekeeping forces; civil disturbance from the Gulf war in the early 1990s was accompanied by a major upsurge of infection in Turkey, Iran and Iraq [11,12].
Prior to the nineteenth century, virus was introduced to Africa through Egypt [13], or spread periodically from east to west through the Sudan and on to Senegal [14], most probably from the eastern African seaboard. Given the relatively low livestock and human densities, the virus apparently did not establish. Expanding livestock-owning communities fuelled these epidemics until the Great Rinderpest Pandemic in the 1890s that devastated human, cattle and wildlife populations in sub-Saharan Africa. These events were followed by endemic virus circulation in the pastoral areas of eastern Africa with occasional outbreaks of disease driven by inter-tribal raiding, especially among the Karamojong tribes resident in contiguous areas of southern Sudan, Ethiopia, Kenya and Uganda. Conflict resolution among these peoples became a major exercise and an important last-mile effort in the fight against rinderpest [15].
(a). Beginnings of rinderpest eradication
Rinderpest eradication started with the drawing up of zoosanitary procedures in the eighteenth century [16]. Much of the early history of eradication was more concerned with suppressing the disease by annual mass vaccination rather than with eradication [11]. The Food and Agriculture Organization (FAO) of the United Nations implemented many control programmes in Eastern Asia in the wake of the 1940s global war; however, the first coordinated international control programme for rinderpest was implemented only from 1963 to 1975 in Africa. The Inter-African Bureau of Epizootic Diseases was founded in 1950 with a plan to eliminate rinderpest from Africa. Heads of African Veterinary Services meeting in Kano, Nigeria, in 1960 pledged to implement a multinational project called Joint Project 15 (JP15) under the aegis of the Organization of African Unity, supported by many individual country aid programmes, especially the United States Agency for International Development (USAID), international organizations and agencies [17]. The aim was for each country to vaccinate all cattle of all ages every year for three successive years. Thereafter, each country undertook to vaccinate all calves annually.
Twenty-two countries were involved in JP15 in the beginning, of which 17 had rinderpest. By the end of 1979, only one country, Sudan, admitted to having the disease. This apparent success led to complacency, and many countries failed both to operate surveillance systems linked to official disease reporting and to maintain vaccination of young animals. JP15 ran out of steam in about 1975 having come close to eliminating rinderpest from Africa. Its major deficit was that it failed to recognize continuing covert circulation in domestic and wild ungulates in West and eastern Africa and, most importantly, persistent reservoirs of infection in the extensive pastoral communities of the Senegal River basin of West Africa and in eastern Africa. In retrospect, it was clear that JP15 had placed too much reliance on pulsed vaccination campaigns without a clearly defined objective or exit plan and had failed to address defects in the monitoring of vaccination programmes and in disease surveillance. Alarm over the inevitable resurgence of rinderpest in sub-Saharan Africa in the early 1980s stimulated establishment of the Pan-African Rinderpest Campaign (PARC) in 1986 under the aegis of the African Union-Inter-African Bureau of Animal Resources (AU-IBAR [18]). Supported largely by the European Commission (EC), this and its successor, the Pan-African Control of Epizootics Programme, were critical to the eventual elimination of rinderpest from Africa.
It was only from 1994 that a programme dedicated to global eradication was conceived to be feasible; FAO rose to the challenge by establishing the Global Rinderpest Eradication Programme (GREP) supported by a Secretariat based in Rome. Key to the GREP concept was the understanding, unlike other earlier rinderpest initiatives, that it was to be time-bound, with a 2010 deadline established for its completion. This review deals primarily with its operations and the final, successful effort.
2. Innovations that led to success
(a). Diagnostics and phylogenetics
An analysis of the achievements and deficits of JP15 identified several technical issues as constraints to success. One of these was a lack of appropriate diagnostic techniques for detecting rinderpest antibodies and capacity to confirm diagnosis and monitor the effectiveness of vaccination programmes. To meet these deficits, two European institutes developed diagnostic assays for rinderpest virus and antibodies in the 1980s and 1990s: the UK Pirbright Institute (formerly the Institute for Animal Health, Pirbright Laboratory), and the Centre de Coopération Internationale en Recherche Agronomique pour le Développement-Élevage et Medicine Vétérinaire des Pays Tropicaux in France.
Supported by the UK Overseas Development Administration and the PARC, scientists at Pirbright laboratory developed an indirect antibody ELISA (enzyme-linked immunosorbent assay) and soon after, a monoclonal-antibody-based competitive ELISA format [19,20] which became the mainstay of serological studies for monitoring of vaccination programmes and surveys for verification and official recognition of freedom. With sensitivity exceeding 70 per cent and specificity of at least 99.5 per cent, the test was fit to meet the needs of GREP. Apparent false-positives met during extensive serosurveillance programmes in the final stages of GREP were greatly reduced when it was realized that maternally derived antibodies could persist for 11 months. Recommending that only a cohort of cattle young enough not to have been vaccinated and old enough to have lost any colostral antibody greatly improved the specificity of the testing procedure. As a proxy for age, the adopted guideline was that cattle and water buffaloes should have at least one erupted pair of incisor teeth, providing a wide margin of safety. Follow-up of clusters of apparent false-positive cattle in Pakistan usually showed that the age criterion had been ignored and field infection could be ruled out.
Both commercial and national laboratory support was critical to the success of GREP. The developed standard tests were made available worldwide through collaboration with a commercial company, and international funding1 enabled technology transfer of essential diagnostic methods to relevant countries in Asia and Africa. Regional networks and regular meetings of veterinary scientists developed technical competence in laboratory technology and in coordinating control programmes.
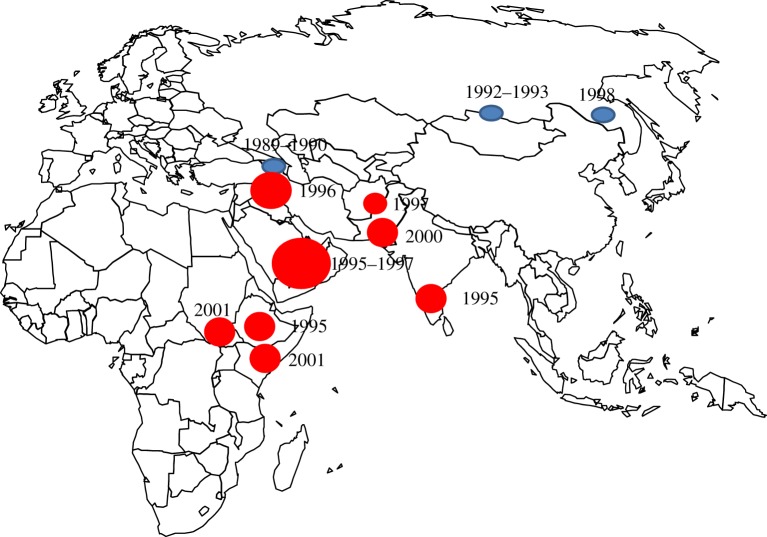
Another major contribution to GREP was the development of genetic characterization methods for morbilliviruses [21]. Although the number of viruses submitted for characterization was never large, the elucidation of viral phylogeny was very informative, demonstrating three largely geographically distinct viral clades: African lineages 1 and 2 and the Asian lineage. Viruses within clades that were indistinguishable serologically showed significant genomic differences, enabling the identification of origins of outbreaks. For example, molecular phylogeny demonstrated that the causal virus in the outbreak in the Tsavo National Park of Kenya in 1994/5 was related closely to lineage 2 viruses that had been circulating in eastern Africa 30 years previously and not the lineage 1 viruses present in the Sudan, as had been assumed. The source was traced to a hitherto undisclosed reservoir of rinderpest in the Somali ecosystem. Molecular epidemiology suggested that outbreaks of rinderpest in the Middle East were derived from viruses repeatedly introduced from Asia and not from Africa as many had assumed and this was confirmed when such outbreaks ceased with the control of rinderpest in the Indian subcontinent [11,22]. Similarly, viruses derived from outbreaks in the vaccination buffer zone surrounding the Soviet Union and the vaccine virus used were shown to be virtually identical, indicating that reversion to virulence of the vaccine on three occasions over 20 years had been responsible for the outbreaks and not a persisting unknown reservoir (figure 2).
Figure 2.
Last occurrence of wild rinderpest virus (red), and outbreaks of vaccine-derived rinderpest (blue).
(b). Vaccine developments
Development of the Plowright tissue culture rinderpest vaccine (TCRV) in 1960 [23] was an important milestone in rinderpest control that gave impetus to the first coordinated effort to eradicate rinderpest from all of Africa. TCRV was one of the finest vaccines ever developed in human or veterinary medicine. It protected against all clades of rinderpest virus, provided lifelong immunity to cattle, was never associated with any adverse reactions, and a single tissue culture infectious dose was immunogenic. The vaccine benefited hundreds of millions of livestock-dependent people and, properly, Plowright received the World Food Prize in 1999. The principal limitation of the vaccine was that it required a strict cold-chain—a significant impediment to field vaccination programmes. The production process using primary bovine kidney cells was a potential source of contaminants and a constraint to large-scale manufacturing.
Production of TCRV was established in national laboratories across Africa, but the annual demand of 50 million doses was insufficient to achieve economies of scale, maintain quality of production or recapitalize their facilities. In 1986, rinderpest vaccines were typically of low quality in terms of both potency and purity. This led to establishment of the Pan-African Veterinary Vaccine Centre that successfully institutionalized independent quality control for rinderpest and other key livestock vaccines for the national production laboratories and assured an ample supply of safe and efficacious rinderpest vaccine for the eradication effort.
Second, a research programme to develop a thermostable formulation of TCRV was initiated by Tufts University School of Veterinary Medicine and the US Department of Agriculture. Within 2 years, the project was producing rinderpest vaccine that retained the international required minimum immunizing dose for up to eight months at 37°C [24,25], and 10 days at 56°C. The new vaccine, named ThermoVax, had a recommended shelf life of 30 days outside the cold-chain and it was required to hold the minimum titre for 14 days at 45°C, sufficient to dramatically extend the reach of field vaccination programmes. The transfer of technology to African production facilities led to the commercial availability of ThermoVax in quantities sufficient for rinderpest eradication by 1992.
The Russian vaccine-associated incidents suggested that the use of attenuated vaccines posed a threat to rinderpest eradication even though TCRV had never been suspected of reversion to virulence. As a result, timely cessation of vaccination became a key element of GREP policy.
(c). Innovation in vaccine delivery and community involvement
To capture the full benefit of ThermoVax vaccine, institutional change was needed that would have far-reaching effects on the relationships and roles of public, private and community animal health service actors [25]. Refrigerating a vaccine requires a network of ice-making capacity, static refrigeration and portable cold boxes that determine the reach of vaccination programmes. The cold-chain essentially requires significant organizational support, logistics and a vehicular transport network, which was one of the single largest costs in the delivery of vaccine. Rinderpest vaccination was one of the principal activities of the public veterinary services in the affected countries in Africa and Asia [11], and a large part of veterinary services budgets. For individual veterinarians and para-professionals, income from rinderpest vaccination activities was a major component of their livelihoods, and involvement with campaigns was also a source of power and prestige. With ThermoVax, vaccine could be delivered on foot, by bicycle or using animal transport by a wide range of stakeholders, and this flexibility was perceived as a threat to the prestige and resource flows to conventional veterinary systems.
Early advocates for change recognized that community-based animal health workers (CBAHWs) could make a major contribution to rinderpest eradication [26]. CBAHWs are livestock owners selected by their communities to be trained and equipped for treating priority animal diseases. Incorporation of vaccination, especially vaccination for a critical disease, was a new concept. With difficulty, permission was obtained from national authorities to conduct pilot programmes to test the reliability of CBAHWs in rinderpest vaccination. These pilots worked with communities to select trainees; they provided training on vaccination against rinderpest and treatment of key diseases, and built supply and supervision networks. Initial programmes incorporated seromonitoring to measure the quality and impact of the vaccination activity. The results demonstrated that CBAHW vaccination programmes were achieving over 80 per cent herd immunity [27], matching or surpassing the levels achieved by national veterinary services in more accessible areas [28].
The reason for the success of community-based vaccination relates largely to incentives. The communities perceived rinderpest as a major threat and access to vaccination was sought by livestock owners. The training of CBAHWs empowered the local community and they were highly respected. They were vaccinating the cattle of their extended families and neighbours (figure 3) and had every reason to work diligently to assure the success of the vaccination. They were able to offer vaccination in safe and easily accessible locations consistent with the movement needs of the herds. Remuneration was based on the quantity of work and, in most cases, provided by the livestock owners. By contrast, government vaccination programmes were implemented by personnel in receipt of daily field allowances; payment was not linked to the quantity or quality of work, but to the number of days spent in the field. In our experience, the single most important factor improving the performance of vaccination programmes is to establish incentive systems that motivate the staff to reinforce the quality and quantity of their outputs. In many settings, veterinarians were possibly not the best-suited actors to deliver effective services in rural Africa and it was clear that a rethinking of veterinary service models could result in a better service to farmers. The result was a new business model that teamed veterinary practitioners with CBAHWs in a synergistic partnership that expanded the opportunities for veterinary practice, gave livestock owners a greater role and enhanced access to services.
Figure 3.

Ethiopian CBAHW of the Afar tribe vaccinating cattle in the early 1990s (courtesy of AU-IBAR).
(d). Strategy developments based on epidemiological understanding
In 1993, FAO and OIE (Office International des Epizooties, i.e. the World Organisation for Animal Health) convened a group to consider how best to provide guidance to countries participating in a GREP, resulting in the ‘OIE pathway’ that outlined a progression from an initial self-declaration of provisional freedom from disease and cessation of vaccination, followed by OIE accredited stages of freedom from disease and freedom from infection. Accreditation, overseen by the OIE Rinderpest Ad Hoc Group, required that a country operate a surveillance system that would be able to detect rinderpest, if it were present. This enabled countries to monitor their own progress towards accredited freedom from infection and to plan their own eradication timelines. This process proved to be very useful for the duration of the global programme, only being modified to a one-step process of accreditation of freedom from infection in 2005 once it became clear that rinderpest disease had not been seen for several years.
During the early 1990s, epidemiological studies in Ethiopia showed that rinderpest had persisted in parts of the pastoral ecosystems of eastern Africa throughout the JP15 campaign and that annual mass vaccination campaigns generally reached less than one-third of the 35 million cattle believed to be present. Official discouragement of investigation of outbreaks and insecurity in the lowland areas which were home to the nomadic pastoralists and their large herds of cattle obscured the true incidence of rinderpest [11].
From 1989 to 1992, repeated outbreaks were studied in the highlands, in the Afar lowlands, in the Rift Valley and in the highland–lowland interface areas to understand the relationship of these outbreaks to each other and to determine the persistence of infection. In the lowland areas, Afar livestock owners were desperate for vaccination to help them control rinderpest outbreaks in cattle less than 3 years of age. Drs W. Asfaw, G. Van't Klooster and P. Roeder conducted field investigations in 1992 focusing on interviewing government veterinary officers and livestock owners, and collecting samples from cattle for testing for rinderpest in the highland–lowland interface and along the border with Eritrea through Wollo and Tigrai provinces. It became clear from this mission that
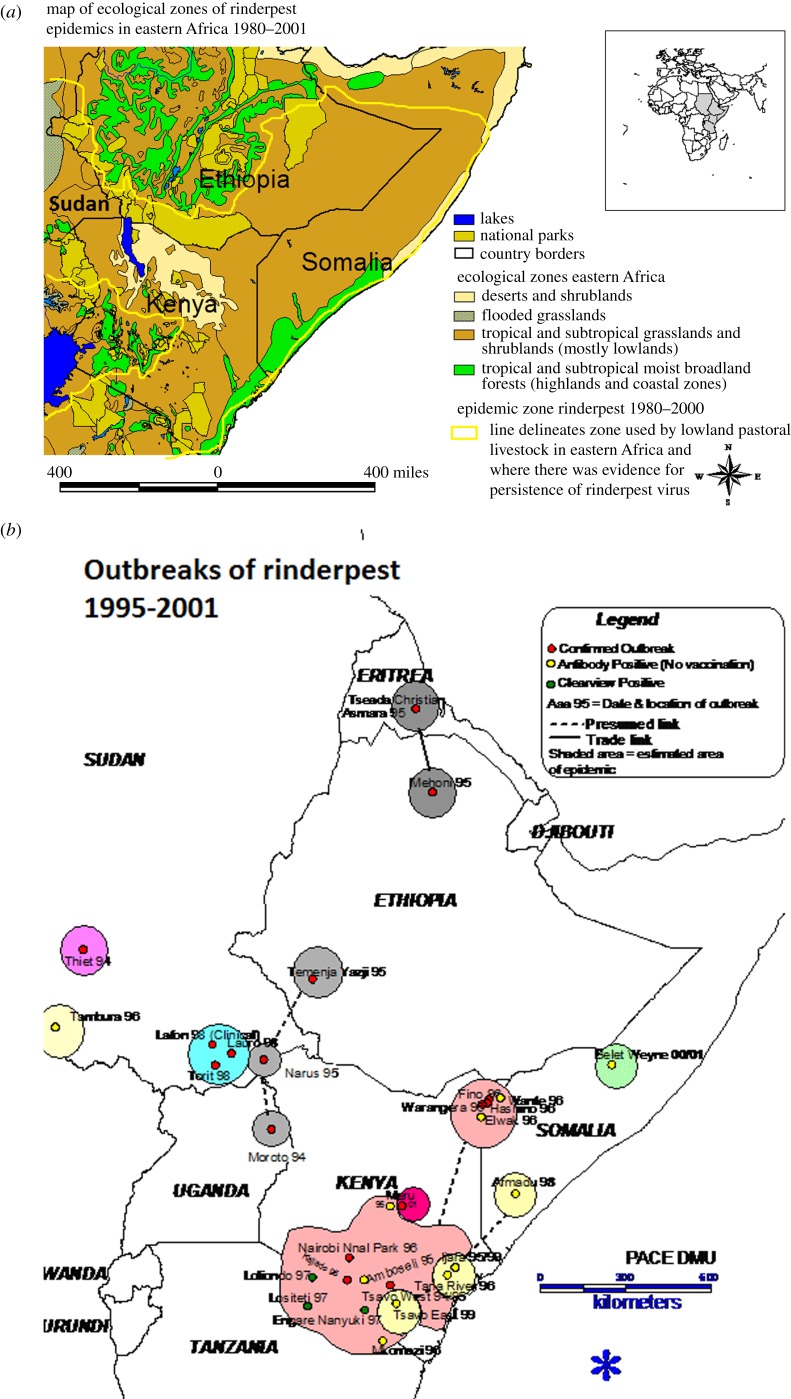
— Rinderpest was endemic within the pastoralists' herds in discrete lowland areas surrounding the highlands: the Afar region to the northeast; between Lake Tana and Sudan to the west; to the southwest on the Sudanese border where pastoralists' cattle seasonally migrated between Sudan and the lowlands of Ethiopia and less clearly in an area to the south of the southern highlands (see figure 4a for a map of the region).
— Rinderpest repeatedly spread into the highland areas adjacent to the lowland pastoral areas by seasonal migrations of herders to access grazing and trade plough oxen in the highland–lowland interface area.
— Outbreaks in the sedentary cattle population in highland areas were spread mainly through livestock markets, moving slowly until they either burnt out or were controlled; highland areas could therefore serve as epidemic indicator areas for rinderpest endemicity in the remote lowland pastoral areas (figure 4b).
— Attempting mass vaccination campaigns for highland cattle was largely a waste of resources; what was needed was to eliminate infection from the pastoralists' cattle. In the endemic pastoralist areas, vaccination could be limited to cattle between 1 and 3 years of age, because older cattle were immune from earlier contact with infection.
— Remote, marginalized areas required a new approach—vaccine delivery through annual, pulsed, mass vaccination requiring cold-chain was not effective here. Involvement of the community and the use of ThermoVax proved critical for the endgame success.
Figure 4.
(a) Map of ecological zones in eastern Africa mentioned in the text showing the rinderpest epidemic zone for 1980–2000. (b) Rinderpest outbreaks in the time period from 1995 to 2001 in eastern Africa.
Based on these observations, a new rinderpest elimination strategy was developed for Ethiopia, the essence of which was to
— cease mass vaccination in most of the highlands of Ethiopia and replace it with improved surveillance and emergency preparedness;
— focus vaccination on the pastoralist areas, where rinderpest was persisting, and create buffer zones in the highland–lowland interface to minimize spread to the highlands; approximately three million cattle were to be targeted;
— train and support CBAHWs to deliver vaccination in the remote, marginalized areas, at that time made especially hazardous by civil strife. ThermoVax was to be used in cattle between 1 and 3 years of age; and
— cease vaccination as soon as there was confidence that rinderpest was no longer circulating and confirm absence of infection by serological and participatory surveillance.
Strategy was refined as more epidemiological information became available to clarify the situation in the Rift Valley, where rinderpest was being introduced by exchange of heifers from the Arssi highlands with Afar plough oxen. West of Lake Tana, vaccination teams were taken into the most inaccessible areas by military helicopters provided by the new government, quickly eliminating this reservoir. The Afar region took longer with the last outbreak occurring in October 1995, the same time as the last incursion from Sudan into western Ethiopia. Since that time, Ethiopia has been free from rinderpest. Once there was growing confidence that rinderpest virus was no longer present, it was possible to progressively reduce the vaccinated zones eventually ceasing vaccination completely and commencing verification of freedom through clinical, participatory and serological surveillance. However, official recognition by OIE of freedom for Ethiopia had to await determination of the freedom of neighbouring countries, which took until 2008 following a regional coordination of strategy.
The other significant event, which contributed to defining the final phase of eradication, was a severe epidemic in wildlife from 1993 to 1996 [29], apparently originating from the coastal lowlands of eastern Kenya, and affecting mainly lesser kudus (T. imberbis), African buffaloes (Syncerus caffer), elands (Tragelaphus oryx) and giraffes (Camelopardalis tragocamelus). Mortality in buffaloes of 60 per cent was estimated from aerial census and possibly reached up to 90 per cent in some kudu populations. The outbreak showed the value of wild animals as sentinels and contributed to defining the final eradication phase, initiating an intensive, regional search for rinderpest infection in wildlife populations [30,31]. Given there were no known foci of rinderpest nearby, and the extent of control in Ethiopia, the outbreak came as a total surprise. It was assumed to be due to virus spreading through livestock trade movements deep into the country from the Sudan–Ethiopia–Kenya border areas. This sparked a mass vaccination campaign in Kenya despite the apparent failure to detect the disease in cattle herds. Experimental studies showed the virus to cause mild disease in indigenous livestock, whereas it was severe in grade cattle [32]. Recorded observations of rinderpest in wild populations over a period of 30 years provided evidence of where the virus had been present across eastern, West and Central Africa. Of profound importance to eradication was the demonstration that a rinderpest virus causing only mild disease in cattle was still persisting [33,34] in the Somali ecosystem, a region comprising contiguous areas of northeastern Kenya, southeastern Ethiopia and southern Somalia (figure 4). An association between mild rinderpest in cattle and severe disease in wild artiodactyls had been observed frequently in East Africa from the early years of the twentieth century [35–37]; Maasai herders even used ‘mild strains’ of rinderpest to immunize their cattle [35].
In retrospect, there were indications of persistent enzootic mild rinderpest in Somalia well before its confirmation in 1996 [33,38]. Somali veterinarians described a syndrome in young cattle they attributed to ‘bovine viral diarrhoea’ (caused by a pestivirus). The apparent independent persistence of this reservoir of infection in wildlife generated pessimistic predictions about the outcome of GREP. Yet, ‘mild’ rinderpest was neither a feature of eastern Africa alone nor of the African lineage 2, as many had proposed. Evidence accrued that the wildlife reservoir was not an insurmountable obstacle to eradication [11]. In the 1960s, seroconversion indicative of endemic rinderpest in the wildlife of the Serengeti National Park in Tanzania became undetectable soon after intensive vaccination campaigns were mounted in the dense cattle herds surrounding the park [39–41]. ‘Mild rinderpest’ caused by African lineage 1 was also recognized in cattle in Egypt in the 1980s and in Iraq, most likely caused by the Asian lineage, in the 1990s. Historical accounts of rinderpest in Vietnam, Laos and Cambodia in the 1950s, which must have been caused by the Asian lineage, bear similarities to that of rinderpest in East Africa at around the same time caused by African lineage 2. Rinderpest in Southeast Asia affecting wild pigs and a diversity of wild ruminants [42,43], spread rapidly and died out spontaneously; local cattle were apparently resistant. Based on studies of rinderpest epidemics in wildlife in eastern Africa in the 1980s and 1990s, an upper limit of 4 years for virus circulation in wildlife was proposed [30].
(e). Accessing community knowledge for surveillance
As a first step in the establishment of CBAHW programmes, participatory rural appraisal (PRA) techniques [44] were used to understand the community perceptions regarding animal health and to prioritize disease problems. It was evident from these activities that the communities had very detailed knowledge of the presentation, gross pathology and epidemiology of many disease problems affecting their livelihoods. Local knowledge included terms for clinical conditions that often translated into specific diseases in modern terminology.
Given the importance of rinderpest to pastoral livestock keepers, the history and behaviour of rinderpest was often an important topic in the oral traditions of communities. Elders and community animal health workers identified rinderpest outbreaks and could describe the risk factors that created the conditions for endemic persistence of the virus. They proved to be key informants often providing more accurate disease intelligence than the formal surveillance systems [45]. For example, Tom Olaka, a CBAHW from Karamoja, Uganda, identified an outbreak of rinderpest and provided information on livestock movements that led to an effective response, enabling the completion of rinderpest eradication from Uganda [25]. A similar participatory disease-searching technique documented aforementioned mild disease in the cattle in the Somali ecosystem [33]. Participatory disease surveillance was further developed in Pakistan and was widely used as a tool to contribute to the validation of rinderpest eradication. Today, participatory epidemiology has become an accepted and valued tool for the veterinary profession [25] and is one of the legacies of rinderpest eradication [16].
(f). The contribution of modelling to rinderpest eradication
Modelling allows data from diverse sources to be integrated into analytical systems that can then be used to assess the probable impact of alternative control scenarios. Combined with field intelligence, models can assist decision-makers to make informed choices and to set intervention targets. In the case of rinderpest, stochastic SEIR (Susceptible, Exposed, Infectious and Recovered) models of transmission were constructed using parameters estimated from the literature and livestock owner information on population contact structure and the clinical behaviour of rinderpest in their herds [46]. Serological surveys facilitated estimation of the basic reproductive number, R0, defined as the number of new cases resulting from the introduction of one infected animal into a susceptible population, which determines the minimum herd immunity required to interrupt transmission (1 − 1/R0). Estimates of R0 ranged from 1.5 for cattle in Somalia (lineage 2) to 4.6 for cattle in Sudan (lineage 1) [46]. Values of R0 for rinderpest transmission in African buffaloes calculated from post-outbreak seroprevalence estimates ranged from 2 to 7.1 [47]. The values of R0 in cattle correspond to a minimum herd immunity requirement in cattle for eradication of between 33 and 78 per cent. The estimate of the herd immunity threshold of 33 per cent for lineage 2 was surprisingly low and greeted with scepticism. However, rinderpest was eradicated from the Somali ecosystem with herd immunity levels that probably never exceeded 50 per cent.
There is no evidence of latent or persistent infection being involved in the maintenance of rinderpest virus; persistence, therefore, requires a constant supply of susceptible animals for the disease to be sustained. If the population is too small to establish sustained chains of transmission, the virus can fade out or be intentionally eliminated relatively easily. Estimates from simulations of the critical community size required to sustain transmission were in the order of 200 000 head of cattle. This meant that communities smaller than 200 000 head did not need to be prioritized in the final stages of eradication as the disease would naturally fade out from these populations [46]. Further, this supported the view that if the disease was controlled in cattle, then it would fade out from wildlife populations as the fragmented populations remaining in Africa are not nearly large enough to sustain infection even if biologically competent to do so.
Rinderpest modelling was shown to be an effective communication tool to engage decision-makers in visualizing important epidemiological processes and choices. Purposefully avoiding unnecessary complexity, the models could serve as demonstration tools and interactive demonstrations were used to illustrate concepts such as fade out of disease from small populations. More importantly, the models illustrated how suboptimal vaccination could contribute to virus persistence. This helped to create a consensus for a strategy of focused vaccination as a necessary route to achieve sufficient herd immunity to interrupt virus persistence as the necessary route to eradication.
3. The last days of rinderpest
(a). Reservoirs and elimination
The epidemiological studies conducted from 1994 to 1998, together with the assessment of global rinderpest distribution described by FAO in 1996, identified seven areas of the world that constituted possible infection reservoirs: (i) contiguous areas of the far east of the Russian Federation, Mongolia and China; (ii) India, Pakistan and Afghanistan; (iii) contiguous areas of Iraq, Iran and Turkey; (iv) Saudi Arabia and Yemen; (v) the northeastern Ethiopian Afar region; (vi) contiguous areas of southern Sudan, Ethiopia, Uganda and Kenya; and (vii) contiguous areas of Kenya, Somalia and Ethiopia. Ultimately, eradication came down to ensuring elimination in each of these seven areas through embarking on elimination campaigns or, alternatively, proving absence of infection through enhanced surveillance. This understanding prompted FAO and partners to launch the Intensified GREP in 1999 [48]. ‘Seek, Contain, Eliminate’ became the slogan for eradication.
Elimination of rinderpest from Sudan, in an insecure and resource-poor environment dominated by civil war, was a difficult and protracted process. Fortunately, mass vaccination during the 1990s by non-governmental organisations (NGOs) working under the umbrella of the UNICEF Operation Lifeline Sudan Household Food Security Programme [49] greatly reduced disease incidence in the pastoral herds of southern Sudan. The last time that rinderpest crossed the border into Ethiopia from Sudan was in October 1995. In 2000, an unconfirmed outbreak occurred in the cattle belonging to the Murle and Jie tribes of southern Sudan, a population amounting to some 800 000 cattle. Widespread surveillance conducted by the staff of the NGO Vétérinaires Sans Frontières (VSF) Belgium strongly suggested that the cattle of other tribes were free from rinderpest at that time, providing a unique opportunity to eliminate this reservoir of infection. An intensive vaccination programme was mounted that reached virtually the entire cattle population; at the same time, vaccination was withdrawn elsewhere despite initial opposition. Subsequent active surveillance indicated that rinderpest ceased to circulate after 2001. Serological testing of a population of over a million migratory white-eared kob (Kobus leucotis) antelopes, in close contact with livestock of many different tribal communities, supported the view that rinderpest had been eliminated. Ironically, the virtual elimination of the large buffalo herds during the military conflict may have removed one source of re-infection at this critical time. Extensive participatory and serological surveillance provided sufficient assurance that freedom from rinderpest was recognized by OIE in 2011.
While sustained transmission in Africa related to pastoral ecosystems, studies in Pakistan and Afghanistan indicated that the Indus River dairy buffaloes of Sindh Province in southern Pakistan acted as a reservoir as did the buffaloes and cattle in rural Tamil Nadu and Karnataka in peninsular India (11). The key to elimination of rinderpest in India, Pakistan and Afghanistan therefore lay in elimination of these persisting foci of infection (6). Progressive reduction in rinderpest infection in the region resulted from increased quality control of Pakistan-produced rinderpest vaccine from 1996, mounting active participatory disease surveillance [50], serological surveillance, and an extension programme for farmers. Surveillance detected rinderpest for the last time in 2000, the year in which a bold decision was taken to withdraw vaccination from the whole country. Participatory surveys conducted in 10 347 villages from 2003 to 2006 representing 20.5 per cent of the total 50 568 villages, found no evidence of infection from 2000 onwards. Extensive serological surveys (table 1) provided clear evidence of rinderpest freedom by 2007, allowing official recognition of freedom by OIE.
Table 1.
Results of a randomized serological survey for rinderpest antibodies in cattle and riverine buffaloes in Pakistan conducted by competitive ELISA [15] as an aide to demonstrating freedom from infection. Data shown by courtesy of the Government of Pakistan.
| year | 2003 |
2004/5 |
2006 |
|||
|---|---|---|---|---|---|---|
| province | positive | tested (%) | positive | tested (%) | positive | tested (%) |
| Azad Jammu and Kashmir | 0 | 760 (0) | 1 | 2394 (0) | 2960 (0) | |
| Balochistan | 7 | 1000 (0.7) | 13 | 6101 (0.2) | 2 | 6960 (0) |
| Islamabad Capital Territory | 2 | 507 (0.4) | 2 | 452 (0.4) | 0 | 1000 (0) |
| northern areas | 2 | 760 (0.3) | 55 | 2462 (2.2) | 12 | 2949 (0.4) |
| North-West Frontier Province | 4 | 1000 (0.4) | 7 | 5800 (0.1) | 6 | 6974 (0) |
| Punjab | 4 | 2107 (0.2) | 6 | 6068 (0.1) | 8 | 7022 (0.1) |
| Sindh | 13 | 2455 (0.5) | 16 | 5939 (0.3) | 23 | 8000 (0.3) |
| total | 32 | 8567 (0.4) | 100 | 29216 (0.3) | 51 | 35865 (0.1) |
(b). Cessation of vaccination
Cessation of vaccination, vigorously opposed by many in these focal areas, combined with livestock owner sensitization and clinical and serological surveillance became keystones of success. Livestock-rearing communities whose lives had been blighted by rinderpest over many years saw vaccination as their saviour and it was crucial that they understood that cessation of vaccination was a necessary step to eradication. Communication with and involvement of communities in the eradication process was most finely developed by VSF under the aegis of the AU-IBAR PACE programme ‘Fight against African lineage 1’ and was crucial to achieving an orderly progress towards rinderpest freedom.
In the absence of unequivocal evidence for the presence of rinderpest in Somalia and Kenya, GREP accented the need to cease vaccination to facilitate surveillance, including serosurveillance. Unfortunately, initial equivocal results from serological studies [51] delayed development of a definitive consensus that rinderpest was no longer present in Somalia and Kenya until 2006 only then allowing OIE to recognize all three countries—Ethiopia, Kenya and Somalia—as free. Rinderpest had last been seen in wild African buffaloes in Meru National Park in Kenya in October 2001, it was mild in nature as the herds were partially immune, with only young animals less than 3–4 years of age susceptible. Clinical disease was not reported; its discovery in a buffalo was a result of the annual serosurveillance and monitoring. Through subsequent careful clinical observation of herds and selection of mildly diseased buffalo, samples were obtained that indicated that African lineage 2 rinderpest virus was still circulating at that time. The source of this outbreak was never proved, but the timing points to the large groups of Somali or Boran livestock entering the Greater Meru ecosystem in the dry season. These animals might have travelled from deep in Somalia or even southern Ethiopia. After this final expression of virus, it gradually became evident that rinderpest ceased to circulate in Africa at about this time.
(c). Peste des petits ruminants
Sadly, this was not the last word on morbillivirus infection in livestock in Africa; historically linked PPR virus continues to cause significant economic losses [52,53]. Subsequent to the apparent eradication of rinderpest virus from the Karamojong cluster, estimated around the late 1990s, serosurveillance in wildlife in Uganda detected seroconversion to PPR virus in buffalo herds in several National Parks, between 2002 and 2004 [47]. Officially, PPR outbreaks in small ruminants in Uganda were not reported to the OIE until 2007 but a single unconfirmed report of PPR was provided to the AU-IBAR ARIS reporting system in 2003 from around Soroti in Karamojong region of Uganda [18]. This was supported by serological evidence for spread of PPR in buffalo herds in Kidepo, Lake Mburo and Queen Elizabeth National Parks between 2003 and 2004 [47]. This was the first evidence for the significant spread of PPR South into East Africa and beyond, which has become a feature in the region in the past decade. PPR is now endemic in eastern Africa and as far south as Angola, southern Democratic Republic of the Congo and in Tanzania, south of Selous National Reserve [52,54]. The possibility that eradication of rinderpest and cessation of vaccination against this virus was a causative factor in the emergence of PPR was raised at the time of the buffalo seroconversion but largely ignored. One possible but debatable explanation for this emergence of PPR might be the reduction in cross protective immunity in potential vector species (cattle, buffalo) and the cessation of the widespread (if illegal) use of rinderpest vaccine in small livestock to protect them against PPR (e.g. vaccination of goats in Ethiopia [47]). Whether removal of one morbillivirus from an ecological niche and host community had a significant impact on PPR emergence remains speculative, but these data are supportive of this hypothesis (see also Lloyd-Smith [55]). Nevertheless, there is a global imperative to seek progressive control and the tools for making progress are available [56].
(d). Political and donor support
The least economically developed countries found it difficult to allocate the funding necessary to mount systematic and sustained rinderpest eradication programmes even when the disease was having a major impact on livelihoods and national economies. The success of an eradication programme for a contagious transboundary animal disease eventually depends on the performance of its weakest partners. However, political imperatives meant that certain key countries did not receive the necessary donor support to enter fully into regional initiatives. Thus, FAO GREP needed to source funding for rinderpest control outside regional rinderpest control programmes. Fortunately, donors appreciated that rinderpest was an issue in many humanitarian crises and its control was included in aid programmes such as Operation Lifeline Sudan and the Iraq Oil for Food Programme. The UN Office for the Coordination of Humanitarian Affairs, the EC and USAID were especially supportive. However, there was marked reluctance to fund the latter stages of the programme when the disease impact was dramatically reduced and surveillance activities were needed to verify freedom. This critical process received major support from the EC through FAO GREP and national projects intended for the strengthening of livestock services.
(e). Cost–benefit analysis
One weakness of GREP was that it lacked in-depth analysis of the socio-economic impact of rinderpest and the benefits likely to accrue from its eradication that would have been useful for persuading economists and other decision-makers that rinderpest eradication merited funding and dissuading detractors of the eradication effort. In the case of rinderpest, it is difficult to accurately estimate the expenditures, because budgets were often subsumed into support for more broadly based issues such as privatization of veterinary services, surveillance and other disease control programmes; financial allocations specifically for rinderpest eradication are difficult to identify. For Chad, the benefit–cost ratio associated with rinderpest control from 1963 to 2002 was estimated to be just over 4 [57]. This analysis takes into account sector-level benefits but excludes macroeconomic and regional benefits. However, rinderpest eradication does not benefit just the livestock sector in terms of mortality and avoided losses. The livestock sector impacts the broader economy and cost–benefit analyses should measure how the whole economy adjusts to an intervention and capture the full productivity dividend from rinderpest eradication. This is carried out by constructing a social accounting matrix and deriving multipliers to apply to the basic cost–benefit analysis [16]. Applying livestock-sector multipliers ranging from 3.5 to 4 yields much higher aggregate benefits. In the case of India, the benefit–cost ratio of the National Programme for Rinderpest Eradication, which brought about eradication in the 1990s, was estimated to be over 60 fuelled by higher market access for livestock exports, which boomed as rinderpest freedom was achieved [46]. In addition, the many unquantifiable indirect benefits extend across participatory epidemiology, CBAHW systems and strengthened veterinary services [16,25].
(f). Rinderpest virus sequestration
One issue that remains after a global declaration of rinderpest eradication is the sequestration of all rinderpest viruses in specialized, secure laboratories and their eventual destruction. The number of laboratories remaining with rinderpest virus is small but the threat of irresponsible or malign reintroduction of the virus into livestock demands vigilance until all viruses are secured. FAO and partners are engaged in identifying remaining virus stocks and preparing the formal framework for their sequestration.
4. Conclusion
Much is being made of the need for a ‘one health’ approach to disease in the twenty-first century [58], and the rinderpest eradication process was perhaps the first collaborative effort that could be described as a success story of this approach. Contributions came from both veterinary and wildlife ecology sectors and these were integrated with socio-ecological approaches to disease investigation and service delivery. The need for eradication programmes to be adaptively managed, time-bound and based on a sound epidemiological understanding of disease in all susceptible species was prime among the lessons learnt; routine, pulsed vaccination programmes alone were found to be inadequate. From a technical viewpoint, the value of vaccine quality assurance was evident as was that of field studies using robust, sensitive and specific diagnostic tests leading into epidemiological clarification supported by molecular epidemiology to provide the sound technical basis to achieve and monitor progress. Two other issues favoured progress; these were support for diagnostic technology transfer to national and regional laboratories, underpinned by the services of a global reference laboratory, and global and regional coordination with the leadership of appropriate global institutions and regional political organizations. However, arguably the most important factor contributing to success was obtaining the support of national and regional organizations and donors; without this, real progress would have been impossible. Although it was not put in place for rinderpest, it became clear that achieving this support could be assisted greatly by having the results of socio-economic studies demonstrating the impact of the disease concerned and its eradication.
Many of the lessons learnt from the rinderpest eradication process are relevant to undertaking control of other livestock plagues. Arguably, the disease most likely to be addressed successfully is that of PPR in sheep and goats [56]. Morbilliviruses are highly labile and this raises concerns that PPR virus, now widespread in Africa and Asia, could evolve to cause disease in other wild and domesticated species. Given this possibility and the severe impact of PPR on livelihoods of farmers and wildlife, its eradication would seem to be justifiable. With the tools available and experience gained from rinderpest, eradication success is virtually guaranteed.
Another prominent but much longer-term candidate is foot-and-mouth disease (FMD) for which FAO and OIE have developed a Progressive Control Pathway emulating the GREP Blueprint [59]. However, the case of FMD, unlike rinderpest and PPR, is very different. It might be possible to eradicate in Asia, but the independent persistence in buffalo and savannah wildlife and the lack of clinical disease complicates the situation in Africa. Eradication of FMD in Africa is, therefore, a much greater challenge, and eradication is perhaps not feasible or even necessary if African livestock production systems evolve to be locally relevant to people rather than to international agribusiness and north-based market trade systems [60]. The relatively mild nature of the disease in indigenous African livestock, and opportunity for trade through commodities which can be rendered free from FMD virus, further suggest alternate strategies [61]. Arguably, more attention should be given to developing the buffalo and wild bovids as alternative resources to domestic cattle in many settings, given proven high economic value and disease resistance [62]. It would be nigh on impossible to control FMD infection in its natural state, in these species.
Acknowledgements
Eradication of rinderpest would not have been possible without the work of many veterinarians dedicated to the success of national, regional and global rinderpest control programmes in the last quarter of the twentieth century. So many people were involved that space precludes a full coverage, but special attention is merited for the vision and seminal contributions of Yoshihiro Ozawa, Walter Plowright, Gordon Scott, Alain Provost, Yves Cheneau, Walter Masiga, Jan Mulder and Mark Rweyemamu from FAO, OIE, the United Kingdom, France, the European Union and the African Union.
Endnote
Pirbright Institute and the FAO/International Atomic Energy Agency (IAEA) Joint Division, supported by funding from the Swedish International Development Agency, the EC, USAID and FAO.
References
- 1.Roelke-Parker ME, et al. 1996. A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379, 441–445 10.1038/379441a0 (doi:10.1038/379441a0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munson L, Terio KA, Kock R, Mlengeya T, Roelke ME, Dubovi E, Summers B, Sinclair ARE, Packer C. 2008. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions. PLoS ONE 3, e2545. 10.1371/journal.pone.0002545 (doi:10.1371/journal.pone.0002545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kock R, Chalmers WS, Mwanzia J, Chillingworth C, Wambua J, Coleman PG, Baxendale W. 1998. Canine distemper antibodies in lions of the Masai Mara . Vet. Rec. 142, 662–665 10.1136/vr.142.24.662 (doi:10.1136/vr.142.24.662) [DOI] [PubMed] [Google Scholar]
- 4.Guardo Di G, Marruchella G, Agrimi U, Kennedy S. 2005. Morbillivirus infections in aquatic mammals: a brief overview. J. Vet. Med. Physiol. Pathol. Clin. Med. 52, 88–93 10.1111/j.1439-0442.2005.00693.x (doi:10.1111/j.1439-0442.2005.00693.x) [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner W, et al. 2003. Canine distemper virus: an agent looking for a new host. Dtsch. Tierarztl. Wochenschr. 110, 137–142 [PubMed] [Google Scholar]
- 6.Furuse Y, Suzuki A, Oshitani H. 2010. Origin of measles virus: divergence from rinderpest virus between the 11th and 12th centuries. Virol. J. 7, 52–55 10.1186/1743-422X-7-52 (doi:10.1186/1743-422X-7-52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo PCY, et al. 2012. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc. Natl Acad. Sci. USA 109, 5435–5440 10.1073/pnas.1119972109 (doi:10.1073/pnas.1119972109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott GR. 2000. The murrain now known as rinderpest. Newsletter Trop. Agric. Assoc. UK 20, 14–16 [Google Scholar]
- 9.Spinage CA. 2003. Cattle plague: a history. New York, NY: Kluwer Academic [Google Scholar]
- 10.Littlewood W. 1905. Cattle plague in Egypt in 1903–04–05. J. Comp. Pathol. 18, 312–321 [Google Scholar]
- 11.Roeder PL, Taylor WP, Rweyemamu MM. 2006. Rinderpest in the 20th and 21st centuries. In Monograph series biology of animal infections, rinderpest and peste des petits ruminants: virus plagues of large and small ruminants (eds Barrett T, Pastoret P-P, Taylor WP.), pp. 105–142 London, UK: Academic Press [Google Scholar]
- 12.Roeder PL, Rich M. 2009. The global effort to eradicate rinderpest. IFPRI Discussion paper 00923; pp70 See http://www.ifpri.org/sites/default/files/publications/ifpridp00923.pdf.
- 13.Salem Bey IF.1947. Cattle plague in Egypt. Technical and Scientific Service Bulletin, Issue 88. Cairo, Egypt: Egypt Government Press.
- 14.Curasson G. 1936. La peste bovine. In Traite de pathologie exotique vétérinaire et compare, ch. 3 1, pp. 28–302 Paris, France: Vigot Fréres [Google Scholar]
- 15.Blench R. 1996. Aspects of resource conflict in semi-arid Africa. Natural Resources Perspectives No. 16 London, UK: Overseas Development Institute [Google Scholar]
- 16.Roeder PL, Rich M. 2009. Rinderpest eradication. In Millions fed: successes in agriculture (eds Spielman D, Pandya-Lorch R.), pp. 109–116 Washington, DC: International Food Policy Research Institute [Google Scholar]
- 17.Rweyemamu MM, Roeder PL, Taylor WP. 2006. Towards the global eradication of rinderpest. In Monograph series biology of animal infections, rinderpest and peste des petits ruminants: virus plagues of large and small ruminants (eds Barrett T, Pastoret P-P, Taylor WP.), pp. 298–322 London, UK: Academic Press [Google Scholar]
- 18.AU-IBAR 2003. Pan African animal health yearbook, p. 23. Nairobi, Kenya: African Union Inter African Bureau for Animal Resources. See http://www.au-ibar.org/index.php?option=com_flexicontent&view=items&id=109:pan-african-animal-health-yearbook&Itemid=56. [Google Scholar]
- 19.Anderson J, McKay JA, Butcher RN. 1991. The use of monoclonal antibodies in competitive ELISA for the detection of antibodies to rinderpest and peste des petits ruminants. In The sero-monitoring of rinderpest throughout Africa: phase I, IAEA publication TECDOC-623, pp. 45–53 Vienna, Austria: Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, International Atomic Energy Agency [Google Scholar]
- 20.OIE 2012. Terrestrial manual, ch. 2.1.15 Rinderpest See: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.15_RINDERPEST.pdf.
- 21.Forsyth MA, Barrett T. 1995. Evaluation of polymerase chain reaction for the detection and characterisation of rinderpest and peste des petits ruminants viruses for epidemiological studies. Virus Res. 39, 151–163 10.1016/0168-1702(95)00076-3 (doi:10.1016/0168-1702(95)00076-3) [DOI] [PubMed] [Google Scholar]
- 22.FAO. 1997. Report of the FAO expert consultation on development of the emergency prevention system for transboundary animal and plant pests and diseases and review of the global Rinderpest eradication programme, Rome, 14 to 16 July 1997. Rome, Italy: FAO.
- 23.Plowright W, Ferris RD. 1962. Studies with rinderpest virus in tissue culture: the use of attenuated culture virus as a vaccine in cattle. Res. Vet. Sci. 3, 172–182 [Google Scholar]
- 24.Mariner JC, House JA, Sollod AE, Stem E, van den Ende MC, Mebus CA. 1990. Comparison of the effect of various chemical stabilizers and lyophilization cycles on the thermostability of a vero cell-adapted rinderpest vaccine. J. Vet. Microbiol. 21, 95–209 10.1016/0378-1135(90)90032-Q (doi:10.1016/0378-1135(90)90032-Q) [DOI] [PubMed] [Google Scholar]
- 25.Mariner JC, House JA, Mebus CA, Sollod AE, Chibeu D, Jones BA, Roeder PL, Admassu B, van't Klooster GM. 2012. Rinderpest eradication: appropriate technology and social innovations. Science 337, 1309–1312 10.1126/science.1223805 (doi:10.1126/science.1223805) [DOI] [PubMed] [Google Scholar]
- 26.Masiga WN, Cheneau Y, Mariner JC, Lefevre PC. 1993. Strategy for the eradication of rinderpest from Africa with thermostable vero cell-adapted vaccine. In Proc. 7th Annual Conf. the Ethiopian Veterinary Association (EVA), October Addis Ababa, Ethiopia: Ethiopian Veterinary Association [Google Scholar]
- 27.Mariner JC, Akabwai DMO, Toyang J, Zoyem N, Ngangnou A. 1994. Community-based vaccination with thermostable Vero cell-adapted rinderpest vaccine (ThermoVax). In Proc. 7th Int. Symp. on Veterinary Epidemiology and Economics, Nairobi, August 15–17 Kenyan Veterinarian; 18, 507–509 [Google Scholar]
- 28.FAO/IAEA 1992. The seromonitoring of rinderpest throughout Africa: phase II. Vienna, Austria: Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, International Atomic Energy Agency [Google Scholar]
- 29.Kock RA, Wambua JM, Mwanzia J, Wamwayi H, Ndungu EK, Barrett T, Kock ND, Rossiter PB. 1999. Rinderpest epizootic in wild ruminants in Kenya 1993–7 . Vet. Rec. 145, 275–283 10.1136/vr.145.10.275 (doi:10.1136/vr.145.10.275) [DOI] [PubMed] [Google Scholar]
- 30.Chardonnet P, Kock R.2000. Report of the African Wildlife Veterinary Project. CIRAD EMVT, Montpellier, France.
- 31.Kock RA. 2006. Rinderpest and wildlife. In Monograph series biology of animal infections: rinderpest and peste des petits ruminants virus plagues of large and small ruminants (eds Barrett T, Pastoret P-P, Taylor WP.), pp. 143–162 London, UK: Academic Press [Google Scholar]
- 32.Wamwayi H. 2002. Responses of cattle to infection with kudu-derived rinderpest virus. In Proc. PACE Wildlife Training Workshop, Mount Meru Hotel, Arusha, 29 November to 3 December 2002, pp. 79–85. Nairobi, Kenya: AU IBAR
- 33.Mariner JC, Roeder PL. 2003. The use of participatory epidemiology in studies of the persistence of rinderpest in East Africa. Vet. Rec. 152, 641–647 10.1136/vr.152.21.641 (doi:10.1136/vr.152.21.641) [DOI] [PubMed] [Google Scholar]
- 34.Barrett T, Forsyth MA, Inui K, Wamwayi HM, Kock RA, Wambua J, Mwanzia J, Rossiter P. 1998. Rediscovery of the second African lineage of rinderpest virus: its epidemiological significance. Vet. Rec. 142, 669–671 10.1136/vr.142.24.669 (doi:10.1136/vr.142.24.669) [DOI] [PubMed] [Google Scholar]
- 35.Plowright W. 1963. The role of game animals in the epizootiology of rinderpest and malignant catarrhal fever in East Africa. Bull. Epizootic Dis. Africa 11, 149–162 [PubMed] [Google Scholar]
- 36.Branagan D, Hammond JA. 1965. Rinderpest in Tanzania: a review. Bull. Epizootic Dis. Africa 13, 225–246 [PubMed] [Google Scholar]
- 37.Macauley JW. 1973. Kenya. In A history of the overseas veterinary services, Part 2 (ed. GP West), pp. 139–161 London, UK: British Veterinary Association [Google Scholar]
- 38.Plowright W, McCulloch B. 1967. Investigations on the incidence of rinderpest virus infection in game animals of N. Tanganyika and S. Kenya 1960/63. J. Hyg. Cambridge 65, 343–358 10.1017/S0022172400045861 (doi:10.1017/S0022172400045861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor WP, Watson RM. 1967. Studies on the epizootiology of rinderpest in blue wildebeest and other game animals of Northern Tanzania and Southern Kenya. J. Hyg. Cambridge 65, 537–545 10.1017/S0022172400046064 (doi:10.1017/S0022172400046064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kock RA, Wamwayi HM, Rossiter PB, Libeau G, Wambwa E, Okori J, Shiferaw FS, Mlengeya TD. 2006. Rinderpest in East Africa: continuing re-infection of wildlife populations on the periphery of the Somali ecosystem. Prev. Vet. Med. 75, 63–80 10.1016/j.prevetmed.2006.01.016 (doi:10.1016/j.prevetmed.2006.01.016) [DOI] [PubMed] [Google Scholar]
- 41.Plowright W. 1982. The effects of rinderpest and rinderpest control on wildlife in Africa. In Symp. Zoological Society of London, number 50, pp. 1–28 London, UK: Zoological Society of London [Google Scholar]
- 42.Hudson DR. 1960. Lutte contre les maladies animales. Rapport aux gouvernements de la Birmanie, du Cambodge, du Laos, de la Thailande et du Viet-nam. FAO Programme Elargi d'Assistance Technique, Report no. 1202. Rome, Italy: FAO
- 43.Jost CC, Mariner JC, Roeder PL, Sawitri E, Macgregor-Skinner GJ. 2007. Participatory epidemiology in disease surveillance and research. Rev. Sci. Tech. Off. Int. Epiz. 26, 537–547 [PubMed] [Google Scholar]
- 44.Chambers R. 1994. The origins and practice of participatory rural appraisal. World Dev. 22, 953–969 10.1016/0305-750X(94)90141-4 (doi:10.1016/0305-750X(94)90141-4) [DOI] [Google Scholar]
- 45.Mariner JC, Paskin R.2000. Participatory epidemiology: methods for the collection of action-oriented epidemiological intelligence. FAO Animal Production and Health Manual No. 10. Rome, Italy: FAO.
- 46.Mariner JC, McDermott JJ, Heesterbeek JAP, Catley A, Roeder P. 2005. A model of lineage 1 and lineage 2 rinderpest virus transmission in pastoral areas of East Africa. Prev. Vet. Med. 69, 245–263 10.1016/j.prevetmed.2005.02.001 (doi:10.1016/j.prevetmed.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 47.Kock RA. 2008. The role of wildlife in the epidemiology of rinderpest in East and Central Africa 1994–2004: a study based on serological surveillance and disease investigation. Thesis for the Degree of Doctor of Veterinary Medicine, University of Cambridge, UK, pp. 157–162 [Google Scholar]
- 48.FAO. 1998. Rinderpest: the challenge ahead. Report of the FAO Technical Consultation on the Global Rinderpest Eradication Programme 28 to 30 September 1998. Rome, Italy: FAO.
- 49.Leyland T. 1996. The world without rinderpest: outreach to the inaccessible areas. The case for a community-based approach with reference to Southern Sudan. In Proc. FAO Technical Consultation on the Global Rinderpest Eradication Programme, FAO Animal Production and Health Paper 129, pp. 109–122 Rome, Italy: FAO [Google Scholar]
- 50.Mariner JC, Hussain M, Roeder PL, Catley A. 2003. The use of participatory disease searching as a form of active disease surveillance in Pakistan for rinderpest and more. In Proc. 10th Int. Symp. Veterinary Epidemiology and Economics, Vina del Mar, Chile, November 17–21 Fort Collins, CO: USDA APHIS [Google Scholar]
- 51.Tempia S, et al. 2010. A sero-survey of rinderpest in nomadic pastoral systems in central and southern Somalia from 2002 to 2003 using a spatially integrated random sampling approach. OIE Revue Sci. Tech. 29, 497–511 [DOI] [PubMed] [Google Scholar]
- 52.Libeau G, Kwiatek O, Lancelot R, Albina E. 2011. Peste des petits ruminants, growing incidence worldwide. OIE Bull. 2, 52–54 [Google Scholar]
- 53.Minet C, Kwiatek O, Keita D, Diallo A, Libeau G, Albina E. 2009. Morbillivirus infections in ruminants: rinderpest eradication and peste des petits ruminants spreading towards the North. Virologie 13, 103–130 [DOI] [PubMed] [Google Scholar]
- 54.Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G. 2010. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J. Gen. Virol. 91, 2885–2897 10.1099/vir.0.025841-0 (doi:10.1099/vir.0.025841-0) [DOI] [PubMed] [Google Scholar]
- 55.Lloyd-Smith JO. 2013. Vacated niches, competitive release and the community ecology of pathogen eradication. Phil. Trans. R. Soc. B 368, 20120150. (doi:10.1098/rstb.2012.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson J, et al. 2011. Rinderpest eradicated; what next? Vet. Rec. 169, 10–11 10.1136/vr.d4011 (doi:10.1136/vr.d4011) [DOI] [PubMed] [Google Scholar]
- 57.Rich KM, Roland-Holst D, Otte J.2012. An assessment of the socio-economic impacts of global rinderpest eradication: methodological issues and applications to rinderpest control programmes in Chad and India. FAO Animal Production and Health Working Paper number 7. Rome, Italy: FAO.
- 58.Zinsstag J, Schelling E, Tanner M. 2011. From ‘one medicine’ to ‘one health’ and systemic approaches to health and well-being. Prev. Vet. Med. 101, 148–156 10.1016/j.prevetmed.2010.07.003 (doi:10.1016/j.prevetmed.2010.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paton DJ, Sumption KJ, Charleston B. 2009. Options for control of foot-and-mouth disease: knowledge, capability and policy. Phil. Trans. R. Soc. B 364, 2657–2667 10.1098/rstb.2009.0100 (doi:10.1098/rstb.2009.0100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallace R, Kock RA. 2012. Whose food footprint? Capitalism and global agriculture. Hum. Geogr. 5, 63–83 [Google Scholar]
- 61.Scoones I, Bishi A, Mapitse N, Moerane R, Penrith ML, Sibanda R, Thomson G. 2010. Foot-and-mouth disease and market access: challenges for the beef industry in southern Africa. Pastoralism: Res. Policy Pract. 1, 135– 164 [Google Scholar]
- 62.Child BA, Musengezi J, Parent GD, Child GFT. 2012. The economics and institutional economics of wildlife on private land in Africa. Pastoralism: Res. Policy Pract. 2, 18. 10.1186/2041-7136-2-18 (doi:10.1186/2041-7136-2-18) [DOI] [Google Scholar]