Abstract
As multiple papers within this special issue illustrate, the dynamics of disease eradication are different from disease control. When it comes to disease eradication, ‘the last mile is longest’. For social and ecological reasons such as vaccine refusal, further ending incidence of a disease when it has reached low levels is frequently complex. Issues of non-compliance within a target population often influence the outcome of disease eradication efforts. Past eradication efforts confronted such obstacles towards the tail end of the campaign, when disease incidence was lowest. This article provides a comparison of non-compliance within polio, measles and smallpox campaigns, demonstrating the tendency of vaccine refusal to rise as disease incidence falls. In order to overcome one of the most intractable challenges to eradication, future disease eradication efforts must prioritize vaccine refusal from the start, i.e. ‘walk the last mile first’.
Keywords: disease eradication, vaccine refusal, polio, measles
1. Introduction
Despite advances in sanitation and immunization initiatives, infectious diseases remain a significant cause of mortality and morbidity worldwide. In low and middle income countries especially, infections rank among the top ten leading causes of mortality. The effect is particularly devastating among communities with low vaccination coverage. The World Health Organization (WHO) estimates that 1.5 million deaths among children under 5 years were due to vaccine-preventable diseases (VPDs), representing 17 per cent of under-five child mortality worldwide [1]. Global eradication of many infectious diseases depends on widespread vaccine coverage. Indeed, the only two diseases to be eradicated thus far have been vaccine preventable—rinderpest and smallpox.
(a). The first eradication: smallpox
Earliest records of smallpox inoculation efforts date back to AD 590–1000, during which time individuals in China [2] are said to have inhaled pulverized smallpox scabs. Historians speculate that inoculation was practised among members of the Chinese population during this time period by scratching infected material into the skin of a healthy individual [2,3]. These methods closely mirror those observed by Edward Jenner in 1796, who famously documented the apparent smallpox immunity of milkmaids who previously contracted cowpox. Jenner differed primarily from his predecessors in that he lacerated patients with cowpox (as opposed to smallpox), and introduced injection as a primary method of inoculation [4–6].
For the next 150 years, the smallpox vaccine played an important role in controlling smallpox rates in Europe and America. It was not until 1958 that the World Health Assembly (WHA) passed a global smallpox eradication resolution. In 1966, WHA endorsed an official campaign plan and budget to support the resolution. A shift in strategy, from mass vaccination to surveillance and containment, sped up eradication efforts considerably and the campaign was completed in just over a decade. The last cases of naturally occurring smallpox were detected in Ethiopia and Sudan between 1976 and 1977, after which continental Africa successfully concluded a centuries-long battle with smallpox variola. The final case of smallpox was detected in 1978, 12 years after programme ratification [7,8].
(b). Vaccines are indispensable to global disease eradication
The success of these prior eradication campaigns demonstrates the ability of vaccines to substantially influence public health practice through infectious disease eradication.
Unlike other methods of infectious disease prevention, vaccines do not typically require drastic and costly changes in human behaviour, sanitation practices or sexual networks. Enabling the human body to defend itself is unarguably one of the most sustainable prevention methods in public health (however, also see articles on polio and guinea worm in this issue [9,10]). Among children, vaccination against common communicable diseases through the Expanded Programme on Immunization (EPI) is one of the most cost-effective, large-impact methods for global reduction of disease mortality and morbidity [11,12].
The WHO has implemented and ratified initiatives for the eradication of polio and for the control of measles and rubella—the Global Polio Eradication Initiative and the Measles and Rubella Initiative. The polio eradication campaign began in 1988; since then, only three endemic countries remain. In 2001, WHA founded a measles control and reduction initiative, now called the Measles and Rubella Initiative. Nevertheless, VPD outbreaks such as measles have become more common in the past century, in part because the success of immunization campaigns depends on vaccine compliance.
(c). Vaccine refusal is a significant obstacle to disease eradication
Without widespread compliance, vaccines have a limited impact on public health. Overcoming vaccine refusal is critical to the eradication of VDPs.
Disease eradication campaigns often shape the progression of anti-vaccination movements. Following the initial introduction of a vaccine into a population, VPD mortality typically drops steadily, followed by a plateau in disease rates and related deaths [13]. The period of time during which disease prevalence remains low enough to escape public notice corresponds to a spike of vaccine refusal as VPDs fall out of public notice and post-vaccine adverse events gain more attention [14]. A myriad of factors influence rates of non-compliance within a population, including popular misconceptions of the hygiene hypothesis [15] and the tendency for individuals to accept the harmful effects of inaction over less harmful acts, i.e. ‘omission bias’ [16]. Psychosocial factors such as risk perception and awareness are influenced by the progress of eradication efforts. This paper examines the trend of increasing vaccine refusal in tandem with low disease prevalence by exploring the role vaccine refusal played in past eradication attempts, its capacity to shape current campaigns, and which strategies should be used to mitigate the impact of vaccine refusal on eradication campaigns.
The tendency for vaccine refusal to fluctuate in accordance with VPD prevalence and, therefore, VPD awareness is critical to global disease eradication today. A detailed understanding of this trend, as well as its driving factors, must be prioritized by future eradication campaigns in order to ensure their success.
2. History of vaccine refusal
Vaccine resistance movements date back to Edward Jenner (1749–1823), whose work with the protective efforts of cowpox virus in conferring immunity to smallpox is credited as the first deliberate, standardized approach to disease eradication using a known vaccine. In 1796, Jenner presented his findings to the Royal Society of London, describing the successful immunization of 13 people following inoculation with live cowpox material. Shortly thereafter, widespread immunization took place across England, significantly driving down smallpox mortality rates in the latter half of the eighteenth century and early in the nineteenth century (figure 1). The integral role played by the smallpox vaccine in reducing disease prevalence compelled the English government to pass the Vaccination Act of 1853, which established compulsory vaccination throughout London [18]. The anti-vaccination movement gained momentum immediately after the passage of the 1853 law; prior to 1853, incidence of smallpox had been quite low, with some fluctuations, for quite some time [19].
Figure 1.
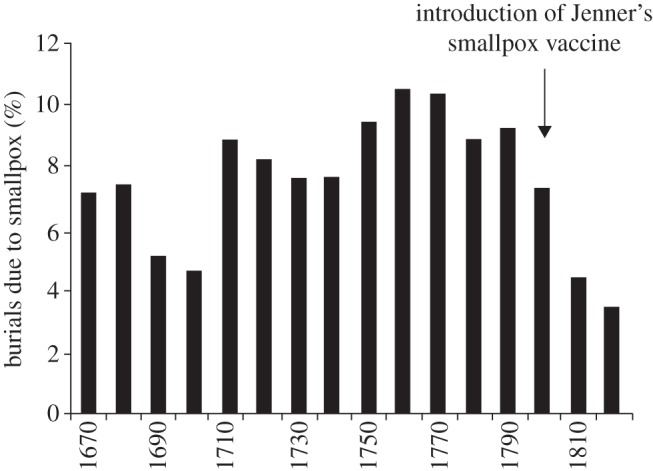
Smallpox as a percentage of all burials in London Bills, by decade. Data from [17].
The Anti-Vaccination League in London formed in 1853. In 1856, a popular pamphlet denouncing compulsory vaccination was widely circulated throughout Europe, encouraging the spread of anti-vaccination sentiment. In The evils of vaccination, with a protest against its legal enforcement, George Gibbs objected to both the compulsory nature of the act as well as the safety and efficacy of the vaccine itself [19]. Anti-vaccination movements today echo much of Gibbs’ own protest.
Anti-vaccination advocates in nineteenth century London disagreed about the true cause of vaccine failure as well as its proposed risk to patients. Prominent medical author Charles Creighton subscribed to the atmospheric theory of disease causation, whereas bacteriologist Edgar Crookshank believed that the prophylactic material was both ineffective against smallpox and responsible for the secondary transmission of syphilis [20]. No clear consensus could be reached on a single scientific paradigm that could explain the presumed danger of Jenner's vaccine, yet members and leaders of the anti-vaccination movement were held together by the strength of their conviction that the vaccine was useless at best and fatal at worst [20]. Similarly, vaccine opposition movements today lack empirical data to support their claims, and encompass a large range of individuals, socio-cultural concerns and justifications for vaccine refusal [21].
Anti-vaccination movements sprouted around the world. In the United States, widespread vaccination of the population drove down smallpox incidence during the early nineteenth century. During a period of low disease prevalence in the 1830s, anti-vaccination movements regained popularity. Renewed susceptibility of the population instigated smallpox epidemics throughout the 1870s, and the anti-vaccination movement subsequently fell out of popularity [18,22,23].
In many ways, vaccine refusal movements of the past parallel those of the present. Dissidents insisted strongly that friends and relatives had suffered from vaccinations, and valued personal narratives above less provocative statistics. Opponents to adverse effects of the vaccine often praised alternative medicine (e.g. homeopathy) as a safer and equally viable alternative to vaccines [4]. They were also suspicious of the profit public health professionals, vaccine developers and physicians stood to gain from widespread vaccination.
3. Smallpox eradication: differences, similarities and reasons for success
Despite historical resistance to widespread vaccination efforts, smallpox was eradicated in the latter half of the twentieth century. The rapid decrease in smallpox-related deaths may have been a factor in the campaign's success. Given the ‘short tail’ of the incidence curve (figure 2), there was insufficient time for a true anti-vaccination movement to gain traction. We hypothesize that disease awareness, trust in vaccine efficacy and the rapid fall in smallpox outbreaks contributed to the scarcity of vaccine opposition.
Figure 2.
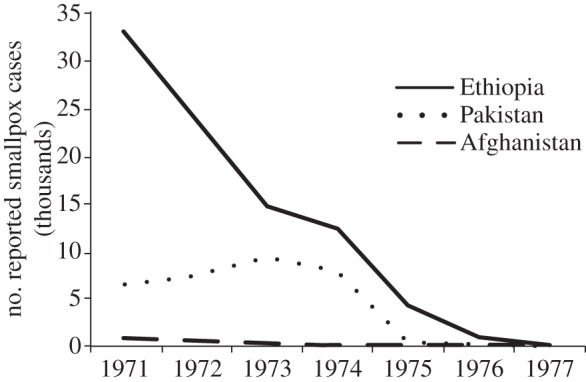
Number of reported smallpox cases from 1971 to 1977 in three endemic countries—Ethiopia, Pakistan and Afghanistan—during the intensified eradication programme. Data from [8].
Unlike future eradication efforts, the smallpox campaign did not contend with a protracted period of low disease prevalence. From 1959 to 1967, the global prevalence of smallpox cases plummeted from 59 to 31 per cent [8]. An intensified eradication programme was implemented by the WHO in 1967. By 1973, endemic smallpox was confined to five countries. The progression of reported smallpox cases in these nations demonstrates the short tail of the eradication campaign and the speed with which the disease was eliminated (figure 2). In the year of 1975 alone, the number of reported smallpox cases in India dropped from 1010 in January to 0 by December. Similarly, the number of reported smallpox cases in Bangladesh fell from 216 to zero between December 1972 and December 1973 [8].
The smallpox eradication campaign benefited from characteristics of the disease itself. Visibly devastating and highly infectious, smallpox was a disease of which most communities were aware. Individuals could tell when a case of smallpox had last occurred by looking for the youngest individuals with the telltale scars. Even schoolchildren could correctly identify cases within the region. Few doubted the severity of the disease or the risk of remaining unprotected. ‘… Unlike many other viruses, smallpox virus simply could not hide. It left too many clues’ [24].
Acute disease awareness was coupled with widespread faith in vaccination as the primary method by which the world could be rid of the disease. In West Africa, Foege notes that individuals historically placed a high value on injections, which he attributes to the success of penicillin injections in reducing yaws during the 1940s. In Southeast Asia, particularly India, government officials felt it was of critical importance to not be the last country with smallpox [24]. National health practitioners felt the pressure of a global eradication effort and sought to match their counterparts in Europe and the Americas that had been smallpox-free for some years [24,25].
As a result, most people targeted in the smallpox eradication effort actively sought inoculation campaigns. In Bangladesh, the WHO Southeast Asia Regional Office reported ‘no organized resistance to vaccination in Bangladesh … in most cases lack of cooperation was due to an ignorance of its benefits' [26]. In an investigation of non-compliance during the smallpox era, Greenough [27] finds little formal report of resistance movements in either India or Bangladesh.
Although literature detailing non-compliance during this time is scarce, there is evidence that vaccine refusal did occur. These instances of refusal occurred primarily in the final years of the campaign, mirroring anti-vaccination movements in nineteenth century Europe and the Americas. In South Asia, resistance peaked between 1973 and 1975 [27], during which time the number of controlled outbreaks surpassed new ones [8]. The rise of anti-vaccination efforts during the tail end of the campaign illustrates the importance of the stage when disease prevalence is typically lowest. The smallpox eradication campaign was able to overcome historical opposition to vaccines in part because of the short tail of the initiative and high levels of disease awareness.
4. Vaccine-preventable disease awareness in non-endemic countries
Eradication programmes seeking to drive down VPD incidence will often inadvertently drive down VPD awareness concurrently. The disappearance of a disease from public eye creates a paradox whereby the success of a public health campaign (widespread vaccination and disease prevention) becomes a serious obstacle to the campaign itself. Diseases such as polio and measles fall out of cultural memory as generations pass without experiencing and remembering their severity [4].
(a). The role of emotion and experience in shaping cultural memory
Communicating the risk posed by VPDs to vaccine-hesitant parents is a difficult task in non-endemic countries. Experience of a relevant event improves public retention of information provided by the media [28]; however, there are few infectious diseases which parents living in industrialized countries have experienced in epidemic form [29]. Similarly, individuals are more likely to seek information about the source of their anxiety if perceived as a realistic threat, although less likely to ascertain the validity of such information [30].
Public anxiety, therefore, has an important dual effect on cultural memory. Many individuals today do not actively worry about polio or measles, and no longer see such diseases as serious threats to their health. A vaccine-hesitant parent who has never experienced the threat of VPDs is also less likely to seek information regarding children's susceptibility to these infectious diseases [31]. On the other hand, parents anxious about post-vaccine adverse events are more likely to seek advice regarding the dangers of vaccination, particularly because they pose a more visible threat [30]. Unfortunately, in a state of heightened anxiety, parents may find it more difficult to judge the validity of vaccine-related news in the media [30].
(b). The role of media reporting in vaccine-preventable diseases awareness and risk communication
Mass media is an important influence on health-seeking behaviour in a population [32]. Television coverage of influenza-related reports increases annual vaccination rates by up to 7.9 per cent among the elderly [33]. Conversely, anti-vaccination media campaigns are associated with decreased vaccine uptake among children whose families are likely to have been exposed [34].
The obstacle of VPD awareness is compounded by a lack of relevant media coverage. Newspaper trends in disease reporting closely follow epidemiological trends of disease mortality. As disease mortality increases, so too does media coverage; a plateau or decrease in disease mortality over a period of time corresponds with a plateau or decrease in coverage of that disease [35]. In countries where a VPD is no longer endemic and its incidence has dropped significantly, news coverage on its severity—and, therefore, on the importance of immunization—may also be lacking.
5. Risk perception and vaccine decision-making
Vaccine-hesitant parents do typically understand that by refusing routine childhood vaccination, they are putting their children at some risk for disease [4]. Furthermore, parents often understand the evolving and nuanced nature of medical knowledge, readily accepting that the choice to vaccinate involves a balance of risk rather than an assumption of zero risk [36]. It is unlikely that oppositionists regard vaccines as completely harmful and without benefit. Instead, they may conclude that their child is more likely to suffer from post-vaccine adverse events than from VPDs.
A significantly greater proportion of vaccine-hesitant parents do not perceive VPDs as a serious threat to the health of their children, as compared with parents who support mandatory vaccination for schoolchildren. A total of 47 per cent of hesitant parents surveyed regarded vaccines as either unsafe or somewhat safe [37].
Studies have found that vaccine campaigns are most successful when they emphasize that VPDs do indeed pose a greater risk to children. A 1994 measles–rubella vaccine campaign in the United Kingdom successfully increased vaccination coverage in part by emphasizing the severity of measles illness [36]. Among a cohort of pro-vaccination parents in the UK, statistical reports on the true morbidity and mortality of these diseases were ranked as the most novel pieces of vaccine information; these same parents expressed that vaccine campaigns ought to emphasize the severity of VPDs [38].
In populations with high levels of immunization, unvaccinated children are often protected from VPDs by benefiting from the herd immunity provided by vaccinated individuals (i.e. ‘free-riding’). Free-riding demonstrates the occasional discrepancy between self-interest and group interest [39], often referred to as ‘the prisoner's Dilemma’: a person may choose to act in opposition to the good of society if doing so benefits the individual [40]. In the case of vaccine refusal, non-compliers reduce overall herd immunity but are protected from both VPDs and post-vaccine adverse events, assuming threshold coverage; however, if coverage falls beneath threshold, herd immunity will cease to protect non-compliers [41].
Under voluntary vaccination programmes, the conflict between group interest and self-interest causes large differences in optimal coverage levels and actual uptake levels [39]. This discrepancy is related to the perceived risks of vaccination and VPDs. When weighing the relative costs and benefits of vaccination, individuals might consider that herd immunity lessens the likelihood of infection for an unvaccinated person. Hence, the risk posed by refusing vaccination and remaining susceptible to infection is further diminished by the concept of free-riding.
6. Vaccine refusal and the polio eradication campaign
(a). Perceived necessity and demand for social amenities
In 2003, a number of official state clerics and residents in Northern Nigeria boycotted the polio vaccine, owing to a multitude of cultural and socioeconomic factors. Many dissidents criticized the federal government for pushing polio eradication while doing little to ameliorate poverty-related issues such as access to food, clean water and electricity [42]. Six Muslim clerics in Suleja, Niger demanded social amenities for their communities, claiming that the federal government had failed to cater to their needs [42]. One cleric, Mallam Aliyu Yakub, stated:
‘Since 1960, when we had our independence, there are five things that government always talk about – water, light, housing, food and health – but up till today, we are still in the same problem…these are the things that make them to die … it is not as if we don't want government to help us but the area we expect them to help us they are not doing it.’ [42]; M. Yahya. Daily Trust Abuja Interview. July 2008
Owing to targeted vaccine campaigning, polio cases and polio-associated deaths were far less visible than death and illness owing to starvation or diarrhea. In one state opposing vaccination, Kaduna, a local butcher complained:
‘Some people have never even seen polio but yet they keep giving us medicine for it. If you look around it is hard to find 2 or 3 people with polio but it is easy to go to the hospital and find 50 people sick with no medicine to buy the medicine they need to be treated with. Help them instead but No! You find a small baby who is well and drop medicine in his mouth, for free!’ ([43]; M. Yahya. Kaduna Interview. July 2005)
The local butcher's frustrations echoed widespread sentiments that children were being treated for a disease which they were unlikely to get, thanks in part to prior success of the polio eradication campaign. Low disease prevalence and a corresponding lack of awareness created a climate in which parents prioritized other threats to children's health. Demand for social amenities was far from the only reason for anti-vaccination sentiment in northern Nigeria. Other sources of dissent were rooted in mistrust of Western political involvement, particularly in relation to a concurrent lawsuit against pharmaceutical company Pfizer for purportedly unethical proceedings during clinical trials of an antibiotic drug in the northern state of Kano [41]. First-hand accounts of vaccine refusals in the area also emphasize the influence of authority figures such as fathers or in-laws on maternal decision-making and a rumor circulated by religious and political leaders that the oral poliovirus vaccine (OPV) was an American conspiracy to sterilize the Muslim population [44]. Nevertheless, timing of the boycott—during the final, intensified stages of the campaign—as well as specific refusal to the polio vaccine rather than broad opposition to any form of Western aid, demonstrates the important role of low disease prevalence and awareness in shaping vaccine uptake and demand.
(b). Religious and cultural objections
Religious and moral objections to vaccination are key to understanding vaccine refusal today. In the Nigeria boycott, for instance, resistance to vaccination was also shaped by the religious politics of predominantly Muslim states in the north. Some residents felt they were being targeted by Western biomedicine as a result of their religion [43]. Increased under-five mortality in Nigeria is also significantly associated with maternal affiliation with a traditional indigenous religion, primarily owing to differential use of maternal and child health services [45].
In Pakistan, vaccine refusals are concentrated among low income individuals of Pashtun ethnicity. Using qualitative interviews with dissenting parents, Khowaja et al. [46] observed important trends in reasons for refusal. Many parents were concerned that OPV caused sterility in adulthood; others suspected the vaccine, developed and distributed by Westerners, was part of a larger conspiracy to sterilize children in Muslim nations [46]. Dissenting parents also expressed concern that the vaccine contained religiously forbidden, or non-halal, ingredients [46]. The authors concluded that, without religious and culturally specific vaccine promotion activities, vaccine hesitancy and mistrust would likely lead to continued poliovirus transmission within the Pashtun community [46].
The association between insular religious communities and under-utilization of health services, in particular routine vaccinations, has been observed in multiple other countries. After 14 years without endemic polio, the Netherlands reported an outbreak of poliomyelitis between 1992 and 1993 [47]. These cases were largely restricted to a religious subpopulation, while no evidence was found of poliovirus circulation outside these risk groups [48].
(c). Passive refusal and ‘missing children’
Parental vaccine hesitancy as well as individual non-compliance later in adulthood constitute forms of active vaccine refusal; however, passive refusal has also posed an important challenge to the polio eradication initiative. In cases of passive refusal, parents typically report that the child is unable to be vaccinated because he or she is ill, too young, sleeping, at school, et cetera, rather than voice an explicit objection to the vaccination.
Passive refusal accounted for a large proportion of ‘missed children’ in endemic countries, far outnumbering the proportion of parents who actively refuse OPV [49]. Sixty-six per cent of missed children in Nigeria were attributable to an unavailable or absent child; however, many absentee children, particularly in the highly endemic northern states, were typically in the playground or at a social event within reach of the household [50]. In Afghanistan, 33 per cent of households with missed children claimed the child could not be reached because he or she was sleeping, ill, or newborn; overt refusals only accounted for 5 per cent of children [51].
Surveillance data from the polio campaigns indicates that even children close by or within the household are missed, in part owing to low demand among caregivers as well as adherence to social norms such as the belief that children should not be woken up for vaccination, or that a sick/newborn child should not be vaccinated [49]. In addition to suboptimal knowledge of immunization, caregivers in polio-endemic countries may also distrust the campaign, find other social amenities more important or have religious objections, but feel uncomfortable vocalizing these sentiments. As a result, dissenting parents may do so not only because of adherence to socio-cultural norms, but for reasons such as vaccine safety and risk perception which affect rates of refusal worldwide.
7. Vaccine refusal and the measles and rubella initiative
(a). Concerns about vaccine safety
Parental concerns about the safety of measles–mumps–rubella (MMR) vaccination in children persist despite overwhelming evidence to the contrary. A 1998 paper by Dr Andrew Wakefield and colleagues falsely linking MMR vaccination to autism alarmed many individuals and parents alike. Shortly after publication, selective MMR vaccine refusal in the United States rose from 0.77 per cent in 1995 to 2.1 per cent in 2000 [52]. Although the paper has since been retracted and no credible evidence exists to support a link between autism and MMR vaccination, parental concerns about vaccine safety linger, leading to suboptimal vaccination coverage and increasing measles outbreaks, particularly in the United States and United Kingdom [53,54].
Brown et al. [55] surveyed the decision-making habits of UK parents in regards to vaccine uptake and the MMR–autism controversy [55]. Results indicated that all parents, whether or not they accepted MMR vaccination for their child, had a tendency to focus on negative aspects of the vaccine, such as possible adverse events and side effects. Even without prompting, many parents remarked on the MMR–autism controversy and remarked that it complicated immunization decision-making. In accordance with the tendency of individuals to focus on threats they have experienced directly, several parents rejecting MMR had a direct experience with autistic children. No parents accepting of MMR had this experience.
Parents who delay or refuse MMR vaccine coverage are also less likely to believe that vaccines are necessary to protect the health of their children [56]. Prior experience with what they believe to be adverse effects of vaccination (i.e. autism, developmental disorders) and little awareness of VPD severity (i.e. fatality rate of measles, mumps or rubella) encourage parents to focus on vaccine safety as the more salient threat to their children. Parents who are less likely to believe that vaccines are safe and that vaccines provide significant health benefit to their children have significantly lower coverage rates for all ten childhood vaccines, including MMR [56].
Parents have also objected to the recommended vaccine schedule of children, many of whom will receive up to 14 vaccines before the age of 2 years [57]. There is no evidence to suggest that a neonate is unable to ‘handle’ combined vaccines [58]. Nevertheless, if given a choice, some parents expressed a desire to follow Dr Andrew Wakefield's recommendation to space out the MMR vaccine into three separate components, rather than follow the recommended vaccine schedule [59].
(b). Religious and moral concerns
As was the case with polio immunization, moral and religious concerns are strongly tied to rates of vaccine refusal among certain communities claiming religious or philosophical exemptions. In May and June of 2005, an outbreak of measles occurred within a small subgroup of an Indiana church congregation. Personal religious objections were among the most cited reasons for refusal to adhere to vaccination, although no formal advice on vaccines had been issued from the church. While most outbreak households reported no change in attitudes towards vaccinations, two out of six reported that the experience had enhanced their opinion of vaccines, demonstrating the potential for refusal to shift to compliance if the severity and likelihood of contracting a VPD is fully understood [60].
8. Specific challenges of disease eradication
(a). Control versus eradication
Mass immunization campaigns have succeeded in driving down the global prevalence of certain VPDs, but complete eradication of a human disease requires an additional set of strategies. As long as herd immunity is maintained in a population, it is possible to halt endemic transmission of a VPD, thereby drastically reducing its prevalence. In the case of disease eradication, however, eliminating pockets of resistance is critical to driving global VPD prevalence down to zero.
Although eradication may appear to be an extension of disease control, the two terms are not interchangeable. Eradication is the deliberate use of interventions to reduce disease incidence to zero, whereas disease control refers to lowering disease incidence to an acceptable level [61]. Aggressive disease control ensures restricted circulation of an infectious agent and prevents future epidemics occurring within a particular geographical area [62]. In contrast, disease eradication on a global scale has specific political and social implications that necessitate additional public health measures. Eradication requires global political cooperation and financial commitment during the same period of time, and must occur within a limited time span—hence the importance of a protracted end stage. Its success depends on deliberate efforts to drive down disease incidence in regions where conditions may not be ideal for campaign efforts. Control efforts, however, may suffice with regulation of high incidence. Small, well-contained outbreaks do not necessarily disrupt control and elimination efforts but have a significant impact on eradication. Statistically small pockets of resistance continue to spark outbreaks in non-immunized children [49]. In polio-endemic regions such as Kano and Sokoto in Nigeria, and Quetta Region in Pakistan, over 60 per cent of annual polio cases were among families refusing OPV [49].
These qualitative differences between eradication and control also mean significant differences in cost; eradication efforts are often costlier and require substantially higher levels of political and financial investment from local leaders, national governments, and global organizations [63]. Many eradication proponents argue that the long-term costs of control exceed those of short term eradication [61], particularly on a cost per additional case prevented basis. However, lengthy eradication efforts may appear unnecessarily expensive and time-consuming to international donors, particularly once incidence is low enough to escape public awareness. The resulting decline in funding, political commitment and public interest further contribute to a protracted period of restricted circulation (disease control) rather than zero incidence (eradication).
Owing to these critical differences in strategy, it is often quite difficult to eradicate a disease that has been controlled. Between 1989 and 2000, the number of polio cases was more than halved; however, this rapid fall in cases does not continue through to the twenty-first century (figure 3). Since 2005, the global prevalence of poliomyelitis has generally hovered between 1000 and 2000 cases, displaying the ‘long tail’ of disease eradication—wherein disease prevalence remains consistently low, rather than the steady decrease typically seen earlier in the campaign. Likewise, the number of measles cases more than halved between 1989 and 2000, but global prevalence has hovered between 300 000 and 400 000 cases since 2005 [64].
Figure 3.
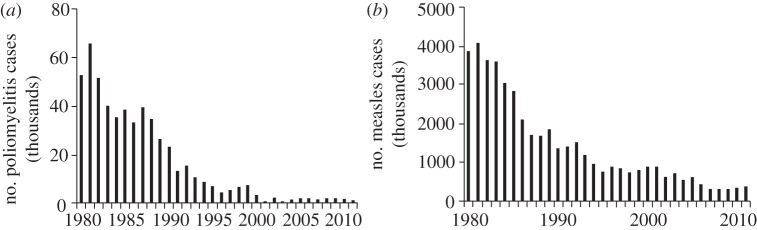
Global incidence of confirmed cases of (a) paralytic polio and (b) measles between 1980 and 2011. Data obtained from World Health Organization; updated July 2012. Accessed September 2012 [64].
9. Walking the last mile first: prioritizing vaccine refusal in twenty-first century disease eradication efforts
The next steps in eradication of VPDs should be shaped by the recognition that rising rates of vaccine refusal pose a considerable threat to global eradication efforts. Eradication campaigns must address issues of vaccine refusal from the onset in order to prevent costly setbacks towards the end of the campaign (e.g. widespread polio resurgence in northern Nigeria). It is essential to preempt and, if possible, avoid widespread non-compliance. In order to do so, eradication campaigns must focus on areas likely to experience refusal in both resource-poor and resource-rich settings.
The boycott of polio vaccines in northern Nigeria is a prime example of refusal in resource-poor settings, where mistrust and marginalization contributed to public perceptions of risk. Instances of children missed for reasons such as ‘sleep’ or illness have risen in polio-endemic countries such as Afghanistan, where nearly one-quarter of children missed are due to these instances of passive refusal. Prior negative experiences with pharmaceuticals and Western campaigns (e.g. allegations of misconduct brought against Pfizer by a Nigerian family, or a CIA effort to send a vaccination team into the bin Laden compound to gather information and DNA samples) fuel widespread non-compliance in resource-poor settings. Nevertheless, global examples of vaccine refusal illustrate that this problem is not isolated to developing countries.
In 2011, the United States experienced its largest measles outbreak in 15 years, with a reported 222 cases, of which 141 were unvaccinated but eligible for vaccination [65]. The WHO European region reported over thirty thousand cases of measles in 2011, nearly a fourfold increase in cases since 2009. More than 90 per cent of cases were concentrated in five countries—France, Italy, Romania, Spain and Germany—with suboptimal vaccine uptake rates [66]. In comparison with 99 per cent uptake of the MMR vaccine in Finland, which reported just 29 measles cases in 2011, France had an uptake rate of just 85 per cent and Germany had a suboptimal uptake rate of 70 per cent [67].
Despite a lack of formal policy to address vaccine refusal in the early stages of eradication campaigns, social mobilization, communication strategies and a focus on areas of probable non-compliance have been proposed as potential methods of controlling vaccine refusal.
10. Strategies to address vaccine refusal in eradication campaigns
(a). Social mobilization
Social mobilization efforts typically use face-to-face communication and interactive discussion with community members in order to raise awareness for a particular health outcome or intervention [68]. A wide range of community members also participate in health training and use their social connections to raise awareness [69]. Social mobilization strategies can improve VPD awareness as well as vaccine-related knowledge, attitudes and practices towards vaccines. A better understanding of how vaccines work and the benefits of immunization, even during periods of low incidence, improves uptake and demand. Eradication campaigns must commit to active engagement with community members and health volunteers, particularly in areas of historically low compliance, from the onset of a campaign rather than towards the end.
(b). Effective communication with public figures
Promoting dialogue with respected community leaders was crucial to overcoming non-compliance during the polio eradication initiative, albeit during its last stages. In March 2011, various clerics in Pakistan helped resolve over 8000 cases of polio vaccine refusal in Khyber-Pakhtunkhwa and the Federally Administered Tribal Areas [70]. During a week-long national immunization campaign, clerics in the districts helped dispel remaining doubts regarding the safety of the polio vaccine and religious objections to the vaccination, during special Friday sermons [70]. The anti-vaccination movement in northern Nigeria, supported by state clerics, further illustrated the importance of religious and cultural leaders in encouraging vaccine uptake. Future eradication initiatives must use the support of community leaders and respected figures in targeted areas early on in the eradication process.
(c). Empowering health professionals to address refusals
Involvement of health professionals at all levels promotes vaccine compliance, particularly in non-endemic countries such as the United States and United Kingdom [4].
Observance of provider–patient interactions is an important step in understanding common approaches to non-compliance among health professionals and potential areas for improvement [71]. Simulation of patient–provider dynamics to assess providers’ response to vaccine refusal can help inform effective and culturally appropriate vaccine promotion strategies. Health professionals in developing and developed regions should have access to resources such as methods of effective communication with vaccine-hesitant patients. Continuing health education for a spectrum of providers should also include updated literature on contributing factors to vaccine refusal as well as evidence-based strategies to combat the issue of non-compliance (e.g. emphasizing disease awareness and acknowledging the validity of some vaccine safety concerns).
(d). Monitoring and surveillance
Monitoring and surveillance of vaccine refusal and hesitancy, as well as implementation of prevention strategies, require substantial financial investment in social mobilization efforts. In many cases, areas of resistance today are the same as those which posed an obstacle to smallpox eradication (i.e. Afghanistan, West Africa—particularly Nigeria—and southeast Asia). Existing surveillance systems such as the Vaccine Confidence Project [20] should serve as the foundation for future vaccine refusal monitoring programmes, by tracking vaccine-related public concerns and global indicators for non-compliance. Without a preemptive understanding of cultural and political factors that make refusal more probable, future efforts will continue to encounter similar obstacles in historically resistant regions of the world.
11. Summary: prioritizing vaccine refusal in the twenty-first century
To walk the last mile first is to recognize the likely rise of vaccine refusal in periods of low disease incidence, as well as other sociopolitical factors, and to preempt such an obstacle in the early stages of any eradication campaign. In the past, vaccine refusal has been addressed only as it becomes a problem towards the end of a campaign. A lag in robust social mobilization has proved costly, both in terms of money spent and spikes in disease incidence.
So long as non-compliance remains an afterthought of eradication strategy, public misinformation and doubt will continue to stymie disease eradication efforts. Future campaigns must lessen the probability that non-compliance will rise as a significant obstacle by accelerating the initiative, thereby preventing a protracted period of low disease incidence. Prioritization of areas that are historically and culturally most likely to resist widespread vaccination efforts is critical in preventing future setbacks. Finally, early strategic use of social mobilization and effective communication with respected community members are critical approaches to addressing issues of risk perception, lack of knowledge or misinformation and disease awareness.
VPD eradication as a public health strategy has strong humanitarian and financial benefits. In order to take full advantage of the life-saving potential of vaccines, campaigns should focus on the challenge of vaccine refusal as one of the most significant components of disease control and prevention.
Acknowledgements
The authors would like to thank Emory University Rollins School of Public Health and the Emory Vaccine Center for their support.
References
- 1.Black RE, et al. ; for the Child Health Epidemiology Reference Group of WHO and UNICEF 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375, 7–14 10.1016/S0140-6736(10)60549-1 (doi:10.1016/S0140-6736(10)60549-1) [DOI] [PubMed] [Google Scholar]
- 2.The College of Physicians of Philadelphia. 2013 The History of vaccines: a project of the College of Physicians of Philadelphia. See http://www.historyofvaccines.org/content/timelines/all
- 3.Allen A. 2007. Vaccine: the controversial story of medicine's greatest lifesaver. New York, NY: W.W. Norton and Company [Google Scholar]
- 4.Kitta A. 2011. Vaccinations and public concern in history: legend, rumor, and risk perception. Florence, KY: Routledge Studies in History of Science, Technology and Medicine [Google Scholar]
- 5.Henderson DA. 1997. Edward Jenner's vaccine. Public Health Rep. 1974 112, 116–121 [PMC free article] [PubMed] [Google Scholar]
- 6.Andre FE. 2003. Vaccinology: past achievements, present roadblocks and future promises. Vaccine 21, 593–595 10.1016/S0264-410X(02)00702-8 (doi:10.1016/S0264-410X(02)00702-8) [DOI] [PubMed] [Google Scholar]
- 7.Hopkins DR. 1988. Smallpox: ten years gone. Am. J. Public Health 78, 1589–1595 10.2105/AJPH.78.12.1589 (doi:10.2105/AJPH.78.12.1589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. 1988. Smallpox and its eradication, ch. 14 Geneva, Switzerland: World Health Organization [Google Scholar]
- 9.Grassly NC. 2013. The final stages of the global eradication of poliomyelitis. Phil. Trans. R. Soc. B 368, 20120140. 10.1098/rstb.2012.0140 (doi:10.1098/rstb.2012.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas G, Sankara DP, Agua-Agum J, Maiga A. 2013. Dracunculiasis (guinea worm disease): eradication without a drug or a vaccine. Phil. Trans. R. Soc. B 368, 20120146. 10.1098/rstb.2012.0146 (doi:10.1098/rstb.2012.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization 2009. State of the world's vaccines and immunizations. Geneva, Switzerland: World Health Organization [Google Scholar]
- 12.World Bank 1993. Investing in Health. World Development Report 1993
- 13.Centers for Disease Control and Prevention 2009. The Pink Book: slide sets, epidemiology and prevention of vaccine preventable diseases, 11th edn See http://198.246.98.21/vaccines/pubs/pinkbook/pink-slides.htm [Google Scholar]
- 14.Chen RT, Rastogi SC, Mullen JR, Hayes SW, Cochi SL, Donlon JA, Wassilak SG. 1994. The vaccine adverse event reporting system (VAERS). Vaccine 12, 542–550 10.1016/0264-410X(94)90315-8 (doi:10.1016/0264-410X(94)90315-8) [DOI] [PubMed] [Google Scholar]
- 15.Schmitt HJ. 2001. Factors influencing vaccine uptake in Germany. Vaccine 20, s2–s4 10.1016/S0264-410X(01)00304-8 (doi:10.1016/S0264-410X(01)00304-8) [DOI] [PubMed] [Google Scholar]
- 16.Asch DA, Baron J, Hershey JC, Kunreuther H, Meszaros J, Ritov I, Spranca M. 1994. Omission bias and pertussis vaccination. Med. Decis. Making 14, 118–123 10.1177/0272989X9401400204 (doi:10.1177/0272989X9401400204) [DOI] [PubMed] [Google Scholar]
- 17.Landers J. 1987. Mortality and the metropolis: the case of London 1675–1825. Popul. Stud. 41, 59–76 10.1080/0032472031000142536 (doi:10.1080/0032472031000142536) [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RM, Sharp LK. 2002. Anti-vaccinationists past and present. BMJ 325, 430–432 10.1136/bmj.325.7361.430 (doi:10.1136/bmj.325.7361.430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbs GS. The evils of vaccination: with a protest against its legal enforcement. London, UK: John Chapman [Google Scholar]
- 20.Porter D, Porter R. 1988. The politics of prevention: anti-vaccinationism and public health in nineteenth-century England. Med. Hist. 32, 231–252 10.1017/S0025727300048225 (doi:10.1017/S0025727300048225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers A, Pilgrim D, Gust ID, Stone DH, Menzel PT. 1995. The pros and cons of immunization-paper one: immunization and its discontents: an examination of dissent from the UK mass childhood immunization programme. Healthcare Anal. Rep. 3, 99–115 10.1007/BF02198210 (doi:10.1007/BF02198210) [DOI] [PubMed] [Google Scholar]
- 22.Kaufman M. 1967. The American anti-vaccinationists and their arguments. Bull. Hist. Med. 41, 463–478 [PubMed] [Google Scholar]
- 23.Stern AM, Markel H. 2005. The history of vaccines and immunizations: familiar patterns, new challenges. Health Affairs 24, 611–621 10.1377/hlthaff.24.3.611 (doi:10.1377/hlthaff.24.3.611) [DOI] [PubMed] [Google Scholar]
- 24.Foege W. 2011. House on fire: the fight to eradicate smallpox. California, UK: University of California Press and Milbank Memorial Fund [Google Scholar]
- 25.Basu RN, Jezek Z, Ward NA. 1979. The eradication of smallpox from India. New Delhi, India: World Health Organization, Southeast Asia Regional Office [Google Scholar]
- 26.Joarder AK, Tarantola D, Tulloch J. 1980. The Eradication of Smallpox from Bangladesh. New Delhi, India: World Health Organization, Southeast Asia Regional Office [Google Scholar]
- 27.Greenough P. 1995. Intimidation, coercion and resistance in the final stages of the South Asian smallpox eradication campaign, 1973–1975. Soc. Sci. Med. 41, 633–645 10.1016/0277-9536(95)00035-6 (doi:10.1016/0277-9536(95)00035-6) [DOI] [PubMed] [Google Scholar]
- 28.Jeffres LW, Perloff RM. 1997. Mass media effects. Waveland, IL: Prospect Heights [Google Scholar]
- 29.May T. 2005. Public communication, risk perception, and the viability of preventive vaccination against communicable diseases. Bioethics 19, 407–421 10.1111/j.1467-8519.2005.00452.x (doi:10.1111/j.1467-8519.2005.00452.x) [DOI] [PubMed] [Google Scholar]
- 30.Valentino NA, Hutchings VL, Banks AJ, Davis AK. 2008. Is a worried citizen a good citizen? Emotions, political information seeking, and learning via the internet. Political Psychol. 29, 247–273 10.1111/j.1467-9221.2008.00625.x (doi:10.1111/j.1467-9221.2008.00625.x) [DOI] [Google Scholar]
- 31.Gino F, Brooks AW, Schweitzer ME. 2012. Anxiety, advice, and the ability to discern: feeling anxious motivates individuals to seek and use advice. J. Person. Soc. Psychol. 102, 497–512 10.1037/a0026413 (doi:10.1037/a0026413) [DOI] [PubMed] [Google Scholar]
- 32.Faase K, Gamble G, Cundy T, Petrie KJ. 2012. Impact of television coverage on the number and type of symptoms reported during a health scare: a retrospective pre-post observational study. BMJ Open 2, e001067. 10.1136/bmjopen-2012-001607 (doi:10.1136/bmjopen-2012-001607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo BK, Holland ML, Bhattacharya J, Phelps CE, Szilagyi PG. 2010. Effects of mass media coverage on timing and annual receipt of influenza vaccination among Medicare elderly. Health Serv. Res. 45, 1287–1309 10.1111/j.1475-6773.2010.01127.x (doi:10.1111/j.1475-6773.2010.01127.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason DW, Donnelly PD. 2000. Impact of a local newspaper campaign on the uptake of the measles mumps and rubella vaccine. J. Epidemiol. Comm. Health 54, 473–474 10.1136/jech.54.6.473 (doi:10.1136/jech.54.6.473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adelman RC, Verbrugge LM. 2000. Death makes news: the social impact of disease on newspaper coverage. J. Health Sci. Behav. 41, 347–367 10.2307/2676325 (doi:10.2307/2676325) [DOI] [PubMed] [Google Scholar]
- 36.Duffell E. 2001. Attitudes of parents towards measles and immunization after a measles outbreak in an anthropological community. J. Epidemiol. Comm. Health 55, 685–686 10.1136/jech.55.9.685 (doi:10.1136/jech.55.9.685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy AM, Brown CJ, Gust DA. 2005. Vaccine beliefs of parents who oppose compulsory vaccination. Public Health Rep. 120, 252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petts J, Niemeyer S. 2004. Health risk communication and amplification: learning from the MMR vaccination controversy. Health Risk Soc. 6, 7–23 10.1080/13698570410001678284 (doi:10.1080/13698570410001678284) [DOI] [Google Scholar]
- 39.Bauch CT, Galvani AP, Earn DJD. 2003. Group interest versus self-interest in smallpox vaccination policy. Proc. Natl Acad. Sci. USA 100, 10 564–10 567 10.1073/pnas.1731324100 (doi:10.1073/pnas.1731324100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhn S. 2009. Prisoner's Dilemma. In The Stanford encyclopedia of philosophy (ed. Zalta EN.), spring 2009 edn. See http://plato.stanford.edu/archives/spr2009/entries/prisoner-dilemma/. [Google Scholar]
- 41.May R. 2000. Simple rules with complex dynamics. Science 287, 60–62 10.1126/science.287.5453.601 (doi:10.1126/science.287.5453.601) [DOI] [PubMed] [Google Scholar]
- 42.Rabiu R. 2008. Nigeria: Six clerics stop polio immunisation in Niger. Daily Trust Abuja Interview, July 2008. See http://allafrica.com/stories/200807281140.html (accessed 28 July 2008).
- 43.Yahya M. 2006. Polio vaccines—difficult to swallow: the story of controversy in Northern Nigeria. Working Paper 261. Brighton, UK: University of Sussex Institute for Development Studies [Google Scholar]
- 44.Larson HJ, Ghinai I. 2011. Lessons from polio eradication. Nature, 473, 446–447 10.1038/473446a (doi:10.1038/473446a) [DOI] [PubMed] [Google Scholar]
- 45.Antai D, Ghilgaber G, Wedren S, Macassa S, Moradi T. 2009. Inequities in under-five mortality in Nigeria: differentials by religious affiliation of the mother. J. Religion Health 48, 290–304 10.1007/s10943-008-9197-7 (doi:10.1007/s10943-008-9197-7) [DOI] [PubMed] [Google Scholar]
- 46.Khowaja AR, Khan SA, Nizam N, Omer SB, Zaidi A. 2012. Parental perceptions surrounding polio and self-reported non-participation in polio supplementary immunization activities in Karachi, Pakistan: a mixed methods study. Bull. World Health Organ. 90, 822–830 10.2471/BLT.12.106260 (doi:10.2471/BLT.12.106260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oostvogel PM, van Wijngaarden JK, van der Avoort HGAM, Mulders MN, Conyn-van Spaendonck MAE, Rumke HC, van Steenis G, van Loon AM. 1994. Poliomyelitis outbreak in an unvaccinated community in the Netherlands, 1992–1993. Lancet 344, 665–670 10.1016/S0140-6736(94)92091-5 (doi:10.1016/S0140-6736(94)92091-5) [DOI] [PubMed] [Google Scholar]
- 48.Conyn-van Spaendonck MAE, Oostvogel PM, van Wijngaarden JK, Kromhout D. 1996. Circulation of poliovirus during the poliomyelitis outbreak in the Netherlands in 1992–1993. Am. J. Epidemiol. 143, 929–935 10.1093/oxfordjournals.aje.a008836 (doi:10.1093/oxfordjournals.aje.a008836) [DOI] [PubMed] [Google Scholar]
- 49.UNICEF 2012. Polio communication quarterly update: October 2012. ‘Missed’. United Nations Children's Fund (UNICEF)
- 50.UNICEF 2012. Overview—Reasons for missed children. Communication in action: Nigeria. PolioInfo: Strengthening Communication for Polio Eradication. UNICEF. See http://polioinfo.org/index.php/communication-in-action-nigeria
- 51.UNICEF 2012. Overview—Reasons for missed children. Communication in action: Afghanistan. PolioInfo: Strengthening Communication for Polio Eradication. UNICEF. See http://polioinfo.org/index.php/afghanistan-q4-review-r2 (accessed January 2013).
- 52.Smith MJ, Ellenberg SS, Bell LM, Rubin DM. 2008. Media coverage of the measles–mumps–rubella vaccine and autism controversy and its relationship to MMR immunization rates in the United States. Pediatrics 121, e836–e843 10.1542/peds.2007-1760 (doi:10.1542/peds.2007-1760) [DOI] [PubMed] [Google Scholar]
- 53.Black C, Kaye JA, Jick H. 2002. Relation of childhood gastrointestinal disorders to autism: nested case–control study using data from the UK General Practice Research Database. BMJ 325, 419–421 10.1136/bmj.325.7361.419 (doi:10.1136/bmj.325.7361.419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godlee F, Smith J, Marcovitch H. 2011. Wakefield's article linking MMR vaccine and autism was fraudulent. BMJ 342, c7452. 10.1136/bmj.c7452 (doi:10.1136/bmj.c7452) [DOI] [PubMed] [Google Scholar]
- 55.Brown KF, Long SJ, Ramsay M, Hudson MJ, Green J, Vincent CA, Kroll JS, Fraser G, Sevdalis N. 2012. UK parents’ decision-making about measles–mumps–rubella (MMR) vaccine 10 years after the MMR–autism controversy: a qualitative analysis. Vaccine 30, 1855–1864 10.1016/j.vaccine.2011.12.127 (doi:10.1016/j.vaccine.2011.12.127) [DOI] [PubMed] [Google Scholar]
- 56.Smith PJ, Humiston SG, Marcuse EK, Zhao Z, Dorell CG, Howes C, Hibbs B. 2011. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Rep. 126, 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatterjee A, O'Keefe C. 2010. Current controversies in the USA regarding vaccine safety. Expert Rev. Vaccines 9, 497–502 10.1586/erv.10.36 (doi:10.1586/erv.10.36) [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention 2001. Simultaneous administration of variolella vaccine and other recommended childhood vaccines—United States 1995–1999. Morbid. Mortal. Wkly Rep. 50, 1058–1061 [PubMed]
- 59.Hilton S, Petticrew M, Hunt K. 2006. ‘Combined vaccines are like a sudden onslaught to the body's immune system’: Parental concerns about vaccine ‘overload’ and ‘immune-vulnerability’. Vaccine 24, 4321–4327 10.1016/j.vaccine.2006.03.003 (doi:10.1016/j.vaccine.2006.03.003) [DOI] [PubMed] [Google Scholar]
- 60.Kennedy AM, Gust DA. 2008. Measles outbreak associated with a church congregation: a study of immunization attitudes of congregation members. Public Health Rep. 123, 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stepan LR. 2011. Eradication: ridding the world of disease forever? Ithaca, NY: Cornell University Press [Google Scholar]
- 62.Barrett S. 2001. Eradication versus control: the economics of global infectious disease policies. Bull. World Health Organ. 82, 639–718 [PMC free article] [PubMed] [Google Scholar]
- 63.Webber R. 2009. Control strategy and organization. In Communicable disease epidemiology and control: a global perspective, 3rd ed Wallingford, Oxfordshire, UK: CABI [Google Scholar]
- 64.World Health Organization Immunization, vaccines and biologicals: immunization surveillance, assessment and monitoring. See http://www.who.int/immunization/documents/monitoring/en/index.html
- 65.Centers for Disease Control and Prevention. 2012 Measles—United States, 2011. Morbid. Mortal. Wkly Rep. 61, 253–257 [PubMed] [Google Scholar]
- 66.European Centre for Disease Prevention and Control 2012. Surveillance report: european monthly measles monitoring, 21 February 2012 Stockholm, Sweden: European Centre for Disease Prevention and Control; See http://ecdc.europa.eu/en/publications/publications/sur_emmo_european-monthly-measles-monitoring-february-2012.pdf (accessed 10 October 2012). [Google Scholar]
- 67.Owens SR. 2002. Injection of confidence: The recent controversy in UK has led to falling MMR vaccination rates. Eur. Mol. Biol. Organ. 3, 406–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.UNICEF. 2013 Communication for development. N.p., 10 Dec. 12. Web. 31 Jan.
- 69.Christie AS, Gay A. 2011. The Measles Initiative: moving toward measles eradication. J. Infect. Dis. 204, S14–S17 10.1093/infdis/jir075 (doi:10.1093/infdis/jir075) [DOI] [PubMed] [Google Scholar]
- 70.APP 2013. Polio eradication: vaccine refusal cases addressed in K-P, FATA. The Express Tribune, 23 March 2013. The Express Tribune News Network
- 71.Bryant KA, Wesley GC, Wood JA, Hines C, Marshall GS. 2009. Use of standardized patients to examine physicians’ communication strategies when addressing vaccine refusal. Vaccine 27, 3616–3619 10.1016/j.vaccine.2009.03.048 (doi:10.1016/j.vaccine.2009.03.048) [DOI] [PubMed] [Google Scholar]


