Abstract
An infectious disease will be eradicated only if it is eliminated everywhere, including in the hardest-to-reach, most vaccine-wary communities. If eradication is successful, it promises a dividend in the form of avoided infections and vaccinations. However, success is never certain unless and until eradication is achieved, and claiming the dividend means bearing the possibly great risk of re-emergence. Economic analysis of eradication evaluates these risks and rewards relative to the alternative of ‘optimal control’, and also exposes the incentives for achieving and capitalizing on eradication. Eradication is a ‘game’, because some countries may be willing to eliminate the disease within their borders only if assured that all others will eliminate the disease within their borders. International financing is also a game, because each country would rather free ride than contribute. Finally, for diseases such as polio, capitalizing on eradication is a game, for should any country continue to vaccinate in the post-eradication era using the live-attenuated polio vaccine, the countries that stop vaccinating will be exposed to the risk of vaccine-derived polioviruses. In the framework developed in this paper, eradication is a seductive goal, its attainment fraught with peril.
Keywords: eradication, infectious diseases, cost–benefit analysis, game theory, global public goods, weakest links
1. Introduction
Eradication of a global scourge is an audacious undertaking. Its achievement depends on the cooperation of nearly every community in every country. It requires pushing a complex biological–social–political system into unchartered territory. It requires belief that the goal is achievable, even though success cannot be guaranteed. It requires trust in the people and organizations leading the effort. It requires patience. It requires resources. It requires luck. Unlike any other public health goal, it is intolerant of error. An eradication effort that reduces cases 99 per cent and no more fails to achieve its goal. Even before an eradication effort begins, attention must focus on how it will end.
If it ends successfully, then eradication can potentially save money as well as lives—money that can be spent on other good causes. Moreover, these dividends will be paid year after year, generation after generation. The rewards from eradication can be immense.
The risks can also be immense. Eradication may fail (previous efforts to eradicate hookworm, yellow fever, yaws and malaria all failed). If it succeeds, and we capitalize on its achievement by ceasing vaccination, then the world will be left more vulnerable than ever to a new outbreak. In terms of risks and rewards, few public policy goals can compare with eradication.
With so much at stake, economic analysis is critical, but many analyses of eradication rest on weak conceptual underpinnings (see [1]). First, some studies (examples include [2,3]) compare eradication with ‘no control’, or a similarly arbitrary alternative, when it should be compared with ‘optimal’ control. Second, virtually all studies assume that eradication is certain to be achieved, when the (subjective) probability of success will always be smaller than one. Finally, very few studies incorporate post-eradication risks or the costs of mitigating these.
In this paper, I develop a framework for economic analysis of eradication, grounded in simple epidemiology. The framework exposes the tensions between private and public interests—tensions that can make elimination difficult to achieve. It assumes that the disease harms and is transmitted by humans, and that the pathogen is controlled, eliminated and possibly eradicated by means of a vaccine. I demonstrate its relevance for three cases: smallpox, poliomyelitis and measles.
Most analyses ask only if the economics of eradication are favourable overall. However, to succeed, pursuit of the goal must also be consonant with the self-interests of every state. Some states will attempt to eliminate a disease only if assured that all other states will eliminate the disease, making eradication a ‘game’. Other states will increase vaccination only if given assistance. The states that gain from eradication should be willing to pay, but each will prefer that others pay, making financing another game. Finally, the incentives to eradicate and to finance eradication depend ultimately on whether vaccination can cease should eradication succeed, for only then will eradication yield a dividend. In the case of polio, if just one state continues to vaccinate with the live-attenuated vaccine in the post-eradication era, then other states may continue to vaccinate to protect against the threat of circulating vaccine-derived polioviruses, preventing a dividend from being realized and making vaccination cessation another game. All of these games are critical and they are also interlinked. A weakness in any one game threatens the others. Eradication is a precarious enterprise.
2. The economics of control versus elimination
The framework developed here is not only grounded in classical epidemiology, but also takes into account behaviour.
As is customary, let R0 denote the basic reproductive rate of a disease—the number of secondary infections expected to occur when one infected individual is introduced into a fully susceptible population. Obviously, for a disease to spread, we must have R0 > 1. For a disease to continue to circulate, it is also essential that the host population is sufficiently large. The value of R0 is disease-specific, and also depends on local conditions, most especially population density, sanitation, climate and established social norms.
Let R represent the effective reproductive rate—the average number of secondary infections produced when an infected individual is dropped into a partially susceptible population. We obviously require R ≤ R0. In a steady state (assuming one exists), if the disease is endemic, then we must have R = 1. Assuming that the host population is homogeneously mixed, we obtain R = R0x, where x is the fraction of the population that is susceptible.1 A steady state thus implies 1 = R0x.
Let p denote the fraction of the population that is immune. This implies p = 1 – x. Upon substituting, we obtain
| 2.1 |
where pc is the critical level of immunity—the value of p which achieves elimination. Equation (2.1) is a key relationship [5, p. 87]. It tells us the fraction of the population that must be immune in order for the disease to be eliminated locally. For example, taking R0 = 5, equation (2.1) implies that a disease can be eliminated if 80 per cent of the population is immune. For the three diseases discussed in this paper, R0 is about 5 for smallpox, 6 for polio and 15 for measles [5]. All else being equal, measles should be the harder disease to eradicate.
To eliminate a vaccine-preventable disease, the vaccine must be highly efficacious (multiple doses may be required) and be given to enough susceptible persons. Mass vaccination was central to the initial strategy for smallpox eradication, and was effective in many countries. However, it was unable to eliminate smallpox in densely populated countries, implying dependence of R0 on population density [5, p. 89]. In countries like India, elimination required a strategy of repetitive active searches and containment [6–8]. For polio, however, only a small fraction of infected persons show symptoms, making mass vaccination essential.
Let λ represent the ‘force of infection’, or the probability that a given susceptible host will become infected within a small interval of time [5, p. 63]. Under certain assumptions [5, p. 91], the steady-state value for λ, λ′, can be related to a given immunization level, p, by the relationship λ′ = μR0(pc − p) for pc≥ p and λ′ = 0 otherwise, where μ is an epidemiological parameter.
Now consider the decision by a susceptible person (labelled i) of whether or not to get vaccinated. If she gets vaccinated, and the vaccine is 100 per cent protective (this assumption can easily be relaxed), then her pay-off will be πi = −c, where c is the cost of vaccination, including the risk of complications. If this person does not get vaccinated, then her pay-off will be πi = −bμR0(pc − p), where b represents the loss she suffers should she become infected. Given these assumptions, person i will wish to be vaccinated if and only if bμR0(pc − p)≥c.
Like c, parameter b is a monetary value. It represents willingness to pay to avoid infection. For example, if the infection were guaranteed lethal (100% case fatality rate), then b would equal the value of a statistical life (‘statistical’, because ex ante we will not know who will actually become infected). These values are unbounded by income, but are generally lower in developing countries than in rich countries [9]. Although standard in cost–benefit analysis, Duintjer Tebbens et al. [10] are alone using such values in a study of eradication. Most eradication studies avoid them. A different approach is to estimate everything else that goes into a cost–benefit analysis of eradication, and then to determine how big b needs to be in order for eradication to be economically justified [11].
Because i's pay-off to getting vaccinated depends on immunity in the population (and, therefore, the decisions of others), vaccination is a ‘game’ (what each player most wants to do depends on what others do or can be expected to do). The Nash equilibrium is a central concept in game theory. In a Nash equilibrium, no player wishes to change his or her behaviour, given the choices made by others. For this game, the Nash equilibrium level of vaccination is unique and equal to
| 2.2 |
Barrett [12] and Bauch & Earn [13] obtain a similar result. p* is the vaccination level that could be expected to arise ‘spontaneously’ in a population in which individuals have private access to vaccine and choose independently whether or not to be vaccinated. Equation (2.2) has two important implications. First, so long as bμ(R0−1) − c ≥ 0, some people will choose to get vaccinated even in the absence of a public health policy. Second, so long as c > 0, individual behaviour will never lead to elimination (i.e. p* < pc). Elimination requires government intervention.2
Assume that the aim of public health policy is to maximize the collective pay-off of a population of size n by choice of the policy variable, p.3 Society gets a pay-off Π = −cpn − bμR0(pc − p)(1 − p)n for p < pc and Π = −cpn for p ≥ pc. The optimal vaccination level for the whole society, p**, is [12]
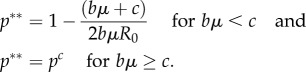 |
2.3 |
Again, two observations stand out. First, public policy requires that vaccination is increased above the level associated with individualistic behaviour (p** > p*) so long as c > 0.4 Second, if bμ > c, countries will wish to eliminate the disease unilaterally. Many countries, for example, eliminated smallpox and poliomyelitis before eradication plans were underway. Many have already eliminated measles.
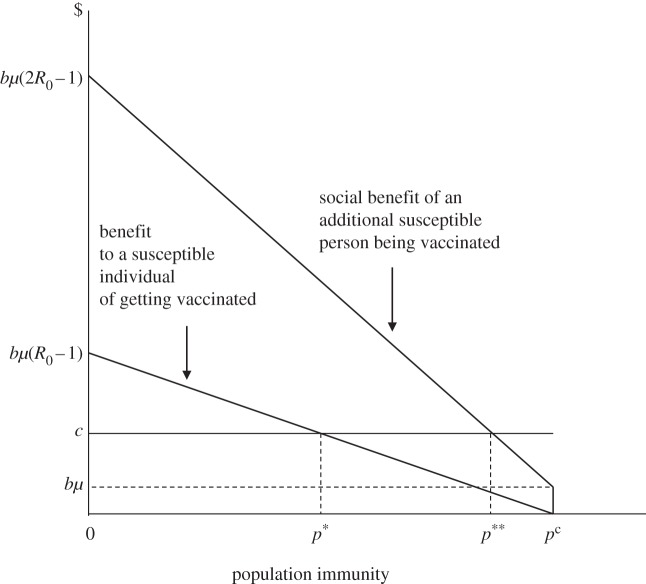
Figure 1 illustrates the simple economics of vaccination. The horizontal axis represents population immunity, the vertical axis economic value (benefits and costs). The lower downward sloping curve shows the benefit a susceptible individual obtains from vaccination. This declines with p, because the chance that any susceptible individual will get infected declines as the proportion of immune persons in the population increases—a phenomenon known as ‘prevalence-dependent demand’ [16]. The probability equals zero for p ≥ pc due to herd immunity. The higher downward sloping curve shows the social benefit of an additional vaccination. This exceeds the individual benefit owing to the vaccination ‘externality’—vaccination of one more person offers a measure of protection to all susceptible persons. The curve is kinked at pc, the point at which all remaining susceptible persons acquire herd immunity. Just to the left of pc, the pathogen continues to circulate, posing a tiny risk to each susceptible individual, but a significant risk to the entire susceptible population. When p ≥ pc, everyone is protected. If the cost line (c) were to lie below bμ, p** would equal pc, making unilateral elimination optimal; where c > bμ, control will be preferred to elimination.
Figure 1.
The vaccination game.
Note that society as a whole is better off at p** than at p*, but that the individuals who must get vaccinated in order to raise population immunity from p* to p** are made worse off. Mandatory vaccination can thus stimulate social resistance; vaccine refusal can be rational.5 Subsidies that lower the private cost of vaccination should help, though they may have little effect if the vaccine poses a risk to the individual, as is true of the live smallpox and polio vaccines.6 In the case of measles, perceived risk may be the greater threat. Opt outs from a mandatory programme may dampen social resistance, but vaccination rates tend to fall as opt outs become more permissive [18].
3. From elimination to eradication
If a population existed in isolation (and the pathogen could only reproduce in humans), then elimination would suffice to eradicate the disease. But countries are interconnected. A disease that circulates in one country can spread to another. The potential for spread is of little importance to endemic countries. However, for the countries that have eliminated the disease, the risk of imports means that vaccination to the level pc must continue year after year if elimination is to be maintained. (If there were local patches in which immunity fell below the critical level, then surveillance and outbreak response would also be needed.)
A disease can be eradicated only if it is eliminated everywhere. If p** = pc for every country, this will happen spontaneously. If, to the contrary, p** < pc for some countries, eradication will need a push; it will require global collective action (see §5).
(a). The eradication dividend
Maintenance of elimination requires that a fraction pc of new births be vaccinated year after year (assuming that immunity in individuals does not wane). If a disease is eradicated, however, and vaccination ceases, the countries that would have eliminated the disease anyway save a value c for every child that no longer needs to be vaccinated. The countries that would not have eliminated the disease on their own also gain. But their gain is different. They not only avoid the need to vaccinate in the future but they also avoid disease in the future. Every country obtains a ‘dividend’ from eradication, but the nature of this gain and its magnitude will vary.
How large is the dividend? Figure 2 shows how its value can be calculated. The gross cost of increasing vaccination from p** to pc is the rectangle c × (pc − p**), also shown as area C + D. However, this additional vaccination protects the people who would have remained susceptible at p**. Some are protected by the vaccine; the rest by herd immunity. The benefit both of these groups obtain from elimination (relative to control) is the area between pc and p** under the marginal social benefit curve (area D), which again indicates the benefit to society of one more susceptible person becoming immune. The net cost of eradication is found by deducting this area from the gross cost, and is represented by the area in dark grey (area C). This amount must be subtracted from the gross benefit of eradication, shown in light grey, which is equal to the avoided cost of vaccinating the individuals who would have been protected in any event (area B).
Figure 2.
The economics of eradication.
Note that the benefits and (net) costs of eradication are calculated relative to optimal vaccination (p**). This is the vaccination level a country should aim for were it not for eradication. If a lower level of control is assumed to be the alternative, then the benefit of eradication will be overstated. Arita et al. [19] make the reverse error. They argue that the alternative to polio eradication is very high control—a case count below 500. However, the eradication effort, backed by unprecedented international support, has struggled to limit the case count to this level, implying that optimal vaccination would allow a much larger number of cases worldwide.
(b). Dynamics and discounting
The dynamics of eradication are important. Eradication will take years to achieve, during which time countries must pay a net cost for the attempt. Once achieved, however, eradication may yield a benefit in terms of avoided vaccinations and disease—a benefit that, once again, must be calculated with respect to the alternative of optimal control. Costs and benefits accruing at different times must be put into comparable units. Future values must be discounted (the rate of discount can be zero or negative but will normally be positive) to yield present values. For example, if the annual discount rate is 3 per cent, then the present value of $1 a year from now is $1/(1.03) = $.97. The rationale for discounting is complicated (see [20]). It clearly reflects impatience—the preference people show for having something now rather than later. But it can also reflect ethics: if future generations are expected to be better off than the present generation, whether or not eradication is achieved, then ethics will commend that a dollar accruing to the future be valued less relative to a dollar accruing to the present. All else being equal, use of a lower discount rate will favour eradication relative to control so long as eradication promises a future dividend [21].
Suppose eradication is expected to take T years to achieve and that, after this, vaccination can cease indefinitely and at no cost. Eradication should then be undertaken if
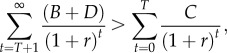 |
3.1 |
where r is the per-period discount rate, and B, C and D are the areas shown in figure 2 and discussed above (of course, the inequality can also be expressed in terms of the underlying parameters).
A look at figure 2 shows that area B + D is much larger than C. Moreover, it is easy to see that this relationship will be robust to changes in the economic and epidemiological parameters. This means that, so long as T and r are not very large, the economics of eradication will appear incredibly attractive.
However, the analysis so far has relied on two strong assumptions: (i) eradication is certain to be achieved; and (ii) once eradication is certified, vaccination can cease without putting the unvaccinated population at risk. Neither assumption is realistic.
(c). The risk of failure
Adjusting for the first assumption is easy. It requires multiplying the left-hand side of (3.1) by the (subjective) probability of success.
Obviously, for eradication to be worth attempting, success must be technically feasible. There must not exist a non-human reservoir. It must be possible, using existing technologies, to lift population immunity above the critical level. To succeed, however, eradication must also be practically feasible. It must be practicable to reach and vaccinate the critical number of susceptible persons—a major problem when a substantial fraction of people cannot be vaccinated, or resist being vaccinated, or when the vaccine has low efficacy.
Technical feasibility can be ascertained before an eradication effort begins. For example, we knew that polio eradication was technically feasible after the disease had been eliminated in the Americas. Practical feasibility, by contrast, can only be known with hindsight. We know that smallpox could be eradicated because it was eradicated!
The campaign to eradicate polio, launched in 1988, was supposed to have been completed by 2000. That deadline and others (most recently, the plan to stop wild poliovirus transmission by the end of 2012) were all missed. We now know that eradication will take more than twice as long as originally expected—and still we cannot be sure it will be achieved. With this track record, why continue? One reason is that the money spent on eradication in the past is sunk; it is irrelevant for the decisions we make today. Another reason is that the programme is better placed today to eradicate polio than it was in 1988; after all, polio has been eliminated from all but a few countries. Finally, the programme has learned from past mistakes. It still faces uncertainties but these are different from the ones anticipated in 1988.
However, pursuit of an eradication goal is costly. Resources spent on eradication cannot be spent on other worthy causes. My analysis has assumed that countries are sovereign, and determine their priorities independently. But as noted by Taylor et al. [22, p. 924], ‘Donors exert great influence on health systems in poor countries’. These authors question whether the opportunity cost of eradication is too great. They consider it ‘short-sighted for donors to use their considerable influence to promote polio eradication if this delays or diverts long-term investment by poor countries in sustainable health systems’.
(d). Post-eradication risks
The assumption about post-eradication risks is trickier to handle. Here, I can only give a sense of the difficulties.
In period T + 1, the value at risk is equal to area A + B + D in figure 2. We can multiply this value (discounted to the present) by the probability of re-emergence, and subtract this from the left-hand side of (3.1), after adjusting for the policies and measures adopted to reduce the impact of re-emergence from the maximum level. The calculations for period T + 2 are more complicated. The choices to be considered in this period depend on what happened previously. If the disease did not re-emerge in T + 1, we can repeat the previous calculation, while recognizing that the susceptible population will have increased with the passage of time. However, if the disease re-emerged in period T + 1, then we would need to ask if the goal of eradication would be abandoned in period T + 2, and replaced by optimal control, or if an attempt would be made to eradicate the disease all over again. And so on for every future period. Finally, the costs of policies and measures adopted to reduce post-eradication risks and their impacts would also need to be deducted from the left-hand side of (3.1), after making adjustments for their effects in all the above calculations.7
To sum up: relying only on inequality (3.1), the economics of eradication are supremely attractive. Taking into account the possibility of failure and post-eradication risks, the decision of whether or not to eradicate is murkier.
4. Analyses of smallpox, poliomyelitis and measles
Let us now use this framework to consider the economics of three important cases, beginning with smallpox.
(a). Smallpox
The economics of smallpox eradication, as revealed in estimates of the costs and benefits supplied by Fenner et al. [24], are truly astonishing. The total benefit to industrialized countries was about $350 million a year (in 1970s dollars), and consisted solely of avoided vaccination costs, as these countries had eliminated smallpox previously. The cost to them of achieving global eradication was equal to their financial contribution to the international effort—about $98 million [24]. Assuming a 3 per cent discount rate, and an infinite flow of benefits, these numbers imply a benefit–cost ratio of ($350 ÷ 0.03)/$98 ≈ 119 : 1. For developing countries, the benefit of eradication was about $1070 million per year, and consisted almost entirely of avoided deaths (in 1967, smallpox killed perhaps 1.5 million people annually; see Fenner et al. [24, p. 1365]), because vaccination coverage in these countries was low at the start of the programme. Eradication cost these countries about $200 million, making their benefit–cost ratio ($1070 ÷ 0.03)/$200 ≈ 178 : 1. Adding up, the benefit–cost ratio for the entire world would have been about (($350 + $1070) ÷ 0.03)/$298 ≈ 159 : 1. These are huge numbers. I would venture to guess that smallpox eradication was probably the greatest global public investment in human history.
The analysis by Fenner et al. [24] is retrospective; when it was prepared, eradication had already been achieved. It is as well to note, however, that the success of the programme was never certain. Indeed, the Director General of the World Health Organization (WHO) was sure it would fail [24, p. 417; 25, p. 62].
Fenner et al. [24] take the alternative to eradication to be the actual level of control prior to eradication. When eradication was first declared in 1959, the virus remained endemic in about 59 poor countries [24, p. 393–394]. At the start of the intensified programme in 1966, this number had shrunk to about 31 countries [24, p. 518]. Getting rid of smallpox in these last strongholds was a formidable undertaking; elimination in many of these countries would not have happened ‘spontaneously’, certainly not within such a short period of time. Eradication required a concerted, focused and determined effort unprecedented in the history of human endeavour.
The earlier-mentioned calculations ignore the post-eradication risks, but there was and remains a chance that smallpox might reappear. Indeed, after circulation of the virus had stopped but before eradication had been certified, smallpox escaped from a laboratory in Birmingham, UK. It infected one person, who went on to infect another (her mother) before the outbreak was contained [24, pp. 1097–1098]. This is why every eradication effort must eliminate or secure all remaining stocks held in laboratories. However, we can never be sure that all remaining stocks have been identified. Virus might even be concealed deliberately. The Soviets launched a covert programme to manufacture smallpox soon after the disease was eradicated—something that became known to the outside world only years later [25]. Bioterrorism is another risk. Finally, scientists have demonstrated that poliovirus could be manufactured from inert chemicals obtained by mail order [26]. This means that, even if the extinction of an infectious agent could be proved beyond any doubt, we could never be sure that the agent would not re-emerge.8
The risk of reintroduction cannot be eliminated, but it can be mitigated. The USA, for example, now maintains a stockpile of 300 million doses of smallpox vaccine, one for every American. The UK maintains its own stockpile, and recently vaccinated more than 500 medical personnel in preparation for the 2012 summer Olympic Games. Of course, measures such as these are costly, and they do not eliminate the risk, but we can be confident that the economics of smallpox eradication remain attractive, because no country has reinstated routine smallpox vaccination.
(b). Poliomyelitis
Certifying polio eradication will be more difficult. In contrast to smallpox, only about one in 200 infections lead to irreversible paralysis. It is relatively easy for the virus to circulate undetected.
The risks of reintroduction are also greater. Smallpox was eradicated using a live virus vaccine, vaccinia, which protects against smallpox but cannot cause smallpox. Polio is being eradicated using a live-attenuated vaccine (also known as the oral polio vaccine or OPV) that in rare cases reverts to virulence and causes infection. These vaccine-derived viruses can, in turn, evolve to resemble the wild virus and spread [29].
Because of the risks of reintroduction, Chumakov et al. [30] believe ‘we may have no other choice than to continue immunization against a non-existing disease to prevent ourselves from becoming hostages to the threat of the devastating return of poliomyelitis’. They say we should achieve and maintain ‘the widest-possible protection against polio… regardless of whether polio has been eradicated’, but they do not comment on why eradication should be pursued if vaccination is to be continued. The policy of universal elimination advocated by Chumakov et al. [30] would only make economic sense if the optimal level of immunization were pc for each and every country, and this is plainly not the case today. If the eradication effort were abandoned, then we should expect an increase in the number of polio cases.
One option Chumakov et al. [30] consider is switching to the inactivated poliovirus vaccine (IPV). OPV is easier to administer (OPV is given in drops, IPV by injection) and better at reducing spread (OPV unlike IPV induces immunity in the gut). OPV is also cheaper. However, IPV, being a killed virus vaccine, cannot cause polio. Rich countries have already switched to IPV, mainly to avoid cases of vaccine-associated paralytic polio.
A recent economic analysis of the Global Polio Eradication Initiative by Duintjer Tebbens et al. [10] found that eradication achieved using OPV results in a higher overall pay-off than routine vaccination with OPV without eradication being attempted—the difference is about $42 billion in present value terms. As shown in figure 2, starting from p** < pc, increasing vaccination only makes sense if by doing so the disease can be eradicated, allowing countries to stop vaccinating in the future. Duintjer Tebbens et al. [10], however, not only assume that vaccination continues, they also assume that in the post-eradication world, countries switch to the more costly IPV. This makes their results surprising.
Like the earlier-mentioned calculations for smallpox, this analysis looks backwards as well as forwards. It is not framed to answer the question of whether the goal of eradication should be pursued today. Its purpose is to evaluate whether the global polio eradication initiative will deliver a net benefit overall, assuming that it ultimately succeeds (the study assumes, incorrectly it turns out, that the wild viruses would be eradicated in 2012). The analysis accounts for post-eradication risks, such as the emergence of circulating vaccine-derived viruses, but assumes that every such outbreak is controlled successfully.
A crucial assumption is the case count corresponding to routine vaccination with OPV, the alternative to eradication. Figure 1 of Duintjer Tebbens et al. [10] suggests roughly zero cases in the future under eradication compared with around 175 000 cases of paralytic polio under the alternative. Recalling that there are about 200 infections per case of paralysis, this implies about 35 million infections a year under the alternative to eradication. Further analysis is required to show if this comes close to optimal vaccination.
Another crucial assumption concerns the post-eradication scenario, especially whether vaccination is continued. Duintjer Tebbens et al. [10] calculate that vaccination cessation in the post-eradication era would raise the net benefits of eradication (compared with the alternative) to $47 billion (from $42 billion with continued vaccination, both numbers expressed in present value terms). It is surprising that vaccination cessation should appear this unimportant, but this analysis begins in 1988, assumes that vaccination ceases in 2016, and terminates in 2035.9 The pre-vaccination-cessation era thus lasts 28 years, whereas the post-vaccination-cessation era lasts just 19 years. On top of this, discounting gives greater weight to history than to the future. Finally, the analysis is conducted only for 104 countries; it leaves out the richest countries and the Americas—countries that the analysis assumes would not be impacted by eradication.
(c). Measles
A recent analysis by Bishai et al. [31] finds that ‘the cost effectiveness of measles eradication is not inferior to control strategies’.10 Why such diffidence? The study calculates the incremental cost effectiveness ratio (incremental relative to the alternative of control) for a variety of scenarios. These ratios are expressed in dollars per disability adjusted life year—an approach that avoids having to put an economic value on avoided cases (b). The study does not calculate optimal control, but it recognizes that many countries have eliminated measles unilaterally. For the other countries, it assumes that vaccination reduces mortality by 95 and 98 per cent compared with the alternative of 90 per cent relative to the 2000 level. The incremental cost calculations for these scenarios and eradication are nearly identical, implying that the marginal cost of reducing cases cannot be much greater than the average cost. Although this study pays close attention to these costs, experience with polio eradication should make us cautious; expenditure has increased substantially over the last decade, without having much impact on the case count, and measles is more easily transmitted than polio. In common with all other eradication studies, Bishai et al. [31] assume that measles eradication is achievable with probability one.
The other reason that the economics of measles eradication do not differ much from high control is that vaccination is assumed to continue indefinitely whether or not the disease is eradicated. In another study of the economics of measles eradication, Levin et al. [32] consider a scenario in which eradication with continued vaccination reduces the need for supplemental immunization and outbreak response. This offers a positive but small dividend. To realize significant savings from eradication, vaccination must be reduced. Bishai et al. [31] consider a partial step in this direction, with countries dropping the second dose of measles vaccine (Klepac et al. [15] demonstrate in a general setting why this kind of policy can be optimal). This saves money for every country, and improves the incremental cost effectiveness ratio dramatically. But of course, this would also expose countries to an increased risk. Bishai et al. [31] do not explore the implications of this risk.
5. The eradication game
Eradication is worth attempting if the expected net benefits are positive. However, eradication has a chance of being achieved only if every country contributes enthusiastically to the effort. An attractive economic calculation is a necessary condition for success; it is not sufficient.
To see this, it will help to treat every country as being symmetric. Consider, then, a situation in which every country chooses unilaterally to control but not to eliminate a disease, but that each country would be even better off if every country eliminated the disease, causing the disease to be eradicated. Just as vaccination is a game played by individuals within every country, so eradication is a game played by countries on the global stage.
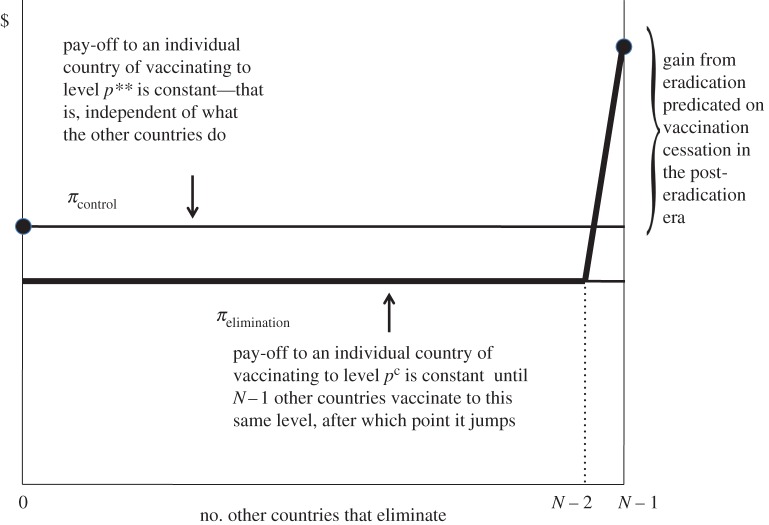
The ‘eradication game’ is shown in figure 3. In figure 3, each country has a binary choice. It can control the disease (i.e. vaccinate to the level p**) or it can eliminate it (vaccinate to the level pc). As depicted in figure 3, each country obtains a higher pay-off (π) when it controls rather than eliminates the disease unless every other country eliminates the disease. The pay-off to elimination jumps when every other country eliminates the disease, on the assumption that once the disease is eradicated vaccination can cease. Note that, in contrast to the game depicted in figure 1, this one has two Nash equilibria. In one, every country controls but does not eliminate the disease. In the other, the disease is eradicated. The latter makes every country better off, but getting there requires international coordination.
Figure 3.
The eradication game. (Online version in colour.)
Coordination should be easy. So long as every country believes that all the others will eliminate the disease, each has an incentive to eliminate it. This assurance can be provided by a resolution adopted unanimously by the World Health Assembly.
However, figure 3 also illustrates the fragility of this arrangement. If just one country fails to eliminate the disease within its borders, for whatever reason, then the others lose the incentive to eliminate the disease within their borders. When vaccination was suspended in northern Nigeria in 2003, the programme nearly crashed. In late 2012 and early 2013, polio vaccinators were murdered in Afghanistan, Pakistan and Nigeria, putting the initiative in jeopardy once again. Eradication is a weakest link global public good [33].
6. The eradication financing game
While all countries can gain from eradication, some will gain more than others, and some have a greater ability to pay. For both reasons, eradication efforts typically involve international financing.
The WHO estimated that eliminating smallpox in its last strongholds would cost just under $100 million. In 1959, the World Health Assembly voted unanimously for eradication, with the expectation that financing would be voluntary. As noted previously, smallpox eradication was probably the greatest single investment in human history. And yet, from 1959 to 1966, only eight countries donated money to the programme, for a total of just $27 345 [24]—a truly sobering illustration of ‘free riding’ behaviour.
In 1966, a different approach was tried. Every year, the World Health Assembly approves an overall budget for the WHO. Before launching the intensified smallpox eradication programme, the WHO asked members to approve a separate budget for smallpox. Each member would pay the amount of the budget times its share as determined by the United Nations scale of assessments. These assessments are calculated based on differences in population and income per capita, and must be approved by the UN General Assembly, usually by consensus. In 1966, the Director General of the WHO proposed a budget for smallpox of $2.415 million. The proposal was approved by the required two-thirds majority, but with just two votes to spare—‘the narrowest margin for the acceptance of a budget in the Organization's history’ [24, p. 416].
With the help of the new fund, the eradication effort was able to move forward. And yet financing of the remaining balance through voluntary contributions was touch-and-go to the end. As noted by Fenner et al. [24, p. 423], ‘Success was never a certainty even during the years immediately preceding the last known cases’.
The irony is that game theory suggests financing should have been easy. Denote the costs of achieving eradication by  (in the smallpox example,
(in the smallpox example, 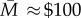 million in terms of external finance) and country i's contribution by mi. Finally, let Δi denote i's eradication dividend. Then, so long as eradication yields an aggregate gain
million in terms of external finance) and country i's contribution by mi. Finally, let Δi denote i's eradication dividend. Then, so long as eradication yields an aggregate gain  ), budget shares can be calculated for each country such that each gains by contributing (Δi > mi∀i), and the budget is exactly met
), budget shares can be calculated for each country such that each gains by contributing (Δi > mi∀i), and the budget is exactly met  . In words, given that all other countries pay their share, it will be in the interests of each country to pay its share—a Nash equilibrium.11
. In words, given that all other countries pay their share, it will be in the interests of each country to pay its share—a Nash equilibrium.11
The problem comes in deciding the shares. Financing is a zero sum game—if one country pays less, others must pay more. Analysis of voluntary contributions to the special account reveals a strong correlation with the UN scale [34], but the incentives to free ride were evidently powerful.
Financing of polio eradication has been remarkably successful, especially given that rich countries will gain little from eradication so long as they plan to vaccinate indefinitely. Between 1988 and 2013, donors contributed more than $9.5 billion to polio eradication. These are voluntary contributions, but the leadership of the global polio eradication initiative has tried to coordinate donations by calculating ‘fair shares’ for every country [35]. Based on the earlier-mentioned analysis, this is an astute move, and yet financing has remained a persistent threat. Indeed, the Independent Monitoring Board [36, p. 6] has said that financing poses the ‘primary risk’ to the global programme.
7. The vaccination cessation game
When smallpox was declared eradicated, countries could stop vaccinating as they pleased; coordination was unnecessary because continued vaccination by any country did not endanger others.
For polio, the risks are different. Since 2000, outbreaks of circulating vaccine-derived polioviruses (cVDPVs) have been detected in 19 different countries, several times spilling across borders. In Nigeria, transmission of type 2 cVDPV has persisted for many years (despite wild-type 2 virus having been eradicated long ago). There is a high probability that, after countries stop vaccinating with OPV, more cVDPVs will emerge, posing a risk to susceptible people everywhere. To reduce this risk, the Global Polio Eradication Initiative recommended that vaccination be increased everywhere, so as to shrink the stock of susceptible persons, and then stopped abruptly everywhere, so as to cut the time during which cVPDVs could emerge. This is important, because the probability of a cVPDV outbreak should fall sharply after vaccination has stopped [37].
Is it reasonable to expect that countries would comply with this plan? Figure 4 depicts the vaccination cessation game. Figure 4 assumes that countries (again, assumed symmetric) have two choices. They can continue to vaccinate to maintain herd immunity in the post-eradication era or they can stop vaccinating. The pay-off to continuing vaccination is constant, because vaccinating to the critical level reduces the risk of infection to zero. By contrast, the pay-off to stopping vaccination declines with the number of countries that continue to vaccinate, because the risk of a cVDPV outbreak increases in the number of countries that continue to vaccinate. As drawn, there are two Nash equilibria. In one, every country continues to vaccinate. In the other, every country stops. The latter outcome is an equilibrium so long as countries reap a windfall by stopping vaccination, given that the disease has been eradicated. Of course, if there is no windfall, there is no economic case for eradication.
Figure 4.
The post-eradication, vaccination cessation game. (Online version in colour.)
In figure 4, there is a tipping point at  . If more than
. If more than  other countries continue to vaccinate, it will pay each country to continue to vaccinate. If fewer than
other countries continue to vaccinate, it will pay each country to continue to vaccinate. If fewer than  other countries continue to vaccinate, every country will prefer to stop vaccinating. The value of
other countries continue to vaccinate, every country will prefer to stop vaccinating. The value of 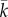 is an empirical matter. For polio, rough calculations suggest that the tipping point is about three [38]. This means that, in the post-eradication era, even if one or two countries continue to vaccinate, then it will still pay the others to stop. However, for reasons of confidence, it is likely that OPV cessation will need to be universal.
is an empirical matter. For polio, rough calculations suggest that the tipping point is about three [38]. This means that, in the post-eradication era, even if one or two countries continue to vaccinate, then it will still pay the others to stop. However, for reasons of confidence, it is likely that OPV cessation will need to be universal.
There is one further problem with OPV cessation. Rich countries switched to IPV long ago, mainly to avoid the very small (about a one in 750 000) risk of vaccine-associated paralytic polio (VAPP) from OPV. When the risk of getting infected from the wild viruses is high, the risk of VAPP can be ignored. Once polio has been eliminated, however, the risk of VAPP stands out. Unfortunately, IPV is costlier than OPV, and few poor countries will want to pay the difference. For rich countries, the calculation is different; they intend to continue to vaccinate with IPV in the post-eradication era. As a consequence, all the risk from stopping OPV will fall on the poor countries. This outcome may be compatible with self-interest, but it seems unjust, particularly as the rich countries may possibly gain by stopping vaccination at a later date, once the risk of cVDPVs has fallen over time.
Rough calculations show that rich countries could subsidize IPV use by poor countries for a few years, until the risk from cVDPVs has shrunk, and still gain from eradication provided they subsequently stop vaccinating [38]. In this case, rich and poor countries would bear similar risks and reap similar rewards from polio eradication. Investing in R&D to lower the cost of IPV for everyone is an even more robust solution. It is just one of many things the Global Polio Eradication Initiative is doing now to give eradication its best chance of succeeding.
8. Conclusions
Eradication is a seductive goal. It promises to rid the world of a scourge, and to free up resources for other good purposes. However, eradication is a high stakes gamble. To succeed, the disease must be eliminated everywhere, including in the most wretched places. To succeed, every state must play its part. Poor states must conduct the required vaccinations; rich states must help to pay for them. All of this depends on expectations being high that eradication will be achieved. Also important is the belief that every state will be able to claim a dividend, which means there must be an expectation that vaccination can cease after eradication has been certified, with little fear of the pathogen (wild or vaccine-derived) re-emerging. Were it not for these risks, the economics of eradication would be remarkably attractive; the decision of whether or not to attempt eradication and to press on to the very end would depend only on technical feasibility. It is because the pursuit of eradication is so perilous that economic considerations are essential, even when devising strategies for the endgame—indeed, even when planning for the post-eradication era.
Acknowledgements
I have benefited enormously over the years from numerous discussions with Bruce Aylward and D. A. Henderson. I am also grateful to D. A. Henderson, Petra Klepac and three anonymous reviewers for their excellent comments on a previous draft.
Endnotes
See Perisic & Bauch [4] for a model of individual vaccine choice when people belong to social networks.
Geoffard & Philipson [14] obtain a similar result.
Klepac et al. [15] offer a more sophisticated approach to understanding optimal vaccination, incorporating dynamics within a spatial framework. Not surprisingly, they obtain richer results.
Note as well that I have assumed c is unchanged as between (2.1) and (2.3). We could, however, interpret c as reflecting perceived risk of the vaccine in (2.2) and actual risk in (2.3). If the former value were greater than the latter, the gap between p* and p** would widen even more.
Manfredi et al. [17] model the game theory of vaccination policy when there are communities divided by ‘pro-’ and ‘anti’-vaccination communities.
Geoffard & Philipson [14] are sceptical of the utility of subsidies for elimination.
Measures to limit risks in the post-eradication era also involve a conflict between individual and collective interests; for an analysis relating to a smallpox bioterrorist attack, see Bauch et al. [23].
There may be other risks. Research suggests that vaccination cessation has enabled human monkeypox virus to spread in the Democratic Republic of Congo [27]; see also Lloyd-Smith [28].
This is another crucial assumption, particularly as regards the economics of vaccination cessation.
A similar analysis by Levin et al. [32] concludes that eradication is more cost-effective than control. Bishai et al. [31] attribute the difference ‘to differences in the way variation in measles transmission was modelled and how variation in costs and effects were analysed’.
For smallpox, the incentives were unusually strong. The dividend for the United States alone was estimated to be $150 million per year [24], greater than the entire international budget for smallpox. With 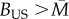 , it should have been in the interests of the US to finance the entire effort, even if no other country contributed.
, it should have been in the interests of the US to finance the entire effort, even if no other country contributed.
References
- 1.Miller M, Barrett S, Henderson DA. 2006. Control and eradication. In Disease control priorities in developing countries (ed. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P.), pp. 1163–1176 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Bart KJ, Foulds J, Patriarca P. 1996. Global eradication of poliomyelitis: benefit–cost analysis. Bull. WHO 74, 35–45 [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn M, Ehreth J. 2003. Costs and benefits of polio eradication: a long-run global perspective. Vaccine 21, 702–705 10.1016/S0264-410X(02)00584-4 (doi:10.1016/S0264-410X(02)00584-4) [DOI] [PubMed] [Google Scholar]
- 4.Perisic A, Bauch CT. 2009. Social contact networks and disease eradicability under voluntary vaccination. PLoS Comput. Biol. 5, e1000280. 10.1371/journal.pcbi.1000280 (doi:10.1371/journal.pcbi.1000280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RM, May RM. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press [Google Scholar]
- 6.Arita I, Wickett J, Fenner F. 1986. Impact of population density on immunization programmes. J. Hyg. 96, 459–466 10.1017/S0022172400066249 (doi:10.1017/S0022172400066249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foege WH. 2011. House on fire: the fight to eradicate smallpox. Berkeley, CA: University of California Press [Google Scholar]
- 8.Henderson DA, Klepac P. 2013. Lessons from the eradication of smallpox: an interview with D. A. Henderson. Phil. Trans. R. Soc. B 368, 20130113. 10.1098/rstb.2013.0113 (doi:10.1098/rstb.2013.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viscusi WK, Aldy JE. 2003. The value of a statistical life: critical review of market estimates throughout the world. J. Risk Uncertain. 27, 5–76 10.1023/A:1025598106257 (doi:10.1023/A:1025598106257) [DOI] [Google Scholar]
- 10.Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, Linkins J, Sutter RW, Aylward RB, Thompson KM. 2011. Economic analysis of the global polio eradication initiative. Vaccine 29, 334–343 10.1016/j.vaccine.2010.10.026 (doi:10.1016/j.vaccine.2010.10.026) [DOI] [PubMed] [Google Scholar]
- 11.Barrett S, Hoel M. 2007. Optimal disease eradication. Environ. Dev. Econ. 12, 627–652 10.1017/S1355770X07003816 (doi:10.1017/S1355770X07003816) [DOI] [Google Scholar]
- 12.Barrett S. 2003. Global disease eradication. J. Eur. Econ. Assoc. 1, 591–600 10.1162/154247603322391224 (doi:10.1162/154247603322391224) [DOI] [Google Scholar]
- 13.Bauch CT, Earn DJD. 2004. Vaccination and the theory of games. Proc. Natl Acad. Sci. USA 101, 13 391–13 394 10.1073/pnas.0403823101 (doi:10.1073/pnas.0403823101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geoffard P-Y, Philipson T. 1997. Disease eradication: private versus public vaccination. Am. Econ. Rev. 87, 222–230 [Google Scholar]
- 15.Klepac P, Laxminarayan R, Grenfell BT. 2011. Synthesizing epidemiological and economic optima for control of immunizing infections. Proc. Natl Acad. Sci. USA 108, 14 366–14 370 10.1073/pnas.1101694108 (doi:10.1073/pnas.1101694108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein E, Laxminarayan R, Smith DL, Gilligan CA. 2007. Economic incentives and mathematical models of disease. Environ. Dev. Econ. 12, 707–732 10.1017/S1355770X0700383X (doi:10.1017/S1355770X0700383X) [DOI] [Google Scholar]
- 17.Manfredi P, Della Posta P, d'Onofrio A, Salinelli E, Centrone F, Meo C, Poletti P. 2009. Optimal vaccination choice, vaccination games, and rational exemption: an appraisal. Vaccine 28, 98–109 10.1016/j.vaccine.2009.09.109 (doi:10.1016/j.vaccine.2009.09.109) [DOI] [PubMed] [Google Scholar]
- 18.Parkins C. 2012. Protecting the herd: a public health, economics, and legal argument for taxing parents who opt-out of mandatory childhood vaccinations. S. Cal. Interdisc. Law J. 21, 437–489 [Google Scholar]
- 19.Arita I, Nakane M, Fenner F. 2006. Is polio eradication realistic? Science 312, 852–854 10.1126/science.1124959 (doi:10.1126/science.1124959) [DOI] [PubMed] [Google Scholar]
- 20.Portney PR, Weyant JP. (eds). 1999. Discounting and intergenerational equity. Washington, DC: Resources for the Future [Google Scholar]
- 21.Murray CJL, Acharya AK. 1997. Understanding DALYs. J. Health Econ. 16, 703–730 10.1016/S0167-6296(97)00004-0 (doi:10.1016/S0167-6296(97)00004-0) [DOI] [PubMed] [Google Scholar]
- 22.Taylor CE, Cutts F, Taylor ME. 1997. Ethical dilemmas in current planning for polio eradication. Am. J. Public Health 87, 922–925 10.2105/AJPH.87.6.922 (doi:10.2105/AJPH.87.6.922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauch CT, Galvani AP, Earn DJD. 2003. Group interest versus self-interest in smallpox vaccination policy. Proc. Natl Acad. Sci. USA 100, 10 564–10 567 10.1073/pnas.1731324100 (doi:10.1073/pnas.1731324100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. 1988. Smallpox and its eradication. Geneva, Switzerland: World Health Organization [Google Scholar]
- 25.Henderson DA. 2009. Smallpox: the death of a disease. Amherst, NY: Prometheus Books [Google Scholar]
- 26.Cello J, Paul AV, Wimmer E. 2002. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. Science 297, 1016–1018 10.1126/science.1072266 (doi:10.1126/science.1072266) [DOI] [PubMed] [Google Scholar]
- 27.Rimoin AW, et al. 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl Acad. Sci. USA 107, 16 262–16 267 10.1073/pnas.1005769107 (doi:10.1073/pnas.1005769107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd-Smith JO. 2013. Vacated niches, competitive release and the community ecology of pathogen eradication. Phil. Trans. R. Soc. B 368, 20120150. 10.1098/rstb.2012.0150 (doi:10.1098/rstb.2012.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grassly NC. 2013. The final stages of the global eradication of poliomyelitis. Phil. Trans. R. Soc. B 368, 20120140. 10.1098/rstb.2012.0140 (doi:10.1098/rstb.2012.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chumakov K, Ehrenfeld E, Wimmer E, Agol VI. 2007. Vaccination against polio should not be stopped. Nat. Rev. Microbiol. 5, 952–958 10.1038/nrmicro1769 (doi:10.1038/nrmicro1769) [DOI] [PubMed] [Google Scholar]
- 31.Bishai D, Johns B, Lefevre A, Nair D, Simons E, Dabbagh A. In press. Measles eradication versus measles control: an economic analysis. J. Vaccines Vaccin. 10.4172/2157-7560.S3-002 (doi:10.4172/2157-7560.S3-002) [DOI] [Google Scholar]
- 32.Levin A, Burgess C, Garrison LP, Jr, Bauch C, Babigumira J, Simons E, Dabbagh A. 2011. Global eradication of measles: an epidemiologic and economic evaluation. J. Infect. Dis. 204(Suppl. 1), S98–S106 [DOI] [PubMed] [Google Scholar]
- 33.Barrett S. 2007. Why cooperate?: the incentive to supply global public goods. Oxford, UK: Oxford University Press [Google Scholar]
- 34.Barrett S. 2006. The smallpox eradication game. Public Choice 130, 179–207 10.1007/s11127-006-9079-z (doi:10.1007/s11127-006-9079-z) [DOI] [Google Scholar]
- 35.Aylward RB, Acharya A, England S, Agocs M, Linkins J. 2003. Polio eradication. In Global public goods for health: health economics and public health perspectives (eds Smith R, Beaglehole R, Woodward D, Drager N.), pp. 33–53 Oxford, UK: Oxford University Press [Google Scholar]
- 36.Independent Monitoring Board. 2012. Every missed child. See http://www.polioeradication.org/Portals/0/Document/Aboutus/Governance/IMB/6IMBMeeting/IMB6_Report.pdf .
- 37.Thompson KM, Duintjer Tebbens RJ. 2008. The case for cooperation in managing and maintaining the end of poliomyelitis: stockpile needs and coordinated OPV cessation. Medscape J. Med. 10, 190. [PMC free article] [PubMed] [Google Scholar]
- 38.Barrett S. 2010. Stop! the polio vaccination cessation game. World Bank Econ. Rev. 24, 361–385 10.1093/wber/lhq018 (doi:10.1093/wber/lhq018) [DOI] [Google Scholar]