Abstract
Many studies have addressed the effects of climate change on species as a whole; however, few have examined the possibility of sex-specific differences. To understand better the impact that changing patterns of snow-cover have on an important resident Arctic mammal, we investigated the long-term (13 years) phenology of hibernating male arctic ground squirrels living at two nearby sites in northern Alaska that experience significantly different snow-cover regimes. Previously, we demonstrated that snow-cover influences the timing of phenological events in females. Our results here suggest that the end of heterothermy in males is influenced by soil temperature and an endogenous circannual clock, but timing of male emergence from hibernation is influenced by the timing of female emergence. Males at both sites, Atigun and Toolik, end heterothermy on the same date in spring, but remain in their burrows while undergoing reproductive maturation. However, at Atigun, where snowmelt and female emergence occur relatively early, males emerge 8 days earlier than those at Toolik, maintaining a 12-day period between male and female emergence found at each site, but reducing the pre-emergence euthermic period that is critical for reproductive maturation. This sensitivity in timing of male emergence to female emergence will need to be matched by phase shifts in the circannual clock and responsiveness to environmental factors that time the end of heterothermy, if synchrony in reproductive readiness between the sexes is to be preserved in a rapidly changing climate.
Keywords: climate change, Urocitellus parryii, Arctic, reproductive phenology, snowmelt
1. Introduction
In Arctic regions, the rate of climate warming is occurring two to three times that of the global average, and warming has accelerated from 0.15–0.17°C decade−1 (1961–1990) to 0.3–0.4°C decade −1 [1–6]. Concurrent with this environmental change, there have been significant changes in the geographical distribution, phenotypes, abundance and the timing of recurring seasonal events (phenology) of Arctic species [5,7]. Animal and plant phenologies are among the best-studied traits in response to climate change, and there is now ample evidence that over the past three decades the phenology of many organisms has advanced in response to warmer springs [8–10].
Although phenological responses at lower latitudes are primarily related to changes in temperature, in snow-dominated environments such as the Arctic, phenology can be more influenced by timing and patterns of snow-cover. For example, studies on plants have shown that later snowmelt in spring, owing to increased snow depth, delayed the phenology of flowering, regardless of temperature [11–14]. In contrast, environments with earlier spring snowmelt are associated with advanced spring events in plants, mammals and arthropods [15–17].
If species that interact across trophic levels respond differently in their phenology in response to trends in climate change, timing mismatches may occur between linked trophic levels. Many studies have shown that climate change has induced mismatches between the timing of breeding in animals and their food source, which can have negative effects on reproductive success ([9,18–20]; but see [21,22]). For example, Post & Forschhammer [23] found that the timing of caribou calving has not kept pace with the advancement of the plant-growing season in West Greenland, and they suggest that this divergence has contributed to the observed decline in caribou reproductive success. Mismatches may also be created between sexes within a species if males and females use different cues to time their phenology or respond to climate change differently. Høye et al. [24] found that earlier snowmelt increased adult body size in wolf spiders but with a skew towards positive sexual size dimorphism, which may have later effects on population dynamics.
The arctic ground squirrel (AGS), Urocitellus parryii, is an excellent species in which to investigate sex-specific phenological responses to environmental differences, as males and females display pronounced phenological differences in annually recurring life-history events [16,25,26]. Ground squirrels experience distinct events that divide each year into a long hibernation season, defined by when animals remain sequestered in their hibernacula, and a conversely short active season, when animals are active, above-ground on a daily basis. During hibernation, ground squirrels are heterothermic, altering their metabolism and body temperature between long periods (two to three weeks) of torpor and spontaneous arousals, when euthermic levels of body temperature briefly (less than 1 day) resume. However, male but not female ground squirrels return to continuous euthermy in spring for two to three weeks before first emerging to the surface, since sustained high body temperatures are necessary for gonadal growth and maturation [27,28]. In contrast, females end heterothermy three to four weeks after males and begin euthermia and emerge within 3–4 days. Females re-enter hibernation in August, up to eight weeks before males [25,26]; this results in active and hibernation seasons that can differ in length between the sexes by three to four months. There can be relative differences in annual timing associated with differences in habitat between populations as well. Using long-term data comparing free-living female AGS in two nearby populations in northern Alaska, we have shown that seasonal differences in snow-cover are associated with significant differences in their phenology [16,29]. At one site, Atigun, where snowmelt occurs on average 26 days earlier than the other site, Toolik, female AGS emerge from hibernation, give birth and enter hibernation significantly earlier than females living at Toolik, only 20 km away [16]. Snow-cover in both autumn and spring may be major factors influencing female phenology [29].
To understand better the impact future changing patterns of snow-cover may have on AGS as a species, we investigated the long-term (13 years) phenology of male AGS living at each site during similar time periods as our investigations of females. We compared phenological differences between the sites in relation to the calendar year, the timing of snow-cover, and in comparison with the emergence date of females. We also examined potential relevant ecological and environmental cues males may use to adjust their phenology. Lastly, we estimated male body weight within 10 days of emergence as an index of individual fitness. Because males engage in intense agonistic interactions during breeding, with significant fighting, wounding and loss in weight, we make the assumption that heavier males would be more successful [30–32].
2. Material and methods
(a). Study area
This study occurred at two nearby sites in northern Alaska, separated by about 20 km: Toolik Lake (68°38′ N, 149°38′ W; elevation 719 m) and Atigun River (68°27′ N, 149°21′ W; elevation 812 m). Despite this proximity, physical and biotic conditions differ between the sites. Atigun is slightly sloping along the northern bank of the Atigun River. The flora is dominated by a mix of low growing Salix sp. (less than 30 cm), Dryas octopetala, Rhododendron lapponicum, Arctostaphylos alpina and Vaccinium uliginosun [16]. Thaw depth of the sandy soil reaches 1–2 m in August, into which AGS have dug a high density of burrows and nest sites and hibernacula. The Toolik area is categorized as cotton grass (Eriophorum) tussock and dry tundra with more than 98 per cent of the vegetative biomass and productivity attributable to only 10 species [33]. The topography is relatively flat, with gently rolling hills underlain by rocky soils with a depth of thaw usually less than 1 m [34]. AGS burrows are less dense and more isolated from each other at Toolik than at Atigun. Entrances of AGS burrows at both sites are randomly oriented and showed no differences in exposure to prevailing environmental conditions, such as solar radiation, shade or wind. Owing to less overwinter accumulation of snow and high winds that disperse snow, spring snowmelt occurs on average 26 days earlier at Atigun than at Toolik, and in autumn snow-cover occurs on average 14 days later at Atigun than at Toolik. Thus, Atigun has a 44-day longer snow-free period.
(b). Animal trapping and handling
Adult males 2 years or older (73 at Atigun over the past 6 years and 43 at Toolik over the past 13 years) were captured using Tomahawk live-traps baited with carrot. Traps were set in the early morning and checked every 1–3 h until closure in the mid-afternoon. Trapped animals were transported to the Toolik Field Station of the University of Alaska Fairbanks. Animals were anesthetized by a 3–5 min exposure to methoxyflurane or isoflurane vapours and uniquely tagged (ear tags, Monel no. 1; pit tags, AVID MUSICC), weighed and sexed. To determine fine-scale data on timing of hibernation and reproduction through the recording of core body temperature (Tb) [35], a subset of 71 adult males (28 at Atigun and 43 at Toolik) were abdominally implanted and explanted 6–12 months later, with temperature-sensitive data loggers (modified TidBit Stowaway model TBICU32–05 + 44, Onset Computer Corporation; or iButtons, Maxim Integrated, that were programmed to record core Tb at either 20 or 120 min intervals) from autumn 1999 through spring 2011 at Toolik and from 2006 to 2011 at Atigun (see [36] for surgical details). The subset of squirrels implanted versus captured at Atigun is due to animal care restrictions on the number of implants per year. Some squirrels were implanted multiple years, but being implanted once or multiple times did not affect Tb changes (data not shown). After surgery, animals were monitored in captivity for 12 h prior to release at their site of capture.
(c). Phenology measurements
We estimated phenological events from patterns of Tb change recorded by the temperature loggers implanted in free-living squirrels, validated by Williams et al. [35]. As shown in figure 1, we determined (a) the time of euthermy: the date (in spring) each male returned to and remained at high, normal levels of Tb (36–37°C), but without circadian patterns; (b) emergence from hibernation: the date male's Tb displays clear diurnal cycles indicating resumption of daily above-ground activity; (c) entrance into hibernation: the date (in autumn) male's daily Tb cycles significantly decreased in amplitude and lost diurnal entrainment; (d) beginning of heterothermy: the first date male's Tb declined to less than 30°C for over 24 h.
Figure 1.
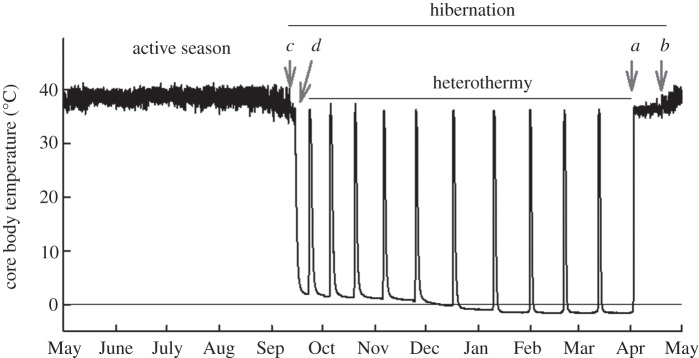
Core body temperature of representative individual free-living male arctic ground squirrel (AGS) in northern Alaska. Patterns of body temperature provide precise measures of the timing of annually recurring life-history events. (a) Return to euthermy and end of heterothermic season; (b) emergence from hibernation; (c) entrance into hibernation; (d) beginning of heterothermy.
(d). Environmental cues
We assessed the relationship between two environmental cues, soil temperature and snow-cover, and phenology. We chose these as the most likely available cues to males because: (i) males remain sequestered within a nest in their burrow 1 m below-ground for six to eight months during hibernation prior to spring emergence and soil temperature is an environmental cue they are exposed to, and (ii) snow-cover limits food availability; thus males may use snow conditions to time emergence in spring or entrance in autumn.
Soil temperature was measured using high-resolution temperature loggers (Hobo Pro Series Temp, ONSET; accuracy ±0.16°C) at each site. Loggers recorded temperature every 4 h from thermistor probes placed at the bottom of a 1 m-deep plastic pipe, backfilled with sand and tamped firm (see [34] for details). For each site, soil temperatures were calculated for day (24 h average) corresponding to the beginning of spring euthermy of males and the day of the penultimate arousal. Soil temperatures were compared by year and if they influence arousal we expect equivalencies in temperature patterns near the time of arousal.
The extent of snow-cover was quantified from images acquired daily at solar noon from 2007 to 2011 using a camera (Campbell Scientific, CC640 Digital Camera System mounted inside a Pelco EH4700 Environmental Enclosure) mounted on a tower facing across each of the study areas. To describe differences in snow-cover among years and between the two sites, we recorded the day each site was first 100 per cent snow-free and remained so for at least 3 days in spring and the day each site was first 100 per cent snow-covered and remained snow-covered for at least 3 days in autumn.
(e). Statistics
To test for phenological differences and body weight differences between the sites we used ANOVAs (year × site) or (site). General linear models were used to test the relationship between the timing of entrance into hibernation and heterothermy (independent variables) and euthermy (dependent); emergence from hibernation (independent variable) and entrance into hibernation (dependent); female emergence (independent variable) and male emergence (dependent); snowmelt date (independent variable) and emergence (dependent); snow-cover date (independent variable) and entrance (dependent). A Kruskal–Wallis ANOVA by Ranks (for non-parametric comparisons) was used to test whether soil temperature was similar when AGS became euthermic. A GLM was then used to assess the relationship between soil temperatures (independent variable) and the timing of euthermy (dependent variables). We also used a GLM to test how soil temperature varied across years and between sites. Models were run at both a landscape level, inclusive of individuals from both sites, and within a site, inclusive of individuals from each site. The assumption of normality was tested with Shapiro–Wilks test, and the assumption of homogeneity of variances was tested with Levene's test. All statistics were performed using the software package STATISTICA v. 10. All data are expressed as means ± 1 s.e., unless otherwise stated. p-Values were included for all analyses and considered significant if p < 0.05; however, may still be biologically relevant if p < 0.1.
3. Results
(a). Phenology
We found no consistent directional trends across years in the timing of male phenology; however, there was an effect of year on the date males became euthermic and when they entered hibernation (euthermy: F5,36 = 2.62, p = 0.04; entrance: F5,39 = 5.16, p = 0.0009; figure 2). Comparing Atigun and Toolik, there was no difference in the date when males ended heterothermy and became euthermic in spring (F1,28 = 0.50, p = 0.48) or entered hibernation in autumn (F1,28 = 2.42, p = 0.13; figure 2). However, males at Atigun consistently emerged from hibernation on average 8 days earlier than those at Toolik (F1,35 = 15.27, p = 0.0004; figure 2).
Figure 2.
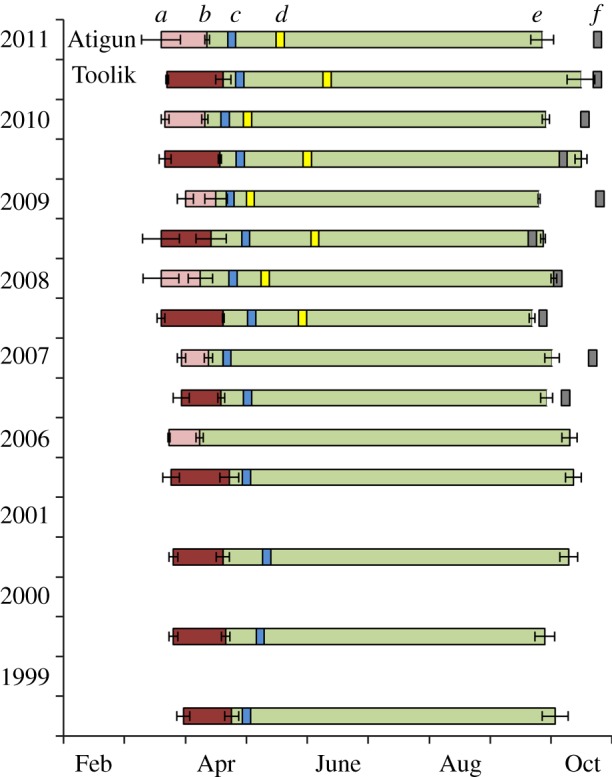
Annual timing in adult male AGS of the (a) return to euthermy, (b) emergence from, and (e) entrance into hibernation (mean ± s.e.) at two nearby sites, Atigun (2006–2011) and Toolik (1999–2011), in northern Alaska, approximately 68°N. The (c) timing of adult female AGS emergence; (d) timing of 100% snowmelt; (f) timing of 100% snow-cover are also shown.
In relation to the timing of spring snowmelt and winter snow-cover, we found that males at Atigun became euthermic and emerged from hibernation closer to the time of snowmelt than those at Toolik (euthermy: F1,6 = 17.64, p = 0.006; emergence: F1,6 = 11.98, p = 0.01; figure 2). At Atigun, males became euthermic and emerged 45 ± 6 days and 26 ± 5 days, respectively, before snowmelt. At Toolik, males became euthermic and emerged 73 ± 5 days and 46 ± 7 days, respectively, before snowmelt. Conversely, at Atigun, males entered hibernation long before the time of snow-cover compared with those at Toolik (F1,8 = 7.71, p = 0.02). At Atigun, males entered hibernation 19 ± 5 days before snow-cover, while those at Toolik entered hibernation 2 ± 4 days before snow-cover.
In relation to female phenology, at Atigun, males became euthermic closer to when females emerged from hibernation compared with males at Toolik (F1,12 = 10.01, p = 0.008; figure 2). At Atigun, males became euthermic 29 ± 3 days before females emerged. At Toolik, males became euthermic 39 ± 2 days before females emerged. However, we found no difference in the timing of when males emerged compared with females between the two sites (F1,12 = 0.71, p = 0.42). At Atigun and Toolik, males emerged 11 ± 1 days and 13 ± 2 days, respectively, before females emerged.
(b). Ecological and environmental cues
We found significant but weak relationships between when male AGS entered hibernation and first became heterothermic in autumn and when they became euthermic in spring, when combining data from both sites (entrance: β = 0.28, F1,65 = 5.39, p = 0.02, r2 = 0.08; heterothermy: β = 0.38, F1,65 = 10.79, p = 0.002, r2 = 0.14; figure 3) and within each site (Atigun entrance: β = 0.35, F1,25 = 3.44, p = 0.07, r2 = 0.12; heterothermy: β = 0.32, F1,25 = 2.81, p = 0.10, r2 = 0.10; Toolik entrance: β = 0.27, F1,38 = 2.97, p = 0.09, r2 = 0.07, heterothermy: β = 0.44, F1,38 = 9.03, p = 0.005, r2 = 0.19; figure 3). Conversely, we found no relationship between when males emerged from hibernation in spring and when they entered hibernation in autumn either combining sites (β = −0.08, F1,13 = 0.09, p = 0.76) or within each site (Atigun β = −0.34, F1,4 = 0.52, p = 0.51; Toolik β = 0.38, F1,7 = 1.17, p = 0.32).
Figure 3.
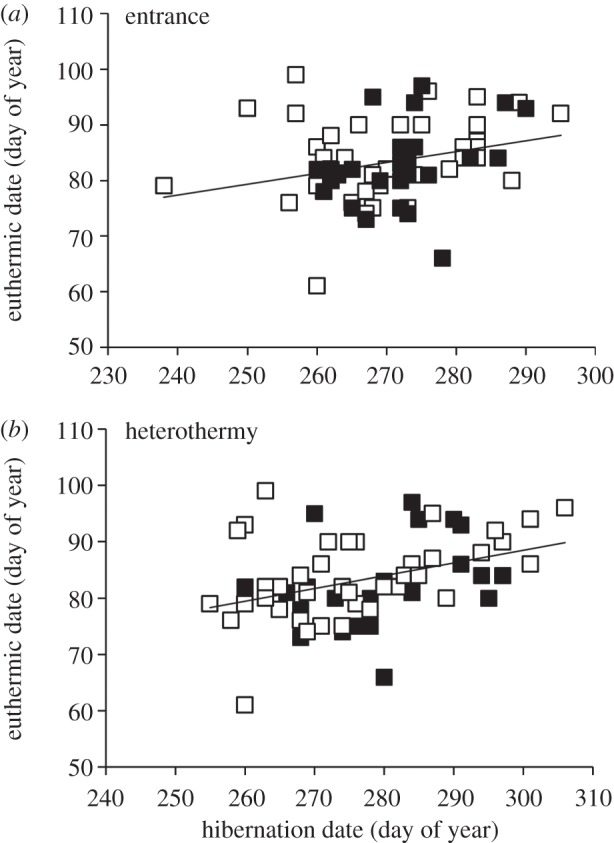
The date adult male AGS (a) enter hibernation (y = 0.2x + 30.58) or (b) become heterothermic (y = 0.2x + 20.73) in autumn is significantly related to the date they become euthermic in spring at two sites in northern Alaska, Atigun (filled squares) and Toolik (open squares).
Combining data from both sites, we found a correlation between the timing of female emergence and male emergence (β = 0.70, F1,12 = 11.33, p = 0.006, r2 = 0.49), but no relationship between female emergence and the date of male euthermy (β = 0.05, F1,12 = 0.03, p = 0.87; figure 4). Within each site, we found no relationship between female emergence and the date of male euthermy (Atigun β = −0.16, F1,3 = 0.07, p = 0.80; Toolik β = 0.20, F1,7 = 0.29, p = 0.61) or emergence (Atigun β = −0.00, F1,3 = 0.00, p = 1.00; Toolik β = 0.37, F1,7 = 0.15, p = 0.70).
Figure 4.
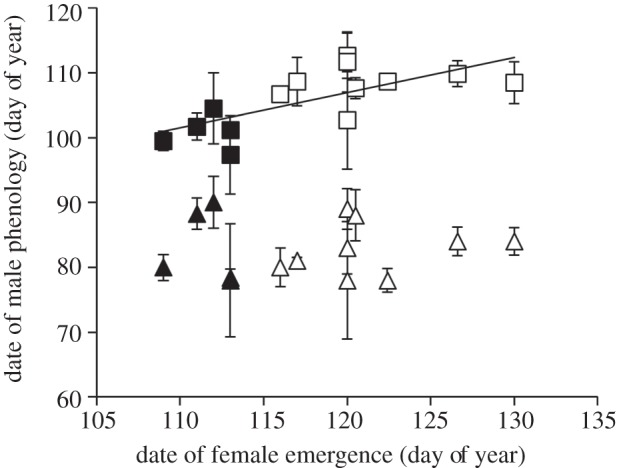
The date of male emergence (square) was significantly related to the date of female emergence at two nearby sites in northern Alaska, Atigun (filled symbols) and Toolik (open symbols). But there was no relationship between the date males became euthermic (triangle) and the date of female emergence.
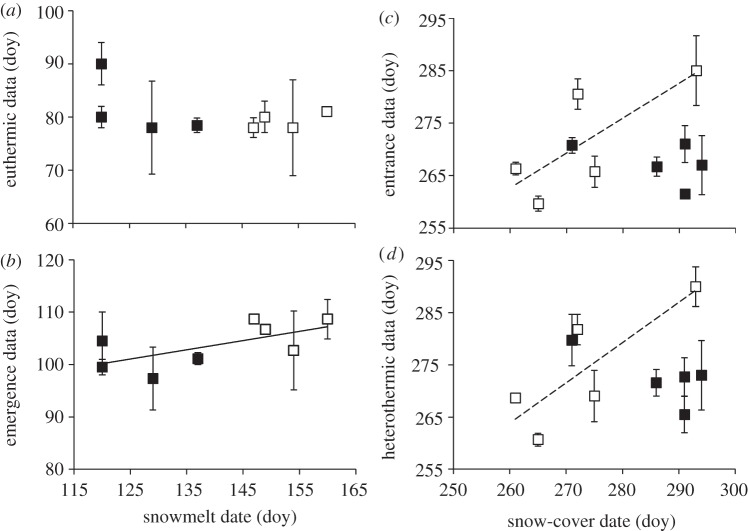
Combining data from both sites, we found a correlation between the timing of snowmelt and the date squirrels emerged from hibernation (β = 0.64, F1,6 = 4.23, p = 0.08, r2 = 0.41), but not the date squirrels became euthermic (β = −0.45, F1,6 = 1.49, p = 0.27; figure 5a,b). Within each site, we did not find a relationship between snowmelt and euthermy (Atigun β = −0.62, F1,2 = 1.26, p = 0.38; Toolik β = 0.59, F1,2 = 1.09, p = 0.41) or emergence (Atigun β = −0.29, F1,2 = 0.17, p = 0.72; Toolik β = −0.04, F1,2 = 0.00, p = 0.96). There was no landscape level, data from both sites, relationship between the timing of snow-cover and the date squirrels entered hibernation (β = 0.23, F1,8 = 0.45, p = 0.52) or became heterothermic (β = 0.30, F1,8 = 0.77, p = 0.41; figure 5c,d). At Atigun, we found no relationship between the timing of snow-cover and the date squirrels entered hibernation (β = −0.46, F1,3 = 0.80 p = 0.44) or became heterothermic (β = −0.75, F1,3 = 3.77, p = 0.14). At Toolik, we found a trend between the timing of snow-cover and the date males entered hibernation (β = 0.76, F1,3 = 4.17, p = 0.10, r2 = 0.58) and became heterothermic (β = 0.82, F1,3 = 6.00, p = 0.09, r2 = 0.67; figure 5c,d).
Figure 5.
The relationship between spring snowmelt and the timing (day of year, doy) of (a) euthermy and (b) emergence from hibernation, and the relationship between autumn snow-cover and the timing of (c) entrance in hibernation and (d) heterothermy in adult male AGS at two nearby sites in northern Alaska, Atigun (filled symbols) and Toolik (open symbols). There was a correlation between snowmelt and emergence (b) at a landscape level (solid line); and a correlation between snow-cover and entrance (c) and heterothermy (d) only at Toolik (dashed line). Note that the solid square that falls along the correlation between snow-cover and entrance (c) and heterothermy (d) is the only year (2008) that snow-cover limited access to food at Atigun; in all other years males entered hibernation 14–26 days before snow-cover.
We found that soil temperatures differed significantly (across years at each site) on the date males became euthermic (Atigun H5,27 = 23.21, p = 0.0003; Toolik H8,37 = 31.95, p = 0.0001) and during the penultimate arousal of hibernation (Atigun H5,32 = 26.26, p = 0.0001; Toolik H8,42 = 34.00, p < 0.0001).When males became euthermic, soil temperatures ranged from −17.64 ± 0.15°C at Atigun in 2007 to −9.32 ± 0.39°C at Toolik in 2001. However, we did find a significant relationship between the time males became euthermic and soil temperatures at their penultimate bout (β = −1.37, F1,35 = 2.43, p = 0.02, r2 = 0.45), and a slight trend between the timing of euthermy and soil temperatures at the time of euthermy (β = −0.76, F1,35 = 1.69, p = 0.11, r2 = 0.37). There was no site or year effect for any of the relationships (p > 0.05). Thus, although soil temperatures were significantly different at the time of euthermy, when soil temperatures were warmer AGS became euthermic earlier (figure 6). Overall, we found no yearly directional trend in average March soil temperatures at either site (Atigun: F1,4 = 3.76, p = 0.12; Toolik: F1,8 = 0.03, p = 0.86) and no difference in March soil temperatures between the two sites (F1,14 = 0.004, p = 0.95).
Figure 6.
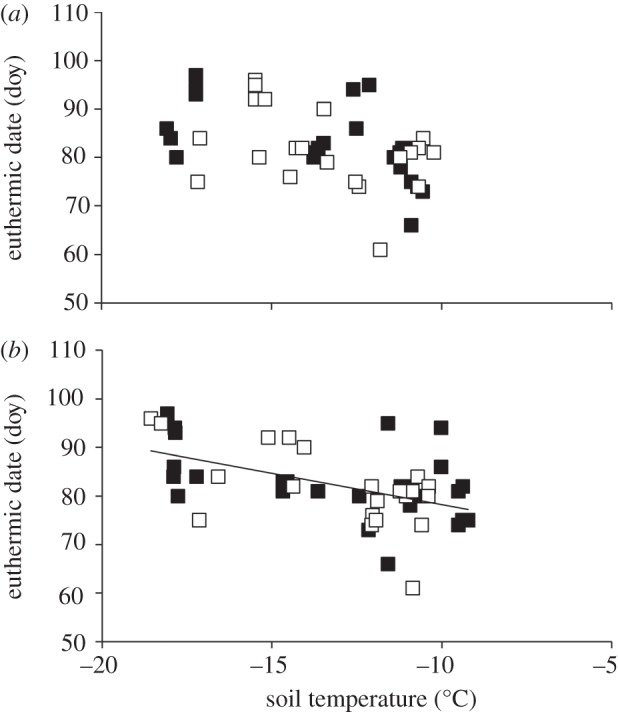
The relationship between the timing of euthermy (doy) and spring soil temperature on the day (24 h average) (a) of euthermy and (b) the penultimate bout of hibernation in adult male AGS at two nearby sites in northern Alaska, Atigun (filled symbols) and Toolik (open symbols). The regression line in (b) denotes a significant relationship (based on data from both sites).
(c). Male body weight
We found a significant effect of year (F4,95 = 3.75, p = 0.007) and site (F1,95 = 9.33, p = 0.003) with male body weight at emergence, and no interaction (F4,95 = 0.36, p = 0.84). Since spring 2007, male body weight at emergence has declined by approximately 15 per cent or 100 g at both sites (figure 7). However, consistent across the years, males at Toolik are 7 per cent heavier in spring than at Atigun, averaging 682 ± 16 g versus 635 ± 9 g, respectively (figure 7).
Figure 7.
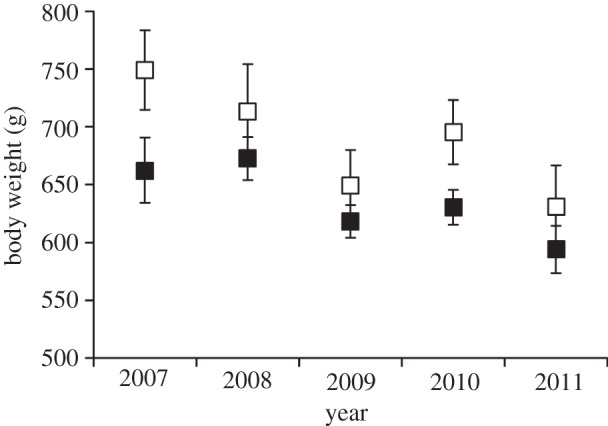
The body weight (mean ± s.e.) of adult male AGS living at two nearby sites in northern Alaska, Atigun (filled symbols) and Toolik (open symbols).
4. Discussion
(a). Phenology
The timing of annual events of reproduction, moult, fattening and hibernation or migration, among others, in the life history of a species is the result of sequencing of physiologically linked events, trade-offs in partitioning benefits between parents and offspring, and optimization to match availability of environmental resources to organismal need [37,38]. In seasonal environments with restricted growing seasons, animals must anticipate the availability of food in timing migration or hibernation to appropriately initiate reproductive readiness. They do so through the use of proximate cues of changing day length that directly stimulate responses in photoperiodic species or indirectly through seasonal entrainment of animals with endogenous, circannual rhythms. However, in environments where climate change is rapidly altering the timing of when food is available, organisms that use photoperiod to time spring reproduction may experience mismatches between when they are prepared to breed and when resources are available to support that reproduction.
Critical to male reproductive success across many taxa is primary access to females. In AGS, the male that first consorts with a newly emerged female fathers 90 per cent of the young within her litter in this multi-male mating system [31]. In response to changing climate, males must adjust their phenology in a manner that will not disrupt the breeding synchrony between the sexes. Previously we have shown that Atigun females emerge earlier in response to earlier spring snowmelt. Here, we found no difference in the duration between male and female emergence between Atigun and Toolik; males have adjusted their timing to emerge 12 days before females at both sites (figure 2). However, we found no difference between sites in the date when males become euthermic in spring (figure 2). Thus, to emerge 12 days prior to females, males at Atigun shorten the time they spend euthermic while remaining in their burrows before emerging from hibernation. This may represent a trade-off between physiological and behavioural preparation for breeding. The time after males emerge to the surface, but prior to female emergence, may be critical for males to locate and scent mark female burrows and for male–male interactions that can define access to females when they emerge. At this time, males can be often seen standing next to each other or leaning on one another, and intense male–male agonistic behaviour occurs in the presence of females in spring [32]. In many other species, males engage in pre-breeding activities, such as sexual displays, songs or combat, nuptial gift gathering, nest building, etc. There is potential for large consequences if the cues males use to time, these activities differ from those females use to time breeding. That males at both Toolik and Atigun conserve a full 12 days of above-ground activity prior to the appearance of females suggests an importance for these social interactions.
Although male AGS are maintaining the time between their emergence and that of females, they are not adjusting the time they become euthermic; males at both sites become euthermic at the same time of year regardless of the timing female emergence (figure 2). A shortening of the euthermic period may mean that Atigun males have to begin expending energy on above-ground male–male interactions before they are fully spermatogenic and fertile. Male ground squirrels resume euthermy early in spring but remain in their burrows while they undergo reproductive maturation, including gonadal growth and the establishment of spermatogenesis, physiological and endocrinological processes that are inhibited by the low tissue temperatures of torpor. For example, in the golden-mantled ground squirrel, Callospermophilus lateralis, there is little to no testicular growth and no advancement of spermatogenic stage during heterothermy (low Tb during hibernation) [27]. Sequential testicular biopsies found that only over the first three weeks of euthermy do testes enlarge, seminiferous tubules expand, and primary spermatocytes divide, mature into elongated spermatids and spermiation occurs. If male AGS have the same developmental time-course, Atigun males would be infertile when they emerge only after 15 days of euthermy. In addition, because male gonads begin regression after 30 days of beginning euthermy [39], a mismatch between males and females would also occur if females delay their breeding as opposed to advancing it.
The timing of entrance into hibernation may be as critical for male reproductive success as the timing of spring euthermy and emergence. To become reproductively mature, owing to the energetics of remaining at high Tb and the necessity to reach and maintain a suitable body size, males rely on a food cache in spring [28]. Because of the extremely low food availability in spring, males gather the cache and reach a threshold body size in autumn [40]. Thus, one would predict that in areas with prolonged autumns and delayed onset of winter snow-cover, such as at Atigun, male AGS would remain active longer. However, we found that males enter hibernation on a similar calendar date between the two sites (figure 2), even though snow-cover occurs much later at Atigun. Males at Atigun enter hibernation approximately 19 days before complete snow-cover. Alternatively, the active season at Toolik is constricted by snow-cover and males enter hibernation only 2 days before complete snow-cover. Why do males at Atigun not take advantage of the prolonged autumn by remaining above-ground longer with the potential of increasing their cache and body size, and increasing their competitive advantage the following spring? One possibility is that the advantages of remaining on the surface are outweighed by the increase in risk of predation. Both et al. [41] suggest that phenological adjustments may be driven by changes in lower trophic levels but may also be explained by predator avoidance. If individual AGS males remain on the surface longer than the average of all males they may increase their risk of predation. Our methods do not allow us to determine whether males engage in this strategy but are then preyed upon (and not re-captured) or whether males do not engage in this strategy at all. Alternatively, males may not take advantage of the prolonged autumn at Atigun, because the date they enter hibernation constrains when they become euthermic in spring. We found a positive relationship between the date males enter hibernation and when they become euthermic in spring; males that enter hibernation later become euthermic later (further discussed below; figure 3). Males that end hibernation late may miss breeding opportunities [31].
(b). Ecological and environmental cues
Many non-tropical animals rely on day length to regulate the coarse timing of their annual rhythms, rather than reacting to environmental conditions as they happen [42–44]. However, other environmental cues also may be important for fine-tuning when breeding occurs. Experimental evidence suggests a strong influence of both food availability and winter snow-insulation, for example, on reproductive timing in photoperiodic collared-lemmings [45]. Alternatively, other species use an endogenous circannual clock to regulate annual cycles [44]. For example, under constant conditions with no changes in daylength or temperature, captive equatorial African stonechats displayed circannual rhythmicity in their reproductive capacity and moult that persist for up to 10 years [46]. However, because under constant conditions circannual clocks free-run with periods usually less than 12 months, information from the environment is required for animals to remain entrained to the calendar year [47]. Lee & Zucker [48] demonstrated that changing photoperiod during summer may provide enough stimulus to roughly synchronize circannual rhythms of weight gain in hibernating golden-mantled ground squirrels. However, in environments experiencing rapid climate change that includes changes in the timing of food availability in spring, animals that are primarily dependent on photoperiod to time reproduction may become desynchronized with periods of optimal food availability.
Here, we assessed whether the timing of snowmelt or spring soil temperature may be associated with the timing of when hibernating male AGS return to euthermy each spring. We found no relationship between the timing of snowmelt and euthermy (figure 5a). Furthermore, we found that although soil temperatures differed significantly at the time of euthermy among years, there was a negative relationship between spring soil temperature and the time males became euthermic; the warmer the soil temperature in spring the earlier males emerged (figure 6). Thus, although there is no across year warming trend in spring soil temperatures at either site, males may have the ability to adjust the timing of euthermy in a warming environment. This is in contrast to results in Williams et al. [49] for female AGS, which appear unresponsive to spring soil temperatures. However, the difference between the studies may relate to the time when soil temperature was assessed. Here, we demonstrate a relationship in soil temperatures during the penultimate bout; Williams et al. [49] did not investigate soil temperatures at this time. We also found a significant positive correlation between when males entered hibernation and became heterothermic in autumn and the time they became euthermic in spring; the earlier males enter hibernation in autumn the earlier they emerge in spring (figure 3). Previously, we demonstrated that autumn may be important in influencing the timing of spring phenological events in female AGS [29]. Environmental cues in autumn are also important in other species and are associated with the onset of winter migration [50] and breeding in numerous ungulates [51,52]. AGS, sequestered in their burrow below the surface for six to eight months of the year, may use a combination of spring soil temperature and an endogenous clock mechanism set the previous summer or autumn to time when they become euthermic.
The timing of male euthermy is related to the date they emerge from hibernation and become active on the surface; however, males at Atigun emerge sooner after they become euthermic (figure 2). Thus, males at each site may use other cues, or respond to cues differently, to set their euthermic–emergence timing relationship. At a landscape level, we found a relationship between the timing of male emergence and snowmelt, and between the timing of male emergence and female emergence (figures 4 and 5b). However, these relationships were not significant at a local, within-site, level. Because of the similar time interval between male and female emergence regardless of site (12 days) we believe that female emergence is a strong cue males use to time their emergence. But the questions remains, how would an AGS male anticipate female emergence? Males may engage in a bet-hedging strategy with respect to female emergence by timing their emergence to coincide with a long-term average of female emergence in a given area. A critical consideration is that males are using two different cues (spring soil temperature and an endogenous clock versus female emergence) to set the timing of their euthermy and their emergence (respectively). Because these cues do not have the same relationship between the two sites (i.e. soil temperature is not different between Toolik and Atigun, but snowmelt, and thus female emergence are significantly different), there may be severe consequences to sequential patterns and length of phenological stages and thus to male reproductive success. This may be a general theme among animals, and it may be very important to understand all the cues used to time the complete set of phenological events and how climate change is affecting them.
On average, males entered hibernation at the same time at both sites, but there was considerable variation across the years (figure 2). We found no significant relationship between any ecological or environmental factor and male's entrance, with the exception of snow-cover date at Toolik, where snow-cover comes very early in the year (figure 5c,d). However, even with little snow-cover, males at Atigun entered hibernation at a similar time and even earlier in some years than males at Toolik. Studies on hibernators have found that animals will only enter hibernation after a certain body mass is attained [53]; however, hibernators may begin preparation for hibernation months prior to entrance [54]. Thus, the timing of entrance into hibernation may be related to a complex interaction of local environmental conditions in autumn, body condition and an endogenous clock set weeks or months prior to entrance. In migratory species, complex interactions may also occur in timing their departure date and considerations may also include the number of broods possible per summer and the length and location of migration [50].
(c). Body size
How environmental change impacts adult size, a key life-history trait, may be highly variable among species and between sexes. In wolf spiders, Høye et al. [24] found that both males and females increased in size in association with earlier snowmelt, but that females grew more. Here, we found the opposite; male AGS at Atigun, where spring conditions occur earlier, weighed less than Toolik males (figure 7). These differing responses may be due to the different spring activity patterns between the species. In wolf spiders, activity begins after snowmelt and earlier snowmelts may lead to increased body size by lengthening the active season of spiders, increasing food availability, and possibly allowing for an additional moult [24]. In AGS, an earlier snowmelt increases male's active season by only 8 days and does not change the time that they become euthermic and begin feeding in spring or when they enter hibernation. However, in areas with an earlier snowmelt, AGS emerge to an environment with increased food availability, which may in effect rescue poor-conditioned individuals. Clearly, it is important to understand how climate-induced seasonal variation will influence life histories of animals prior to making predictions on how it will impact body condition.
5. Conclusion
Because of the inherently different life-history strategies of males and females, resulting in different selection pressures, the two sexes will probably respond differently to sustained changes in environmental conditions. Furthermore, their phenological response to change may differ if the timing of seasonal events is set by different environmental cues. We show that males emerge from hibernation 12 days before females, which may be important for males to assess competition, acquire breeding territories and locate females. However, in areas with earlier snowmelt males spend less time sequestered in the burrows at high body temperature prior to emergence; thus they emerge at an earlier stage of reproductive maturation, and may be less physiologically prepared for breeding when females first emerge. The timing of when males return to high body temperature in spring is influenced by spring soil temperatures and an endogenous circannual clock set the previous summer through photoperiodic entrainment, while emergence date appears to be flexible relative to female emergence date. Conversely, in females the return to high body temperature and timing of emergence, which occur nearly at the same time, does not seem to be affected by spring soil temperature [49] and may be influenced by the timing of spring snowmelt and winter snow-cover [29]. Given that reproduction relies on appropriate interactions between the sexes and this is the driving force behind all biological decisions, understanding how climate change will impact each sex and their interactions is of critical importance.
Acknowledgement
We thank Franzisca Kohl and all the Team Squirrel volunteers and assistants who helped with fieldwork. Procedures were approved by the University of Alaska Fairbanks Institutional Animal Care and Use Committee, and fieldwork was conducted under Alaska state permits.
Funding statement
Funding was provided by the National Science Foundation (0732763, 0732755, 1147232 and 1147187) to C.L.B. and B.M.B., US Army Medical Research and Materiel Command grant no. 05178001 to B.M.B., Natural Sciences and Engineering Research Council Post-Doctoral Fellowship to M.J.S.
References
- 1.Serreze MC, et al. 2000. Observational evidence of recent change in the northern high latitude environment. Clim. Change 46, 159–207 (doi:10.1023/A:1005504031923) [Google Scholar]
- 2.Hinzman LD, et al. 2005. Evidence and implications of recent climate change in northern Alaska and other Arctic regions. Clim. Change 72, 251–290 (doi:10.1007/s10584-005-5352-2) [Google Scholar]
- 3.ACIA 2005. Arctic climate impact assessment. Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Trenberth KE, et al. 2007. Observations: surface and atmospheric climate change. In Climate change 2007: The physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change (eds Solomon S, Qin D, Manning M, Chen Z, Marquis M, Everyt KB, Tignor M, Miller HL.), pp. 236–336 Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Post E, et al. 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355–1358 (doi:10.1126/science.1173113) [DOI] [PubMed] [Google Scholar]
- 6.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (doi:10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 7.IPCC 2007. Climate Change 2007: Mitigation of climate change. In Contribution of Working Group III to the fourth assessment report of the Intergovernmental Panel on Climate Change, 2007 (eds Metz B, Davidson OR, Bosh PR, Dave R, Meyer LA.). Cambridge, UK: Cambridge University Press, pp. 1–851 [Google Scholar]
- 8.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Høgh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395 (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 9.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 10.Root TL, Price JL, Hall KR, Schneider SH, Rosenzweig C, Pounds AJ. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (doi:10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 11.Walker MD, et al. 1999. Long-term experimental manipulation of winter snow regime and summer temperature in Arctic and alpine tundra. Hydrol. Process. 13, 2315–2330 (doi:10.1002/(SICI)1099-1085(199910)13:14/15<2315::AID-HYP888>3.0.CO;2-A) [Google Scholar]
- 12.Starr G, Oberbauer SF, Pop EW. 2000. Effects of lengthened growing season and soil warming on the phenology and physiology of Polygnum bistorta. Glob. Change Biol. 6, 357–369 (doi:10.1046/j.1365-2486.2000.00316.x) [Google Scholar]
- 13.Borner AP, Kielland K, Walker MD. 2008. Effects of simulated climate change on plant phenology and nitrogen mineralization in Alaskan Arctic tundra. Arct. Antarct. Alp. Res. 40, 27–38 (doi:10.1657/1523-0430(06-099)[BORNER]2.0.CO;2) [Google Scholar]
- 14.Cooper EJ, Dullinger S, Semenchuk P. 2011. Late snowmelt delays plant development and results in lower reproductive success in the high Arctic. Plant Sci. 180, 157–167 (doi:10.1016/j.plantsci.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 15.Høye TT, Post E, Meltofte H, Schmidt NM, Forschhammer MC. 2007. Rapid advancement of spring in the high Arctic. Curr. Biol. 17, R449–R451 (doi:10.1016/j.cub.2007.04.047) [DOI] [PubMed] [Google Scholar]
- 16.Sheriff MJ, Kenagy GJ, Richter M, Lee T, Tøien Ø, Kohl F, Buck CL, Barnes BM. 2011. Phenological variation in annual timing of hibernation and breeding in nearby population of Arctic ground squirrels. Proc. R. Soc. B 278, 2369–2375 (doi:10.1098/rspb.2010.2482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iler AM, Høye TT, Inouye DA, Schmidt NM. 2013. Nonlinear flowering responses to climate: are species approaching their limits of phenological change? Phil. Trans. R. Soc. B 368, 20120489 (doi:10.1098/rstb.2012.0489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cresswell W, McCleery R. 2003. How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. J. Anim. Ecol. 72, 356–366 (doi:10.1046/j.1365-2656.2003.00701.x) [Google Scholar]
- 19.Both C, Visser ME. 2005. The effect of climate change on the correlation between avian life-history traits. Glob. Change Biol. 11, 1606–1613 (doi:10.1111/j.1365-2486.2005.01038.x) [Google Scholar]
- 20.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. 2010. The effects of phenological mismatches on demography. Phil. Trans. R. Soc. B 365, 3177–3186 (doi:10.1098/rstb.2010.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485 (doi:10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed TE, Jenouvrier S, Visser ME. 2012. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J. Anim. Ecol. 82, 131–144 (doi:10.1111/j.1365-2656.2012.02020.x) [DOI] [PubMed] [Google Scholar]
- 23.Post E, Forchhammer MC. 2008. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2369–2375 (doi:10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Høye TT, Hammel JU, Fuchs T, Toft S. 2009. Climate change and sexual size dimorphism in an Arctic spider. Biol. Lett. 5, 542–544 (doi:10.1098/rsbl.2009.0169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLean IG, Towns AJ. 1981. Differences in weight changes and the annual cycle of male and female Arctic ground squirrels. Arctic 34, 249–254 [Google Scholar]
- 26.Buck CL, Barnes BM. 1999. Temperatures of hibernacula and changes in body composition of Arctic ground squirrels over winter. J. Mammal. 80, 1264–1276 (doi:10.2307/1383177) [Google Scholar]
- 27.Barnes BM, Kretzmann M, Licht P, Zucker I. 1986. The influence of hibernation on testis growth and spermatogenesis in the golden-mantled ground squirrel, Spermophilus lateralis. Biol. Reprod. 35, 1289–1297 (doi:10.1095/biolreprod35.5.1289) [DOI] [PubMed] [Google Scholar]
- 28.Barnes BM. 1996. Relationships between hibernation and reproduction in male ground squirrels. In Adaptations to the Cold: Tenth International Hibernation Symposium (eds Geiser F, Hulbert AJ, Nicol SC.), pp. 71–80 Armidale, Australia: University of New England Press [Google Scholar]
- 29.Sheriff MJ, Buck CL, Barnes BM. In press. Phenological consequences of an early spring: autumn as a back seat driver. J. Anim. Ecol. [Google Scholar]
- 30.Carl EA. 1971. Population control in Arctic ground squirrels. Ecology 52, 395–413 (doi:10.2307/1937623) [Google Scholar]
- 31.Lacey EA. 1991. Reproductive and dispersal strategies of male Arctic ground squirrels (Spermophilus parryii plesius). PhD Thesis University of Michigan, Ann Arbor, MI, USA [Google Scholar]
- 32.Buck CL, Barnes BM. 2003. Androgen in free-living Arctic ground squirrels: seasonal changes and influence of staged male-male aggressive encounters. Horm. Behav. 43, 318–326 (doi:10.1016/S0018-506X(02)00050-8) [DOI] [PubMed] [Google Scholar]
- 33.Shaver GR, Chapin F, III, Gartner BL. 1986. Factors limiting seasonal growth and peak biomass accumulation in Eriophorum vaginatum in Alaska tussock tundra. J. Ecol. 74, 257–278 (doi:10.2307/2260362) [Google Scholar]
- 34.Buck CL, Barnes BM. 1999. Annual cycle of body composition and hibernation in free-living Arctic ground squirrels. J. Mammal. 80, 430–442 (doi:10.2307/1383291) [Google Scholar]
- 35.Williams CT, Sheriff MJ, Schmutz JA, Kohl F, Tøien Ø, Buck CL, Barnes BM. 2011. Data logging of body temperatures provides precise information on phenology of reproductive events in a free-living Arctic hibernator. J. Comp. Physiol. B 181, 1101–1109 (doi:10.1007/s00360-011-0593-z) [DOI] [PubMed] [Google Scholar]
- 36.Long RA, Hut RA, Barnes BM. 2007. Simultaneous collection of body temperature and activity data in burrowing mammals: a new technique. J. Wildl. Manag. 71, 1375–1379 (doi:10.2193/2006-399) [Google Scholar]
- 37.Stearns SC. 1976. Life history tactics: a review of the ideas. Q. Rev. Biol. 5, 3–47 (doi:10.1086/409052) [DOI] [PubMed] [Google Scholar]
- 38.Kerby J, Post E. 2013. Capital and income breeding traits differentiate trophic match–mismatch dynamics in large herbivores. Phil. Trans. R. Soc. B 368, 20120484 (doi:10.1098/rstb.2012.0484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes BM, York AD. 1990. Effects of winter high temperatures on reproduction and circannual rhythms in hibernating ground squirrels. J. Biol. Rhythms 5, 119–130 (doi:10.1177/074873049000500204) [DOI] [PubMed] [Google Scholar]
- 40.Barnes BM. 1984. Influence of energy stores on activation of reproductive function in male golden-mantled ground squirrels. J. Comp. Physiol. B 154, 421–425 (doi:10.1007/BF00684449) [Google Scholar]
- 41.Both C, van Asch M, Bijlsma RG, van den Burg AB, Visser ME. 2009. Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J. Anim. Ecol. 78, 73–83 (doi:10.1111/j.1365-2656.2008.01458.x) [DOI] [PubMed] [Google Scholar]
- 42.Bronson FH. 2009. Climate change and seasonal reproduction in mammals. Phil. Trans. R. Soc. B 364, 3331–3340 (doi:10.1098/rstb.2009.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walton JC, Weil ZM, Nelson RJ. 2011. Influence of photoperiod on hormones, behavior, and immune function. Front. Neuroendorcrinol. 32, 303–319 (doi:10.1016/j.yfrne.2010.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helm B, Ben-Schlomo R, Sheriff MJ, Hut R, Foster R, Barnes BM, Dominoni D. 2013. Annual rhythms that underlie phenology: biological timekeeping meets environmental change. Proc. R. Soc. B 280, 20130016 (doi:10.1098/rspb.2013.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gower BA, Nagy TR, Stetson MH. 1997. Alteration of testicular response to long photoperiod by transient exposure to short photoperiod in collard lemmings (Dicrotonyx groenlandicus). J. Reprod. Fertil. 109, 257–262 (doi:10.1530/jrf.0.1090257) [DOI] [PubMed] [Google Scholar]
- 46.Gwinner E. 1996. Circadian and circannual programmes in avian migration. J. Exp. Biol. 199, 39–48 [DOI] [PubMed] [Google Scholar]
- 47.Zucker I. 2001. Circannual rhythms. In Handbook of behavioural neurobiology, vol. 12 (eds Takalhashi S, Turek FW, Moore RY.), pp. 509–528 New York, NY: Kluwer Academic [Google Scholar]
- 48.Lee TM, Zucker I. 1991. Suprachiasmatic nucleus and photic entrainment of circannual rhythms in ground squirrels. J. Biol. Rhythms 6, 315–330 (doi:10.1177/074873049100600403) [DOI] [PubMed] [Google Scholar]
- 49.Williams CT, Sheriff MJ, Kohl F, Barnes BM, Buck CL. 2012. Interrelationships among timing of hibernation, reproduction, and warming soil in free-living female Arctic ground squirrels. In Living in a seasonal world: thermoregulatory and metabolic adaptations (eds Ruf T, Bieber C, Arnold W, Millesi E.), pp. 63–72 Berlin, Germany: Springer [Google Scholar]
- 50.Jenni L, Kéry M. 2003. Timing of autumn bird migration under climate change: advances in long-distance migrants, delays in short distance migrants. Proc. R. Soc. Lond. B 270, 1467–1471 (doi:10.1098/rspb.2003.2394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skogland T. 1989. Comparative social organization of wild reindeer in relation to food, mates and predator avoidance. Adv. Ethol. 29, 1–74 [Google Scholar]
- 52.Post E. 2003. Timing of reproduction in large mammals. Climate and density-dependent influences. In Phenology: an integrative environmental science (ed. Schwartz MD.), pp. 437–449 Dordrecht, The Netherlands: Kluwer Academic [Google Scholar]
- 53.Morton ML, Maxwell CS, Wade CE. 1974. Body size, body composition, and behavior of juvenile Belding's ground squirrels. Great Basin Natural 34, 121–134 [Google Scholar]
- 54.Sheriff MJ, Williams CT, Kenagy GJ, Buck CL, Barnes BM. 2012. Thermoregulatory changes anticipate hibernation onset by 45 days: data from free-living Arctic ground squirrels. J. Comp. Physiol. B 182, 841–847 (doi:10.1007/s00360-012-0661-z) [DOI] [PubMed] [Google Scholar]