Abstract
Arctic wildlife is often presented as being highly at risk in the face of current climate warming. We use the long-term (up to 24 years) monitoring records available on Bylot Island in the Canadian Arctic to examine temporal trends in population attributes of several terrestrial vertebrates and in primary production. Despite a warming trend (e.g. cumulative annual thawing degree-days increased by 37% and snow-melt date advanced by 4–7 days over a 23-year period), we found little evidence for changes in the phenology, abundance or productivity of several vertebrate species (snow goose, foxes, lemmings, avian predators and one passerine). Only primary production showed a response to warming (annual above-ground biomass of wetland graminoids increased by 123% during this period). We nonetheless found evidence for potential mismatches between herbivores and their food plants in response to warming as snow geese adjusted their laying date by only 3.8 days on average for a change in snow-melt of 10 days, half of the corresponding adjustment shown by the timing of plant growth (7.1 days). We discuss several reasons (duration of time series, large annual variability, amplitude of observed climate change, nonlinear dynamic or constraints imposed by various rate of warming with latitude in migrants) to explain the lack of response by herbivores and predators to climate warming at our study site. We also show how length and intensity of monitoring could affect our ability to detect temporal trends and provide recommendations for future monitoring.
Keywords: tundra food web, climate change, ecological response, long-term monitoring, Arctic vertebrates, trophic interaction
1. Introduction
The planet is currently warming and it is in the polar regions that this warming is proceeding at the fastest pace [1–3]. The effects of climate warming on the physical environment of the polar regions are well documented and include melting of icecaps and glaciers, deepening of the summer thawing of permafrost and decrease in snow and sea-ice cover [2,4–7]. Although some studies have documented similar impacts on Arctic biological systems [8–12], evidence remains limited and generally biased toward lower trophic levels (e.g. plants).
Arctic wildlife is often presented as being highly at risk in the face of current climate warming [1,13,14]. However, studies documenting clear, unequivocal impact of warming on Arctic wildlife are still scant, though exceptions exist (e.g. impact of receding sea ice on southern populations of polar bears, Ursus maritimus [15–17]). Several reasons may explain the paucity of evidence. First, the most studied taxa of tundra wildlife are endothermic animals, which are generally buffered from the direct impacts of temperature changes, unlike lower trophic levels (i.e. plants or detritivores). Second, animals show phenotypic plasticity that allows them to cope with short-term environmental changes, though within some limits [18]. Third, the majority of breeding species are long-distance migrants that only come to the Arctic during the summer. For the rest of the year, they are exposed to different climatic regimes that may be changing at a different pace than in the Arctic [19,20]. Fourth, considering the large inter-annual variability in climate and the multiple drivers of animal populations besides climatic conditions [21–23], detecting temporal trends in animal populations (or their attributes, e.g. phenology) requires long-term datasets, which are rare in the Arctic [24].
We argue that a sound assessment of the impact of climate warming on Arctic wildlife requires three key elements. First, it must be based on long-term datasets. Second, it requires the intensive monitoring of several population attributes, in particular population size, demography and phenology. Third, it should involve multiple species and ideally key players at all levels of the food web (i.e. plants, herbivores and predators). The last point is important because, for endotherms inhabiting the tundra, it has been suggested that the most severe impact of climate warming may be indirect, through alterations in trophic interactions such as change in food availability, predation pressure, apparition of new competitors or mismatch between trophic levels [25–27]. Therefore, a food web approach is essential in order to fully understand the impact of climate warming on individual species [28].
Long-term monitoring of wildlife populations are especially scant in the vast Canadian Arctic but one site where such records are available is Bylot Island, Nunavut. This high Arctic site is located in the eastern Canadian Arctic Archipelago and several key components of the tundra food web have intensively been monitored during the past two decades [29–31]. Like many other Arctic sites, this region has experienced warming for the past 40 years [30]. We use the long-term records available at this site to first examine temporal trends in population attributes (primarily phenology, abundance and productivity) of several herbivorous, insectivorous and carnivorous species of terrestrial birds and mammals, as well as in primary production. Second, we determine to what extent climatic conditions (primarily temperature and snow) can explain annual variation in these population attributes and, when detected, in temporal trends, and we discuss how this should impact these populations and ultimately the whole food web in the future. This also leads us to emphasize the difficulties associated with the detection of temporal trends or the attribution of annual variations and temporal trends to climatic variation. Third, considering the costs and efforts required to maintain long-term programmes in the Arctic, we also investigate how length and intensity of monitoring could affect our conclusions and we provide some recommendations for future monitoring programmes.
2. Material and methods
(a). Study site
Bylot Island is an 11 100 km2 island located at the northern tip of Baffin Island, Nunavut, in the Canadian Arctic (figure 1). The island is dominated by mountains culminating at almost 2000 m and a large icecap except for the 1600 km2 South Plain, which is bordered to the north by the mountains and to the south by the sea (Navy Board Inlet and Eclipse Sound). This portion of the island is characterized by flat lowlands and upland plateaus dissected by valleys, with elevation generally below 350 (m).a.s.l. It is covered by relatively lush tundra vegetation for the latitude due to the generally southern exposure and the relative protection offered by the mountains from the cold, northerly winds. Average annual temperature is −15°C [32]. The coldest month of the year is February and the warmest is July (average temperature during these two months is −34°C and +6°C, respectively). Snow typically covers the ground from late September to mid-June.
Figure 1.
Localization of the study area. Light grey: South Plain of Bylot Island; horizontal hatch: Qarlikturvik Valley (main study area); vertical hatch: study area for foxes; black area: snow goose nesting colony.
We can recognize three main plant communities on Bylot Island, which are largely determined by soil moisture and elevation. Wetlands occur in low-lying areas such as along streams and shallow ponds and, most commonly, in low-centre tundra polygons created by the growth of ice wedges in the permafrost. These sites are typically moss-covered fens dominated by grasses and sedges such as Dupontia fisheri, Carex aquatilis and Eriophorum scheuchzeri. Mesic tundra covers most of the landscape on plateaus and gentle slopes and frequently forms hummocks. The most common plants of the mesic tundra are prostrate shrubs (Salix arctica, Vaccinium uliginosum) and forbs (e.g. Cassiope tetragona, Luzula spp.) along with some grasses (Arctagrostis latifolia, Poa arctica) and mosses. Finally, exposed areas with dry, gravel soil such as ridges or high-elevation sites have a very sparse vegetative cover consisting of only a few plant species such as Dryas integrifolia or Saxifraga oppositifolia [33,34].
The main herbivore at the study site during the summer is the colonial-nesting greater snow goose (Anser caerulescens atlanticus). During nesting, geese are restricted to a small area but during brood-rearing families disperse throughout the South Plain of the island [35]. Two species of small mammals are present, the brown (Lemmus trimucronatus) and collared (Dicrostonyx groenlandicus) lemmings. The former species has regular, high-amplitude cycles of abundance with a 3–4-year periodicity whereas the collared has weak, low-amplitude cycles [36]. Other herbivores such as the rock ptarmigan (Lagopus mutus) and Arctic hare (Lepus arcticus) are also present but in low numbers. Large mammalian herbivores such as muskox (Ovibos moschatus) or caribou (Rangifer tarandus) are absent. Insectivorous migratory birds are abundant and include a few passerines (mostly Lapland longspur, Calcarius lapponicus) and several species of shorebirds (primarily Calidris spp. and Pluvialis spp. [30]). The mammalian predator guild is dominated by two species that are year-round residents, the Arctic fox (Vulpes lagopus) and the stoat (Mustela erminea). Red foxes (Vulves vulpes) have been present in low numbers in the region since the 1950s [37]. Numerous avian predators are present during the summer, especially the snowy owl (Bubo scandiacus), rough-legged hawk (Buteo lagopus), long-tailed jaeger (Stercorarius longicaudus), parasitic jaeger (S. parasiticus) and glaucous gull (Larus hyperboreus), though the abundance of the first three species can vary considerably with the phases of lemming cycle [38].
Our activities are conducted primarily at two sites on the island, the large Qarlikturvik Valley (approx. 100 km2; 73° 08′ N; 80° 00′ W), and a secondary site 30 km to the south (approx. 30 km2; 72° 53′ N, 79° 55′ W) in the centre of the large greater snow goose nesting colony (figure 1). However, some of the monitoring activities (e.g. for foxes and for some avian predators) encompass a 60-km long coastal area (approx. 520 km2) including these two sites.
(b). Field methods
We have maintained since 1994 a fully automated weather station operating year-round in the Qarlikturvik Valley (located at 20 (m).a.s.l.). The station recorded on an hourly basis air temperature and humidity (at 2 m), soil temperature (10 cm deep), wind speed and direction (at 3 m), snow depth and solar radiation [39]. Snow-melt was monitored from 1 June until snow disappearance by visually estimating snow cover in the Qarlikturvik Valley from a vantage point at 2-day intervals. We also retrieved weather data from the Environment Canada weather station located at the Pond Inlet airport on Baffin Island (72°41′ N, 77°59′ W; 80 km southeast from the Qarlikturvik Valley weather station). Weather data from our automated station and Pond Inlet over the period 1995–2004 were highly correlated and thus could be used to extend the time series of climatic data from our station (once properly adjusted, following the approach of Dickey et al. [40]). We also retrieved monthly values of the North Atlantic Oscillation (http://www.cpc.ncep.noaa.gov/products/precip/CWlink/pna/norm.nao.monthly.b5001.current.ascii.table) to look at temporal trends for the months of interest (May to September).
We have monitored the annual plant production and goose grazing impact in wetlands of the Qarlikturvik Valley since 1990. Each year, 12 new exclosures (1 × 1 m) made of chicken wire and spread over approximately 1 km2 area were installed in late June to prevent goose grazing. At the end of the plant growing season (i.e. mid-August), we sampled the vegetation by removing a 20 × 20 cm piece of turf inside and outside of each exclosure. Live above-ground plant biomass was cut, sorted out, dried and weighed. Live above-ground biomass inside exclosures in mid-August is a good measure of annual production of graminoids, which account for more than 90 per cent of the vascular plant biomass in wetlands [41]. Goose grazing impact was assessed by the difference in biomass inside and outside exclosures [42]. In some years, graminoids (E. scheuchzeri and D. fisheri) were also sampled inside exclosures at 10- to 14-day intervals and dried samples were analysed for nitrogen concentration to determine seasonal variations in plant nutritive quality (see [43] for details).
An annual index of lemming abundance in the Qarlikturvik Valley has been obtained in mid-July using snap-traps (greater than or equal to 1000 trap-nights each year) since 1993. Since 2004, monthly density of brown and collared lemmings has been estimated via capture–mark–recapture methods using live-trapping data from two 11-ha grids in the same area. Each grid had 144 traps laid out every 30 m in a 12 × 12 Cartesian plan (see [36,44] for details). Using the relationship between annual lemming density estimated from live recapture (N) in July and the abundance index (I) derived from snap-trapping over the period 2004–2011 (log(N) = 1.33 log(I) + 0.55; r2 = 0.82), we obtained a time series of annual lemming density from 1993 to 2012 [31].
We have monitored annually the reproductive activity of a large number of birds. The longest time series available are for the snow goose (since 1989), snowy owl (since 1993), Lapland longspur (since 1995) and the long-tailed jaeger (since 2004). Nests of these species were found during systematic searches of the study area, except for longspurs for which nests were found opportunistically. Because geese nest colonially, nest density in this case refers to the central portion of the colony that was monitored annually. Nests were revisited periodically to determine their content (clutch size), phenology (laying date) and success (number of young hatched or fledged). For snow geese, arrival date in early June was also monitored by counting birds on the first snow-free areas at 2-day intervals (see [29,45] for methods). These data provide information on annual abundance, phenology and reproductive success of these species.
We monitored fox dens opportunistically from 1993 to 2002 and systematically throughout the study area since 2003 [46]. Known dens were checked for breeding activity of Arctic and red foxes every year in summer [47]. Since 2003, adult and juvenile Arctic foxes were captured, marked with ear tags and sampled (blood and hairs) in summer for analysis of carbon and nitrogen isotopic ratios [48].
(c). Data analysis
The following variables were extracted from the field data. Cumulative thawing degree-days (TDD) was defined as the sum of the daily mean temperature above 0°C up to a given date. Daily snow depth values from the first snowfall until snow-melt were averaged to obtain mean winter snow depth (see [49] for details). Laying date was defined as the day that the first egg of a nest was laid and clutch size as the maximum number of eggs recorded in a nest after the start of incubation. Hatching success was the probability that at least one egg would hatch in a clutch and nesting success was the probability that at least one young would successfully leave the nest.
Doiron et al. [43] showed that date of 50 per cent maximum normalized difference vegetation index (NDVI) was a very good predictor of date of peak nitrogen concentration in graminoids at our study site. Because the former variable was measured during only 10 years, we extended the time series by retrieving annual NDVI values from our study site and applying the equation of Doiron et al. [43] to predict date of peak nitrogen concentration in graminoid plants from the date of 50 per cent maximum NDVI. However, NDVI was not a good predictor of the actual peak nitrogen concentration and thus we could not extend the time series for the latter variable.
For birds, abundance estimates are based on nest density and thus incorporate only the breeding segment of the population. In some species, annual variation in reproductive effort can be large and may depend on abiotic (e.g. temperature in spring for geese [40]) or biotic factors (e.g. lemming abundance for predators [29]). Therefore, variation in breeding density may not always be indicative of genuine change in population size. In the case of the snowy owl, as breeding occurs only when lemming abundance is high and non-breeding adults are rarely seen [29], we excluded years without nesting owls from the analysis.
Arctic fox productivity is defined as the proportion of monitored dens where an Arctic fox litter emerged (natal dens). The nitrogen isotopic ratio in fox blood is used here as a trophic index, assuming that higher δ15N generally indicates foraging at higher trophic levels [50]. On Bylot Island, a higher δ15N also reflects stronger reliance of foxes on marine food [48].
We used linear regression to examine temporal trends in the data. However, temporal series are often subject to autocorrelation problems, which could bias the parameter estimates of the regression [51]. To test for that, we computed the Durbin–Watson statistic and we adjusted first- and second-order autoregressive models (procedure autoreg in SAS, [52]). We used the AICc to determine whether a model with autoregressive terms fitted our data better than a simple regression model. We present parameter values of the best fitting model (i.e. either with or without the autoregressive terms). When needed, data were log-transformed (or arcsine-transformed for proportion) to respect normality and homogeneity of variance.
Data used in this paper have been deposited in the Polar Data Catalogue (http://www.polardata.ca).
3. Results
(a). Climate
Average monthly temperature over the past 24 years showed a strong warming trend in both spring and summer on Bylot Island (see the electronic supplementary material, figure S1). The increasing trends ranged from 0.3°C per decade in June to 1.1°C per decade in May and August. The average number of frost-free days also increased from 97 in 1989 to 106 in 2011 (see the electronic supplementary material, figure S2) and the average annual cumulative number of TDD by 37 per cent (from 381 to 521 TDD; figure 2). Cumulative TDD to 15 August, when we sampled plants, showed the same trend. Temperature at nearby Pond Inlet revealed similar warming trends over the same period with generally steeper slopes (see the electronic supplementary material, table S1). However, annual mean ground temperature at 10 cm did not show any trend on Bylot Island (β = 0.002°C per year; overall mean, −10.9°C).
Figure 2.
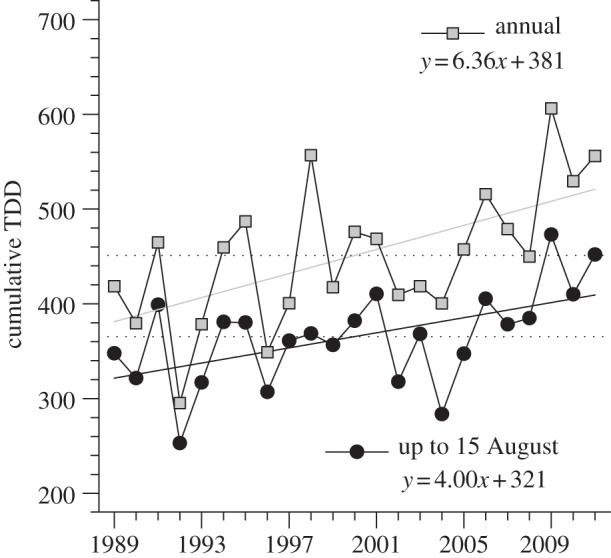
Cumulative thawing degree-days (TDD = degree-days above 0°C) in the Qarlikturvik Valley of Bylot Island from 1989 to 2011. The dotted lines show the mean for the whole period. The regression lines show the temporal trends (1989 = year 0 in the regression equations).
The North Atlantic Oscillation (NAO), a large-scale climatic index that has previously been shown to affect wildlife populations on Bylot Island [40,53], has changed during the spring and summer over the same time period. Average monthly NAO from May to September showed a strong decreasing trend over the past 24 years (see the electronic supplementary material, table S1). On average, it decreased from 0.36 in 1989 to −0.77 in 2012. Negative NAO values are associated with warm temperature on Bylot Island [53], which fits well with the observed warming trend.
Mean winter snow depth increased by 48 per cent from 1994 to 2010 (figure 3a). Despite this deeper snow cover, there was a trend for an advance in snow-melt; average date at which the ground was 50 per cent snow-free advanced by 4.1 days from 1989 to 2012 (figure 3b). However, one point (i.e. the very early snow-melt in 1993) is highly influential in this relationship; excluding this point, the advance in snow-melt date was 6.6 days.
Figure 3.
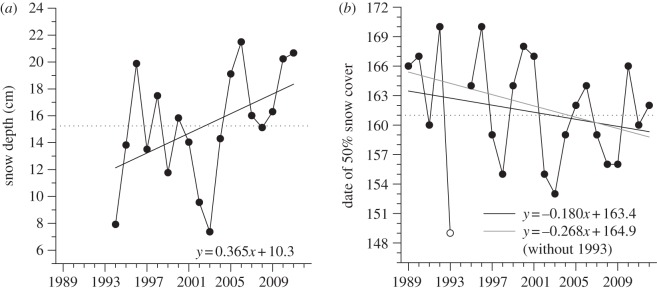
(a) Mean annual winter snow depth (cm) and (b) date of 50% snow cover (day of the year) in the Qarlikturvik Valley of Bylot Island from 1989 to 2012. The dotted lines show the mean for the whole period. The regression lines show the temporal trends (1989 = year 0 in the regression equations).
(b). Phenology
Date of peak nitrogen concentration of graminoid plants showed large inter-annual variability but no clear temporal trend (table 1 and figure 4a). Despite the trend for warmer spring and earlier snow-melt in recent years, we found little evidence for changes in the phenology of nesting birds. Laying date of snow geese, the species for which we had the longest record (24 consecutive years), did not show any temporal trend (figure 5a). We found similar results for snowy owl and Lapland longspur laying dates (table 1). Peak arrival date of snow geese in spring showed a weak trend to occur later over the past 17 years (2.0 days per decade) but the relationship was not statistically significant (table 1).
Table 1.
Temporal trends in biological parameters monitored on Bylot Island. The t-statistic is for the slope parameter of interest. Autoregressive terms (ARs) were retained in the model when significant. The r2 is only for the regression parameter of interest and excludes the autoregressive component when significant (1989 = year 0 in the regression equations). n.s., non-significant (p > 0.05).
| variable | period | n | mean | CV | r2 | intercept | slope | s.e. | t | p | AR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| phenology | |||||||||||
| graminoids—datea of peak nitrogen concentration | 1991–2010 | 18 | 181 | 3.60 | 0.006 | 182 | −0.089 | 0.290 | 0.31 | 0.764 | n.s. |
| snow geese—peak arrival datea | 1996–2012 | 17 | 161 | 3.27 | 0.037 | 163 | −0.201 | 0.264 | 0.76 | 0.458 | n.s. |
| snow geese—median laying datea | 1989–2012 | 24 | 164 | 2.07 | 0.009 | 163 | 0.046 | 0.100 | 0.46 | 0.651 | n.s. |
| Lapland longspur—median laying datea | 2000–2012 | 13 | 169 | 2.28 | 0.004 | 168 | 0.066 | 0.298 | 0.22 | 0.829 | n.s. |
| snowy owl—median laying datea | 1993–2010 | 7 | 141 | 3.34 | 0.004 | 142 | −0.045 | 0.328 | 0.14 | 0.896 | n.s. |
| abundance | |||||||||||
| graminoids—primary production (g m−2) | 1990–2012 | 22 | 49.5 | 31.2 | 0.343 | 29.0 | 1.731 | 0.558 | 3.10 | 0.006 | AR1e |
| brown lemming–summer density (nb ha−1)b | 1993–2012 | 20 | 4.74 | 147 | 0.075 | 1.84 | −0.051 | 0.042 | 1.21 | 0.243 | n.s. |
| brown lemming—summer density (3-year running mean, nb ha−1)b | 1993–2012 | 18 | 4.24 | 73.4 | 0.147 | 2.13 | −0.050 | 0.030 | 1.66 | 0.116 | n.s. |
| snow geese—nest density (nb ha−1) | 1993–2012 | 19 | 4.17 | 58.0 | 0.099 | 2.20 | 0.132 | 0.097 | 1.37 | 0.190 | n.s. |
| long-tailed jaeger—nest density (nb ha−1) | 2004–2012 | 8 | 0.61 | 54.5 | 0.083 | 0.42 | 0.007 | 0.011 | 0.67 | 0.530 | AR1,2f |
| snowy owl—nest density (nb km−2)c | 1993–2010 | 7 | 0.11 | 84.3 | 0.362 | 0.23 | −0.009 | 0.005 | 1.69 | 0.153 | n.s. |
| productivity | |||||||||||
| snow geese—clutch size | 1989–2012 | 24 | 3.7 | 8.54 | <0.001 | 3.7 | 0.0003 | 0.009 | 0.03 | 0.978 | n.s. |
| snow geese—nesting successd | 1989–2012 | 24 | 0.66 | 32.1 | 0.015 | 0.91 | 0.004 | 0.007 | 0.58 | 0.568 | n.s. |
| snow geese—young-adult ratio near fledging | 1990–2012 | 23 | 1.04 | 22.5 | 0.009 | 1.08 | −0.003 | 0.007 | 0.43 | 0.673 | n.s. |
| Lapland longspur—clutch size | 1995–2012 | 18 | 5.4 | 5.17 | 0.009 | 5.5 | −0.005 | 0.013 | 0.38 | 0.708 | n.s. |
| Lapland longspur—nesting success | 1995–2012 | 18 | 0.48 | 49.0 | 0.049 | 0.61 | −0.010 | 0.010 | 0.91 | 0.375 | n.s. |
| Lapland longspur—hatching success | 1995–2012 | 18 | 0.64 | 31.7 | 0.010 | 0.58 | 0.004 | 0.009 | 0.40 | 0.696 | n.s. |
| snowy owl—clutch size | 1993–2010 | 7 | 7.0 | 8.79 | 0.523 | 7.9 | −0.069 | 0.030 | 2.34 | 0.064 | n.s. |
| Arctic fox—proportion of natal dens | 1995–2012 | 18 | 0.12 | 0.73 | 0.048 | 0.07 | 0.003 | 0.004 | 0.82 | 0.380 | n.s. |
| other attributes | |||||||||||
| Graminoids—peak nitrogen concentration (%) | 1991–2010 | 10 | 3.12 | 10.5 | 0.027 | 3.21 | −0.007 | 0.016 | 0.47 | 0.653 | n.s. |
| goose—grazing impact (% biomass removed) | 1990–2012 | 22 | 30.4 | 54.0 | 0.339 | 48.3 | −1.438 | 0.449 | 3.20 | 0.005 | n.s. |
| Arctic fox—isotopic signature (δ15N, ‰) | 2003–2011 | 9 | 8.42 | 18.4 | 0.062 | 11.0 | −0.141 | 0.207 | 0.682 | 0.517 | n.s. |
aDate expressed in day of the year (1 January = 1).
bData were transformed (ln(y)) for regression analysis.
cExclude years where snowy owls did not nest due to low lemming abundance.
dData were arcsine-transformed for regression analysis.
eFirst-order autoregressive coefficient significant (AR1 = −0.55, p = 0.011).
fFirst- and second-order autoregressive coefficients significant (AR1 = 1.09, p = 0.006; AR2 = 0.91, p = 0.003).
Figure 4.
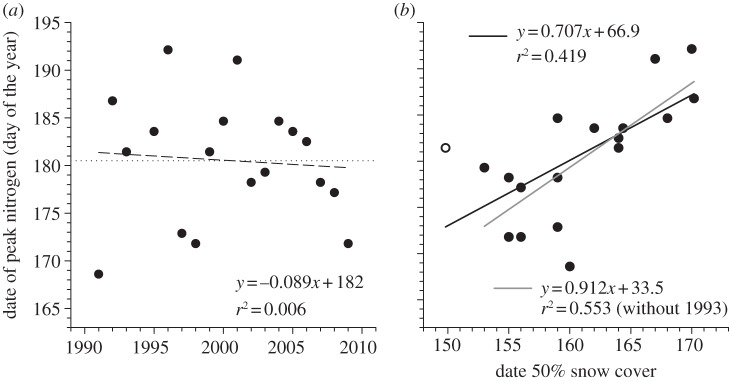
(a) Annual date of peak nitrogen concentration (day of the year) in wetland gramioids and (b) relationship between date of peak nitrogen concentration and date of 50%snow cover (day of the year) in the Qarlikturvik Valley of Bylot Island from 1990 to 2010. The dotted line shows the mean for the whole period. The regression lines show the temporal trends (1989 = year 0 in the regression equations). The white dot in (b) is year 1993.
Figure 5.
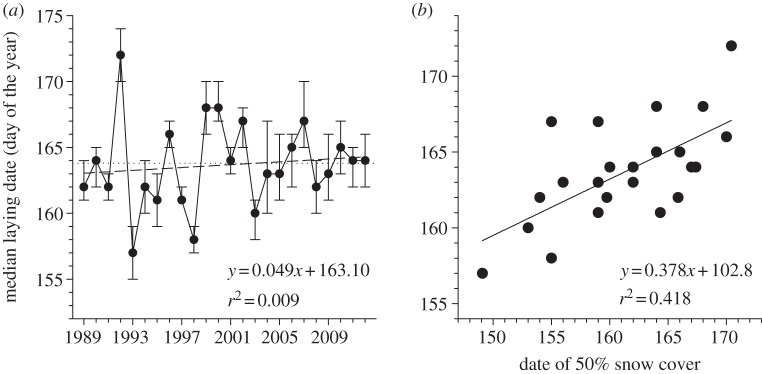
(a) Annual median goose laying date (day of the year) with 25% and 75% percentiles and (b) relationship between median goose laying date and date of 50% snow cover (day of the year) in the Qarlikturvik Valley of Bylot Island from 1989 to 2012. The dotted line shows the mean for the whole period. The regression lines show the temporal trends (1989 = year 0 in the regression equation with year).
Considering the lack of temporal trends in our phenological data, we examined how inter-annual variability in the timing of snow-melt affected phenology, focusing on the datasets for which we had the longest record, snow geese and their graminoid food plants. There was a positive relationship between the date of 50 per cent snow cover in spring and annual median laying date of geese (F1,21 = 15.1, p < 0.001, figure 5b) but the slope was only 0.38 (95% CI = 0.18–0.58), significantly less than unity. Geese undercompensated since, for a change in snow-melt of 10 days, they adjusted their laying date by only 3.8 days. Date of peak nitrogen concentration in plants was also related to date of 50 per cent snow cover (F1,16 = 11.5, p = 0.004) but this time the slope did not differ significantly from unity (β = 0.71, 95% CI = 0.27–1.15; when excluding 1993, an outlier, β = 0.91, 95% CI = 0.46–1.36; figure 4b). Thus, contrary to geese, the timing of plant growth appears to track rather well the timing of snow-melt in spring.
(c). Abundance
Primary production in wetlands showed a strong and unequivocal increasing trend over time. Average above-ground graminoid biomass increased by 123 per cent over 23 years (table 1 and figure 6a). Annual variation in plant biomass was associated with summer climatic conditions. Indeed, graminoid biomass was positively related to cumulative TDD until 15 August (r2 = 0.285, F1,19 = 7.95, p = 0.011, y = 0.149x − 7.15) and negatively to the July–August NAO (r2 = 0.409, F1,19 = 14.6, p = 0.001, y = −13.3x + 46.1).
Figure 6.
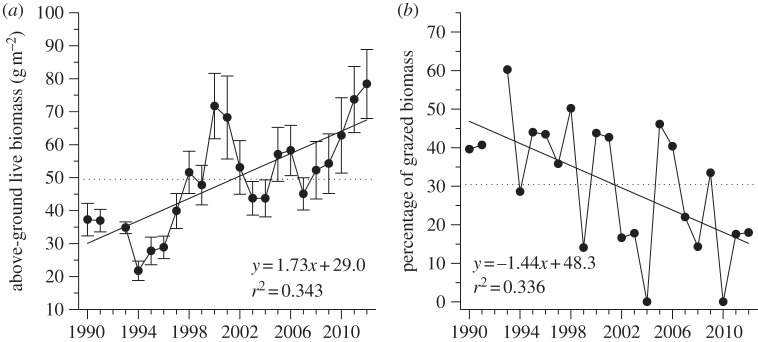
(a) Annual above-ground production of wetland graminoid plants (mean ± s.e.) and (b) percentage of above-ground biomass of wetland graminoid plants removed by geese in the Qarlikturvik Valley of Bylot Island from 1990 to 2012. The dotted lines show the mean for the whole period. The regression lines show the temporal trends (1989 = year 0 in the regression equations).
There was little evidence of a temporal trend in lemming density (table 1) but the large inter-annual variability in density due to cyclic population fluctuations makes the detection of such a trend difficult (figure 7). To smooth out those variations, we looked at the 3-year running mean of density. This analysis suggested a possible decreasing trend in summer lemming density (table 1). Lemming populations were especially low during the period 2002–2009 but we note that the most recent peak (2011) was relatively high (figure 7). Characteristic of the snow cover can account for some of the annual variations in lemming density at our study site [49].
Figure 7.
Annual abundance of two lemming species estimated by snap-trapping (up to 2003) and live-trapping (after 2003) in the Qarlikturvik Valley of Bylot Island from 1993 to 2012. The inset shows the 3-year running mean abundance for brown lemmings over the same period. The regression line shows the temporal trend (1989 = year 0 in the regression equations).
Annual nesting density of snow geese and long-tailed jaegers showed no temporal change (table 1). Although not statistically significant, nesting snowy owl abundance tended to decrease over time, which is consistent with the apparent decreasing trend in lemming density (table 1).
(d). Productivity
In snow geese, the clutch size, nesting success and ratio of young to adults near fledging did not show any temporal trend (table 1). Similarly, no temporal trend in the clutch size or nesting and hatching success of Lapland longspurs was detected. There was evidence for a decreasing trend in snowy owl clutch size (a reduction of 0.7 egg per decade or 10% over 18 years) though this was based on only 7 years of data (table 1). However, snowy owl clutch size was not related to lemming density (F1,5 = 0.85, p = 0.400).
The productivity of Arctic foxes did not show any significant temporal trend (table 1 and figure 8). Productivity was strongly linked to annual lemming abundance (r2 = 0.619, F1,16 = 26.3, p < 0.001, y = 0.080 log(x) + 0.11) and thus showed large fluctuations according to the phase of the lemming cycle. Annual fox productivity was not related to climatic variables such as the timing of snow-melt and the number of TDD. We also tested for a temporal trend while accounting for the effect of lemming abundance on fox productivity, but there was still little evidence of change over time despite a slight positive trend (r2 = 0.113, F1,16 = 2.03, p = 0.17, y = 0.0046x − 0.066).
Figure 8.
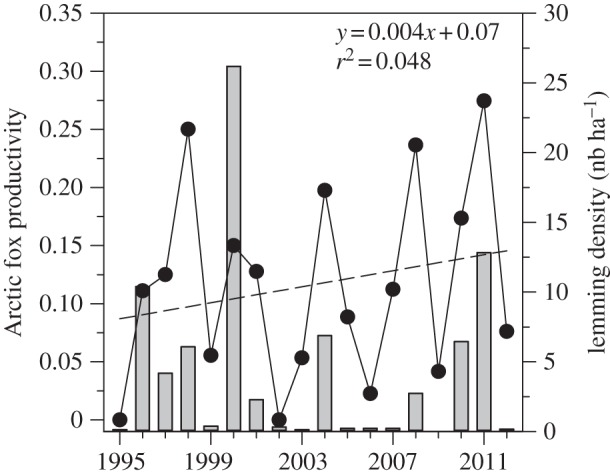
Annual productivity of Arctic foxes (proportion of monitored dens with fox litter) on Bylot Island from 1995 to 2012. The regression line shows the temporal trend in productivity (1989 = year 0 in the regression equations). Grey bars show annual lemming density.
(e). Other attributes
The nitrogen concentration of graminoid plants at its peak did not show any temporal trend for the 10 years of data available spanning a 20-year period (table 1). Goose grazing impact lessened over time as the average percentage of wetland graminoids consumed by snow geese decreased from 47 per cent in 1990 to 15 per cent in 2012 (table 1, figure 6b), although the absolute amount of biomass grazed did not change over time (r2 = 0.003, F1,20 = 0.07, p = 0.79).
The annual Arctic fox trophic level, as indexed by its 15N isotopic signature, was highly variable among years and did not show any temporal trend over a 9-year period (table 1). This large annual variability was due to lemming cycles as lemming abundance was strongly linked to this index (r2 = 0.818, F1,7 = 31.4, p < 0.001, y = −1.65 log(x) + 8.31).
At the community level, the number of red fox natal dens did not change through time, since we never detected more than one pair of red fox breeding in the study area and their presence (in 1996, 1998–2001, 2004, 2008 and 2011–2012) was relatively stable.
(f). Length and intensity of monitoring
To explore how length or intensity of monitoring could have affected our ability to detect significant biological trends, we re-analysed subsets of the most significant temporal trend that we detected, namely the increase in graminoid biomass in wetlands (figure 6a). Significant positive trends were still detected when the length of monitoring was reduced by half (11 years), though slopes were inflated (see the electronic supplementary material, table S2). However, when monitoring was reduced to 7 or 8 years, a significant positive trend was detected only in the most recent period whereas the middle period showed an almost significant negative trend. Sampling only every other year would have yielded similar results to those obtained with annual monitoring.
4. Discussion
The most surprising outcome of our analyses has been that, generally, we found little evidence for changes in the phenology, abundance or productivity of several species of vertebrates on Bylot Island despite the warming trend that has affected this area over the past three decades. The only clear change that we detected in the food web over the past 20 years has been with primary production, which more than doubled in wetland vascular plants. Similar increases in plant production have recently been reported elsewhere in the Canadian Arctic [8–10]. These increases are probably linked to improved growth conditions brought by climate warming as we found a strong association between annual climatic conditions (both at local and regional scales) and plant biomass on Bylot Island.
Why, then, are we not detecting temporal changes in population attributes of higher trophic levels (i.e. herbivores and predators) at our study site, especially considering the relatively large spatial scale of our monitoring? Phenology, for instance, has been shown to respond rapidly to climate warming in many areas [54–56] even sometimes at the genetic level [57]. We offer several explanations to this apparent paradox.
First, our time series are perhaps too short to detect trends. For plants, which are likely to be quite responsive to environmental conditions, we showed that 11 years of monitoring would have been sufficient to detect the increasing trend in biomass found with the full time series, but generally not with shorter time series. The majority of our datasets (16 out of 22) met this criterion, with some series spanning two decades or more (e.g. geese, lemmings). For cyclic populations such as small rodents and the predators that depend upon them, a higher number of years may be needed to detect trends, given the large inter-annual variability in population densities. However, extreme changes such as a total collapse of cycles [58] are probably easier to detect from relatively short time series and we clearly did not observe such changes.
Second, inter-annual variation may be especially large in the Arctic. For instance, annual reproductive success in geese can vary enormously, with alternating ‘boom’ and ‘bust’ years [53], and this may contribute to masking long-term changes in the data. This also raises the issue of the statistical significance of a trend. In presence of high annual variability, it will be difficult to demonstrate the statistical significance of weak or even moderate trends (in terms of slope parameter value) unless sample size is large (e.g. more than 40 years). In such situations, we would argue that discussing a trend based solely on the magnitude of its slope parameter, even if the confidence interval overlaps zero, may be justified, though within some limits. Indeed, this should apply to situations where the measurement error of the variable of interest is small relative to the mean observed change and to time series that are reasonably long (e.g. more than 10 years; see above). A related issue is the relative importance of making type I versus type II errors when it comes to detecting effects of climate change. If important conservation issues are at stake, then type II error is as damageable as type I error [59], and it may be wise to raise the threshold of statistical significance from 0.05 to 0.10 or 0.20 in those cases.
Third, the amplitude of the observed climate change may not be large enough yet to elicit a response. In arthropods, species at higher latitudes have broader thermal tolerance than those at lower latitudes and they are living in climates that are currently cooler than their physiological optima [60]. A similar phenomenon may occur in some of the vertebrates we are monitoring in the high Arctic as the climate changes may have not yet exceeded their phenotypic plasticity and thus their capacity to cope with these changes [18]. It is also possible that the benefits associated with a response to some environmental changes, for instance, in terms of advancement in phenology, may not yet outweigh the potential costs, and thus the lack of response could be beneficial under certain circumstances [61].
Fourth, the tundra food web may be slower to respond to changes than other biomes. This could be especially true in the high Arctic where the inertia could be greater than in the low Arctic, for instance, owing to the greater stability of some physical components of the environment such as the permafrost or snow cover. Responses to environmental changes in many Arctic species may not be linear but could occur rather suddenly when some thresholds are exceeded [56,62,63]. For instance, abrupt changes could occur when the annual ground temperature increases above 0°C, resulting in disappearance of the permafrost, or when the ecosystem switches from a herbaceous to an erect shrub-dominated vegetation [64]. At our site, permafrost has rather been stable so far as ground temperature showed little temporal trend, probably because winter temperature has not warmed up over the past 40 years [30].
Fifth, constraints can prevent short-term adjustments in some species. In migratory birds, population attributes can be strongly affected by environmental factors encountered away from the Arctic breeding grounds [53,65,66]. Spring migration phenology, and hence timing of breeding, may be constrained by the timing of life-cycle phases preceding migration, by different climatic variations in the wintering areas and along the migratory route, or by depleted genetic variance in migration traits [67]. For instance, delays in snow goose spring migration resulting in lagged arrival on the breeding grounds with respect to snow-melt may be partly driven by different rates of advancement of spring between the Arctic and temperate terrestrial biomes.
Despite the relative stability of the Bylot Island vertebrate food web over the past two decades, our analyses still provide some insights into which components are most likely to be affected by future climate warming and how. By relating reproduction phenology with environmental conditions using a common metric (i.e. date), we showed that snow geese undercompensated in response to change in the timing of snow-melt as they laid after the 50 per cent snow-melt date in early years but before it in late years. This probably illustrates two different constraints faced by geese at each end: in early years, they do not arrive early enough to take advantage of the good conditions and in late years, they have to start laying even if conditions are not quite right owing to the shortness of the summer (if they delay more, they may run out of time at the end to complete their breeding cycle, [45]). In contrast, the timing of growth of their graminoid food plants (in terms of peak nitrogen concentration) seems to adjust rather well to annual change in snow-melt date (i.e. slope close to unity). In years of very early snow-melt, there is evidence that this leads to a reduction in growth of goslings, probably due to a mismatch between the timing of gosling hatch and the timing of peak plant nutritive quality [40]. Thus, as climate continues to warm, we should expect a greater mismatch between geese and their food plants, with negative consequences for the population. Increased mismatch between the timing of breeding of insectivorous shorebirds and the timing of emergence of their insect prey in years with early springs, which led to reduced chick growth, has also been reported at our site [68].
In the case of predators such as the Arctic fox, future change in their population may be more related to changes in the absolute abundance of their prey rather than on the seasonal timing of their availability. We found that both their diet and annual productivity were strongly affected by cyclic fluctuations in lemming abundance, as found elsewhere [69–71]. Even though lemming peaks may have been less pronounced during the 2000s than in the previous decade on Bylot Island, this had no long-term effect on fox productivity. This may not be surprising considering that the effect of lemming abundance on fox productivity was nonlinear as it was only at the lowest lemming density that it declined abruptly (see also [29]). In some regions of the Arctic, lemming cycles have recently collapsed [58] and change in the duration and quality of the snow cover has been invoked to explain this [72,73]. Although snow depth and density can affect the amplitude of lemming oscillations at our study site, the usually dry snow pack of the Canadian high Arctic has probably been less affected so far by climate warming than in regions exposed to the warm water of the northeast Atlantic Ocean [49]. Nonetheless, any serious disruption of lemming cycles due to future climate warming could have devastating effects for Arctic foxes, as observed in Fennoscandia [74].
Invasion by new competitors such as the red fox is another threat faced by tundra species. The red fox breeds on Bylot Island where it competes with Arctic foxes, but its abundance has not increased over the past two decades. A similar stability was also observed in the western Canadian Arctic (Yukon) despite recent warming [75].
5. Conclusion
We suggest that the most serious impacts of climate warming on Arctic vertebrate species will probably be indirect, due to disruption of trophic interactions caused by mismatches, decline in prey abundance and appearance of new competitors or predators [28]. Recent findings provide evidence that migratory birds are becoming ecologically mismatched in temperate regions and that failure to respond to climate change can have severe negative impacts on their populations [76]. Increasing trophic mismatch between migrating herbivores or insectivores and their food resources may thus be a widespread threat to Arctic food webs. It will be important to track migratory species during their entire annual cycle to identify potential constraints preventing adjustment to rapid changes happening on their Arctic-nesting grounds.
Because of their simplicity, Arctic food webs may especially be prone to exhibit nonlinear dynamics in response to climate warming and to show abrupt changes owing to threshold effects and feedback processes [56,62,63]. Therefore, great care is needed when trying to extrapolate future ecological responses (or lack of them) observed at the early stage of warming, especially considering that upcoming warming should greatly exceed what has been observed so far [1]. In order to better understand the complex mechanisms and processes behind the responses of individual species to climate change, increased efforts should be devoted to understanding the interactions and strengths of linkages among species.
There is currently an international effort underway to implement a coordinated, circumpolar programme to monitor future changes in Arctic ecosystems [77]. Our results can provide some guidance on how such programmes should be implemented. For instance, our analysis suggests that length of monitoring is critical and that conclusions based on short time series should be viewed with caution. Monitoring spanning a decade appears to be a minimum before drawing some conclusions, and probably more for cyclic species and predators that depend upon them. For some components of the environment showing moderate year-to-year variability such as primary production, we showed that less intensive monitoring (e.g. every other year rather than annual) could be sufficient to detect long-term trends, which could reduce costs. However, this recommendation would not apply to cyclic species, which require annual monitoring because cycles typically show irregularities in periodicity.
Acknowledgement
We thank Toke Høye and Eric Post for inviting us to participate in this special thematic issue.
Data accessibility
We are especially grateful to the many people who helped us with field work over more than two decades and the Mittimatalik Hunters and Trappers Organization and Park Canada's staff for their assistance. All animal manipulations were approved by the Animal Care Committees of Université Laval and Université du Québec à Rimouski.
Funding statement
Our long-term study was funded by (alphabetical order): Canada Research Chairs Program, Canadian Wildlife Service, Fonds québécois de la recherche sur la nature et les technologies, International Polar Year program of Indian and Northern Affairs Canada (INAC), Natural Sciences and Engineering Research Council of Canada, Network of Centers of Excellence of Canada ArcticNet, Northern Ecosystem Initiative Program (Environment Canada), Northern Scientific Training Program (INAC), Parks Canada, Polar Continental Shelf Program (Natural Resources Canada), Université du Québec à Rimouski, and Université Laval.
References
- 1.ACIA 2005. Arctic climate impact assessment: scientific report. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Barber DG, Lukovich JV, Keogak J, Baryluk S, Fortier L, Henry GHR. 2008. The changing climate of the Arctic. Arctic 61, 7–26 [Google Scholar]
- 3.Kaufman DS, et al. 2009. Recent warming reverses long-term Arctic cooling. Science 325, 236–1239 (doi:10.1126/science.1173983) [DOI] [PubMed] [Google Scholar]
- 4.Kerr RA. 2007. Climate change: is battered Arctic sea ice down for the count? Science 318, 33–34 (doi:10.1126/science.318.5847.33a) [DOI] [PubMed] [Google Scholar]
- 5.Smith SL, Romanovsky VE, Lewkowicz AG, Burn CR, Allard M, Clow GD, Yoshikawa K, Throop J. 2010. Thermal state of the permafrost in North America: a contribution to the International Polar Year. Permafrost Periglac. 21, 117–135 (doi:10.1002/ppp.690) [Google Scholar]
- 6.Sharp M, Burgess DO, Gogley JG, Ecclestone M, Labine C, Wolken GJ. 2011. Extreme melt on Canada's Arctic ice caps in the 21st century. Geophys. Res. Lett. 38, L11501 (doi:10.1029/2011GL047381) [Google Scholar]
- 7.Derksen C, Brown R. 2012. Spring snow cover extent reductions in the 2008–2012 period exceeding climate model projections. Geophys. Res. Lett. 39, L19504 (doi:10.1029/2012GL053387) [Google Scholar]
- 8.Tape K, Sturm M, Racine C. 2006. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Glob. Change Biol. 12, 686–702 (doi:10.1111/j.1365-2486.2006.01128.x) [Google Scholar]
- 9.Hudson JMG, Henry GHR. 2009. Increased plant biomass in a High Arctic heath community from 1981 to 2008. Ecology 90, 2657–2663 (doi:10.1890/09-0102.1) [DOI] [PubMed] [Google Scholar]
- 10.Hill GB, Henry GHR. 2011. Responses of High Arctic wet sedge tundra to climate warming since 1980. Glob. Change Biol. 17, 276–287 (doi:10.1111/j.1365-2486.2010.02244.x) [Google Scholar]
- 11.Jia GJ, Epstein HE, Walker DA. 2009. Vegetation greening in the Canadian Arctic related to decadal warming. J. Environ. Monitor. 11, 2231–2238 (doi:10.1039/B911677J) [DOI] [PubMed] [Google Scholar]
- 12.Post E, et al. 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355–1358 (doi:10.1126/science.1173113) [DOI] [PubMed] [Google Scholar]
- 13.Gilg O, et al. 2012. Climate change and the ecology and evolution of Arctic vertebrates. Ann. NY Acad. Sci. 1249, 166–190 (doi:10.1111/j.1749-6632.2011.06412.x) [DOI] [PubMed] [Google Scholar]
- 14.Post E, Brodie J. 2012. Extinction risk at high latitudes. In Saving a million species: extinction risk from climate change (ed. Hannah L.), pp. 121–137 Washington, DC: Island Press [Google Scholar]
- 15.Regehr EV, Hunter CM, Caswell H, Amstrup SC, Stirling I. 2010. Survival and breeding of polar bears in the southern Beaufort Sea in relation to sea ice. J. Anim. Ecol. 79, 117–127 (doi:10.1111/j.1365-2656.2009.01603.x) [DOI] [PubMed] [Google Scholar]
- 16.Wiig Ø, Aars J, Born EW. 2008. Effects of climate change on polar bears. Sci. Prog. 91, 151–173 (doi:10.3184/003685008X324506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stirling I, Derocher AE. 2012. Effects of climate warming on polar bears: a review of the evidence. Glob. Change Biol. 18, 2694–2706 (doi:10.1111/j.1365-2486.2012.02753.x) [DOI] [PubMed] [Google Scholar]
- 18.Berteaux D, Réale D, McAdam AG, Boutin S. 2004. Keeping pace with fast climate change: can Arctic life count on evolution? Integr. Comp. Biol. 44, 140–151 (doi:10.1093/icb/44.2.140) [DOI] [PubMed] [Google Scholar]
- 19.Carey C. 2009. The impacts of climate change on the annual cycles of birds. Phil. Trans. R. Soc. B 364, 3321–3330 (doi:10.1098/rstb.2009.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Both C, Van Turnhout CAM, Bijlsma RG, Siepel H, Van Strien AJ, Foppen RPB. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B 277, 1259–1266 (doi:10.1098/rspb.2009.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton I. 1998. Population limitation in birds. London, UK: Academic Press [Google Scholar]
- 22.Sinclair ARE, Kreb CJ. 2002. Complex numerical responses to top-down and bottom-up processes in vertebrate populations. Phil. Trans. R. Soc. Lond. B 357, 1221–1231 (doi:10.1098/rstb.2002.1123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Post E. 2005. Large-scale spatial gradients in herbivore population dynamics. Ecology 86, 2320–2328 (doi:10.1890/04-0823) [Google Scholar]
- 24.Magurran AE, Baillie SR, Buckland ST, Dick JMcP, Elston DA, Scott EM, Smith RI, Somerfield PJ, Watt AD. 2010. Long-term datasets in biodiversity research and monitoring: assessing change in ecological communities through time. Trends Ecol. Evol. 25, 574–582 (doi:10.1016/j.tree.2010.06.016) [DOI] [PubMed] [Google Scholar]
- 25.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. 2010. The effects of phenological mismatches on demography. Phil. Trans. R. Soc. B 365, 3177–3186 (doi:10.1098/rstb.2010.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Putten WH, de Ruiterb PC, Bezemera TM, Harveya JA, Wassenb M, Wolters V. 2004. Trophic interactions in a changing world. Basic Appl. Ecol. 5, 487–494 (doi:10.1016/j.baae.2004.09.003) [Google Scholar]
- 27.Kerby J, Post E. 2013. Capital and income breeding traits differentiate trophic match–mismatch dynamics in large herbivores. Phil. Trans. R. Soc. B 368, 20120484 (doi:10.1098/rstb.2012.0484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Putten WH, Macel M, Visser ME. 2010. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Phil. Trans. R. Soc. B 365, 2025–2034 (doi:10.1098/rstb.2010.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gauthier G, Bêty J, Giroux J-F, Rochefort L. 2004. Trophic interactions in a high Arctic snow goose colony. Integr. Comp. Biol. 44, 119–129 (doi:10.1093/icb/44.2.119) [DOI] [PubMed] [Google Scholar]
- 30.Gauthier G, Berteaux D, Bêty J, Tarroux A, Therrien J-F, McKinnon L, Legagneux P, Cadieux M-C. 2011. The tundra food web of Bylot Island in a changing climate and the role of exchanges between ecosystems. Ecoscience 18, 223–235 (doi:10.2980/18-3-3453) [Google Scholar]
- 31.Legagneux P, et al. 2012. Disentangling trophic relationships in a high Arctic tundra ecosystem through food web modeling. Ecology 93, 1707–1716 (doi:10.1890/11-1973.1) [DOI] [PubMed] [Google Scholar]
- 32.Cadieux M-C, Gauthier G, Gagnon CA, Lévesque E, Bêty J, Berteaux D. 2008. Monitoring the environmental and ecological impacts of climate change on Bylot Island, Sirmilik National Park—2004–2008 final report. Centre d’études nordiques, Université Laval [Google Scholar]
- 33.Gauthier G, Rochefort L, Reed A. 1996. The exploitation of wetland ecosystems by herbivores on Bylot Island. Geosci. Canada 23, 253–259 [Google Scholar]
- 34.Duclos I. 2002. Milieux mésiques et secs de l’Île Bylot, Nunavut (Canada): caractérisation et utilisation par la grande oie des neiges. MSc thesis, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada [Google Scholar]
- 35.Mainguy J, Gauthier G, Giroux J-F, Bêty J. 2006. Gosling growth and survival in relation to brood movements in greater snow geese (Chen caerulescens atlantica). Auk 123, 1077–1089 (doi:10.2307/25150221) [Google Scholar]
- 36.Gruyer N, Gauthier G, Berteaux D. 2008. Cyclic dynamics of sympatric lemming populations on Bylot Island, Nunavut, Canada. Can. J. Zool. 86, 910–917 (doi:10.1139/Z08-059) [Google Scholar]
- 37.Gagnon CA, Berteaux D. 2009. Integrating traditional ecological knowledge and ecological science: a question of scale. Ecol. Soc. 14, 19 [Google Scholar]
- 38.Therrien J-F. 2012. Réponses des prédateurs aviaires aux fluctuations d'abondance de proies dans la toundra. PhD thesis, Université Laval, Québec, Canada [Google Scholar]
- 39.CEN 2013. Environmental data from Bylot Island, Nunavut, Canada, v. 1.0 (1992–2012). Nordicana D2 (doi:10.5885/45039SL-EE76C1BDAADC4890) [Google Scholar]
- 40.Dickey M-H, Gauthier G, Cadieux M-C. 2008. Climatic effects on the breeding phenology and reproductive success of an Arctic-nesting goose species. Glob. Change Biol. 14, 1973–1985 (doi:10.1111/j.1365-2486.2008.01622.x) [Google Scholar]
- 41.Gauthier G, Hughes RJ, Reed A, Beaulieu J, Rochefort J. 1995. Effect of grazing by greater snow geese on the production of graminoids at an Arctic site (Bylot Island, NWT, Canada). J. Ecol. 83, 653–664 (doi:10.2307/2261633) [Google Scholar]
- 42.Valéry L, Cadieux M-C, Gauthier G. 2010. Spatial heterogeneity of primary production as both cause and consequence of foraging patterns of an expanding greater snow goose colony. Ecoscience 17, 9–19 (doi:10.2980/17-1-3279) [Google Scholar]
- 43.Doiron M, Legagneux P, Gauthier G, Lévesque E. 2013. Broad-scale satellite normalized difference vegetation index data predict plant biomass and peak date of nitrogen concentration in Arctic tundra vegetation. Appl. Veg. Sci. 16, 343–351 (doi:10.1111/j.1654-109X.2012.01219.x.) [Google Scholar]
- 44.Gruyer N, Gauthier G, Berteaux D. 2010. Demography of two lemming species on Bylot Island, Nunavut, Canada. Polar Biol. 33, 725–736 (doi:10.1007/s00300-009-0746-7) [Google Scholar]
- 45.Lepage D, Gauthier G, Menu S. 2000. Reproductive consequences of egg-laying decisions in snow geese. J. Anim. Ecol. 69, 414–427 (doi:10.1046/j.1365-2656.2000.00404.x) [Google Scholar]
- 46.Szor G, Berteaux D, Gauthier G. 2008. Finding the right home: distribution of food resources and terrain characteristics influence selection of denning sites and reproductive dens in Arctic foxes. Polar Biol. 31, 351–362 (doi:10.1007/s00300-007-0364-1) [Google Scholar]
- 47.Cameron C, Berteaux D, Dufresne F. 2011. Spatial variation in food availability predicts extrapair paternity in the Arctic fox. Behav. Ecol. 22, 1364–1373 (doi:10.1093/beheco/arr158) [Google Scholar]
- 48.Tarroux A, Bêty J, Gauthier G, Berteaux D. 2012. The marine side of a terrestrial carnivore: intra-population variation in use of allochthonous resources by Arctic foxes. PLoS ONE 7, e42427 (doi:10.1371/journal.pone.0042427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bilodeau F, Gauthier G, Berteaux D. In press The effect of snow cover on lemming population cycles in the Canadian high Arctic. Oecologia. (doi:10.1007/s00442-012-2549-8) [DOI] [PubMed] [Google Scholar]
- 50.Post DM. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718 (doi:10.2307/3071875) [Google Scholar]
- 51.Box GEP, Jenkins GM, Reinsel GC. 1994. Time series analysis: forecasting and control, 3rd edn Upper Saddle River, NJ: Prentice-Hall [Google Scholar]
- 52.Freund RJ, Littell RC. 2000. SAS system for regression, 3rd edn Cary, NY: SAS Institute [Google Scholar]
- 53.Morrissette M, Bêty J, Gauthier G, Reed A, Lefebvre J. 2010. Climate, indirect trophic interactions, carry-over and density-dependent effects: which factors drive High Arctic snow goose productivity? Oikos 119, 1181–1191 (doi:10.1111/j.1600-0706.2009.18079.x) [Google Scholar]
- 54.Høye T, Post E, Meltofte H, Schmidt NM, Forchammer MC. 2007. Rapid advancement of spring in the high Arctic. Curr. Biol. 17, R449–R451 (doi:10.1016/j.cub.2007.04.047) [DOI] [PubMed] [Google Scholar]
- 55.Oberbauer SF, et al. 2013. Phenological response of tundra plants to background climate variation tested using the International Tundra Experiment. Phil. Trans. R. Soc. B 368, 20120481 (doi:10.1098/rstb.2012.0481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walther GR. 2010. Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B 365, 2019–2024 (doi:10.1098/rstb.2010.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Réale D, McAdam AG, Boutin S, Berteaux D. 2003. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. Lond B 270, 591–596 (doi:10.1098/rspb.2002.2224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ims RA, Henden J-A, Killengreen ST. 2008. Collapsing population cycles. Trends Ecol. Evol. 23, 79–86 (doi:10.1016/j.tree.2007.10.010) [DOI] [PubMed] [Google Scholar]
- 59.Taylor BL, Gerrodette T. 1993. The uses of statistical power in conservation biology: the vaquita and northern spotted owl. Conserv. Biol. 7, 489–500 (doi:10.1046/j.1523-1739.1993.07030489.x) [Google Scholar]
- 60.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Both C, van Asch M, Bijlsma RG, van den Burg AB, Visser ME. 2009. Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J. Anim. Ecol. 78, 73–83 (doi:10.1111/j.1365-2656.2008.01458.x) [DOI] [PubMed] [Google Scholar]
- 62.Groffman PM, et al. 2006. Ecological thresholds: the key to successful environmental management or an important concept with no practical application? Ecosystems 9, 1–13 (doi:10.1007/s10021-003-0142-z) [Google Scholar]
- 63.Callaghan TV, et al. 2013. Ecosystem change and stability over multiple decades in the Swedish sub-Arctic: complex processes and multiple drivers. Phil. Trans. R. Soc. B 368, 20120488 (doi:10.1098/rstb.2012.0488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ims RA, Henden JA. 2012. Collapse of an Arctic bird community resulting from ungulate-induced loss of erect shrub. Biol. Cons. 149, 2–5 (doi:10.1016/j.biocon.2012.02.008) [Google Scholar]
- 65.Descamps S, Yoccoz NG, Gaillard J-M, Gilchrist GH, Erikstad KE, Hanssen SA, Cazelles B, Forbes MR, Bêty J. 2010. Detecting population heterogeneity in effects of north Atlantic oscillations on seabird body condition: get into the rhythm. Oikos 119, 1526–1536 (doi:10.1111/j.1600-0706.2010.18508.x) [Google Scholar]
- 66.Legagneux P, Fast P, Gauthier G, Bêty J. 2012. Manipulating individual state during migration provides evidence for carry-over effects modulated by environmental conditions. Proc. R. Soc. Lond B 279, 876–883 (doi:10.1098/rspb.2011.1351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Møller AP, Fiedler W, Berthold P. (eds). 2010. Effects of climate change on birds. Oxford, UK: Oxford University Press [Google Scholar]
- 68.McKinnon L, Picotin L, Bolduc E, Juillet C, Bêty J. 2012. Timing of breeding, peak food availability, and effects of mismatch on chick growth in birds nesting in the High Arctic. Can. J. Zool. 90, 961–971 (doi:10.1139/z2012-064) [Google Scholar]
- 69.Angerbjörn A, Tannerfeldt M, Erlinge S. 1999. Predator–prey relationships: Arctic foxes and lemmings. J. Anim. Ecol. 68, 34–49 (doi:10.1046/j.1365-2656.1999.00258.x) [Google Scholar]
- 70.Eide NE, Stien A, Prestrud P, Yoccoz NG, Fuglei E. 2011. Reproductive responses to spatial and temporal prey availability in a coastal Arctic fox population. J. Anim. Ecol. 81, 640–648 (doi:10.1111/j.1365-2656.2011.01936.x) [DOI] [PubMed] [Google Scholar]
- 71.Samelius G, Alisauskas RT, Larivière S. 2011. Seasonal pulses of migratory prey and annual variation in small mammal abundance affect abundance and reproduction by Arctic foxes. Polar Biol. 34, 1475–1484 (doi:10.1007/s00300-011-1005-2) [Google Scholar]
- 72.Kausrud KL, et al. 2008. Linking climate change to lemming cycles. Nature 456, 93–97 (doi:10.1038/nature07442) [DOI] [PubMed] [Google Scholar]
- 73.Gilg O, Sittler B, Hanski I. 2009. Climate change and cyclic predator–prey population dynamics in the high Arctic. Glob. Change Biol. 15, 2634–2652 (doi:10.1111/j.1365-2486.2009.01927.x) [Google Scholar]
- 74.Henden JA, Bardsen BJ, Yoccoz NG, Ims RA. 2008. Impacts of differential prey dynamics on the potential recovery of endangered Arctic fox populations. J. Appl. Ecol. 45, 1086–1093 (doi:10.1111/j.1365-2664.2008.01515.x) [Google Scholar]
- 75.Gallant D, Slough BG, Reid DG, Berteaux D. 2012. Arctic fox versus red fox in the warming Arctic: four decades of den surveys in north Yukon. Polar Biol. 35, 1421–1431 (doi:10.1007/s00300-012-1181-8) [Google Scholar]
- 76.Saino N, et al. 2011. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. R. Soc. B 278, 835–842 (doi:10.1098/rspb.2010.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christensen T, et al. 2011. Terrestrial expert monitoring plan—background paper. A Supporting Publication to the CBMP Framework Document. CAFF International Secretariat, CAFF Monitoring Series Report no. 6
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We are especially grateful to the many people who helped us with field work over more than two decades and the Mittimatalik Hunters and Trappers Organization and Park Canada's staff for their assistance. All animal manipulations were approved by the Animal Care Committees of Université Laval and Université du Québec à Rimouski.




