Abstract
Predicting impacts of global warming requires understanding of the extent to which plant biomass and production are controlled by bottom-up and top-down drivers. By annually monitoring community composition in grazed control plots and herbivore-free exclosures at an Arctic location for 15 years, we detected multiple biotic interactions. Regular rodent cycles acted as pulses driving synchronous fluctuations in the biomass of field-layer vegetation; reindeer influenced the biomass of taller shrubs, and the abundance of plant pathogenic fungi increased when densities of their host plants increased in exclosures. Two outbreaks of geometrid moths occurred during the study period, with contrasting effects on the field layer: one in 2004 had marginal effects, while one in 2012 severely reduced biomass in the control plots and eliminated biomass that had accumulated over 15 years in the exclosures. The latter was followed by a dramatic decline of the dominant understory dwarf-shrub Empetrum hermaphroditum, driven by an interaction between moth herbivory on top buds and leaves, and increased disease severity of a pathogenic fungus. We show that the climate has important direct and indirect effects on all these biotic interactions. We conclude that long time series are essential to identify key biotic interactions in ecosystems, since their importance will be influenced by climatic conditions, and that manipulative treatments are needed in order to obtain the mechanistic understanding needed for robust predictions of future ecosystem changes and their feedback effects.
Keywords: plant community composition, herbivory, voles, lemmings, reindeer, moth
1. Introduction
Understanding the factors regulating the abundance of plants has been the central goal within ecology for more than a century, since they form the energy basis of most food webs and drive global carbon cycles. However, plant abundance and net primary production are highly variable in space and time. Spatial variations of net primary production are related to factors such as climate, vegetation distribution and land use across the planet from local to global scales. Temperature and precipitation are key climatic variables, and both are, in general, positively related to plant production in most ecosystems [1]. Between-year variations in temperature and climate are thus important drivers of variation in plant abundance and primary production in space and time [2].
Top-down effects from herbivores and pathogens can also have strong effects on plant biomass [3,4]. Spatial variation in herbivores has, for example, large consequences for species composition and structure of plant communities worldwide [5,6], and dramatic fluctuations in herbivore abundance have also been found to drive corresponding fluctuations in plant abundance in natural ecosystems [7–10].
Numerous studies have investigated vegetation changes and related them to a changing climate [11–16]. However, in order to do so, it is important to understand natural patterns of fluctuations in plant communities. Most existing long time series of population dynamics or community composition reveal large fluctuations [4,17–20], and that the importance of different factors causing these fluctuations often vary over time [4,18,21].
Arctic and alpine regions are examples of currently changing ecosystems. Since these regions have become warmer during the past century [11,22,23], recent observations of shifts in the composition and abundance of Arctic and alpine plants have been interpreted as responses to a warmer climate [12,13]. In many locations, the ranges of thermophilic species such as trees and shrubs [14,15,16], and tall forbs [12,13], have expanded beyond their recent altitudinal or latitudinal limits. Warming experiments support the hypothesis that higher temperatures could drive these vegetation shifts [24,25].
Although the current range of expansion of thermophilic species in Arctic and alpine ecosystems is circumpolar [12,13], such changes vary in space and time [13,26], and in some areas [13,26], no changes or even decreases in the abundance of thermophilic plants have been observed. This indicates that local differences in geology, topography, climate or land-use can strongly influence observed patterns [26]. Moreover, changes in plant community composition are not constant over time [26,27] and herbivores disrupt the linear relationship between summer temperature and shrub growth [17]. Bottom-up regulators such as temperature [24] and nutrient availability [28,29] are indeed major drivers of primary production and plant community composition in nutrient-poor, cold Arctic and alpine ecosystems [30,31]. However, this does not mean that top-down forces such as herbivory and pathogen attacks are unimportant. Numerous exclosure studies have revealed that mammalian herbivores such as voles and reindeer strongly influence the vegetation [7,28,32,33]. Moreover, outbreaks of moth species [8,9,33] and plant pathogens [4] can also cause dramatic changes in Arctic and alpine ecosystems. Many of these bottom-up and top-down forces are likely to interact. For instance, responses of plants to warming are enhanced by nutrient addition [34–36], while herbivores may reduce increases of plant biomass following fertilization [28,29,37] and warming [33,38].
Annual recordings of plant biomass and community composition in open, grazed control plots and herbivore-free exclosures in a subarctic ecosystem in the Abisko region have demonstrated that the regular interannual density fluctuations of voles and lemmings drive synchronous interannual fluctuations in the biomass of field-layer plants, as well as the relative abundance of various species [7]. The effects of combined vole and lemming peaks were clearly visible in satellite images, more specifically in reductions in normalized difference vegetation indices obtained from images covering a 770 km2 area in the following year [7]. Moreover, the total plant biomass almost doubled when all mammalian herbivores were excluded for 14 years [7,17].
Here, we use data from the experiment described above to assess how different top-down and bottom-up forces influence the plant community. More specifically, we assess how the biomass of the six most common plant species of the field-layer changed during the period 1998–2012, in grazed control plots, large mesh-size exclosures and small mesh-size exclosures in two contrasting habitats (forest and tundra). The ecosystem experienced considerable variation in temperatures including an extreme winter warming event [26], four vole peaks, three lemming peaks, two geometrid moth outbreaks and at least two outbreaks of plant pathogenic fungi during this period. One of the outbreaks of geometrid moths, in 2012, had severe effects, including rapid and extensive decline of the dominant understory species Empetrum hermaphroditum. We show here how each of these events separately or interactively influenced the plant community.
2. Material and methods
(a). Study area
The study was carried out in the proximity of the Paddus cliff, 550 m.a.s.l. approximately 4 km southwest of the Abisko Scientific Research Station, in the Torneträsk region, northernmost Sweden (68°19′23″ N, 18°51′57″ E). The bedrock here is nutrient-poor and the forest is dominated by mountain birch (Betula pubescens ssp. czerepanovii), which is typical for Fennoscandian treelines. The mean annual air temperature and precipitation from 1960 to 1990 at the Abisko Scientific Research Station were −0.8°C and 304 mm, respectively [26,39]. The ecosystems at the study location consist of a mixture of forest and tundra patches. The birch density in the forest patches was 747 ± 68 trees per ha in 1998. Reindeer graze in the area mainly in spring and autumn, but solitary reindeer can be found throughout the summer. Climate warming in the region has led to temperatures exceeding those of earlier warm periods since the start of the new millennium and crossing of the 0°C mean annual temperature threshold [26]. In addition, since the 1980s, a previous century-long trend of increasing snow depth has been replaced by an accelerating reduction [39].
(b). Experimental design
The analysis reported here is based on data from three study sites established in proximity to the Paddus cliff. The distance between sites varies between 0.2 and 2 km. Each site consists of a birch forest and adjacent tundra. The forest and tundra were chosen to be as similar as possible, except for the presence of trees. At all of these sites the field-layer vegetation consists of a dwarf-shrub heath, but grasses and herbs are more common inside the forest. The large herbivore guild is totally dominated by semi-domesticated reindeer, while voles and lemmings are the main small vertebrate herbivores [40,41]. Three 8 × 8 m experimental plots were established in 1998 in each of the three forest and three tundra sites. These three experimental plots were randomly assigned to the following treatments: ‘large mesh-size exclosure’ (excluding ungulates and mountain hares), ‘small mesh-size exclosure’ (excluding all mammalian herbivores) and a no-exclosure control treatment (allowing all herbivores unrestricted access to the vegetation). Steel wire sheep netting (1.2 m high with a 10 × 10 cm mesh size), fastened 10–20 cm above the ground, was used for the large mesh-size exclosures and galvanized net (1 m high with a 1.2 × 1.2 cm mesh size), inserted 10–30 cm into the mineral soil, for the small mesh-size exclosures. As voles and lemmings are the most important small vertebrate herbivores in the region the small mesh-size exclosure plots are referred to hereafter as rodent exclosures. The original experiment also included three other locations in northernmost Sweden and Norway [7,17,40,41]. However, only the southern continental location close to Abisko is considered here.
(c). Vegetation recording
We recorded the plant community composition (vascular plants, mosses and lichens) in three permanent subplots (0.5 × 0.5 m, more than 1 m apart) within each exclosure and grazed control plot, and estimated plant biomass non-destructively with a modified point intercept method (100 pins per plot). We carried out the measurements annually from 1998 to 2012, in late July–early August, using a transparent Plexiglas table, 0.5 × 0.5 m, with 100 randomly distributed 4 mm holes. We lowered a pin of the same diameter through each hole, and recorded the number of contacts the pin made for each vascular plant species. Plants of E. hermaphroditum and Vaccinium vitis-idaea showing visible disease symptoms were recorded separately (see §2(e)). To convert the point intercept data to biomass for the permanent vegetation plots, 20 additional representative plots were selected in the summer of 2011 in close proximity to the grazed control plots, surveyed by the point intercept method in the same way as the permanent subplots, and harvested at the peak of the growing season (early August). The plant biomass was sorted into functional groups, dried to constant mass (48 h at 60°C) and then weighed. Only data for the most abundant species are reported here. For older data see [7,17,40,41].
(d). Herbivore damage and visible disease symptoms of Empetrum hermaphroditum
To identify drivers of the extensive and rapid death of E. hermaphroditum in the forest following the moth outbreak in 2012, we sampled five shoots from each of 10 replicated subplots in three microtopographic positions (mounds, intermediate and depressions) across a 50 m long transect in each of the three forest sites at the end of August 2012. The three microtopographic positions reflect large differences in snow cover from snow-poor mounds to snow-rich depressions, which means that timing of snowmelt may differ for several weeks. The mounds are often located around tree trunks. The altitudinal differences between mounds and depressions are about 1 m. The sampled shoots were examined in detail under a stereomicroscope. Top buds were categorized into three classes: intact, killed by parasitic fungi and eaten by herbivores. Thereafter, from each shoot we randomly sampled 10 leaves from each of four age classes (C is the current year's shoot, C + 1, C + 2 and C + 3). Annual stem length increments were identified under a stereomicroscope by the scars left by each year's bursting bud. The sampled leaves were scored for herbivore damage (feeding scars) and presence/absence of pathogenic fungi.
(e). Parasitic fungi
Species identifications of parasitic fungi are based on microscopic examinations and follow [42–44]. Nomenclature follows [45]. In the vegetation recordings with the point intercept method (see above), we only scored disease on dead plant parts. We identified one fungal pathogen, Arwidssonia empetri, on E. hermaphroditum, while disease on V. vitis-idaea was caused by three different pathogens Eupropolella vaccinii, Lophodermium melaleucum and Myxothyrium leptideum. Although E. vaccinii was by far the most abundant, they were treated collectively in the field scoring. Both A. empetri and E. vaccinii are so-called snow-blight fungi with extensive mycelial growth under an isolating snow pack in winter. Attacked plant parts are killed and appear as brownish patches immediately after snowmelt (for further information see [4,46], respectively). The detailed scorings of individual leaves in the Empetrum study differ in detail because disease on both living and dead leaves was identified in the laboratory. We found two common pathogenic fungi, Epipolaeum sulcicola and A. empetri. As the latter was of more limited importance in the birch forest, we only present data for E. sulcicola in figure 7. Epipolaeum sulcicola is a hemibiotrophic pathogen, i.e. it is biotrophic early in the life cycle and eventually switches over to necrotrophic nutrition [47,48]. Following successful inoculation it will take about 1 year until any disease symptoms allowing accurate identification become visible. That is when the first small incipient ascocarps have been formed. This time lapse between successful infection and possible identification of about 1 year is, for obvious reasons, not visible in our data (figure 7). In contrast to both A. empetri and E. vaccinii, E. sulcicola has a more restricted mycelial growth, limiting infection to younger leaf cohorts of the ramet. Lophodermium melaleucum is also hemibiotrophic [46], while the life cycle of the anamorph M. leptideum is not resolved [44].
Figure 7.
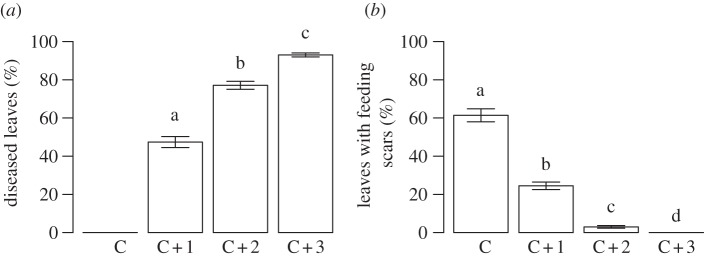
Percentages of leaves of four age cohorts (C, current year; C + 1; C + 2; and C + 3) of E. hermaphroditum with (a) visible disease symptoms of the pathogenic fungus E. sulcicola, and (b) feeding scars in transects from the forest in areas accessible to herbivores. Data from 2012. Groups not sharing letters differ significantly.
(f). Rodent densities
We thoroughly surveyed the sites for signs of rodents in June 1998, before the exclosures were constructed. Tracks, droppings, damaged plants and bodies of dead voles and lemmings were found. All these signs indicated that there was a rodent peak in the autumn of 1997. We subsequently monitored spring and autumn densities of rodents using the small quadrate method [49]. The spring trapping took place as soon after the snowmelt as possible (mid-June) and the autumn trapping during the first two weeks of September. A quadrate of 15 × 15 m was marked, and 12 traps of galvanized steel were placed as clusters of three in each corner. Distances between quadrates were approximately 100 m. Traps were baited with small pieces of Finnish rye bread, set for 48 h and checked twice (after 24 h and 48 h). Each quadrate thus represents 24 trap nights. We used five small quadrates in a forest and five small quadrates in an open heathland (240 trap nights in total) close to the sites (0.2–2 km) where exclosures were built in all locations. More than 80 per cent of all caught voles were Myodes rufocanus individuals. In addition, Norwegian lemmings, Lemmus lemmus, were abundant during peak years. A few individuals of Myodes rutilus and Microtus agrestis were also caught. This species composition is typical for dry heathlands in the Fennoscandinan forest–tundra ecotone [50]. Moth population dynamics were not surveyed in any systematic way. Population peaks were easily distinguished as a binary variable, since the birches were almost totally defoliated in the whole research area during these years, while damage levels did not exceed 20 per cent in the other years.
(g). Statistical analyses
Changes in biomass of the total community or individual species in the grazed controls were tested using linear regression with years as a continuous factor. Relationship between biomass of species and years since a rodent peak, mean temperatures and precipitation were tested using linear regression. The models were simplified and the models with the lowest AIC were selected. The effects of excluding herbivores on plant biomass were analysed using repeated measure ANOVA. The linearity of the species responses in the exclosures over time were tested by comparing linear and asymptotic models (y ∼ a − b × exp(−c)) with an ANOVA. The fit of the linear model and nonlinear model was tested with AIC, and by comparing the variance explained by the two models with an ANOVA. Herbivore damage and pathogen abundance on E. hermaphroditum were analysed using two-way ANOVA. The effect of different types of herbivores (small and large mesh-sized exclosures) were tested with a two-way ANOVA followed by a Tukey HSD test to separate individual treatments.
3. Results
(a). Climate and herbivore dynamics
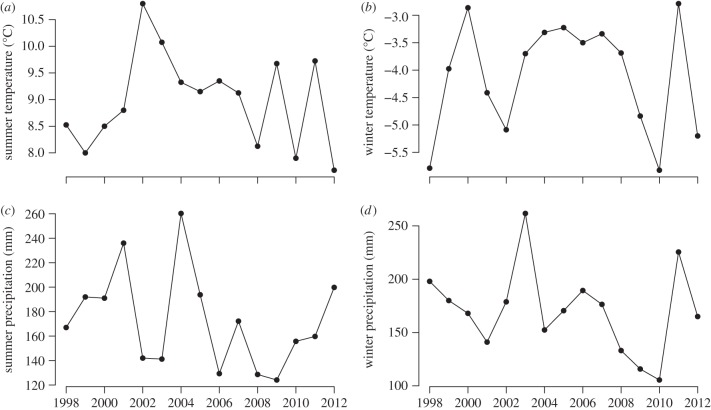
The past two decades have been warmer in the study region than in the rest of the twentieth century. Summer temperatures peaked in 2002, but all summers between 2002 and 2007 were fairly warm. However, summer temperatures have tended to decline during the past 10 years in Abisko, and the two coldest summers during the past 15 years were in 2010 and 2012 (figure 1). As previously presented [7], the density of voles fluctuated with a 3-year cycle, with peaks in 2001, 2004, 2007 and 2010 (figure 2). Lemming densities peaked in synchrony with those of voles, except in 2004 when no lemmings were caught, but there was a large variation in the amplitude of the peaks. There were two geometrid moth outbreaks during these 15 years and trees in the mountain birch forest were completely defoliated in both 2004 and 2012. During the 2004-peak, only Epirrita autumnata was recorded, while the 2012-peak was a combined E. autumnata and Operophthera brumata outbreak. However, although large parts of the surroundings were totally defoliated, our study sites in the upper part of the forest–tundra ecotone were only 50–80% defoliated in 2004 while the defoliation was close to 100 per cent in 2012 (J. Olofsson, personal observation).
Figure 1.
(a) Mean summer temperature (May–August), (b) mean winter temperature (September–April), (c) summer precipitation (May–August) and (d) winter precipitation (September–April) in Abisko during the period 1998 to 2012. Data provided by the Abisko Scientific Research Station.
Figure 2.
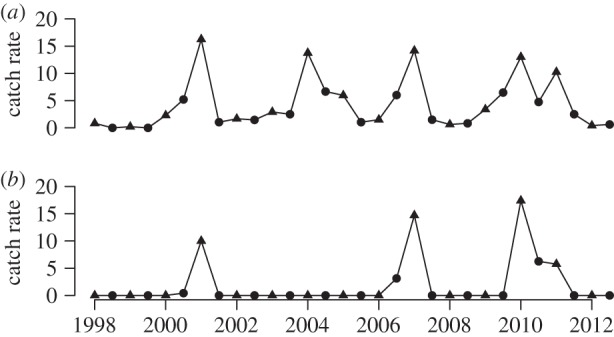
(a) Vole catch rates (number caught per 100 trap nights: circles, spring; triangles, autumn). (b) Lemming catch rates (number caught per 100 trap nights: circles, spring; triangles, autumn) in the study area during the period 1998–2012.
(b). Vegetation changes in grazed controls
The total vascular plant biomass in the grazed control plots fluctuated with a 3- to 4-year cycle in response to the vole and lemming cycles (cf. [7], statistical analyses not repeated here). The biomass of most common plant species changed over time. The biomass of Betula nana and V. vitis-idaea increased in the tundra (p = 0.017, p = 0.002), but did not change in the forest (p = 0.133, p = 0.312). The biomass of E. hermaphroditum increased in the forest (p = 0.015), but did not change in the tundra (p = 0.079). Vaccinium uliginosum decreased in the forest (p < 0.001), but did not change in the tundra (p = 0.090). The biomass of Deschampsia flexuosa increased (p = 0.045) and the biomass of Vaccinium myrtillus decreased in the forest (p < 0.001). Both these species were too rare for statistical tests in the tundra.
The biomass of plants did not experience linear changes during these 15 years, but rather fluctuated dramatically depending on climatic conditions and rodent dynamics. The total field-layer biomass increase was statistically significant with years since the last rodent peak both in the forest and in the tundra, but there were no statistically significant effects of temperature and precipitation (table 1). On the level of individual species, the increase in biomass of V. myrtillus and V. uliginosum was statistically significant with years since a rodent peak in the forest, and years since a rodent peak was also included with a positive value in the best models (lowest AIC) for most species (table 1). Precipitation outside the growing season had a negative effect on the biomass of B. nana in the forest, and precipitation both within and outside the growing season had a negative effect on V. uliginosum in the tundra. There was also a positive effect of higher temperatures outside the growing season for B. nana and D. flexuosa.
Table 1.
Effect of years since a rodent peak (YRP), precipitation within the growing season (May–August, PreM-A), precipitation outside the growing season (September–April, PreS-A), temperature within the growing season (May–August, TempM-A) and temperature outside the growing season (September–April, TempS-A) on plant biomass in the grazed controls during 15 years. Variable coefficients are given in the table and significant relationships are marked in italics. Non-significant relationships that were included in the model with the best fit (lowest AIC) are also shown in the table (not italics).
| habitat | YRP (g/year) | PreM-A (g/mm) | PreS-A (g/mm) | TempM-A (g/°C) | TempS-A (g/°C) | |
|---|---|---|---|---|---|---|
| total plant biomass | forest | 24.9 | 15 | |||
| tundra | 16.3 | –2.0 | ||||
| Betula nana | forest | –0.4 | –0.4 | 1.7 | ||
| tundra | ||||||
| Deschampsia flexuosa | forest | –3.0 | –0.7 | 4.4 | ||
| Empetrum hermaphroditum | forest | 3.5 | ||||
| tundra | 5.8 | |||||
| Vaccinium myrtillus | forest | 18.9 | 13.0 | |||
| Vaccinium uliginosum | forest | 5.3 | 0.7 | |||
| tundra | 2.3 | –1.0 | –1.3 | 1.9 | ||
| Vaccinium vitis-idaea | forest | 0.8 | 1.0 | 3.0 | ||
| tundra | 2.2 | 0.2 |
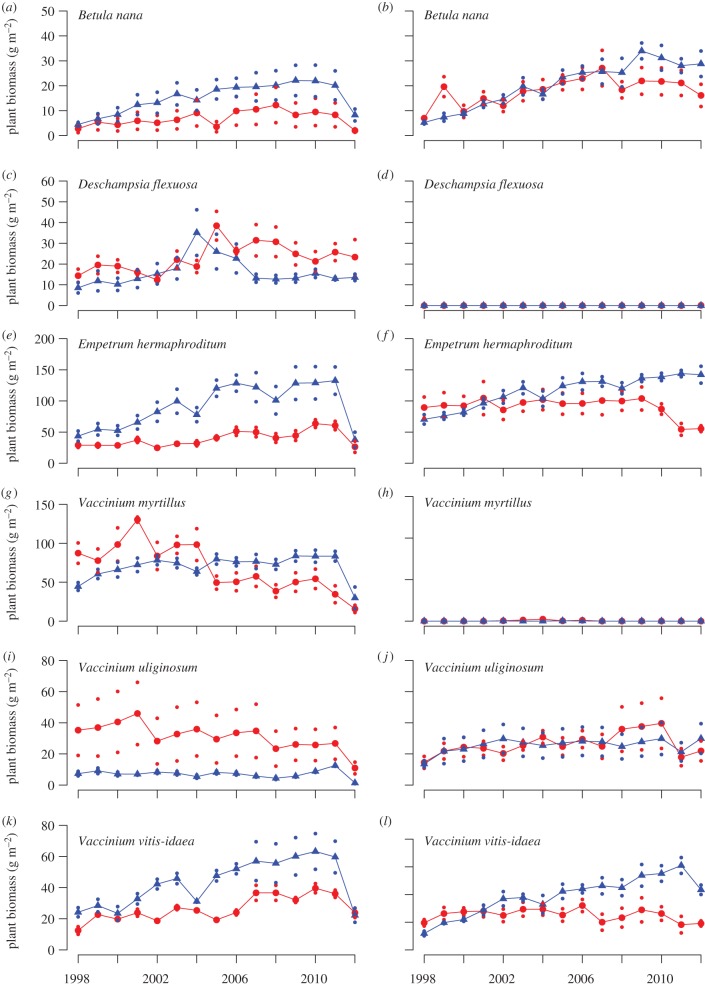
However, the severe outbreak of geometrid moths in 2012 dramatically interrupted these trends in the forest by reducing the biomass of all common plants except for the graminoid D. flexuosa (figure 4c). The moth outbreak reduced the biomass of B. nana, E. hermaphroditum, V. myrtillus, V. uliginosum and V. vitis-idaea by 76, 56, 54, 59 and 34 per cent, respectively (figure 4a–k) compared with the year before. In contrast to the dramatic effects in 2012, the geometrid moth outbreak in 2004 had only marginal, although observable, effects on the field-layer vegetation (figure 4).
Figure 4.
The plant biomass in grazed control plots (red, dots) and mammalian herbivore-free exclosures (blue, triangles) of the six dominant plant species in the forest (a,c,e,g,i,k) and the tundra (b,d,f,h,j,l) during the period 1998–2012. The small dots represent ±1 s.e.m.
(c). Vegetation changes in vertebrate herbivore exclosures
Excluding rodents (that is the small mesh-size exclosures) influenced the total plant biomass (table 2), and there were no statistically significant differences between the habitats (there were no statistically significant year (Y) × habitat (H) × treatment (T) interactions; table 2 and figure 3). Rodents influenced all dominant plant species, except the grass D. flexuosa, either by affecting their long-term trends in biomass or by inducing interannual fluctuations (tables 1 and 2, figure 4). We found no indications that the responses of any species to rodents differed between the forest and tundra in habitat-specific analysis of the data (there were no statistically significant H × T or Y × H × T interactions, table 1). Four of the six dominant vascular plant species (B. nana, E. hermaphroditum, V. myrtillus and V. vitis-idaea) increased in biomass in response to herbivore exclosure (table 2, figure 4). Initially, all these species increased linearly in the exclosures both in the forest and in the tundra. This increase was clearly interrupted by the dramatic die-off by many species following the moth outbreak in 2012 in the forest. However, this increase declined towards the end of the study period in the exclosures in the tundra as well (figures 3 and 4), and asymptotic linear regression functions provide significantly better fits for all these species (B. nana, p = 0.025; E. hermaphroditum, p = 0.008; V. uliginosum, p = 0.001; V. vitis-idaea, p = 0.024). The disruption to increases of E. hermaphroditum (figure 5a) and V. vitis-idaea (figure 5b) in the exclosures coincide with increases in the severity of damage caused by a number of host-specific parasitic fungi: A. empetri (figure 5a) and the sum of E. vaccinii, L. melaleucum and M. leptideum (figure 5b). The differences in disease severity between treatments were only statistically significant for E. hermaphroditum (F1,4 = 63.9, p = 0.001) and not for V. vitis-idaea due to large spatial variation (F1,4 = 1.3, p = 0.314). In the forest, the long period of linear increase in plant biomass is interrupted by a dramatic drop in 2012, since the total plant biomass and biomass of all common plants, except D. flexuosa, decreased in the exclosures in response to the geometrid moth outbreak in 2012 (figures 3b and 4). The decline in total plant biomass, and of the six common species, was dramatically stronger in the mammalian herbivore exclosures than in the grazed controls. In these exclosures, biomass that had accumulated over 14 years was eliminated within a few weeks, and consequently no statistically significant between-treatment differences remained for any species in August 2012 (B. nana, p = 0.221; D. flexuosa, 0.547; E. hermaphroditum, p = 0.690; V. myrtillus, p = 0.608; V. uliginosum, p = 0.219; V. vitis-idaea, p = 0.876).
Table 2.
F-values from statistical analyses (repeated measure ANOVA) of the responses of the six most common plant species to exclusion of all mammalian herbivores during 15 years in forest and nearby tundra. H = habitat, T = treatment, Y = year.
| H1,8 | T1,8 | H × T1,8 | Y14,112 | Y × H14,112 | Y × T14,112 | Y × H × T14,112 | |
|---|---|---|---|---|---|---|---|
| total plant biomass | 3.5 | 2.8 | 0 | 8.7*** | 2.5** | 7.2*** | 1.5 |
| Betula nana | 2.0 | 0.9 | 0.6 | 60.1*** | 9.3** | 13.4*** | 1.5 |
| Deschampsia flexuosa | 0.5 | 4.2* | 1.5 | ||||
| Empetrum hermaphroditum | 4.4 | 4.1 | 0.6 | 5.6*** | 1.2 | 3.0*** | 1.4 |
| Vaccinium myrtillus | 10.8* | 2.8*** | 0.6 | ||||
| Vaccinium uliginosum | 0.2 | 0.6 | 0.6 | 0.7 | 1.4 | 0.6 | 0.5 |
| Vaccinium vitis-idaea | 0.5 | 14.8** | 0.1 | 4.2*** | 0.5 | 1.8* | 0.6 |
*p > 0.05, **p > 0.01, ***p > 0.001.
Figure 3.
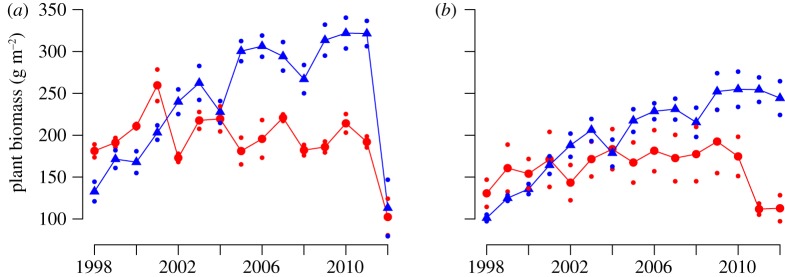
The plant biomass in grazed control plots (red, triangles) and mammalian herbivore-free exclosures (blue, dots) in (a) the forest and (b) the tundra during the period 1998–2012. The small dots represent ±1 s.e.m.
Figure 5.
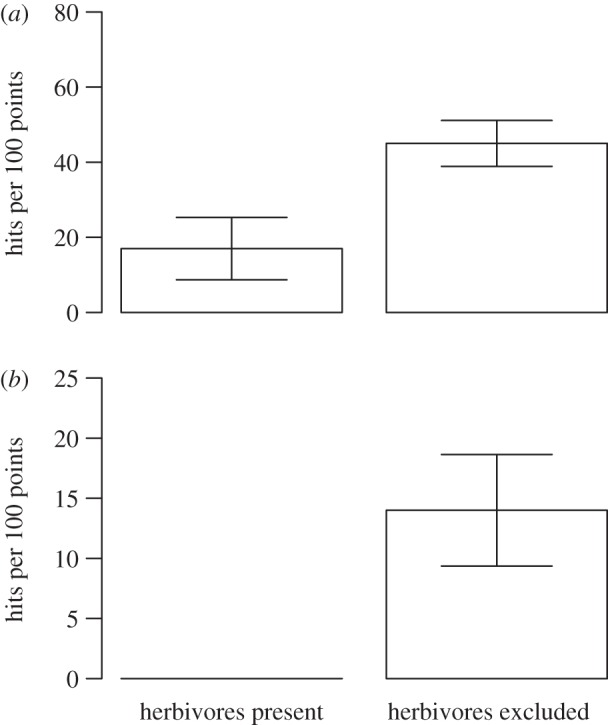
Cover index of (a) diseased E. hermaphroditum, attacked by the host-specific pathogenic fungus A. empetri, and (b) diseased V. vitis-idaea, attacked by three different host-specific pathogens, in grazed controls and mammalian herbivore-free exclosures in the tundra. Data from 2012. Error bars represent±1 s.e.m.
(d). Moth outbreak and Empetrum hermaphroditum browning
Although the moth outbreak caused E. hermaphroditum to decline in the forest as much as many other species, its mechanism differed since plants of the species rapidly browned, in spite of relatively modest feeding damage. Furthermore, the detailed analyses of sampled shoots in 2012 revealed that the response of E. hermaphroditum plants to the moth outbreak depended on their small-scale topographic position. The frequency of intact buds was over 25 per cent on small mounds around the birch tree trunks and 0 per cent in depressions (F2,83 = 33.4, p < 0.001, figure 6a). The direct moth herbivory was severe, but it differed along topographic gradients; more than 50 per cent of the top buds were damaged by herbivory on mounds, while only about 25 per cent were damaged in the depressions (figure 6b). However, even more top buds were killed by parasitic fungi, but the pattern was reversed with only 21.6 per cent of top buds diseased on mounds, and 73.4 per cent of the top buds diseased in depressions (F2,83 = 52.8, p < 0.001, figure 6c). About 60 per cent of the leaves from the current year's growth (C leaves) had feeding scars, fewer than 25 per cent of the leaves on the 1-year-older cohort showed any damage, and feeding signs on older leaf cohorts were negligible (figure 7b). E. empetri, which entirely dominated the pathogens in the forest, showed the opposite pattern: almost 100 per cent of the 3- and 2-year-old leaf cohorts were diseased, while less than 50 per cent of 1-year-old leaves showed any visible disease symptoms (F2,174 = 200.3, p < 0.001; figure 7a).
Figure 6.
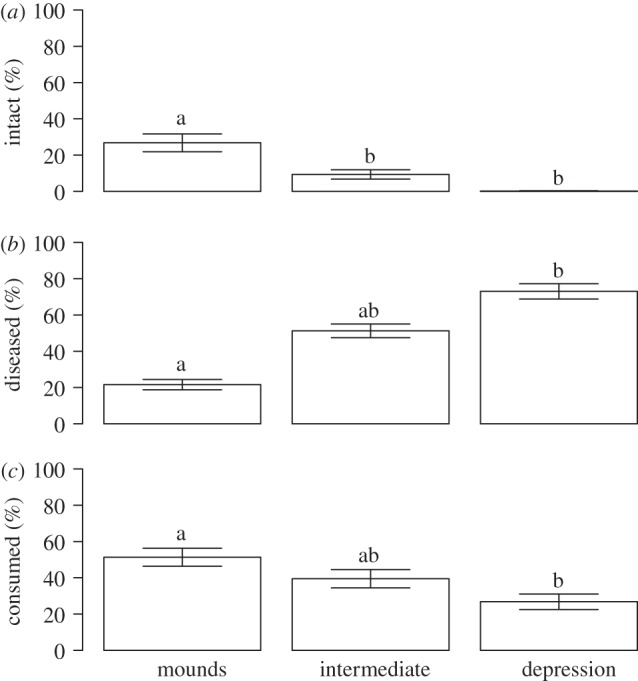
Percentages of top buds of E. hermaphroditum that were (a) intact, (b) killed by pathogenic fungi and (c) killed by herbivores in three different micro-topographic positions (mounds, intermediate and depressions) in transects from the forest in areas accessible to herbivores. Data from 2012. The error bars represent±1 s.e.m. Groups not sharing letters differ significantly.
(e). Large and small herbivores
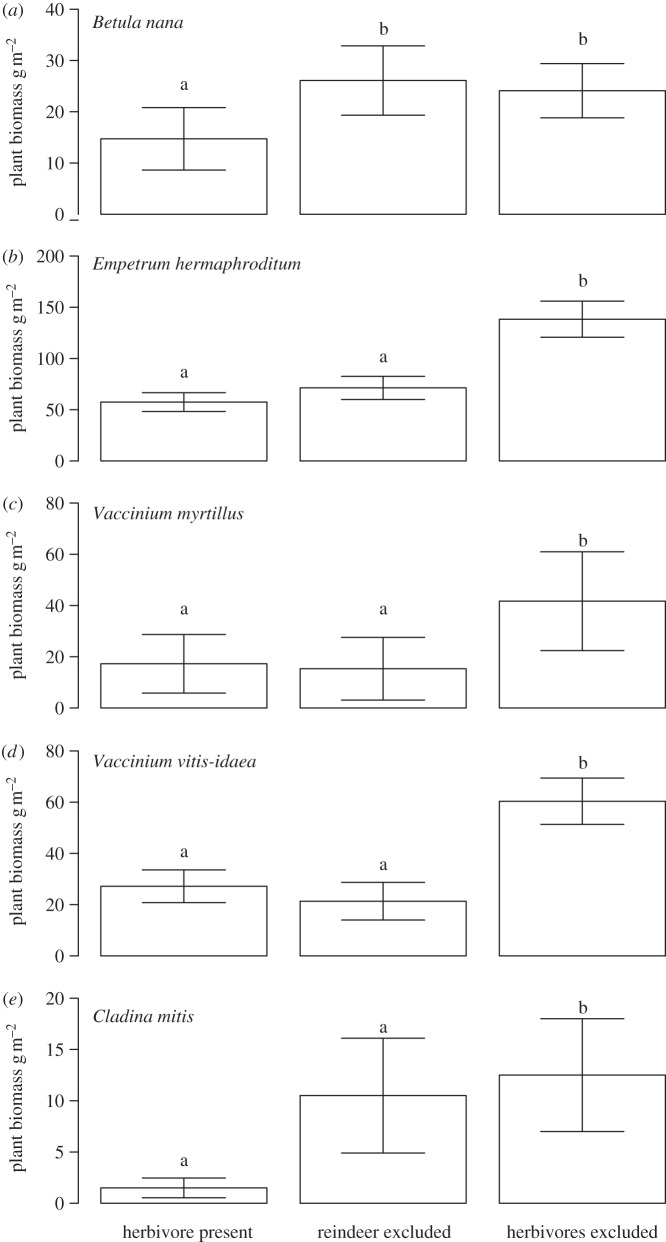
All data presented above are from the small mesh-size exclosures, excluding all mammalian herbivores. By contrasting large mesh-size exclosures and grazed controls, and small-mesh size exclosures with large mesh-size exclosures, we were able to analyse the effects of large and small herbivores separately. In 2012, after 15 years of herbivore exclusion, large herbivores, presumably reindeer, reduced the biomass of B. nana (figure 8a, F2,12 = 6.2, p = 0.048) and the lichen Cladina mitis (F2,12 = 18.7, p < 0.001; figure 8e). Small vertebrate herbivores, presumably voles and lemmings, reduced the biomass of E. hermaphroditum (F2,12 = 6.2, p = 0.014; figure 8b), V. myrtillus (F2,12 = 13.1, p = 0.003; figure 8c) and V. vitis-idaea (F2,12 = 5.6, p = 0.019; figure 8d).
Figure 8.
The plant biomass in grazed control plots, reindeer-free exclosures and all herbivore-free exclosures (rodent free + reindeer free) of (a–d) four of the six dominant plant species for which statistically significant differences were found, and (e) for the mat-forming lichen C. mitis in the tundra. Data from 2012. Error bars represent± 1 s.e.m. Groups not sharing letters differ significantly.
4. Discussion
This unique time-series demonstrates the importance of vertical biotic interactions such as herbivores and pathogens for the plant community composition. However, it also indicates that climate has strong direct effects on plants and pathogens, and how they interact. Our data show that the studied ecosystem has been profoundly affected by gradual changes in biotic and abiotic variables, regular pulses of biotic disturbance and sudden irregular biotic disturbances during the past 15 years. The vole and lemming cycles, which reduce plant biomass and change plant community composition every third to fourth year, are the most obvious examples of regular pulses, while the severe outbreaks of geometrid moths and plant pathogens are examples of sudden irregular events that profoundly affect the field-layer vegetation, at least within the time frame considered in this study.
As previously shown, the regular interannual density fluctuations of voles and lemmings drive synchronous fluctuations in the biomass of field-layer vegetation, and of NDVI estimates obtained from satellite images covering a 770 km2 area [7]. By analysing the data separately for the two habitats, we show here that the cycles in total plant biomass are more pronounced in the forest than in the open tundra, although year since the last rodent peak was still the only statistically significant predictor of total plant biomass, with no significant effect of temperature or precipitation in any of the two habitats. This finding may appear to conflict with a previous report that voles and lemmings influence low-productivity tundra vegetation more than productive forest floor vegetation, since the densities of herbivores are limited by predators in the latter habitat [32]. However, in our study the forests and tundra were separated by less than 100 m and did not differ in productivity, so significant differences in predation pressures between the two habitats are unlikely. Moreover, since the effects of the voles and lemmings on individual species did not differ between the two habitats, their impact is probably weaker in the tundra than in the forest because the tundra vegetation in this study is dominated by relatively unpalatable evergreen species.
This study confirms that voles and lemmings have stronger effects on the vegetation than reindeer by consuming and damaging more biomass, especially during the winter, when plants are unable to compensate for the lost tissue [7,17,40], although the succession towards a dominance of larger shrubs (i.e. B. nana) observed in the exclosures indicates that reindeer could be more important for the long-term vegetation changes observed in this area than previously assumed. Various density-dependent processes will increase in importance in response to increasing densities of host plants in exclosures, eventually resulting in outbreaks of biotrophic pathogens [4,46]. Thus, the initial increases of ericaceous dwarf shrubs in the absence of small rodents will after some time be inhibited by pathogens, while taller shrubs and lichens (which both are mainly influenced by reindeer), will continue to increase provided that climatic conditions are suitable [17,33,51,52]. Such shrubification may have cascading effects on the tundra ecosystem, since shrubs will increase the snow depth, thereby raising winter soil temperatures, microbial activity and plant-available nitrogen supplies [15]. In addition, it is likely to reduce biodiversity [52] and contribute to the regional warming by decreasing land surface albedo [34].
Outbreaks of caterpillars are important disturbance factors in boreal forest, subarctic forest and Arctic tundra [21,33,53,54]. Outbreaks of at least one of the geometrid moth species present in the study area, E. autumnata, occur at regular intervals of about 10 years, but the intensity of the outbreaks varies dramatically [26,53–55]. Severe outbreaks of E. autumnata and Operophthera brumata completely defoliate the birches and the larvae then move down and consume many of the common species in the understory [9,53,56–59], while less severe outbreaks only have minor effects on the forest structure and understory [53,59]. These previous findings are confirmed by our data, since the moth outbreak in 2004 only had minor effects on the field layer, while the outbreak in 2012 reduced plant biomass by more than 50 per cent. However, based on the level of defoliation of the birches, even the 2004 outbreak was actually quite severe [54], so most outbreaks may perhaps be expected to have only fairly limited effects on the field-layer vegetation. The latest outbreak that had severe effects on both the forest structure and field-layer vegetation in this study location took place in 1955 [57]. Our study confirms previous findings about vegetation responses to moth outbreaks, except for the dramatic decrease of V. vitis-idaea, which has been reported to be unaffected during earlier outbreaks [57], and the lack of increase of graminoids, which has often been reported [53,56,57,59] and which also took place during the 2004 outbreak. The latter may have been due to the cold summer of 2012 preventing graminoids from responding to the increased light following the extensive defoliation [60].
This study compares, for the first time, the impact of rodent peaks and geometrid moth outbreaks on field-layer vegetation of subalpine birch forest. The vegetation seems to respond to the two rodents and moth larvae in similar ways. Although deciduous shrubs were the preferred food for both types of herbivores, evergreen dwarf shrubs suffered more from the damage they caused. The only major difference was that B. nana was severely defoliated by the moth species, while voles and lemmings had minor effects on the species. However, although the severity of herbivore damage differed among rodent peaks, the difference in severity among moth outbreaks was much larger. Moreover, the effects of the moths were even more severe in the exclosures than in the grazed control plots, as biomass accumulated over 15 years in the absence of vertebrate herbivores was lost in only a few weeks during the moth outbreak. The stronger impact of moth in exclosures could be the result of excluding moth predators, since rodents potentially can predate on the moth larvae, but since rodent densities were low in 2012, this is unlikely. Instead, we believe that the higher density of food plants favoured both larvae and pathogens in the exclosures and thus increased their impact on the vegetation there.
Although the unpalatable evergreen E. hermaphroditum was much less defoliated than other dwarf shrubs in 2012, it suffered as much as other species from the geometrid moth outbreak; although it was only partly defoliated it rapidly browned within a few weeks. A similar rapid dieback was first observed in a moth outbreak in the same region in 1955 [57], and has been reported several times in northernmost Fennoscandia during the latest outbreak wave [9,59,61]. We show here that the dieback of E. hermaphroditum is related to the combined effects of moth herbivory and a common plant pathogen. The moth feeds on the leaves of the youngest age cohorts, while the pathogen persists on older leaf cohorts lower down in E. hermaphroditum thickets, and together they seem to have a dramatic effect on the sink–source dynamics of the host. Since the moth feeding was mainly confined to the current-year leaves and left older (C + 2 and C + 3) leaves almost untouched (figure 7), and leaves of the latest 3–4 cohorts are normally green (alive) in this area, E. hermaphroditum would presumably have remained predominantly green if subjected to herbivory alone. In the absence of the pathogen, the mortality of undamaged leaves would probably have been low, and carbon and nitrogen would have been translocated to surviving leaf cohorts, as observed in pines following outbreaks of various pest species [62–64]. However, the moth outbreak in 2012 coincided with very high levels of disease caused by a hemibiotrophic plant pathogen, E. sulcicola. During infection, plant pathogenic fungi synthesize and secrete proteins that suppress the immune system and reprogramme the infected tissue to become a source of nutrients needed for mycelial growth and reproduction of the fungus [65]. Thus, parasitic fungi, biotrophic and hemibiotrophic, act as sinks interfering with photosynthesis, respiration and translocation [66,67], and heavily diseased leaves may act as net importers of carbon and nitrogen [68]. It seems probable that the observed browning is a result of a decrease in net assimilation due to a direct negative source effect from moth defoliation in combination with a strong pathogen sink. Further, as defoliation of younger leaves often results in allocation of C and N to older leaves in evergreen plants [62,63,69], it seems reasonable that a similar response also in E. hermaphroditum, might favour the growth of E. sulcicola, and maybe drive its switch from biotrophic to necrotrophic nutrition. The moth outbreak could also have influenced the pathogen via massive deposition of moth faeces (frass), thereby greatly raising available nitrogen contents on the leaf surface, in the host plant, forest floor and soil [9,70]. This nitrogen fertilization could have direct positive effects on both pathogen infection [71] and pathogen growth [21,46]. In our case, it is probable that this fertilization has affected the environment on the leaf surface and resulted in enhanced infection success (cf. [71]). However, if so, that effect will not be visible until the following year when the first ascocarps start to appear.
Another striking pattern is that the impact of parasitic fungi varies with the topographic position. In depressions, almost all top buds were diseased, either by E. sulcicola or A. empetri, most probably because the thicker snow cover facilitates the growth of the mycelia [4]. With increasing snow cover, not only the lower part of the plants, but also the top buds will become available for infection, leading to shoot mortality. This shows that the importance of these pathogens varies within landscapes and that their impact on the plants is highly dependent on climatic conditions [4].
The observed changes in species' biomass between years and among treatments are likely to have large consequences for interactions between plants and ecosystem function in these ecosystems. Especially, the dramatic decrease of the dominant field-layer species, E. hermaphroditum, is expected to be important [4]. It frequently dominates the field-layer vegetation in boreal and subalpine forest, and Arctic and alpine tundra, it makes a major contribution to the productivity of the understory vegetation, and it is associated with retrogressive succession due to the accumulation of polyphenolic compounds it releases into the soil [72]. Thus, changes in the biomass of E. hermaphroditum are likely to have cascading effects on the functioning of the whole ecosystem [4,73]. The decrease of E. hermaphroditum is also expected to influence the occurrence of other plant species, but the direction is hard to predict, since both competitive and facilitative interactions are common in these ecosystems [74]. However, it is probable that the already existing graminoids, especially D. flexuosa [60,75], and lichens [76] will benefit from the increased light and nutrient availability following the disappearance of E. hermaphroditum and other dwarf shrubs. Moreover, E. hermaphroditum is known to prevent establishment and growth of other plants via allelopathic effects [75]. However, although existing experiments show a dominance of positive responses of other plants to removal of dominant dwarf shrubs in tundra heathlands, the response is often weak and the direction varies with abiotic conditions [75,77,78].
We show here that several biotic drivers, including plant pathogens and herbivores, influence plant abundance and community composition. These changes may in turn influence gross primary production and net ecosystem exchange [79–81]. However, climatic conditions also have strong effects on northern plant communities. Temperature and precipitation are known to drive plant growth in these cold ecosystems [12,13,24], which is also evident since these climatic variables explain part of the variation in plant biomass observed in this study. Climatic conditions also have strong indirect effects on the dynamics of the plant community, via influencing herbivores and pathogens. The population dynamics of Arctic herbivores, including voles, lemmings and reindeer, is known to be influenced by winter conditions [27,82,83]. Low winter temperatures are also known to kill moth eggs and limit plant damage [84], and snow conditions and temperatures are major factors regulating many plant pathogens [4]. Moreover, as exemplified by our data, climatic conditions can also influence the resilience and susceptibility of plants to herbivores and pathogens. The dynamics observed during the past 15 years suggests that warmer temperatures increase the capacity of plants to regrow following herbivore outbreaks. Increased temperatures can also decrease nutrient levels and influence the levels of defence substances in dwarf-shrub species [85]. The devastating browning of E. hermaphroditum seems to have been a result of climatic conditions not only favouring both the moth and the pathogen, but also contributing to a dramatic alteration of the nutrient source–sink dynamic in the host plant. This extensive E. hermaphroditum browning is most probably a rare event, and has not been observed in Abisko between 1955 [57] and 2012. Still, it is important to characterize these phenomena because both moths and pathogens have the potential to push Arctic and alpine ecosystems across tipping points [86], causing irreversible changes in the field-layer vegetation [4] and forest structure [26]. Furthermore, such changes may affect the carbon storage of these ecosystems more than decades of gradual climate change [4,73]. Since the importance of different biotic interactions varies not only due to intrinsic population cycles but also with the climatic conditions, long time series recording responses of many different biotic components of the ecosystems are needed in order to identify the key biotic interactions in ecosystems. Furthermore, to get a mechanistic understanding of the importance of various biotic interactions, manipulative experiments are needed. To understand how biotic interactions and climatic conditions interactively influence the dynamics of plant species and communities will be critical for our ability to predict future change. Variations in the climate have the potential to dramatically alter host—natural enemy interactions; however, critical weather conditions are often not easy to identify in standard analyses, since conditions during a few critical days might be more important than seasonal averages [87,88].
Acknowledgements
The authors are grateful to staff at Abisko Scientific Research Station for their essential assistance during this study. Detailed comments by the editor and two referees have been much appreciated.
Data accessibility
Data are available from the Dryad Digital Repository (doi:10.5061/dryad.38s21).
Funding statement
The work was financially supported by the Nordic Centre of Excellence TUNDRA, funded by the Norden Top-Level Research Initiative ‘Effect Studies and Adaptation to Climate Change’, the European Commission (ENV4-CT97–0586), the Swedish Research Council for Environment, Agricultural Science and Spatial Planning (2006–1539 and 2012–230) and Göran Gustafssons Stiftelse för Natur och Miljö i Lappland.
References
- 1.Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. 2011. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob. Change Biol. 17, 927–942 (doi:10.1111/j.1365-2486.2010.02301.x) [Google Scholar]
- 2.Zhao MS, Running SW. 2010. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329, 940–943 (doi:10.1126/science.1192666) [DOI] [PubMed] [Google Scholar]
- 3.Schmitz OJ, Hambäck PA, Beckerman AP. 2000. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 155, 141–153 (doi:10.1086/303311) [DOI] [PubMed] [Google Scholar]
- 4.Olofsson J, Ericson L, Torp M, Stark S, Baxter R. 2011. Carbon balance of Arctic tundra under increased snow cover mediated by a plant pathogen. Nat. Clim. Change 1, 220–223 (doi:10.1038/NCLIMATE1142) [Google Scholar]
- 5.Côte SD, Rooney TP, Tremblay JP, Dussault C, Walter DM. 2004. Ecological impacts of deer over abundance. Annu. Rev. Ecol. Evol. Syst. 35, 113–147 (doi:10.1146/annurev.ecolsys.35.021103.105725) [Google Scholar]
- 6.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306 (doi:10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 7.Olofsson J, Tømmervik H, Callaghan TV. 2012. Vole and lemming activity observed from space. Nat. Clim. Change 2, 880–883 (doi:10.1038/nclimate1537) [Google Scholar]
- 8.Jepsen JU, Kapari L, Hagen SB, Schott T, Vindstad OPL, Nilssen A, Ims RA. 2011. Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with subarctic birch. Global Change Biol. 17, 2071–2083 (doi:10.1111/j.1365-2486.2010.02370.x) [Google Scholar]
- 9.Kaukonen M, Ruotsalainen AL, Wäli PR, Männistö MK, Setälä H, Saravesi K, Huusko K, Markkola A. 2013. Moth herbivory enhances resource turnover in subarctic mountain birch forests. Ecology 94, 267–272 (doi:10.1890/12-0917.1) [DOI] [PubMed] [Google Scholar]
- 10.Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L. 2008. Mountain pine beetle and forest carbon feedback to climate change. Nature 452, 987–990 (doi:10.1038/nature06777) [DOI] [PubMed] [Google Scholar]
- 11.Serreze MC, et al. 2000. Observational evidence of recent change in the northern high-latitude environment. Climate Change 46, 159–207 (doi:10.1023/A:1005504031923) [Google Scholar]
- 12.Elmendorf SC, et al. 2012. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Change 2, 453–457 (doi:10.1038/NCLIMATE1465) [Google Scholar]
- 13.Gottfried M, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2, 111–115 (doi:10.1038/NCLIMATE1329) [Google Scholar]
- 14.Kullman L. 2002. Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. J. Ecol. 90, 68–77 (doi:10.1046/j.0022-0477.2001.00630.x) [Google Scholar]
- 15.Tape K, Sturm M, Racine C. 2006. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biol. 12, 686–702 (doi:10.1111/j.1365-2486.2006.01128.x) [Google Scholar]
- 16.Tømmervik H, Johansen B, Riseth JA, Karlsen SR, Solberg B, Hogda KA. 2009. Above ground biomass changes in the mountain birch forests and mountain heaths of Finnmarksvidda, northern Norway, in the period 1957–2006. Forest Ecol. Manage. 257, 244–257 (doi:10.1016/j.foreco.2008.08.038) [Google Scholar]
- 17.Olofsson J, Oksanen L, Callaghan TV, Hulme PE, Oksanen T, Suominen O. 2009. Herbivores inhibit climate-driven shrub expansion on the tundra. Glob. Change Biol. 15, 2681–2693 (doi:10.1111/j.1365-2486.2009.01935.x) [Google Scholar]
- 18.Salama NKG, Van den Bosch F, Edwards GR, Heard MS, Jeger MJ. 2012. Population dynamics of a non-cultivated biennial plant Tragopogon pratensis infected by the autoecious demicyclic rust fungus Puccinia hysterium. Funct. Ecol. 5, 530–542 (doi:10.1016/j.funeco.2011.12.009) [Google Scholar]
- 19.Laine K, Henttonen H. 1983. The role of plant-production in microtine cycles in northern Fennoscandia. Oikos 40, 407–418 (doi:10.2307/3544313) [Google Scholar]
- 20.Tast J, Kalela O. 1971. Comparisons between rodent cycles and plant production in Finnish Lapland. Ann. Acad. Fenn. A. IV Biologica 186, 1–14 [Google Scholar]
- 21.Nordin A, Strengbom J, Forsum A, Ericson L. 2009. Complex biotic interactions drive long-term vegetation change in a nitrogen enriched boreal forest. Ecosystems 12, 1204–1211 (doi:10.1007/s10021-009-9287-8) [Google Scholar]
- 22.Overpeck J, et al. 1997. Arctic environmental change of the last four centuries. Science 278, 1251–1256 (doi:10.1126/science.278.5341.1251) [Google Scholar]
- 23.Kohler J, Brandt O, Johansson M, Callaghan TV. 2006. A long record of Arctic snow-depth measurements from Abisko, northern Sweden, 1913–2002. Polar Res. 25, 91–113 (doi:10.1111/j.1751-8369.2006.tb00026.x) [Google Scholar]
- 24.Walker MD, et al. 2006. Plant community responses to experimental warming across the tundra biome. Proc. Natl Acad. Sci. USA 103, 1342–1346 (doi:10.1073/pnas.0503198103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaarlejärvi E, Eskelinen A, Olofsson J. In press. Herbivory prevents positive responses of lowland plants to warmer and more fertile conditions at high altitudes. Funct. Ecol. (doi:10.1111/1365-2435.12113) [Google Scholar]
- 26.Callaghan TV, et al. 2013. Ecosystem change and stability over multiple decades in the Swedish subarctic: complex processes and multiple drivers. Phil. Trans. R. Soc. B 368, 20120488 (doi:10.1098/rstb.20120488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Post E, et al. 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355–1359 (doi:10.1126/science.1173113) [DOI] [PubMed] [Google Scholar]
- 28.Gough L, Moore JC, Shaver GR, Simpson RT, Johnson DR. 2012. Above- and below-ground responses of Arctic tundra ecosystems to altered soil and nutrients and mammalian herbivory. Ecology 93, 1683–1694 (wos:000306829300019) [DOI] [PubMed] [Google Scholar]
- 29.Eskelinen A, Harrison S. 2012. Plant traits mediate consumer and nutrient control on plant community productivity and diversity. Ecology 93, 2705–2718. (10.1890/12–0393.1) [DOI] [PubMed] [Google Scholar]
- 30.Theodose TA, Bowman WD. 1997. Nutrient availability, plant abundance, and species diversity in two Alpine tundra communities. Ecology 78, 1861–1872 (doi:10.1890/0012-9658(1997)078) [Google Scholar]
- 31.Hobbie SE, Nadelhoffer KJ, Högberg P. 2002. A synthesis: the role of nutrients as constraints on carbon balances in boreal and Arctic regions. Plant Soil 242, 163–170 (doi:10.1023/A:1019670731128) [Google Scholar]
- 32.Aunapuu M, et al. 2008. Spatial patterns and dynamic responses of Arctic food webs corroborate the exploitation ecosystem hypothesis (EEH). Am. Nat. 171, 249–262 (doi:10.1086/524951) [DOI] [PubMed] [Google Scholar]
- 33.Post E, Pedersen C. 2008. Opposing plant community responses to warming with and without herbivores. Proc. Natl Acad. Sci USA 105, 12 353–12 358 (doi:10.1073/pnas.0802421105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapin FS, et al. 2005. Role of land-surface changes in Arctic summer warming. Science 310, 657–660 (doi:10.1126/science.1117368) [DOI] [PubMed] [Google Scholar]
- 35.Klanderud K, Ø Totland. 2005. Simulated climate change altered dominance hierarchies and diversity of an Alpine biodiversity hotspot. Ecology 86, 2047–2054 (doi:10.1111/j.1365-2745.2005.01000.x) [Google Scholar]
- 36.Klanderud K. 2008. Species-specific responses of an Alpine plant community under simulated environmental change. J. Veg. Sci. 19, 363–372 (doi:10.3170/2008-8-18376) [Google Scholar]
- 37.Grellmann D. 2002. Plant responses to fertilization and exclusion of grazers on an Arctic tundra heath. Oikos 98, 190–204 (doi:10.1034/j.1600-0706.2002.980202.x) [Google Scholar]
- 38.van der Wal R, Sjögersten S, Woodin SJ, Cooper EJ, Jonsdottir IS, Kuijper D, Fox TAD, Huiskes AD. 2007. Spring feeding by pink-footed geese reduces carbon stocks and sink strength in tundra ecosystems. Global Change Biol. 13, 539–545 (doi:10.1111/j.1365-2486.2006.01310.x) [Google Scholar]
- 39.Johansson C, Pohjola VA, Jonasson C, Callaghan TV. 2011. Multi-decadal changes in snow characteristics in subarctic Sweden. Ambio 40, 566–574 (doi:10.1007/s13280-011-0164-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olofsson J, Hulme PE, Oksanen L, Suominen O. 2004. Importance of large and small mammalian herbivores for the plant community structure in the forest–tundra ecotone. Oikos 106, 324–334 (doi:10.1111/j.1365-2486.2009.01935.x) [Google Scholar]
- 41.Olofsson J, Hulme PE, Oksanen L, Suominen O. 2005. Effects of mammalian herbivores on revegetation of disturbed areas in the forest-tundra ecotone in northern Fennoscandia. Landsc. Ecol. 20, 351–359 (doi:10.1007/s10980-005-3166-2) [Google Scholar]
- 42.Eriksson B. 1970. On ascomycetes on Diapensales and Ericales in Fennoscandia. I. Discomycetes. Symbolae Botanicae Upsaliensis, 19, 1–71 [Google Scholar]
- 43.Eriksson B. 1974. On ascomycetes on Diapensales and Ericales in Fennoscandia. II. Pyrenomycetes. Svensk Botanisk Tidskrift 68, 192–234 [Google Scholar]
- 44.Eriksson B. 1974. On deuteromycetes on Diapensales and Ericales in Fennoscandia. Svensk Botanisk Tidskrift 68, 235–253 [Google Scholar]
- 45.Eriksson OE. 2009. The non-lichenized ascomycetes of Sweden. Department of Ecology and Environmental Science, Umeå University [Google Scholar]
- 46.Wiedermann MM, Nordin A, Gunnarsson U, Nilsson MB, Ericson L. 2007. Global change shifts vegetation and plant–parasite interactions in a boreal mire. Ecology 88, 454–464 (doi:10.1890/05-1823) [DOI] [PubMed] [Google Scholar]
- 47.Perfect SE, Green JR. 2001. Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol. Plant Pathol. 2, 101–108 (doi:10.1016/j.pmpp.2007.07.007) [DOI] [PubMed] [Google Scholar]
- 48.Vargas WA, Sanz Martín JM, Rech GE, Rivera LP, Benito EP, Díaz-Mínguez JM, Thon MR, Sukno SA. 2012. Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in maize. Plant Physiol. 158, 1342–1358 (doi:10.1104/pp.111.190397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myllymäki A, Paasikallio AUH. 1971. Analysis of a ‘standard trapping’ of Microtus agrestis (L.) with triple isotope marking outside the quadrat. Ann. Zool. Fenn. 8, 22–34 [Google Scholar]
- 50.Ekerholm P, Oksanen L, Oksanen T. 2001. Long-term dynamics of voles and lemmings at the timberline and above the willow limit as a test of hypotheses on trophic interactions. Ecography 24, 555–568 (doi:10.1034/j.1600-0587.2001.d01-211.x) [Google Scholar]
- 51.Ravolainen VT, Bråthen KA, Ims RA, Yoccoz NG, Henden JA, Killengreen ST. 2011. Rapid, landscape scale responses in riparian tundra vegetation to exclusion of small and large mammalian herbivores. Basic Appl. Ecol. 12, 643–653 (doi:10.1016/j.baae.2011.09.009.) [Google Scholar]
- 52.Pajunen A, Virtanen R, Roininen H. 2012. Browsing-mediated shrub canopy changes drive composition and species richness in forest–tundra ecosystems. Oikos 121, 1544–1552 (doi:10.1111/j.1600-0706.2011.20115.x) [Google Scholar]
- 53.Tenow O.1972. The outbreaks of Oporinia autumnata Bkh. and Operophthera spp. (Lep., Geometridae) in the Scandinavian mountain chain and northern Finland in 1862–1968. Zoologiska bidrag från Upsala, Supplement 2, Uppsala.
- 54.Babst F, Esper J, Parlow E. 2010. Landsat TM/ETM plus and tree-ring based assessment of spatiotemporal patterns of the autumnal moth (Epirrita autumnata) in northernmost Fennoscandia. Remote Sens. Environ. 114, 637–646 (doi:10.1016/j.rse.2009.11.005) [Google Scholar]
- 55.Young AB, Cairns DM, Lafon CW, Moen J. In preparation. Autumnal moth (Epirrita autumnata) outbreaks and their climatic relations in northern Sweden.
- 56.Lehtonen J, Heikkinen RK. 1995. On the recovery of mountain birch after Epirrita damage in Finnish Lapland, with a particular emphasis on reindeer grazing. Ecoscience 2, 349–356 [Google Scholar]
- 57.Sandberg G. 1963. Växtvärlden i Abisko nationalpark. In Natur i Lappland. Svensk Natur (ed. Curry-Lindahl (red) K.), pp. 885–908 Uppsala, Sweden: Almqvist & Wiksells [Google Scholar]
- 58.Kallio P, Mäkinen Y. 1978. Vascular flora in Inari Lapland. IV. Betulaceae. Rep. Kevo Subarctic Res. Station 14, 38–63 [Google Scholar]
- 59.Jepsen JU, Biuw M, Ims RA, Kapari L, Schott T, Vindstad OPL, Hagen SB. 2013. Ecosystem impacts of a range expanding forest defoliator at the forest–tundra ecotone. Ecosystems 16, 561–575 (doi:10.1007/s10021-012-9629-9) [Google Scholar]
- 60.Strengbom J, Näsholm T, Ericson L. 2004. Light, not nitrogen, limits growth of the grass Deschampsia flexuosa in boreal forests. Can. J. Bot. 82, 430–436 (doi:10.1639/0044-7447(2005)034[0020:NDATBO]2.0.CO;2) [Google Scholar]
- 61.Karlsen SR, Jepsen JU, Odland A, Ims RA, Elvebakk A. 2013. Outbreaks by canopy-feeding geometrid moth cause state-dependent shifts in understory plant communities. Oecologia. (doi:10.1007/s00442-013-2648-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ericsson A, Hellqvist C, Långström G, Larsson S, Tenow O. 1985. Effects on growth of simulated and induced shoot pruning by Tomicus piniperda as related to carbohydrate and nitrogen dynamics in Scots pine. J. Appl. Ecol. 22, 105–124 (doi:10.2307/2403331) [Google Scholar]
- 63.Palacio S, Hernández R, Maestro-Martínez M, Camarero JJ. 2012. Fast replenishment of initial carbon stores after defoliation by the pine processionary moth and its relationship to the re-growth ability of trees. Trees—Struct. Funct. 26, 1627–1640 (doi:10.1007/s00468-012-0739-y) [Google Scholar]
- 64.Lyytikäinen-Saarenmaa P. 1999. The response of Scots pine, Pinus sylvestris, to natural and artificial defoliation stress. Ecol. Appl. 9, 469–474 (doi:10.1890/1051761(1999)009[0469:TROSPP]2.0.CO;2) [Google Scholar]
- 65.Koeck M, Hardman AR, Dodds PN. 2011. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell Microbiol. 13, 1849–1857 (doi:10.1111/j.1462-5822.2011.01665.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farrar JF. 1992. Beyond photosynthesis: the translocation and respiration of diseased leaves. In Pests and pathogens: plant responses to foliar attack (ed. Ayres PG.), pp. 107–127 Oxford, UK: Bios Scientific [Google Scholar]
- 67.Bancal MO, Hansart A, Sache I, Bancal P. 2012. Modeling fungal sink competitiveness with grains for assimilates in wheat infected by a biotrophic pathogen. Ann. Bot. 110, 113–123 (doi:10.1093/aob/mcs094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robert C, Bancal MO, Ney B, Lannou C. 2005. Wheat leaf photosynthesis loss due to leaf rust, with respect to lesion development and leaf nitrogen status. New Phytol. 165, 227–241 (doi:10.1093/aob/mcm163) [DOI] [PubMed] [Google Scholar]
- 69.Ericsson A, Larsson S, Tenow O. 1980. Effects of early and late season defoliation on growth and carbohydrate dynamics in Scots pine. J. Appl. Ecol. 17, 747–769 (doi:10.2307/2402653) [Google Scholar]
- 70.Frost CJ, Hunter MD. 2004. Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology 85, 3335–3347 (doi:10.1890/04-0003) [Google Scholar]
- 71.Strengbom J, Nordin A, Näsholm T, Ericson L. 2002. Parasitic fungus mediates change in nitrogen-exposed boreal forest vegetation. J. Ecol. 90, 61–67 (doi:10.1046/j.0022-0477.2001.00629.x) [Google Scholar]
- 72.Nilsson MC, Wardle DA. 2005. Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 3, 421–428 (doi:10.1890/1540-9295(2005)003[0421:UVAAFE]2.0.CO;2) [Google Scholar]
- 73.Hartley IP, Garnett MH, Sommerkorn M, Hopkins DW, Fletcher BJ, Sloan VL, Phoenix GK, Wookey PA. 2012. A potential loss of carbon associated with greater plant growth in the European Arctic. Nat. Clim. Change 2, 875–879 (doi:10.1038/NCLIMATE1575) [Google Scholar]
- 74.Brooker RW, et al. 2008. Facilitation in plant communities: the past, the present, and the future. J. Ecol. 96, 18–34 (doi:10.1111/j.1365-2745.2007.01295.x) [Google Scholar]
- 75.Nilsson MC, Wardle DA, Zackrisson O, Järderud A. 2002. Effects of alleviation of ecological stresses on an Alpine tundra community over an eight-year period. Oikos 97, 3–17 (doi:10.1034/j.1600-0706.2002.970101.x) [Google Scholar]
- 76.Cornelissen JHC, et al. 2001. Global change and Arctic ecosystems: is lichen decline a function of increases in vascular plant biomass? J. Ecol. 89, 984–994 (doi:10.1111/j.1365-2745.2001.00625.x) [Google Scholar]
- 77.Gerdol R, Brancaleoni L, Menghini M, Marchesini R. 2000. Response of dwarf shrubs to neighbor removal and nutrient addition and their influence on community structure in a subalpine heath. J. Ecol. 88, 256–266 (doi:10.1046/j.1365-2745.2000.00445.x) [Google Scholar]
- 78.Bret-Harte MS, Garcia EA, Sacre VM, Whorley JR, Wagner JL, Lippert SC, Chapin FS. 2004. Plant and soil responses to neighbor removal and fertilization in Alaskan tussock tundra. J. Ecol. 92, 635–647 (doi:10.1111/j.0022-4477.2004.00902.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaver GR, Rastetter EB, Salmon V, Street LE, van de Weg MJ, Rocha A, van Wijk MT, Williams M. 2013. Pan-Arctic modelling of net ecosystem exchange of CO2. Phil. Trans. R. Soc. B 368, 20120485 (doi:10.1098/rstb.2012.0485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shaver GR, Street LE, Rastetter EB, Van Wijk MT, Williams M. 2007. Functional convergence in regulation of net CO2 flux in heterogeneous tundra landscapes in Alaska and Sweden. J. Ecol. 95, 802–817 (doi:10.1111/j.1365-2745.2007.01259.x55) [Google Scholar]
- 81.Cahoon SMP, Sullivan PF, Shaver GR, Welker JM, Post E. 2012. Interactions among shrub cover and the soil microclimate may determine future Arctic carbon budgets. Ecol. Lett. 15, 1415–1422 (doi:10.1111/j.1461-0248.2012.01865.x) [DOI] [PubMed] [Google Scholar]
- 82.Kausrud KL, et al. 2008. Linking climate change to lemming cycles. Nature 456, 93 (doi:10.1038/nature07442) [DOI] [PubMed] [Google Scholar]
- 83.Hansen BB, Grotan V, Aanes R, Saether BE, Stien A, Fuglei E, Ims RA, Yoccoz NG, Pedersen AO. 2013. Climate events synchronize the dynamics of a resident vertebrate community in the High Arctic. Science 339, 313–315 (doi:10.1126/science.1226766) [DOI] [PubMed] [Google Scholar]
- 84.Niemelä P. 1979. Topographical delimitation of Oporinia-damages: experimental evidence of the effect of winter temperature. Rep. Kevo Subarctic Res. Station 15, 33–36 [Google Scholar]
- 85.Kaarlejärvi E, Baxter R, Hofgaard A, Hytteborn H, Khitun O, Molau U, Sjögersten S, Wookey P, Olofsson J. 2012. Effects of warming on shrub abundance and chemistry drive ecosystem-level changes in a forest–tundra ecotone. Ecosystems 15, 1219–1233 (doi:10.1007/s10021-012-9580-9) [Google Scholar]
- 86.Scheffer M, et al. 2012. Anticipating critical transitions. Science 338, 344–348 (doi:10.1126/science.1225244) [DOI] [PubMed] [Google Scholar]
- 87.Shaw MW, Osborne TM. 2011. Geographic distribution of plant pathogens in response to climate change. Plant Pathol. 60, 31–43 (doi:10.1111/j.1365-3059.2010.02407.x) [Google Scholar]
- 88.Burdon JJ, Thrall PH, Ericson L. 2006. The current and future dynamics of disease in plant communities. Ann. Rev. Phytopath. 44, 19–39 (doi:10.1146/annurev.phyto.43.040204.140238) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Dryad Digital Repository (doi:10.5061/dryad.38s21).