Abstract
The subarctic environment of northernmost Sweden has changed over the past century, particularly elements of climate and cryosphere. This paper presents a unique geo-referenced record of environmental and ecosystem observations from the area since 1913. Abiotic changes have been substantial. Vegetation changes include not only increases in growth and range extension but also counterintuitive decreases, and stability: all three possible responses. Changes in species composition within the major plant communities have ranged between almost no changes to almost a 50 per cent increase in the number of species. Changes in plant species abundance also vary with particularly large increases in trees and shrubs (up to 600%). There has been an increase in abundance of aspen and large changes in other plant communities responding to wetland area increases resulting from permafrost thaw. Populations of herbivores have responded to varying management practices and climate regimes, particularly changing snow conditions. While it is difficult to generalize and scale-up the site-specific changes in ecosystems, this very site-specificity, combined with projections of change, is of immediate relevance to local stakeholders who need to adapt to new opportunities and to respond to challenges. Furthermore, the relatively small area and its unique datasets are a microcosm of the complexity of Arctic landscapes in transition that remains to be documented.
Keywords: subarctic environment, climate change impacts, ecosystem stability
1. Introduction
Although climate change is occurring in the Arctic twice as fast as in most other places on the Earth [1,2], it is not the only driver of ecosystem change in northern lands [3]. Habitat fragmentation, resource extraction, pollution, changes in land use and ultraviolet-B radiation increases all act together to affect ecosystem services. These services are essential for Arctic residents (provisioning services, such as game and berries) and for the global community (regulatory services, such as carbon capture and release). Although changes in Arctic ecosystems have been documented [4–11], attribution to a particular driver remains uncertain in some cases [12,13]. This is because of the multiple co-occurring drivers and also because any one driver such as climate warming can act on various processes within an organism and on interacting species among trophic levels [4].
Recently, it has been shown that ecosystem responses to climate change vary among northern regions [10,14] and even within one area undergoing the same climatic changes [15,16]. Furthermore, events that disturb ecosystems can counterbalance or even over-compensate for long-term changes [17,18]. In fact, less than 40 per cent of the Arctic has ‘greened’ in the past 30 years despite widespread warming [11]. With this complexity in mind, our aim here is to compile and analyse multi-decadal changes in a range of terrestrial ecosystems from the subarctic of northern Sweden. This area has an unrivalled history of northern environmental observation over 100 years [19,20]. We show that many and diverse responses of ecosystems to climatic and other changes can be observed in one catchment, that climate acts directly and indirectly on ecosystems, and that past land use and short-lived (hours to weeks) extreme events can also have long-term impacts (annual to decadal) that sometimes over-ride long-term, multi-decadal trends driven by climate change. We show conclusively that local knowledge is essential for understanding the cause and potential futures of ecosystems at the wider pan-Arctic scale. This study also develops a platform and new geo-referenced baseline against which future projections of climate-driven ecosystem change can be validated and refined [21–23] as a tool to help local residents and authorities to adapt to climate change and their impacts [24].
2. Methods and study area
This synthesis is based on numerous papers, unpublished reports and theses, historical records, old photographs, site re-visits, data from control plots of long-term experiments, dendrochronolgy and remote sensing (aerial photography and satellite imagery). In addition, some of the sources used are themselves syntheses of multiple studies performed as contributions to the International Polar Year Project ‘Back to the Future’ [6]. The text summarizes the results while the methods behind each study are presented in electronic supplementary material, table S1. Furthermore, the results from each study in terms of change or stability of a variable are geo-referenced and presented in a series of figures (figures 2–4) as well as digitally in electronic supplementary material, table S1. The study starts with an overview of changes in important drivers of change in ecosystems and then presents the complexity of numerous types of vegetation response to these drivers.
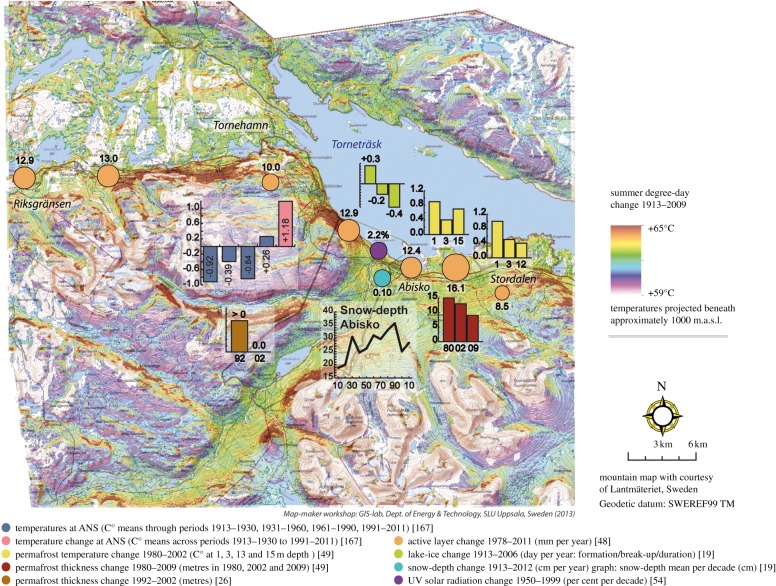
Figure 2.
Abiotic changes in the Abisko region, subarctic Sweden in relation to changes in summer degree days between 1913 and 2009. The location of objects is not exact in congested areas, but optimized to retain overall neighbourhood relationships while maximizing the number of exact positions. See electronic supplementary material, table S1 for exact positions, methodology and data sources. Map prepared by T. Thierfelder.
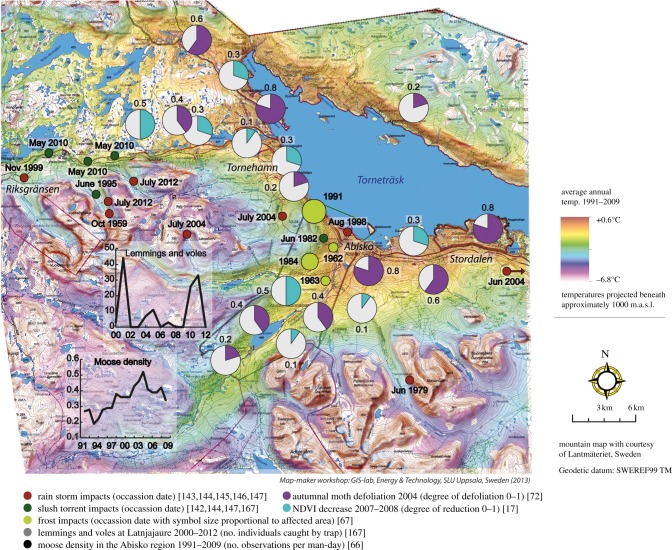
Figure 4.
Impact of rapid events in the Abisko region, subarctic Sweden in relation to average mean annual temperatures 1991–2009. The location of objects is not exact in congested areas, but optimized to retain overall neighbourhood relationships while maximizing the number of exact positions. See electronic supplementary material, table S1 for exact positions, methodology and data sources. Map prepared by T. Thierfelder.
We compile data for a region that includes the northwest catchment of Lake Torneträsk and some areas lying on the border of northern Norway and Sweden (see figure 1 and electronic supplementary material, table S1). The area is diverse topographically (342 m.a.s.l. to more than 1900 m.a.s.l. [25]) and climatically with a northwest–southeast oceanic-continental gradient and local rain shadow effects owing to the Scandes mountains. Climate has been monitored since 1913 at the Abisko Scientific Research Station (ANS) [26]. Currently (2002–2011), mean annual temperature is +0.49°C while the seasonal mean temperatures from spring to winter are −0.82, +10.9, +1.1 and −9.2°C, respectively (ANS data). The ANS has also been the centre of many dispersed observations of climate and ecosystems over the past 50 years or more [19,20]. For example, climate at the mid-alpine Latnjajaure Field Station, one of the satellite stations of the Abisko Station, has been monitored since 1990 (year-round since 1992 [27]). Climate downscaling to 50 m resolution for the Abisko area shows that temperature at the 50 m scale is determined mainly by topography in summer and proximity to the large lake (332 km2) in winter [28,29]. Overall, the results show important long-term trends (see figure 3 and electronic supplementary material, table S1) as well as periodic extreme events (see figure 4 and electronic supplementary material, table S1; see below).
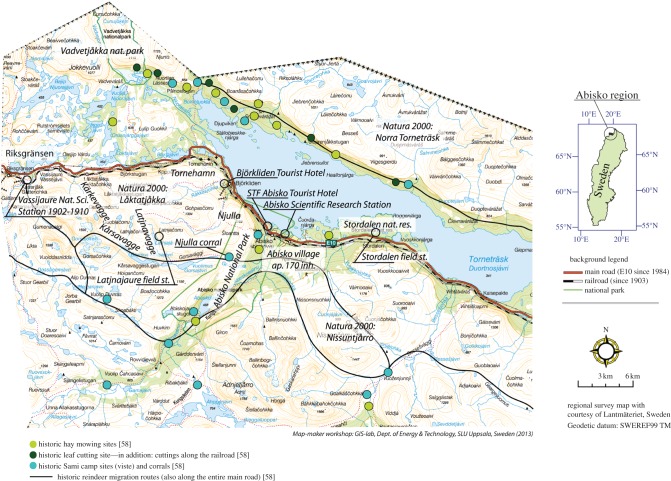
Figure 1.
Land use and infrastructures in the Abisko region, subarctic Sweden. The location of objects is not exact in congested areas, but optimized to retain overall neighbourhood relationships while maximizing the number of exact positions. See electronic supplementary material, table S1 for exact positions, methodology and data sources. Map prepared by T. Thierfelder.
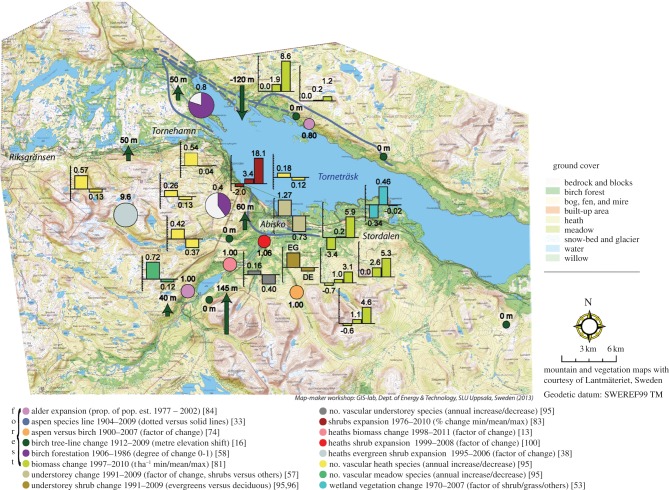
Figure 3.
Vegetation change in the Abisko region, subarctic Sweden through the last century in relation to the distribution of vegetation types. The location of objects is not exact in congested areas, but optimized to retain overall neighbourhood relationships while maximizing the number of exact positions. See electronic supplementary material, table S1 for exact positions, methodology and data sources. Map prepared by T. Thierfelder.
The latest succession stage for the warmest parts of the region is represented by needle-leaf coniferous forest. In the warmer, southern part of the region, the forest is dominated by Norway spruce (Picea abies (L.) Karst.) but this species is absent from the study area. In contrast, Scots Pine (Pinus sylvestris) exists as an exclave population in the warm inner Abisko valley [30,31]. Most of the forest is deciduous and dominated by birch (Betula pubescens ssp. czerepanovii) that can grow in either the polycormic form (multiple stems) or as single-stemmed trees (monocormic) on warmer, well-drained and nutrient-rich sites [32]. The birch forest reaches an altitudinal limit of approximately 600 m.a.s.l. in the western parts and approximately 800 m.a.s.l. in the eastern parts of the Torneträsk catchment [30]. Within the birch forest, isolated clones of aspen (Populus tremula L.) and individuals of alder (Alnus incana (L.) Moench.), rowan (Sorbus aucuparia L.), species of willow (Salix spp.) and bird cherry (Prunus padus L.) occur [33]. The soils are dominated by till, colluvium and glaciofluvial deposits, although peat occurs in lowland depressions.
Soil nutrient content decreases from the west to east but the soils are nutrient-rich in the central part [34]. These climatic and edaphic conditions are associated with a distinctive vegetation composition and distribution: the most widespread plant communities are the birch forest (B. pubescens ssp. czerepanovii), which occurs either as a heath or meadow-type according to the understorey vegetation, heath vegetation (e.g. Arctostaphylos alpina (L.) Sprengel, Arctostaphylos uva-ursi (L.) Sprengel, Betula nana L., Empetrum nigrum L., Vaccinium myrtillus L., Vaccinium vitis-idaea L., Vaccinium uliginosum L., Phyllodoce caerulea (L.) Bab., Juncus trifidus L.) [34], meadow (e.g. Bistorta vivipara (L.) Gray, Calamagrostis lapponica (Wahlenb.) Hartm., Filipendula ulmaria (L.) Maxim., Geranium sylvaticum L., Potentilla crantzii (Crantz) Beck ex Fritsch, Ranunculus acris L., Rumex acetosa L., Trollius europaeus L.) [34,35], and snowbed communities (e.g. Carex bigelowii Torr. ex Schwein, Cassiope hypnoides (L.) D. Don, Gnaphalium supinum L., Sibbaldia procumbens L.) [36], with other sparse plant communities such as bogs (with mosses such as Dicranum fuscescens, Kiaeria starkei, Oligotrichum hercynicum, Pohlia spp., Polytrichum hyperboreum) [37]. Remnants of the typical low Arctic tussock tundra are found in the mid-alpine zone in flat areas on mineral soil with permafrost (now degrading [38]).
The Abisko area has a rich avifauna [39] and there are several mammal species including reindeer (Rangifer tarandus), moose (Alces alces), European brown bear (Ursus arctos), red fox (Vulpes vulpes), wolverine (Gulo gulo), lynx (Lynx lynx), mountain hare (Lepus timidus), Norwegian lemming (Lemmus lemmus) and voles (predominantly Myodes rufocanus). The species that most affect vegetation and predators are reindeer, moose, lemmings and voles, and two geometrid moth species. Small rodents, especially voles and lemmings, are herbivores in northern ecosystems [13,40,41]. In most boreal and Arctic ecosystems, rodents normally experience regular inter-annual population density cycles of 3–5 years [42]. The two geometrid moth species that naturally disturb the birch forest vegetation in the Abisko area are the autumnal moth (Epirrita autumnata) and the winter moth (Operopthera brumata). Both species exhibit a more or less cyclic population fluctuation pattern.
3. Changing drivers of ecosystem structure and function
(a). Temperature
Statistically smoothed climate trends between 1913 and 2006 show a warm period in the late 1930s and early 1940s but a recent warming that statistically significantly exceeds the earlier warming by 0.88°C over the year, by 1.3°C in spring and by 0.40°C in autumn [19]. Over the whole period 1913–2006, mean annual air temperatures increased by 2.5°C, winter and spring temperatures each increased by 2.9°C and the autumn temperature increased by 1.6°C: summer temperatures have not risen significantly (95% confidence limits) in the recent warming or over the twentieth century despite an increase of 1.7°C. At Latnjajaure, the mean annual air temperature has increased steadily since 1992 at a rate of ca 1°C per decade [43]. Temperature extremes in the Abisko area have been most notable in winter when extreme warming events for just a few days result in brief excursions above 0°C, snow-thaw and re-freeze to create ice layers [17]. Records of layers of ice and hard snow in snow profiles from ANS show that the frequency and intensity of winter thaws have increased between 1960 and 2010 [44]. At fine-scale resolution (50 m grid cells), modelled summer degree-day temperature change [28] ranges between 59°C and 65°C (see figure 2 and electronic supplementary material, table S1). Also at this fine scale, the average annual temperature between 1991 and 2009 varies from −6.8 to +0.6°C across the study area (see figure 4 and electronic supplementary material, table 1). There is a notable diminishing of differences in the rate of warming between north- and south-facing slopes (see figure 2 and electronic supplementary material, table S1). Solar radiation (at high northern latitudes) primarily heats south-facing slopes, so as there was less sunshine owing to increased cloudiness over time (ANS data), warming of southern slopes by solar radiation was reduced relative to north-facing slopes. The increased cloudiness at Abisko is consistent with pan-Arctic modelling studies that show an increase in Arctic cloudiness [45,46]. To some extent, these findings mitigate against the concept that fine-scale temperature heterogeneity will create refuges for resident plant species and prolong their existence during warming [47].
(b). Precipitation
Summer precipitation was relatively constant for the first half of the twentieth century and then increased significantly (p = 0.09) from 117 mm in 1959 to 143 mm in 2006 [19]. Currently (2002–2011), mean annual and mean summer precipitation totals are 332 and 138 mm yr−1, respectively (ANS data). Extreme rainfall events (more than 20 mm d−1) have been recorded throughout the twentieth century. However, ‘extremes of extremes’—more than 30 mm per day—occur at intervals of approximately 15 years (with wide variation) [48] and damage landscapes (see figure 4 and electronic supplementary material, table S1) and infrastructure (see figure 1 and electronic supplementary material, table S1). Since 1956, each ‘extreme of extremes’ has been a new record for the instrumental period [48] and in 2004, for instance, over 61 mm of rainfall (about 20% of the annual average) fell in one day [19].
(c). Snow
Snow depth increased during the twentieth century [49] but since the 1980s, there has been an accelerating decrease ([19], see figure 2 and electronic supplementary material, table S1). Between 2002 and 2011, the mean snow depth during the main snow cover season (November–April) is 25.6 cm (ANS data). Snow duration has decreased significantly by 0.1 week yr−1 at high elevations between 1978 and 2007 to 27 weeks (p = 0.018), while at low altitude, spring snow thaw has become significantly earlier by 0.12 week yr−1 over the same period (p = 0.001) [47]. Snow stratigraphy, representing winter weather, has changed since 1960: the incidence of increased and more complete thaw events led to an increase in hard snow/ice layers in the snow pack between 1961 and 2009 [44].
(d). Permafrost and lake ice
Since the 1980s there has also been an accelerating loss of lowland permafrost [50] and lake ice [19] (see figure 2 and electronic supplementary material, table S1). Monitoring of permafrost (by measuring active layer thickness and permafrost temperature) at nine mires along an east–west transect shows increases in active layer thickness (determined by probing) and decreases in permafrost thickness (determined from increases in permafrost temperature): complete loss of permafrost occurred at three mires ([50]; figure 2, electronic supplementary material, table S1). Borehole temperature measurements at three of those mires between 1980 and 2002 show increased temperature (by between 0.4°C and 1°C) and decreased permafrost thickness through thaw at the surface and at depth (probably because of ground water flow) [51]. Aerial colour infrared images from 1970 and 2000 show thawing permafrost at the lowland Stordalen Mire that has resulted in an increase in areas of open water [52], the expansion of wet habitats and a decrease in dry, hummock sites [53]. In contrast, permafrost thaw at higher altitudes has resulted in the rapid drainage of shallow lakes on periglacial rocky silt: there are two cases in the Latnja valley and another lower one in Kårsavagge; draining proceeded rapidly between 2006 and 2010 and the former lake bottoms remain almost unvegetated (Ulf Molau 2012, personal observation). These contrasting local changes in surface hydrology mirror those occurring at much larger geographical scales throughout the Arctic [54,55]. The changes in surface hydrology associated with thawing permafrost directly drive changes in plant communities (see §4a(v)).
Lake ice on the large Lake Torneträsk has melted earlier and formed later each year giving an overall reduction in duration of lake ice of about 40 days between 1913 and 2006 (figure 2; [19]). The dynamics of ice on the large lake are likely to affect vegetation via the temperature regime on land as winter temperatures on land surrounding the lake are affected more by the lake than by topography in winter [28,29]. This effect could become stronger as ice cover decreases further and is similar to the large-scale effect of loss of sea ice that enhances the greening of the Arctic [5].
(e). Ultraviolet-B radiation
Models of UV at Abisko show a statistically significant increase of 2.2 per cent per decade from 1950 to 1999, resulting mainly from thinning stratospheric ozone but also to some extent from varied sunshine duration and snow albedo [56]. These increases could potentially affect the local ecosystems [57].
(f). Land-use and infrastructure development
The land-use of the Torneträsk area by a relatively small population was dominated by fishing and hunting up to the seventeenth century, but more intensive reindeer husbandry developed between the seventeenth century and ca 1920 [58] with a peak in reindeer population of 120 000 around 1890 [59] compared with the current (2010) reindeer population of 50 000 [60]. Although reindeer densities have fluctuated over time, data show no consistent trend of increasing or decreasing densities over the past century. The intensive period of reindeer husbandry had a substantial impact on both the vegetation's productivity and structure as well as on the treeline location (see §4a(i)), as the Sami reindeer herders often stayed close to the treeline with their animals [58]; figure 1; electronic supplementary material, table S1 and figure S1). During the past ca 100 years, extensive reindeer husbandry has resulted in a larger proportion of the landscape being used by reindeer, and thus a more even but reduced grazing pressure. Also, parts of the Torneträsk area have been grazed by goats (especially around the reindeer herding camps) and cattle around farms [58].
Farming also affected the vegetation during the last part of the nineteenth century and the beginning of twentieth century, but ceased in the 1940s. Hay cutting on both mires and on man-made meadows as well as birch leaf cutting was extensive ([58]; figure 1; electronic supplementary material, table 1).
The Abisko area is important for its fishing and hunting resources. The two most common important prey species are moose (Alces alces) and ptarmigan (Lagopus spp.). Ptarmigan hunting attracts many visitors from Sweden and abroad. Most lakes and rivers are accessible by licence to fishing tourists and the local public. The main catch is Arctic char (Salvelinus alpinus), brown trout (Salmo trutta) and grayling (Thymallus thymallus). Recent climate warming has made it possible for pike (Esox lucius) to invade waters to the west from the eastern part of the Abisko area [61].
The Abisko region is attractive as an area for berry picking, in particular for cloudberry (Rubus chamaemorus), lingon berry (V. vitis-idaea) and billberry (V. myrtillus). Berry picking on public or private land is free in Sweden. Berry picking is carried out both for private consumption and for commercial (foreign and domestic) purposes.
During the construction of the railway between Kiruna and Narvik in the first years of the twentieth century, extensive areas of the birch forest were cut in an estimated 1000 m wide zone along the railway [62] and pine trees were harvested from the Abisko valley [58,62]. Much later, in the 1940s, tall birch tree trunks were cut on the north shore of Lake Torneträsk and brought across the frozen lake by horses and trucks to provide fuel for Abisko and even Kiruna (Anders Eriksson 2000, personal communication).
When the railway was opened, the first tourist hotel was built in Abisko in 1903. The environmental impact of tourism (wild-life experiences, mountain-walks and cross-country skiing) during the first half of the twentieth century was small, although tourism from the 1940s to 1980 steadily increased [58,63]. From the 1940s, tourist complexes developed at only a few locations because the area was accessible only by rail. During the 1950s and 1960s, major downhill ski constructions were built in Abisko, Björkliden and Riksgränsen. A new road between Kiruna and Narvik was constructed between 1979 and 1984. The road occupied about 40 ha km−1, in total 530 ha for the Swedish part of the road, but secondary expansions at ski and hotel areas reached a further ca 340 ha by 1987 [64]. Despite early concerns about possible environmental impacts (Mats Sonesson 1976, unpublished data), subsequent research has revealed limited effects so far [64] and the road has stimulated tourism and the local economy.
The study area is a major centre for conservation with two national parks and two nature reserves. The Abisko National Park (77 km2; figure 1) was inaugurated in 1909. More than half its area is mountain birch forest. Vadvetjåkka National Park (26 km2; figure 1), an area mostly of treeless mountains, was inaugurated in 1920 but unlike the Abisko National Park, it has neither trails nor amenities. The two nature reserves (figure 1), Stordalen (1980, 11 km2) and the area around ANS (1982, 55 ha), were created to protect rich bird-life and to secure areas for field-based research, respectively. Together, these four protected areas are part of the Natura 2000 network, that also includes two areas south of Lake Torneträsk (Låktatjåkka and Nissuntjårro), as well as the entire mountain area north of the lake.
As early as 1902, a research station was developed at Katterjokk, west of Abisko a few kilometres from the Norwegian border, in a disused railway building. When the building burned down in 1910 a new facility was located at Abisko, i.e. the Abisko Scientific Research Station. Meteorological monitoring and scientific research started in 1913. Currently, a wide range of research (plant ecology, climate impacts, climatology and geo-science) is carried out by several hundred visiting scientists per year from around the world. Most of the research is carried out in the valleys and mountains south of Lake Torneträsk. Many manipulation studies have been established. They focus on impacts of increased temperature, atmospheric carbon dioxide concentration and UV-B radiation, as well as snow accumulation [20,65].
(g). Animals
Statistics show that from the middle of the nineteenth century to the mid-1990s the number of reindeer within the Norrbotten County (the northernmost county in Sweden) varied between 100 000 and 200 000 [66]. In the Sámi villages of Talma, Gabna and Laevas in the Abisko area, intensively managed reindeer densities peaked at approximately 15 000 in about 1860, while extensively managed reindeer densities peaked at approximately 35 000 in the 1930s [16,58].
Moose populations increased in the area during the twentieth century [67]. Increases in the latter part of the twentieth century were probably owing to the removal of the top predator, wolves (Canis lupus), in the 1960s and earlier [68] and perhaps also by climate warming that has increased forage availability. Statistics from annual observations of moose by hunters in the Abisko area show an increase from 1991 to 2009 of approximately 0.2–0.5 observations per man-day with a peak of 0.52 in 2004 [69]. Although it is difficult to translate this index into numbers of individual moose, other observations indicate that 70–110 moose currently live in the Abisko valley in wintertime. Approximately, one quarter of these animals stay in the valley year-round and the others migrate into neighbouring valleys and even to the Norwegian coastline in summer (Caroline Stolter 2012, personal communication).
The two geometrid moth species (the autumnal moth, E. autumnata, and the winter moth, O. brumata) reach a population density peak approximately every 9–11 years [70]. During most population peaks, tree defoliation levels by the moth's caterpillars do not exceed 15 per cent [71]. However, dating back to 1800, dendrochronological analysis, historical reports and field surveys have identified several moth population peaks that reached outbreak densities [41,72–75]. On average, a birch forest area will experience a severe outbreak every 60–70 years causing high tree or stem mortality [74,76].
In most boreal and Arctic ecosystems, rodents normally experience regular interannual population density cycles of 3–5 years [42], but the amplitude and regularity of these cycles have been reported to be declining during the past decades in response to warmer winter climate and denser snow [42,77]. Clear evidence for this is, however, limited to rodents at the southern edge of their distribution [77,78]. At Abisko, regular rodent trapping has been carried out only since 1998 ([13]; Ulf Molau 2012, personal observation), but in various heath and meadow plant communities. However, during this period, vole densities (predominantly Myodes rufocanus) fluctuated with a regular 3–4 year cycle, and lemming populations peaked during three of the vole peaks, albeit with a more variable amplitude. There are no indications that the cycle characteristics are changing at Abisko, as has been reported for other northern ecosystems [13,41].
4. Responses to changing drivers
(a). Ecosystem responses to long-term trends in drivers
(i). Forest tree distribution and growth
Evergreen needle-leaf trees spruce and pine. Changes in species composition have been relatively small, though important locally. Norway spruce has not yet entered the study area. Scots pine, which expanded during the first warm period of the twentieth century when saplings were ‘shooting up everywhere’ [79], showed no recorded increased distribution by 1996 [31] although on the south slope of Slåttatjåkka, some 2 m tall pine individuals have been found at 45 m higher elevation in the past decade [80]. In contrast, and consistent with [31], small declines in pine were recorded between 1997 and 2010 by Hedenås et al. [81]. The general lack of expected response of pine to recent warming is probably an effect of browsing by increased populations of moose. Pine sown from seeds are now 1 m high in herbivore exclosures whereas they are less than 20 cm high in grazed control plots (Johan Olofsson 2012, personal observation). Furthermore, continued suppression of tree crowns to below winter snow height exposes the trees to heavy infection by the fungus Phacidium infestans (snow blight) [31].
Aspen. Changes in aspen distribution have shown complex trends (see figure 3 and electronic supplementary material, table S1). Overall, aspen increased its distributional range during the twentieth century by spreading northwestwards and by increasing in altitude [33,82]. Over the past 100 years, it became 16 times more abundant and has reached tree-size at the alpine treeline, an ecotone that has been dominated by birch for approximately the past 4000 years. In one location in the Abisko valley (figure 3; electronic supplementary material, table S1), aspen was found to cover 15.4 m2 in 2010 in plots that together cover 7500 m2, whereas the species was absent in 1976/1977 [83]. Reduction in competition by birch that was periodically defoliated by moth caterpillars was in general important for aspen establishment in the subalpine zone. In contrast, some aspen clones in the lowlands have been remarkably stable in at least one area between 1978 and 2008 despite higher recruitment and growth rates in aspen than in neighbouring birch ([74]; figure 3; electronic supplementary material, table S1). Like the pine population in the Abisko valley, the lowland aspen population is heavily browsed by moose. However, in contrast to birch that periodically is defoliated by insects, aspen is only slightly impacted (100-year-old birch trees suffered on average 9.0 years of significantly reduced growth compared with only 1.4 years in aspen: see below). The net result appears to be a dynamic during a warming climate when insect defoliation of birch reduces competition and allows aspen recruitment but subsequent increases in moose browsing of aspen prevent further expansion ([74]; figure 3; electronic supplementary material, table S1).
Alder, willows and rowan. Grey alder (A. incana) has increased its elevational and distributional ranges since 1977 [84] in the west of the study area (figure 3; electronic supplementary material, table S1). In 1977, it was not recorded in transects that were later revisited in 2006 when the trees were seen. It had much faster growth than neighbouring birch and had a similar growth rate to aspen. In more extensive sampling, alder showed an increase in growth from 150 to 210 kg ha−1 between 1997 and 2010 [81]. In the same study, rowan (S. aucuparia) showed a decrease from 8 to 1 kg ha−1 over the same period and willow (Salix spp.) also decreased, from 64 to 52 kg ha−1. Over a smaller area of 150 m2 studied by Rundqvist et al. [83], rowan less than 3.5 cm in DBH increased its overall area from 17.5 to 23.6 m2, an increase of 28 per cent , between 1976/1977 and 2009/2010 (although the number of stems larger than 3.5 cm decreased from a total of six to zero). Recent uphill range extension of rowan has also been documented in nearby Kårsavagge [82].
Birch. Since the beginning of the twentieth century, the forest has become denser and treelines have risen in elevation, partly as a response to changing reindeer husbandry practices [16,58,62,74,85,86]. An extensive and rigorous recording of changes in vegetation dominated by birch between 1997 and 2010 showed that the percentage cover of ‘birch forest of heath type with mosses’ increased significantly from 8.7 to 22.9 per cent [81]. As the changes were mainly in existing birch forest, this can be seen as forest densification. A densification of the birch forest near treeline, as recorded in general in northern Norway [87] was observed as early as the early 1930s [79] in the early twentieth century's first warm period [19]. The densification was also documented in 1977 at the start of the current warming period at the north and south sides of Lake Torneträsk [88] and near Pålnoviken within the Abisko area [58] (figure 3; electronic supplementary material, table S1). This was attributed to the early twentieth century warm period (1930s and 1940s) and reduced reindeer grazing (1960s and 1970s). Later, local densification in the Abisko valley between 1976/1977 and 2009/2010 reached a 600 per cent increase in the area of birch (a total area increase in plots from 122 to 809 m2, [83], figure 3; electronic supplementary material, table S1). Although recent climate warming has probably stimulated growth and regeneration of birch, post-1976 densification of the birch forest near the treeline is also mainly attributed to a continued slow and long-term recovery from intense reindeer browsing damage. In contrast, on the north shore of Lake Torneträsk, forest death has occurred following a winter moth (O. brumata) outbreak in 1964/1965 ([70]; see below).
Birch treeline dynamics also show contrasting patterns and drivers as elsewhere in the Arctic [89]. Maximum increase in altitude of the subalpine birch forest reached 145 m since 1912 at a rate of 1.5 m yr−1 vertically and 2.7 m yr−1 in actual distance ([16]; figure 3; electronic supplementary material, table S1) and birch seedlings have recently invaded alpine vegetation [82,90,91]. Defining treeline as the elevational limit of the highest 2 m tall birch, upslope shifts up to 225 m (3 m yr−1 vertically) have been reported between 1950 and 2010 [80]. In other areas (figure 3; electronic supplementary material, table S1), the subalpine birch forest elevational limit decreased as a result of a winter moth outbreak in 1964/1965 on the north side of Lake Torneträsk, while in still other areas (figure 3; electronic supplementary material, table S1) this vegetation boundary has been stable for nearly 100 years because of steep mountain slopes and unsuitable soils for tree growth [16]. Overall, in four out of eight treeline sites studied by Van Bogaert et al. [16] the subalpine birch forest had advanced and the net displacement was an increase in elevation of 24 m at an average rate of +0.2 m yr−1. Surprisingly, this overall expansion of the birch forest is correlated more with release from heavy reindeer grazing pressure and more intensive herding practices several decades before recent warming (even if evidence of this impact is decreasing in visibility: electronic supplementary material, figure S1) than with recent climate warming in summer [16] that has not been significant (see §3a). However, there may be a synergy between the two processes. In addition, treeline advance can be facilitated by the ascending shrub zone (see §4a(ii)) that can protect tree seedlings from damage from high solar radiation and herbivory [92]. Within the climate warming of the twentieth century, treeline has therefore shown all three possible responses: upward expansion, stability and downward retreat, each resulting from a different local driver and with climate warming playing a relatively minor and sometimes secondary effect. An important conclusion, however, is that local knowledge of site history is an essential factor in interpreting mechanisms of vegetation change.
There are few measurements of northern deciduous tree growth in the study area, and indeed elsewhere in northern Fennoscandia. However, re-sampling of an extensive programme of recording tree and understorey growth in 1997 [93] has shown substantial overall growth (figure 3; electronic supplementary material, table S1) but variable small-scale trends [81]. Tree (mainly birch) growth in the alpine-birch forest ecotone averaged a 19 per cent increase in biomass, from 3507 kg ha−1 to 4176 kg ha−1. However, values for individual plots (radius 5 m) ranged from a decrease of −3.4 t ha−1, through values of no change to substantial increases of 8.6 t ha−1 [81]. Other tree species comprised about 7 per cent of the 1997 total tree biomass values. Much of the variability was again probably associated with site history in that an E. autumnata outbreak defoliated much of the birch area in 2004 ([75]; figure 4; electronic supplementary material, table S1; see below) and tree growth in some areas was reduced compared with uninterrupted growth in areas not defoliated.
Understorey plant community. In 1983, species composition was recorded at a birch forest site [94] which was re-sampled in 2008 by Hedenås et al. [95], who found a decrease in species richness that could be attributed to the dramatic increases in tree and shrub growth demonstrated by Rundqvist et al. [83] for nearby plots. Data collected from an ongoing experiment in the birch understorey between the ANS and the southern lake shore [96] has shown that although the dwarf shrub understorey communities have been exposed to periodic insect outbreaks (e.g. 2004 and 2012) the percentage cover of three out of four dwarf shrub species has increased in recent years (Dylan Gwynn-Jones 2012, personal observation): a total of nearly 27 per cent increase in dwarf shrub cover (V. vitis-idea, 16.1%, E. nigrum, 0.2% and V. uliginosum 11.2% (V. myrtillus cover decreased by 1%)) was recorded between 1991 and 2009. This has meant a reduction in bare ground and cover of other species including mosses and lichens.
At a birch forest site to the southeast of the Abisko village (figure 3), comparison of baseline site data taken in 1991 (prior to experimental manipulations) in a dwarf shrub heathland under birch [97] with another survey taken in 2009 at the same site [98] suggests different trends: the main evergreen dwarf shrub E. nigrum has declined in abundance over this time period although, as in the study above, the other evergreen dwarf shrub, V. vitis-idaea, has increased. The main deciduous dwarf shrubs, V. myrtillus and V. uliginosum have shown rather subtle decreases in abundance, V. myrtillus trends agreeing with those in the previous study whereas the V. uliginosum trends strongly contrasted with this study. However, grasses, Cladonia lichens (the latter again contrasting with the previous study) and the main bryophyte Hylocomium splendens show some indications of increases in cover over this time period. Caution in interpretation of the changes should be taken since many of them are not large and sometimes involve infrequent species, so they could readily arise from differences in observers between years. Overall, the differences in trends of the dwarf shrub species cover from site to site and the increase in lichens are not totally consistent with the changes expected [87,99,100].
These changes at the community level at the birch forest site to the southeast of the Abisko village were not supported by measurements of annual stem growth and leaf mass per shoot in the dwarf shrubs measured in 1992 [101] and again in 2008 [102] in control plots from the same manipulation study. Only leaf mass per shoot in E. nigrum showed some increase between these years, though such single-season shoot-level measurements will be heavily influenced by inter-annual variation in climate that reduces any signal of long-term climate trend changes.
(ii). Tall shrub distribution and growth
It is perhaps the expansion of tall shrubs in the tundra [8,10,103–105] that has recently focused attention on vegetation change in the Arctic. In the Abisko area, tall shrubs are important in the birch forest at treeline and beyond, and in riparian habitats within the forest. Probably, the first recorded increase in shrub distribution at treeline (between 1937 and 1959) was recorded by Sandberg [79]. More recently, a general increase in shrub distribution has been documented throughout the area depicted in figure 2 by several studies.
Throughout an extensive area from treeline to lake on both shores of Lake Torneträsk ([81]: figure 3; electronic supplementary material, table S1), the mean percentage cover increases of shrubs between 1997 and 2010 include 7.8–8.9 for willow, 14.0–20.1 for dwarf birch, 1.4–2.1 for juniper and 0.2–0.3 for other shrub species. In a longer term study (1976/1977 to 2009/2010) of three 50 × 50 m plots on the east-facing slope of the Abisko valley, overall shrub expansion was again documented: dwarf willow area increased by an average of 107 per cent and willows increased by an average of 189 per cent [83]. In recent years, saplings of the normally subalpine willow Salix phylicifolia L. have established in nutrient-rich snowbed meadows at Latnjajaure in the mid-alpine zone [36,38]. However, in contrast to the general increase in shrub cover, juniper cover decreased by 19 per cent. This decrease in measured juniper cover contrasts with its upward extension at or above treeline inferred from dendrochronological analyses by Hallinger et al. [106].
Although Hallinger et al. [106] showed positive correlations between treeline juniper growth and summer warming, this area is recovering from intensive reindeer grazing and human impacts, and the increased shrub growth is likely to be a combination of both effects, particularly as summer temperature increases have been modest [19]. Furthermore, the upward shrub expansion (mainly Salix) recorded by Sandberg [79] occurred in a period without warming trends. Also, experimental exclosure of small rodents and reindeer in the same area has shown conclusively that herbivores moderate the response of vegetation in general to climate warming [12,13]. Since reindeer grazing probably is less intense in the areas close to the treeline [16,62], it can be expected that at least some component of the shrub responses is owing to reduced grazing pressure. Also, if small rodent population peaks should decline in the future as they have done in the south of Norway [77], this could lead to increase of plant biomass and changes in plant community structure.
(iii). Heaths
In 1997, heaths covered 54 per cent of the extensive area sampled by Dahlberg et al. [93]. By 2010, this area was 59 per cent [81] but the increase was not significant. Possible changes in species composition were studied in five heaths. Plant species were recorded in 1984 by Headley et al. [107] on Mount Njulla, in 1984 by Carlsson & Callaghan [108] near the summit of Mount Slåttatjåkka, by Svensson et al. [109] in 1992 near the ANS, by Emanuelsson [110] in 1977–1979 in Kärkevagge and in 1989 by the Swedish Environmental Protection Agency programme for monitoring of environmental quality (Program för övervakning av miljökvalitet; PMK) [94] again at Mount Slåttatjåkka. The vegetation studied at Slåttatjåkka [94] included some elements of low-herb meadow, and the vegetation at Kärkevagge studied by Emanuelsson [110] contained some elements of low-herb meadow and snowbeds. All the sites were re-sampled in either 2008 or 2009 [95].
The re-sampling of the Headley et al. [107] site showed that between 1984 and 2008, one species was lost of a total of 41 original species whereas 13 species were gained. At the Carlsson & Callaghan [108] site, three of the original nine species were lost and six were gained between 1986 and 2009. At the Svensson et al. [109] site, between 1992 and 2009, two of the original 30 species were lost and three were gained. At the Emanuelsson [110] site, four species of the original 37 were lost between 1977/1979 and 2009 whereas 17 were gained. At the PMK [94] Slåttatjåkka site, between 1989 and 2008, seven of the original 36 species were lost and eight were gained.
Overall, and including an open meadow site (see §4a(iv)), total species number increased over the sampling period and substantially at some sites. This contrasts with the overall decrease expected from warming experiments [99] although observations on species changes in cold region open vegetation vary between increases and decreases [95]. Although an increase in thermophilic species would be expected, no overall increase was observed [95]. Cover changes were species-specific with no overall trends, but two graminoid species (C. lapponica and Carex vaginata) and a low shrub (Salix reticulata) increased in cover as would be expected from warming experiments [99–101].
In a climate manipulation study in a wet heath southwest of Abisko ([111]: figure 3), it was found that the total shrub cover in the control plots (deciduous plus evergreen) increased by 6 per cent between 1999 and 2008 [10], while total graminoid cover declined. This was consistent with vegetation responses in the manipulated plots exposed to a decade of summer warming [111].
(iv). Meadows
Meadows covered 5.1 per cent of the extensive and representative area surveyed by Dahlberg et al. in 1997 [93] and 7.5 per cent of the area in 2010 [81], but once again the increase was not statistically significant. The re-sampling of PMK plots established in a meadow at Påtjujaure in 1983 by Hedenås et al. [95] in 2008 showed a significant increase in 11 species and a decrease in seven. Equisetum arvense and E. scirpoides/variegatum decreased in cover [95], perhaps in response to recent decreases in snow depth (see [112] which describes this effect for the area surrounding Wellington Bay on southeastern Victoria Island, Canada). At Latnjajaure also, the species turnover in alpine meadow vegetation has been very significant over the past 20 years [10].
(v). Wetland vegetation
Wetland vegetation including bogs, fens and mosaic mires is estimated by Christensen et al. [113] to cover 3.9 per cent of the entire Torneträsk catchment. This vegetation covered 4.0 per cent of the area sampled by Dahlberg et al. [93] in 1997 and 4.2 per cent when re-sampled in 2010 by Hedenås et al. [81]. However, a survey of the vegetation changes at the subarctic Sphagnum mire at Stordalen (figure 3; electronic supplementary material, table S1) by aerial photography and ground survey in 1970 and 2000 showed changes in surface stability resulting in large increases in wetland habitats and decreases in dry hummock vegetation [52,53,114]. The increased area of wetlands was associated with an increase in graminoids whereas changes in the hummocks resulted in a decrease in evergreen dwarf shrubs and mosses, but an increase in lichens. Increases in wetland vegetation were associated with permafrost degradation whereas decreases in hummock vegetation were related to changes in spring temperatures and decreases in snow cover. These permafrost/vegetation changes have profound effects on ecosystem functioning in terms of the atmospheric exchanges of greenhouse gases such as carbon dioxide and methane [114]. The stability of the lowland permafrost in palsa mires has been found to be strongly dependent on snow cover [115,116]. An increase in snow cover depth or duration insulates permafrost from low winter temperatures thereby raising its temperature. The increased moisture resulting from increased snow amount and permafrost thaw affect vegetation productivity and composition. An analysis of the effect of the permafrost thaw on ecosystem functioning shows increased productivity in the treatment plots of snow accumulation experiments despite a longer lying snow cover and, hence, shorter growing season. Increased moisture availability, greater active layer thickness and subsequent changes in species composition caused the thawing plots to be more productive in terms of carbon uptake when considering the growing season as a whole [117].
In the Abisko area there are a few remaining patches of tussock tundra dominated by Arctic cottongrass (Eriophorum vaginatum), which is a main component of the circumpolar low Arctic vegetation cover [118]. These patches are found in flat areas on mineral soil (silt dominated) close to mid-alpine lakes, the largest one located at Latnjajaure. The tussock tundra at Latnjajaure has been monitored since 1992 within the ITEX project. Here, permafrost was still present in the early 1990s but totally gone 10 years later. Repeated analysis of the plant cover in control plots in 1995 and 2006 revealed a drastic change in the plant community from a total dominance of E. vaginatum to a more boreal heathland community. During the 12-year study period of final permafrost degradation, the boreal dwarf shrub V. vitis-idaea increased 10-fold in biomass in control plots, a response similar to that observed for this species in the lowland birch understorey (see §4a(i)). During the same period, formerly water-filled boulder pits were drained and vegetated by a pioneer bryophyte community dominated by Dicranella subulata [27].
(vi). Changes in snowbed habitats and their vegetation
Snowbed habitats and their vegetation are likely to be particularly vulnerable to the increased warming and reduced snow cover duration and depth (that result in summer moisture stress) recorded in the past decades at Abisko (figure 2). The extensive re-survey of vegetation by Hedenås et al. [81] showed that both snowbeds at their margins (‘moderate snowbeds’) and snowbeds on rocks and boulders mainly at high altitude (‘extreme snowbeds’) had decreased in cover from 13.9 to 7.4 per cent and from 0.37 to 0 per cent, respectively, between 1997 and 2010. The demise of snowbed vegetation was seen through a densification of the vegetation and ingression of surrounding species [36,119,120]. Although climate change must play a major role in the demise of snowbed vegetation, snowbeds are preferred habitats of lemmings, and reduced lemming grazing owing to the unfavourable snow conditions documented above could have led to the expansion of graminoids [1,121]. Snowbeds are also important in reindeer husbandry as they provide downhill meadows with a constant water supply throughout the summer, and are used as retreats for herds during warm days with particularly intense flying insect activity [36].
Two bird species depending on snowbeds for their foraging, snow bunting (Plectrophenax nivalis) and ptarmigan (Lagopus muta), have decreased drastically in the mid-alpine Latnja valley since 1990; the breeding population of the former has decreased by more than 50 per cent [27]. In contrast, in the same area, there is a marked increase in the breeding population of the bluethroat (Luscinia svecica) [27]. However, studies in general of the bird life in the Torneträsk region over the past decades show no general or obvious trends in population pattern of eight species but some variability [122].
(vii). Colonization of glacier forefields
Within the Abisko area, the Kårsa glacier has been retreating at least over the past century and has retreated dramatically over recent decades according to ground and aerial photography as well as ground surveys [123–125]. Although the terminal moraines have been estimated to be of several hundred years of age using lichenometry [126], and the glacial front has retreated approximately 300 m over the last 100 years, no information is yet available on the vegetation succession that has occurred there during recent climatic warming.
(viii). High alpine vegetation
Although the vegetation composition of the mountain summits was to some extent described by Fries [127] and Du Rietz [128] in the early twentieth century, the precise locations of the observations are difficult to re-locate as early measurements of altitude are flawed [84]. In later years, a more precise long-term monitoring programme for alpine vascular plants has been initiated within the network GLORIA (GLobal Observatory Research Initiative in Alpine areas). In a study including all major European mountain areas, a small but significant increase in the number of species was noted in the high alpine sites around Abisko (Latnjatjårro at 1300 m and Mount Kårsatjåkka at 1560 m.a.s.l. between 2001 and 2008 [129,130]. These changes are small compared with the species turnover observed in the Alps and the Pyrenees and in many other plant communities in the Abisko area.
(b). Ecosystem responses to drivers occurring as events
Changes in vegetation and animal populations are driven relatively slowly by long-term climate change but tipping points may be reached quickly by events such as extreme weather, fire, insect pest and disease outbreaks. In the Abisko area, fire has been unimportant in shaping the vegetation for thousands of years [131].
(i). Abiotic environmental events
Warm winters. Harsh winter weather conditions have regularly led to crashes in the reindeer populations of northern Sweden [132,133]. A typical weather pattern leading to a population crash is a winter thaw event after the first snowfall. This causes ice encapsulation of the ground vegetation, blocking the reindeer's access to fodder resources. Snow accumulation after ground-icing together with wind action increases the hardness of the snowpack which reduces the accessibility of reindeer fodder and may lead to reindeer starvation and death [133]. Herders may move the reindeer or feed them to avoid loss of animals, but especially before the herding practices were modernized, this was often impossible, thereby leading to large losses such as during the crisis winters of 1905/1906 and 1934/1935 [134]. Despite modernization, this type of winter climate continues to cause population crashes in reindeer herds [135].
Lemming and vole populations are also affected by extreme winter warming events [77]. An extreme event with heavy rainfall in January 2002 formed an ice crust in the snow pack that abruptly ended an ongoing population peak of the Norwegian lemming (Ulf Molau 2012, personal observation).
Experimental and observational determinations of the impacts of extreme winter thaw events on vascular plants [17,136], mosses and lichens [137], fungi [138], soil arthropods [139] and ecosystem processes [136] have become evident only recently. Results from experimental thaws during winter were validated by a natural thaw in northern Norway and Sweden in 2007 that reduced NDVI (Normalized Difference Vegetation Index) by almost 26 per cent over an area of at least 1400 km2 [17], although the rodent peak [13] and a plant pathogen outbreak [140] may have also contributed to this decline. However, the recovery from most of this vegetation damage took only 2 years [141]. Experiments and the natural event observed in 2007 showed that the evergreen dwarf shrub E. nigrum was particularly damaged with up to 34 per cent loss of its biomass [136]. The co-occurring dwarf shrubs V. myrtillus and V. vitis-idaea were also heavily impacted whereas V. uliginosum and the grass Deschampsia flexuosa were more resilient. The species-specific nature of the responses of the species to winter thaw events and the small-scale heterogeneity of snow thaw might play a role in the patterning of the subarctic plant communities [138]. Thaw events affected the moss H. splendens (but not the lichen Peltigera aphthosa: [137]) and also reduced the abundance of fungal fruiting bodies [138].
Slope processes and flooding. The geomorphological and hydrological activity in the Torneträsk region is fairly intense and very unevenly spread over the year as in most humid, periglacial and mountainous areas [142]. The geomorphological and societal importance of extreme events has been studied since the 1950s [143,144] (Rolf Nyberg 1985, unpublished data) showing that the two major periods of activity are during snowmelt and late summers/autumns with heavy rainfall.
Intense snowmelt periods have triggered slush torrents, but the spatial and temporal distributions of these very intense, sudden and very brief processes are relatively unknown [145]. However, they have a major impact on local landscape development and vegetation cover, and they are a potential hazard to people and infrastructures. Minor slush avalanches/torrents are frequent within vast areas of the Torneträsk mountains but there have also been recent, but rare, major events that affect infrastructures including the road and railway [146]. The last major slush torrents in the Torneträsk area occurred in 1982 (Mount Njulla), 1995 (Kärkevagge) [147] and 2010 (Låktavagge, Kärkevagge) (Christer Jonasson 2012, personal observation) (figure 4; electronic supplementary material, table 1). The events are not associated with years of deep snow and they seem to have been triggered stochastically by short-term weather conditions operating over a few days rather than by climatic trends (Christer Jonasson 2012, personal observation).
In the Torneträsk region, heavy rainstorms in late summer or autumn have caused considerable flooding, increased sediment transport and damage to infrastructure. Several major severe flooding/erosion events have occurred during the past decades; October 1959, June 1979, July 1983 [147], August 1998 [148], November 1999 [148], July 2004 [19,149] and July 2012 (Christer Jonasson 2012, personal observation). During the 1999 and 2004 events, there was heavy damage to the railway and road [146,150] and slope detachment on many mountain sides. The frequency of these extreme climate events appears to have remained stable over the instrumental period but the intensity has increased [48]. It can be expected, therefore, that such damage will increase in the future: adaptation measures need to be taken to protect infrastructure, whereas knowledge is needed on the dynamic interaction between vegetation development related to climate trends, the extent of damage owing to individual events and the rate of recovery during climate warming.
Temperature inversions. Extreme temperature inversions occur in the Abisko valley: temperature differences between nearby localities at altitudes of 379 and 655 m.a.s.l. could be as much as 24°C [151]. Such inversions can cause frost damage to birch trees and pine [70,152]. Dieback of birch in an altitudinal zone of 410–430 m.a.s.l. on Mount Njulla in 1991 is thought to have been caused by such inversions and similar damage has been recorded in neighbouring localities in 1962 and 1985 [70]. Although it would be expected that the inversions would be less intense as the climate continues to warm, the differential warming of south- and north-facing slopes (figure 2; electronic supplementary material, table S1) will probably result in complex, currently unknown effects on inversions.
(ii). Biotic environmental events
Insect pests. Although geometrid damage to forests in the Abisko area is more or less in cyclical events every 10–11 years [70], on average, a birch forest area will experience a very severe insect outbreak every 60–70 years, causing high tree or stem mortality [74,76]. In extreme cases such as in 1964–1965, these short-term moth outbreak events [76] can override a long-term trend of forest expansion and densification [16,81].
A typical moth outbreak results in the defoliation of the birch trees and some understorey species such as B. nana, V. myrtillus, V. vitis-idaea and E. nigrum [72]. The disturbance is usually followed by complete recovery although accelerated nutrient cycling from insect frass can cause a change in plant community species composition by the stimulation of grass growth over dwarf shrub re-growth [153,154].
In the mid-twentieth century two severe outbreaks were recorded in the western part of the Torneträsk area: one in 1954–1955 (E. autumnata) on both sides of the lake and one in 1964–1965 on the north side of the lake (O. brumata) [70]. These outbreaks had particularly large impacts on treeline, forest density and understorey growth. In the former event, Tenow [70] estimated that 780 kg D.W. ha−1 of birch leaves were eaten over 6000–7000 ha. The latter event killed birch trees in an altitudinal belt stretching more than 10 km horizontally. This forest area has not recovered and the treeline has retreated 120 m downwards ([16]; see above and figure 3; electronic supplementary material, table S1).
There were also severe outbreaks of E. autumnata in both 2004 and 2012. In 2004, birch trees and ground vegetation were damaged over large areas on both sides of Lake Torneträsk ([75]; figure 4). Population densities of 940 caterpillars per m2 were recorded in late June 2004 (Dylan Gwynn-Jones 2012, personal observation) and damage was so severe as to convert the forest from a carbon sink to a carbon source [155]. Although recovery occurred, tree growth in areas of the greatest damage was limited compared with that outside areas of intense damage (compare tree growth in figure 3 with 2004 insect damage in figure 4). The 2012 event had more severe impacts on the understory than the 2004 defoliation, but its effect on the treeline and forest structure is not known yet [41] whereas the 2004 outbreak had no lasting effect on the sub-alpine birch forest or treeline (Rik Van Bogaert 2012, personal communication).
Recent large-scale observations show that O. brumata, associated with a relatively moderate coastal climate, and Argyresthia retinella, currently not important in the area, are spreading northeastwards into traditionally colder regions [156,157]. Models of future distributions of the species project further range expansions and increased outbreak frequencies during continued climate warming [158]. This scenario is particularly probable because geometrid moth eggs (e.g. of E. autumnata) are killed by low winter temperatures, a major control on population density [159], and the frequency of winters in which temperatures are low enough to kill the moths' eggs is expected to further decrease [160]. In contrast, high UV-B radiation [161] and warmer summers [162,163] result in reduced foliage quality that might offset increases owing to enhanced egg survival. Furthermore, tree recovery should increase during warmer summers as the damage in 1965 was high because of exceptionally low summer temperatures [162]. However, warmer summers could equally well increase the probability that more thermophilic tree species such as aspen will replace birch following severe outbreaks [33].
In addition to projected increases in damage from insect outbreaks, models project an increase in birch damage from ‘background,’ i.e. non-outbreak, insect herbivory in the scenario of continued climate warming [164]. Over the long term, background herbivory could even result in more negative growth impacts compared with that from outbreak herbivore activity, although tree mortality is unlikely [164].
Voles and lemmings. The rodent cycles drive corresponding cycles in the biomass of field-layer vegetation. Plant biomass in tundra heath and forest understorey vegetation was between 12 and 24 per cent lower during the year after a vole peak than in the year before, and the combined vole and lemming peaks are visible as a reduced normalized difference vegetation index in satellite images over a 770 km2 area in the following year ([13,41]; figure 3). Studies from other regions suggest that rodents should have even stronger effects on the vegetation in high altitude snowbeds [120,165], but no studies have been published in these habitats in Abisko. However, Björk & Molau [36] state that snowbeds are important wintering habitats for microtine rodents, and most lemming winter nests are found in snowbeds. Three population peaks occurred during the period of observations (2000–2012) in heath and meadow habitats at Latnjajaure: 2001, 2004–2005 (weak) and 2010–2011 (Ulf Molau 2012, personal observation; figure 4). The peak in 2010–2011 ended in a situation of severe over-exploitation of the plant cover. Despite the very low lemming population density in the following year, the grazing impact on the vegetation was still very marked in August 2012. Tenow et al. [76] suggested that recovery of the monocormic birch forest on the northern side of Lake Torneträsk is possibly hampered by an abundant vole population living in the deeper snow cover on that side of the lake, and the importance of rodents for tree establishment is supported by ongoing experiments in the Abisko region (Johan Olofsson 2012, personal communication).
Although lemmings and voles have population peaks that can be considered as ‘events’ that drive vegetation structure and productivity, their populations are themselves driven by events in climate. For example, the lemming population build-up in 2001 at Latnjajaure ended abruptly in January 2002, when 150 mm of precipitation fell as rain during 2 days (10–11 January) followed by a marked drop in temperature and the formation of a 10 cm thick pure ice layer in the snow-pack at about 0.5 m above the ground surface (Ulf Molau 2012, personal observation). Hundreds of dead lemmings then appeared during snowmelt in June 2002.
Disease and invasive species. Studies on relationships between climate change and plant disease are almost totally lacking but new studies have demonstrated the effect of increased snow accumulation on a higher incidence of the fungal pathogen, Arwidssonia empetri, on E. nigrum in the subarctic vegetation of the Abisko area [140]. The incidence of snow mould seems to be related to both snow conditions and mild and wet conditions before the onset of snow [140,141]. Consequently, although long-term changes in the occurrence of snow mould have not yet been determined, observed changes in precipitation (see §3b) suggest that snow moulds should be monitored in future. Furthermore, the incidence of the fungal pathogen Exobasidium sp. on V. vitis-idaea was observed by Skinner (Laura J. Skinner 2002, unpublished data) to increase along an altitudinal gradient as temperatures increased (figures 2 and 4; electronic supplementary material, table S1), suggesting that the incidence of this pathogen may increase during climate warming.
Little is known about the incidence of diseases in animals in the area, although it is known that zoonotic diseases could increase during climate warming and range expansion of southern species [3].
Despite the large climatic changes documented in the Abisko area, invasions of southern species are relatively isolated. Exceptions are the invasion of aspen at treeline (see §4a(i)) and the invasion of some boreal species above treeline (see §4a(v)). Also, juvenile plants of S. phylicifolia, normally not growing above the treeline, have appeared in nutrient-rich snowbeds in the mid-alpine zone in the northern Scandes during the past five years [36]. These willow plants are all young and have not yet reached fertile age. Other boreal plant species now expanding far above the treeline include Epilobium angustifolium [38], Salix arbuscula and Viola epipsila (Ulf Molau 2012, personal observations).
Documented invasions of animal species are difficult to find, although moose populations have increased significantly in the twentieth century [166]. Also, the northern red-backed vole (Myodes rutilus), a subalpine boreal species, is now established in the mid-alpine zone far above the treeline and was caught for the first time at Latnjajaure in 2005 (Ulf Molau 2012, personal observation).
5. Conclusions
This compilation of multiple, geo-referenced environmental changes in the Abisko area, a landscape in transition, represents a microcosm of the complexity of changes throughout the Arctic, a region in transition [10,11,14,100,167], but the long-term perspective and multiple studies are unique.
Overall, the results demonstrate that abiotic environmental changes have been dramatic and changes in land use have been significant locally (for example, expansion of infrastructure and clearing of forests) and throughout most of the area in terms of reindeer herding practices. The results show an overall change in many vegetation types, species and growth responses that would be expected during climate warming (e.g. upward expansion of birch treeline, range expansion of aspen, increase in tall and dwarf shrubs, decline in cryptogams, etc.). However, evidence is also presented of stable systems (that are counterintuitive) including the thermophilic aspen clones and pine stands in the Abisko valley. Also evident were indirect effects of climate warming that could contribute to counter direct effects such as the lowering of the birch treeline location owing to climate effects on geometrid moth egg survival. It should also be highlighted that climate change is not the only driver of vegetation change operating in this area: herbivory, human disturbance and impacts of steep, rocky slopes are also evident. Furthermore, short-term events (hours to weeks) can override long-term trends (multiple decades) in vegetation change parameters, and even the long-term trends in temperature are more complex than usually considered when modelled across the landscape at 50 m resolution for 100 years.
This complexity, understandable at the local scale, provides a great challenge for scaling up, e.g. for interlinkage with climate-model output, particularly as the diverse drivers of change and different directions of change operate within individual vegetation types during the same climatic changes. This further confounds the complexity of vegetation responses described by Elmendorf et al. [10,100] for the pan-Arctic. These authors showed that differences in vegetation response were associated with different climate conditions. Furthermore, the complexity of the findings presented for the Abisko area show that simple correlations between vegetation changes recorded at the pan-Arctic scale and climate change have little predictive power because the correlations may hide the actual causes of change and the mechanisms (direct and indirect) of change caused by climate warming.
Although the long-term (approx. 100 years) data presented here represent a unique source, they were not collected in a standardized way, and response variables differ throughout the study. For these reasons, a standard statistical meta-analysis is hard to perform. Instead, we have focused on highlighting the complexity of responses, local drivers and interactions for a single location with great biological and geomorphological complexity that is a microcosm of the wider Arctic area. Meta analyses may reveal broad effects and a veneer of understanding but this approach would not help us to understand the assemblage of systems and their interactions with each other and human activity. The local people as stakeholders require more specific details of change than would be provided via a meta-analysis. For this reason, the information contained in this study is presented as a new baseline for future climate change impact analysis and modelling that will be used to help local stakeholders adapt to changes in environment and ecosystem services (e.g. the projects ‘Climate change, impacts and adaptation in the subarctic: a case study from the northern Swedish mountains’ (214-2008-188) and ‘Advanced Simulation of Arctic climate change and impact on Northern regions’ (214-2009-389), both projects supported by the Swedish research council FORMAS.
The main consequences of the results have substantial implications for adaptation to, and mitigation of climate change, as well as fundamental research and environmental monitoring. These implications include:
- — Fundamental research
- (i) experimentally determining the causal mechanisms underpinning vegetation change, particularly first- and second-order effects of climate on individual species and on species assemblages.
- (ii) further developing dynamic vegetation models and other systems approach models that include the complexity of drivers presented in this paper.
- — Adaptation
- (i) a need to plan for more extreme events.
- (ii) a need to develop new conservation measures, if possible, to focus on tree and shrub encroachment on alpine habitats and to protect snowbed habitats.
- (iii) a need to adapt reindeer herding practices to account for reduced summer grazing areas and more frequent extreme winter warming events.
- (iv) a careful reassessment of hunting and fishing regulations—if moose quotas are reduced and populations increase, this might slow down the expansion of shrubs, birch, aspen and pine.
- — Mitigation
- (i) developing improved models of carbon dynamics based on extreme events as well as long-term trends in soil, vegetation and herbivores.
- (ii) using conservation to offset carbon emissions.
Acknowledgements
We gratefully acknowledge each of the numerous researchers and observers at ANS on whose dedication we draw, two anonymous reviewers and Nils Åke Andersson for suggesting improvements to the paper.
Funding statement
All authors wish to thank the many national and international funding bodies that have funded the projects synthesized in this paper. T.V.C. and C.J. wish to thank FORMAS for funding the projects ‘Climate change, impacts and adaptation in the subarctic: a case study from the northern Swedish mountains’ (214-2008-188) and ‘Advanced simulation of Arctic climate change and impact on Northern regions’ (214-2009-389) to which this paper contributes. J.W.B., H.T., T.V.C. and S.B. gratefully acknowledge the Research Council of Norway for grant number 216434/E10, ‘Extreme winter warming in the High North and its biological effects in the past, present and future’. The study also contributes to the EU Framework 7 Infrastructure Project ‘INTERACT’ (www.eu-interact.org).
References
- 1.Anisimov OA, Vaughan DG, Callaghan TV, Furgal C, Marchant H, Prowse TD, Vilhjálmsson H, Walsh JE. 2007. Polar regions (Arctic and Antarctic). In Climate change 2007: impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the Intergovernmental Panel on Climate Change (eds Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.AMAP. 2011. Snow, water, ice and permafrost in the Arctic (SWIPA): climate change and the cryosphere. Arctic monitoring and assessment programme (AMAP), Oslo, Norway.
- 3.ACIA 2005. Arctic climate impact assessment. Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Post E, et al. 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355–1358 (doi:10.1126/science.1173113) [DOI] [PubMed] [Google Scholar]
- 5.Bhatt US, et al. 2010. Circumpolar Arctic tundra vegetation change is linked to sea ice decline. Earth Interactions 14, 1–20 (doi:10.1175/2010EI315.1) [Google Scholar]
- 6.Callaghan TV, Tweedie CE. (eds) 2011. Multi-decadal changes in tundra environments and ecosystems: the International Polar Year Back to the Future Project Ambio 40, 555–716 (doi:10.1007/s13280-011-0162-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein HE, Raynolds MK, Walker DA, Bhatt US, Tucker CJ, Pinzon JE. 2012. Dynamics of aboveground phytomass of the circumpolar Arctic tundra during the past three decades. Environ. Res. Lett. 7, 015506 (doi:10.1088/1748-9326/7/1/015506) [Google Scholar]
- 8.Myers-Smith IH, et al. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ. Res. Lett. 6, 045509 (doi:10.1088/1748-9326/6/4/045509) [Google Scholar]
- 9.Vincent WF, Callaghan TV, Dahl-Jensen D, Johansson M, Kovacs KM, Michel C, Prowse TD, Reist JD, Sharp M. 2011. Ecological implications of changes in the Arctic cryosphere. Ambio 40(Suppl. 1), 87–99 (doi:10.1007/s13280-011-0218-5) [Google Scholar]
- 10.Elmendorf S, et al. 2012. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Change 2, 453–457 (doi:10.1038/nclimate1465) [Google Scholar]
- 11.Xu L, et al. In press. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Change. (doi:10.1038/nclimate1836) [Google Scholar]
- 12.Olofsson J, Oksanen L, Callaghan TV, Hulme PE, Oksanen T, Suominen O. 2009. Herbivores inhibit climate-driven shrub expansion on the tundra. Glob. Change Biol. 15, 2681–2693 (doi:10.1111/j.1365-2486.2009.01935.x) [Google Scholar]
- 13.Olofsson J, Tømmervik H, Callaghan TV. 2012. Vole and lemming activity observed from space. Nat. Clim. Change 2, 880–883 (doi:10.1038/nclimate1537) [Google Scholar]
- 14.Callaghan TV, et al. 2011. Multi-decadal changes in tundra environments and ecosystems: synthesis of the International Polar Year Back to the Future Project. Ambio 40, 705–716 (doi:10.1007/s13280-011-0179-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danby RK, Hik DS. 2007. Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. J. Ecol. 95, 352–363 (doi:10.1111/j.1365-2745.2006.01200.x) [Google Scholar]
- 16.Van Bogaert R, Haneca K, Hoogesteger J, Jonasson C, De Dapper M, Callaghan TV. 2011. A century of tree line changes in sub-Arctic Sweden show local and regional variability and only a minor role of 20th Century climate warming. J. Biogeogr. 38, 907–921 (doi:10.1111/j.1365-2699.2010.02453.x) [Google Scholar]
- 17.Bokhorst S, Bjerke JW, Tømmervik H, Callaghan TV, Phoenix GK. 2009. Winter warming events damage sub-Arctic vegetation: consistent evidence from an experimental manipulation and a natural event. J. Ecol. 97, 1408–1415 (doi:10.1111/j.1365-2745.2009.01554.x) [Google Scholar]
- 18.Mack MC, Bret-Harte MS, Hollingsworth TN, Jandt RR, Schuur EAG, Shaver GR, Verbyla DL. 2011. Carbon loss from an unprecedented Arctic tundra wildfire. Nature 475, 489–492 (doi:10.1038/nature10283) [DOI] [PubMed] [Google Scholar]
- 19.Callaghan TV, Bergholm F, Christensen TR, Jonasson C, Kokfelt U, Johansson M. 2010. A new climate era in the sub-Arctic: accelerating climate changes and multiple impacts. Geophys. Res. Lett. 37, L14705 (doi:10.1029/2009GL042064) [Google Scholar]
- 20.Jonasson C, Sonesson M, Christensen TR, Callaghan TV. 2012. Environmental monitoring and research in the Abisko area—an overview. Ambio 41, 175–177 (doi:10.1007/s13280-012-0300-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf A, Callaghan TV, Larson K. 2008. Future changes in vegetation and ecosystem function of the Barents Region. Clim. Change 87, 51–73 (doi:10.1007/s10584-007-9342-4) [Google Scholar]
- 22.Kaplan JO, New M. 2006. Arctic climate change with a 2°C global warming: timing, climate patterns and vegetation change. Clim. Change 79, 213–241 (doi:10.1007/s10584-006-9113-7) [Google Scholar]
- 23.Miller PA, Smith B. 2012. Modelling tundra vegetation response to recent Arctic warming. Ambio 41(Suppl. 3), 281–291 (doi:10.1007/s13280-012-0306-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adger WN, Agrawala S, Mirza MMQ, Conde C, O'Brien K, Pulhin J, Pulwarty R, Smit B, Takahashi K. 2007. Assessment of adaptation practices, options, constraints and capacity. In Climate change 2007: impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE.), pp. 717–743 Cambridge, UK: Cambridge University Press [Google Scholar]
- 25.Andersson NA, Callaghan TV, Karlsson PS. 1996. The Abisko Scientific Research Station. Ecol. Bull. 45, 11–14 [Google Scholar]
- 26.Abisko Scientific Research Station (ANS) data See http://www.polar.se/abisko.
- 27.Molau U. 2010. Recent changes of vegetation pattern in the mountains of northern Sweden. In Europe's ecological backbone: recognising the true value of our mountains. European Environment Agency Report no. 6. Box 8.4:159
- 28.Yang Z, Hanna E, Callaghan TV. 2011. Modelling surface-air-temperature variation over complex terrain around Abisko, Swedish Lapland: uncertainties of measurements and models at different scales. Geogr. Ann.: Ser. A Phys. Geogr. 93, 89–112 (doi:10.1111/j.1468-0459.2011.00005.x) [Google Scholar]
- 29.Yang Z, Hanna E, Callaghan TV, Jonasson C. 2012. How can meteorological observations and microclimate simulations improve understanding of 1913–2010 climate change around Abisko, Swedish Lapland? Meteorol. Appl. 19, 454–463 (doi:10.1002/met.276) [Google Scholar]
- 30.Berglund BE, Barnekow L, Hammarlund D, Sandgren P, Snowball IF. 1996. Holocene forest dynamics and climate changes in the Abisko area, northern Sweden—the Sonesson model of vegetation history reconsidered and confirmed. Ecol. Bull. 45, 15–30 [Google Scholar]
- 31.Stöcklin J, Körner C. 1999. Recruitment and mortality of Pinus sylvestris near the Nordic treeline: the role of climate change and herbivory. Ecol. Bull. 45, 168–177 [Google Scholar]
- 32.Wielgolaski FE. 2005. History and environment of the Nordic mountain birch. In Plant ecology, herbivory, and human impact in nordic mountain birch forests, vol. 180 (ed. Wielgolaski FE.), pp. 3–18 New York, NY: Springer [Google Scholar]
- 33.Van Bogaert R, Jonasson C, De Dapper M, Callaghan TV. 2010. Range expansion of thermophilic aspen (Populus tremula L.) in the Swedish Subarctic. Arctic, Antarctic Alpine Res. 42, 362–375 (doi:10.1657/1938-4246-42.3.362) [Google Scholar]
- 34.Sonesson M, Lundberg B. 1974. Late Quaternary forest development of the Torneträsk area, North Sweden, I. Structure of modern forest ecosystems. Oikos 25, 121–133 (doi:10.2307/3543633) [Google Scholar]
- 35.Jägerbrand AK, Lindblad KEM, Björk RG, Alatalo JM, Molau U. 2006. Bryophyte and lichen diversity under simulated environmental change compared with observed variation in unmanipulated tundra. Biodiv. Conserv. 15, 4453–4475 (doi:10.1007/s10531-005-5098-1) [Google Scholar]
- 36.Björk RG, Molau U. 2007. Ecology of alpine snowbeds and the impact of global change arctic. Antarctic Alpine Res. 39, 34–43 (doi:10.1657/1523-0430(2007)39[34:EOASAT]2.0.CO;2) [Google Scholar]
- 37.Bruun HH, Moen J, Virtanen R, Grytnes JA, Oksanen L, Angerbjorn A. 2006. Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. J. Vegetat. Sci. 17, 37–46 (doi:10.1111/j.1654-1103.2006.tb02421.x) [Google Scholar]
- 38.Molau U. 2010. Long-term impacts of observed and induced climate change on tussock tundra near its southern limit in northern Sweden. Plant Ecol. Div. 3, 29–34 (doi:10.1080/17550874.2010.487548) [Google Scholar]
- 39.Andersson NÅ. 1993. Fågelfaunan I Torneträskområdet—främst Abiskotrakten. Fåglar i Norrbotten 12, 1 [Google Scholar]
- 40.Oksanen T, Oksanen L, Olofsson J. 2008. Arctic lemmings, Lemmus spp. and Dicrostonyx spp.: integrating ecological and evolutionary perspectives. Evol. Ecol. Res. 10, 415–434 [Google Scholar]
- 41.Olofsson J, te Beest M, Ericson L. 2013. Complex biotic interactions drive long-term vegetation dynamics in a subarctic ecosystem. Phil. Trans. R. Soc. B 368, 20120486 (doi:10.1098/rstb.2012.0486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ims RA, Henden JA, Killengreen ST. 2008. Collapsing population cycles. Trends Ecol. Evol. 23, 79–86 (doi:10.1016/j.tree.2007.10.010) [DOI] [PubMed] [Google Scholar]
- 43.Björk RG, Majdi H, Klemedtsson L, Lewis-Jonsson L, Molau U. 2007. Long-term warming effects on root morphology, root mass distribution, and microbial activity in two dry tundra plant communities in northern Sweden. New Phytol. 176, 862–873 (doi:10.1111/j.1469-8137.2007.02231.x) [DOI] [PubMed] [Google Scholar]
- 44.Johansson C, Pohjola VA, Callaghan TV, Jonasson C. 2011. Multi-decadal changes in snow characteristics in sub-Arctic Sweden. Ambio 40, 566–574 (doi:10.1007/s13280-011-0164-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Key JR, Liu Z, Wang X, Vavrus SJ. 2012. A cloudier Arctic expected with diminishing sea ice. Geophys. Res. Lett. 39, L05705 (doi:10.1029/2012GL051251) [Google Scholar]
- 46.Vavrus S, Holland MM, Bailey DA. 2011. Changes in Arctic clouds during intervals of rapid sea ice loss. Clim. Dyn. 36, 1475–1489 (doi:10.1007/s00382-010-0816-0) [Google Scholar]
- 47.Scherrer D, Körner Ch. 2011. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 38, 406–416 (doi:10.1111/j.1365-2699.2010.02407.x) [Google Scholar]
- 48.Rudvik A. 2012. Dependence structures in stable mixture models with an application to extreme precipitation. Thesis for the Degree of Licentiate of Engineering. Department of Mathematical Sciences. Division of Mathematical Statistics. Chalmers University of Technology and University of Gothenburg. Gothenburg, Sweden [Google Scholar]
- 49.Kohler J, Brandt O, Johansson M, Callaghan TV. 2006. A long record of Arctic snow-depth measurements from Abisko, northern Sweden, 1913–2002. Polar Res. 25, 91–113 (doi:10.1111/j.1751-8369.2006.tb00026.x) [Google Scholar]
- 50.Åkerman HJ, Johansson M. 2008. Thawing permafrost and thicker active layers in sub-arctic Sweden. Permafrost Periglacial Process. 19, 279–292 (doi:10.1002/ppp.626) [Google Scholar]
- 51.Johansson M, Åkerman J, Keuper F, Christensen TR, Lantuit H, Callaghan TV. 2011. Redrilling of boreholes: past and present permafrost temperatures in the Abisko area. Ambio 40, 558–565 (doi:10.1007/s13280-011-0163-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christensen TR, Johansson T, Åkerman J, Mastepanov M, Malmer N, Friborg T, Crill P, Svensson BH. 2004. Thawing subarctic permafrost: effects on vegetation and methane emissions. Geophys. Res. Lett. 31, L04501 (doi:10.1029/2003GL018680) [Google Scholar]
- 53.Malmer N, Johansson T, Olsrud M, Christensen TR. 2005. Vegetation, climatic changes and net carbon sequestration in a North-Scandinavian subarctic mire over 30 years. Glob. Change Biol. 11, 1895–1909 [Google Scholar]
- 54.Hinzman LD, et al. 2005. Evidence and implications of recent climate change in northern Alaska and other Arctic regions. Clim. Change 72, 251–298 (doi:10.1007/s10584-005-5352-2) [Google Scholar]
- 55.Smith LC, Sheng Y, MacDonald GM, Hinzman LD. 2005. Disappearing Arctic lakes. Science 308, 1429–1429 (doi:10.1126/science.1108142) [DOI] [PubMed] [Google Scholar]
- 56.Lindfors A, Holmgren B, Hansen G. 2006. Long-term erythemal UV at Abisko and Helsinki estimated using total ozone, sunshine duration, and snow depth. In Remote sensing of clouds and the atmosphere XI, vol. 6362 (eds Slusser JR, Schäfer K, Comeron A.) Proceedings of SPIE See http://spie.org/app/Search/InFront?Dy=1&No=60&Nrpp=20&Ntt=Remote%20Sensing%20of%20Clouds%20and%20the%20Atmosphere%20XI&Nty=1. [Google Scholar]
- 57.Gwynn-Jones D, et al. 2012. Enhanced UV-B and elevated CO2 impacts on sub-arctic shrub berry abundance, quality and seed germination. Ambio 41, 256–268 (doi:10.1007/s13280-012-0311-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emanuelsson U. 1987. Human influence on vegetation in the Torneträsk area during the last three centuries. Ecol. Bull. 38, 95–111 [Google Scholar]
- 59.SSB. 1890. Norrbottens län—BISOS H. Kungl. Maj:ts befallningshavandes femårsberättelser. Ny följd 7 Åren 1886–1890.
- 60.Tømmervik H, Riseth JÅ. 2011. Historiske tamreintall i Norge fra 1800-tallet fram til i dag. NINA Rapport 672: 35 pp. Norsk Institutt for Naturforskning, Trondheim
- 61.Hein CL, Englund G, Öhlund G. 2011. Dispersal through stream networks: modelling climate-driven range expansions of fishes. Div. Distrib. 17, 641–651 (doi:10.1111/j.1472-4642.2011.00776.x) [Google Scholar]
- 62.Hofgaard A. 1999. The role of ‘natural’ landscapes influenced by man in predicting responses to climate change. Ecol. Bull. 47, 160–167 [Google Scholar]
- 63.Bäck L, Hedlund L. 1982. Vandringsturismen i Norrbottensfjällen 1980. Statens Naturvårdsverk PM 1572 [Google Scholar]
- 64.Bäck L, Jonasson C. 1998. The Kiruna-Narvik road and its impact on the environment and on recreational land use. Ambio 27, 345–350 [Google Scholar]
- 65.Bernhard GC.1989. Abisko Scientific Research Station. Bidrag till KVA:s historia XVIII.
- 66.Bäck L. 1993. Reindeer management in conflict and cooperation. A geographic land use and simulation study from northernmost Sweden. Nomadic Peoples 32, 65–80 [Google Scholar]
- 67.Sonesson M. 1970. Studies on mire vegetation in the Torneträsk area, northern Sweden. iii. Communities of the poor mires. Opera Botanica 26, 1–122 [Google Scholar]
- 68.Östlund L, Zackrisson O, Axelsson A-L. 1997. The history and transformation of a Scandinavian boreal forest landscape since the 19th century. Can. J. Forest. Res. 27, 1198–1206 (doi:10.1139/x97-070) [Google Scholar]
- 69.Jagareforbundet. 2012. See http://www.jagareforbundet.se/Jagareforbundet-Norr/Norrbotten/Algar-och-algjakt/Algobs-i-Norrbotten/ .
- 70.Tenow O. 1996. Hazards to a mountain birch forest – Abisko in perspective. Ecol. Bull. 45, 104–114 [Google Scholar]
- 71.Bylund H. 1995. Long-term interactions between the autumnal moth and mountain birch—the roles of resources, competitors, natural enemies and weather. PhD thesis, Department of Entomology, Swedish University of Agricultural Sciences, Uppsala [Google Scholar]
- 72.Tenow O. 1972. The outbreaks of Oporinia autumnata bkh. and Operophthera spp. (lep.: Geometridae) in the Scandinavian mountain chain and northern Finland 1862–1968. Zoologiska Bidrag från Uppsala (Suppl. 2), 1–107 [Google Scholar]
- 73.Eckstein D, Hoogesteger J, Holmes RL. 1991. Insect-related differences in growth of birch and pine at northern treeline in Swedish Lapland. Holarctic Ecol. 14, 18–23 [Google Scholar]
- 74.Van Bogaert R, Jonasson C, De Dapper M, Callaghan TV. 2009. Competitive interaction between aspen and birch moderated by invertebrate and vertebrate herbivores and climate warming. Plant Ecol. Div. 2, 221–232 (doi:10.1080/17550870903487456) [Google Scholar]
- 75.Babst F, Esper J, Parlow E. 2010. Landsat TM/ETM+ and tree-ring based assessment of spatiotemporal patterns of the autumnal moth (Epirrita autumnata) in northernmost Fennoscandia. Remote Sensing Environ. 114, 637–646 (doi:10.1016/j.rse.2009.11.005) [Google Scholar]
- 76.Tenow O, Bylund H, Nilssen AC, Karlsson PS. 2005. Long-term influence of herbivores on northern birch forests. In Plant ecology, herbivory, and human impact in Nordic mountain birch forests (ed. Wielgolaski FE.), pp. 166–181 Berlin, Germany: Springer [Google Scholar]
- 77.Kausrud KL, et al. 2008. Linking climate change to lemming cycles. Nature 456, 93–97 (doi:10.1038/nature07442) [DOI] [PubMed] [Google Scholar]
- 78.Hörnfeldt B. 2004. Long-term decline in numbers of cyclic voles in boreal Sweden: analysis and presentation of hypotheses. Oikos 107, 376–392 (doi:10.1111/j.0030-1299.2004.13348.x) [Google Scholar]
- 79.Sandberg G.1965. Abisko (Eng ed.) K Domänstyrelsen 1, 44 pp.
- 80.Öberg L, Kullman L. 2012. Recent glacier recession—a new source of postglacial treeline and climate history in the Swedish Scandes. Landscape Online 26, 1–38 [Google Scholar]
- 81.Hedenås H, Olsson H, Jonasson C, Bergstedt J, Dahlberg U, Callaghan TV. 2011. Changes in tree growth, biomass and vegetation changes over a 13-year period in the Swedish sub-Arctic. Ambio 40, 672–682 (doi:10.1007/s13280-011-0173-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sundqvist MK, Björk RG, Molau U. 2008. Establishment of boreal forest species in alpine dwarf-shrub heath in subarctic Sweden. Plant Ecol. Div. 1, 67–75 (doi:10.1080/17550870802273395) [Google Scholar]
- 83.Rundqvist S, Hedenås H, Sandström A, Emanuelsson U, Eriksson H, Jonasson C, Callaghan TV. 2011. Tree and shrub expansion over the past 34 years at the tree-line near Abisko, sub-Arctic. Ambio 40, 683–692 (doi:10.1007/s13280-011-0174-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Bogaert R. 2010. Recent treeline dynamics in sub-Arctic Sweden: a multidisciplinary landscape assessment. PhD thesis, Geography Department, Ghent University, Ghent [Google Scholar]
- 85.Cairns DM, Moen J. 2004. Herbivory influences treelines. J. Ecol. 92, 1019–1024 (doi:10.1111/j.1365-2745.2004.00945.x) [Google Scholar]
- 86.Dalen L, Hofgaard A. 2005. Differential regional treeline dynamics in the Scandes Mountains. Arctic Antarctic Alpine Res. 37, 284–296 (doi:10.1657/1523-0430(2005)037[0284:DRTDIT]2.0.CO;2) [Google Scholar]
- 87.Tømmervik H, Johansen B, Riseth JA, Karlsen SR, Solberg B, Høgda KA. 2009. Above ground biomass changes in the mountain birch forests and mountain heaths of Finnmarksvidda, northern Norway, in the period 1957–2006. Forest Ecol. Manage. 257, 244–257 (doi:10.1016/j.foreco.2008.08.038) [Google Scholar]
- 88.Sonesson M, Hoogesteger J. 1983. Recent tree-line dynamics (Betula pubescens Ehrh. ssp. tortuosa (Ledeb.) Nyman) in northern Sweden. Nordicana 47, 47–54 [Google Scholar]
- 89.Virtanen R, Luoto M, Rämä T, Mikkola K, Hjort J, Grytnes J-A, Birks HJB. 2010. Recent vegetation changes at the high-latitude tree line ecotone are controlled by geomorphological disturbance, productivity and diversity. Global Ecol. Biogeogr. 19, 810–821 (doi:10.1111/j.1466-8238.2010.00570.x) [Google Scholar]
- 90.Molau U, Larsson E-L. 2000. Seed rain and seed bank along an alpine altitudinal gradient in Swedish Lapland. Can. J. Bot. 78, 728–747 [Google Scholar]
- 91.Truong C, Palme AE, Felber F. 2006. Recent invasion of the mountain birch Betula pubescens ssp. tortuosa above the treeline due to climate change: genetic and ecological study in northern Sweden. J. Evol. Biol. 20, 369–380 (doi:10.1111/j.1420-9101.2006.01190.x) [DOI] [PubMed] [Google Scholar]
- 92.Grau O, Ninot JM, Blanco-Mporeno JM, van Logtestijn RSP, Cornelissen JHC, Callaghan TV. 2012. Shrub-tree interactions and environmental change drive treeline dynamics in the Subarctic. Oikos 121, 1680–1690 (doi:10.1111/j.1600-0706.2011.20032.x) [Google Scholar]
- 93.Dahlberg U, Berge TW, Petersson H, Vencatasawmy CP. 2004. Modelling biomass and leaf area index in a sub-arctic Scandinavian mountain area. Scandinavian J. Forest Res. 19, 60–71 (doi:10.1080/02827580310019266) [Google Scholar]
- 94.Bernes C. 1980. Monitor 1980 en presentation av PMK—Programmet för övervakning av miljökvalitet. Stockholm: Naturvårdsverket [Google Scholar]
- 95.Hedenås H, Carlsson BÅ, Emanuelsson U, Headley A, Jonasson C, Svensson BM, Callaghan TV. 2012. Changes versus homeostasis in alpine and sub-alpine vegetation over three decades in the sub-Arctic. Ambio 41, 187–196 (doi:10.1007/s13280-012-0312-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johanson U, Gehrke C, Björn LO, Callaghan TV. 1995. The effects of enhanced UV-B radiation on the growth of dwarf shrubs in a subarctic heathland. Funct. Ecol. 9, 713–719 (doi:10.2307/2390243) [Google Scholar]
- 97.Wookey P, Parsons A, Welker JM, Potter J, Callaghan TV, Lee JA, Press MC. 1993. Comparative responses of phenology and reproductive development to simulated environmental change in sub-arctic and high arctic plants. Oikos 67, 490–502 (doi:10.2307/3545361) [Google Scholar]
- 98.Sloan VL. 2011. Plant roots in Arctic ecosystems: stocks and dynamics, and their coupling to above-ground parameters. PhD thesis, Department of Animal and Plant Sciences, University of Sheffield, UK
- 99.Walker MD, et al. 2006. Plant community response to experimental warming across the tundra biome. Proc. Natl Acad. Sci. USA 103, 1342–1346 (doi:10.1073/pnas.0503198103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elmendorf SC, et al. 2012. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol. Lett. 15, 164–175 (doi:10.1111/j.1461-0248.2011.01716.x) [DOI] [PubMed] [Google Scholar]
- 101.Parsons AN, Welker JM, Wookey PA, Press MC, Callaghan TV, Lee JA. 1994. Growth responses of four sub-arctic dwarf shrubs to simulated environmental change. J. Ecol. 82, 307–318 (doi:10.2307/2261298) [Google Scholar]
- 102.Koller EK. 2011. Impacts of environmental change on subarctic dwarf shrub communities: landscape gradient and field manipulation approaches. PhD thesis, Department of Animal and Plant Sciences, University of Sheffield, UK
- 103.Sturm M, Racine C, Tape K. 2001. Climate change—increasing shrub abundance in the Arctic. Nature 411, 546–547 (doi:10.1038/35079180) [DOI] [PubMed] [Google Scholar]
- 104.Tape K, Sturm M, Racine C. 2006. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biol. 12, 686–702 (doi:10.1111/j.1365-2486.2006.01128.x) [Google Scholar]
- 105.Forbes BC, Fauria MM, Zetterberg P. 2009. Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Global Change Biol. 15, 1–13 (doi:10.1111/j.1365-2486.2008.01704.x) [Google Scholar]
- 106.Hallinger M, Manthey M, Wilmking M. 2010. Establishing a missing link: warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytol. 186, 890–899 (doi:10.1111/j.1469-8137.2010.03223.x) [DOI] [PubMed] [Google Scholar]
- 107.Headley AD. 1986. The comparative autecology of some European species of Lycopodium sensao lato. PhD thesis, University of Manchester, UK [Google Scholar]
- 108.Carlsson BÅ, Callaghan TV. 1991. Positive plant interactions in tundra vegetation and the importance of shelter. J. Ecol. 79, 973–983 (doi:10.2307/2261092) [Google Scholar]
- 109.Svensson BM, Carlsson BÅ, Karlsson PS, Nordell KO. 1993. Comparative long-term demography of three species of Pinguicula. J. Ecol. 81, 635–645 (doi:10.2307/2261662) [Google Scholar]
- 110.Emanuelsson U. 1984. Ecological effects of grazing and trampling on mountain vegetation in northern Sweden. PhD thesis, University of Lund, Sweden [Google Scholar]
- 111.Sorensen PL, Michelsen A. 2011. Long-term warming and litter addition affects nitrogen fixation in subarctic heath. Global Change Biol. 17, 528–537 (doi:10.1111/j.1365-2486.2010.02234.x) [Google Scholar]
- 112.Schaeffer JA, Messier F. 1995. Scale-dependent correlations of Arctic vegetation and snow cover. Arctic Alpine Res. 27, 38–43 (doi:10.2307/1552066) [Google Scholar]
- 113.Christensen TR, et al. 2007. A catchment-scale carbon and greenhouse gas budget of a subarctic landscape. Phil. Trans. R. Soc. A 365, 1643–1656 (doi:10.1098/rsta.2007.2035) [DOI] [PubMed] [Google Scholar]
- 114.Johansson T, Malmer N, Crill PM, Friborg T, Akerman JH, Mastepanov M, Christensen TR. 2006. Decadal vegetation changes in a northern peatland, greenhouse gas fluxes and net radiative forcing. Global Change Biol. 12, 2352–2369 (doi:10.1111/j.1365-2486.2006.01267.x) [Google Scholar]
- 115.Johansson M, Christensen TR, Akerman HJ, Callaghan TV. 2006. What determines the current presence or absence of permafrost in the Torneträsk region, a subarctic landscape in Northern Sweden? Ambio 35, 190–197 (doi:10.1579/0044-7447(2006)35[190:WDTCPO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 116.Johansson M, Callaghan TV, Bosiö J, Åkerman HJ, Jackowicz-Korcynski M, Christensen TR. Submitted. Rapid responses of permafrost and vegetation to experimentally increased snow cover in sub-arctic Sweden. Environ. Res. Lett. [Google Scholar]
- 117.Bosiö J, Johansson M, Callaghan TV, Johansen B, Christensen TR. 2012. Future vegetational patterns in subarctic palsa mires: a simple modelling approach using high resolution land cover mapping. Clim. Change 115, 379–398 (doi:10.1007/s10584-012-0445-1) [Google Scholar]
- 118.Walker DA, et al. 2005. The circumpolar Arctic vegetation map. J. Vegetat. Sci. 16, 267–282 (doi:10.1111/j.1654-1103.2005.tb02365.x) [Google Scholar]
- 119.Heegaard E. 2002. A model of alpine species distribution in relation to snowmelt time and altitude. J. Vegetat. Sci. 13, 493–504 (doi:10.1111/j.1654-1103.2002.tb02076.x) [Google Scholar]
- 120.Heegaard E, Vandvik V. 2004. Climate change affects the outcome of competitive interactions—an application of principal response curves. Oecologia 139, 459–466 (doi:10.1007/s00442-004-1523-5) [DOI] [PubMed] [Google Scholar]
- 121.Virtanen R. 2000. Effects of grazing on above-ground biomass on a mountain snowbed, NW Finland. Oikos 90, 295–300 (doi:10.1034/j.1600-0706.2000.900209.x) [Google Scholar]
- 122.Andersson NÅ. 2006. Fågelfauna i förändring. Några tankar och funderingar om fågelfaunan i västra Torneträskområdet. Fåglar i Norrbotten 25, 14–17 [Google Scholar]
- 123.Holmlund P, Karlén W, Grudd H. 1996. Fifty years of mass balance and glacier front observations at the Tarfala research station. Geogr. Ann. Ser. A Phys. Geogr. 78, 105–114 (doi:10.2307/520972) [Google Scholar]
- 124.Holmlund P, Jansson P. 1999. The Tarfala mass balance programme. Geogr. Ann. Ser. A Phys. Geogr. 81, 621–631 (doi:10.1111/j.0435-3676.1999.00090.x) [Google Scholar]
- 125.Rippin DM, Carrivick J, Williams C. 2011. Evidence towards a thermal lag in the response of Kårsaglaciären, northern Sweden, to climate change. J. Glaciol. 57, 895–903 (doi:10.3189/002214311798043672) [Google Scholar]
- 126.Karlén W. 1973. Holocene glacier and climatic variations, Kebnekaise mountains, Swedish Lapland. Geogr. Ann. 55A, 29–63 (doi:10.2307/520485) [Google Scholar]
- 127.Fries TCE. 1925. Ökologische und phänologische Beobachtungen bei Abisko in den Jaheren 1917–1919. I.Svenska Växtsociologiska Sällskapets Handlingar 5, 1–171 [Google Scholar]
- 128.Du Rietz GE. 1925. Studien über die Höhengrenzen der hochalpinen Gefässpflanzen in nördlichen Lappland. Veröffentlichungen des Geobotanischen Insituts Rübel in Zürich 3, 67–86 [Google Scholar]
- 129.Gottfried M, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2, 111–115 (doi:10.1038/nclimate1329) [Google Scholar]
- 130.Pauli H, et al. 2012. Recent plant diversity changes on Europe's mountain summits. Science 336, 353–355 (doi:10.1126/science.1219033) [DOI] [PubMed] [Google Scholar]
- 131.Jankovska V, Kocianova M. 2010. Palaeo-reconstruction of vegetation development of the landscape and of origin, development and collapse of a dome-shaped palsa in Abisko in the Torneträsk area (northern Sweden) in the holocene. Opera Corcontica 47, 111–128 [Google Scholar]
- 132.Hamberg HE. 1912. Om skare och flen i Lappland. Kungliga Svenska Vetenskapsakademiens Årsbok 10, 225–303 [Google Scholar]
- 133.Riseth JÅ, et al. 2011. Sámi traditional ecological knowledge as a guide to science: snow, ice and reindeer pasture facing climate change. Polar Rec. 47, 202–217 (doi:10.1017/S0032247410000434) [Google Scholar]
- 134.Ruong I. 1937. Fjällapparna i Jukkasjärvi socken. Geographica 3, 1–76 + 2 maps [Google Scholar]
- 135.Lie I, Riseth JÅ, Holst B. 2008. Reindrifta i et skiftende klimabilde. Norut Alta Rapport 6, 98 pp [Google Scholar]
- 136.Bokhorst S, Bjerke JW, Street L, Callaghan TV, Phoenix GK. 2011. Impacts of multiple extreme winter warming events on sub-Arctic heathland: phenology, reproduction, growth, and CO2 flux responses. Global Change Biol. 17, 2817–2830 (doi:10.1111/j.1365-2486.2011.02424.x) [Google Scholar]
- 137.Bjerke JW, Bokhorst S, Zielke M, Callaghan TV, Bowles FW, Phoenix GK. 2011. Contrasting sensitivity to extreme winter warming events of dominant sub-Arctic heathland bryophyte and lichen species. J. Ecol. 99, 1481–1488 (doi:10.1111/j.1365-2745.2011.01859.x) [Google Scholar]
- 138.Bokhorst S, Bjerke JW, Tømmervik H, Preece C, Phoenix GK. 2012. Ecosystem response to climatic change: the importance of the cold season. Ambio 41(Suppl. 3), 246–255 (doi:10.1007/s13280-012-0310-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bokhorst S, Phoenix GK, Bjerke JW, Callaghan TV, Huyer-Brugman F, Berg MP. 2012. Extreme winter warming events more negatively impact small rather than large soil fauna: shift in community composition explained by traits not taxa. Global Change Biol. 18, 1152–1162 (doi:10.1111/j.1365-2486.2011.02565.x) [Google Scholar]
- 140.Olofsson J, Ericson L, Torp M, Stark S, Baxter R. 2011. Carbon balance of Arctic tundra under increased snow cover mediated by a plant pathogen. Nat. Clim. Change 1, 220–223 (doi:10.1038/nclimate1142) [Google Scholar]
- 141.Bokhorst S, Tømmervik H, Callaghan TV, Phoenix GK, Bjerke JW. 2012. Vegetation recovery following extreme winter warming events in the sub-Arctic estimated using NDVI from remote sensing and handheld passive proximal sensors. Environ. Exp. Bot. 81, 18–25 (doi:10.1016/j.envexpbot.2012.02.011) [Google Scholar]
- 142.Embleton C, King CAM. 1975. Glacial geomorphology, 573 pp New York, NY: Wiley [Google Scholar]
- 143.Rapp A. 1960. Recent development of mountain slopes in Kärkevagge and surroundings, Northern Scandinavia. Geogr. Ann. 42, 71–200 (doi:10.2307/520126) [Google Scholar]
- 144.Strömquist L. 1985. Geomorphic impact of snowmelt on slope erosion and sediment production. Z. Geomorphol. 29, 129–138 [Google Scholar]
- 145.Gude M, Scherer D. 1995. Snowmelt and slush torrents—preliminary report from a field campaign in Kärkevagge, Swedish Lappland. Geogr. Ann. 77A, 199–206 (doi:10.2307/521329) [Google Scholar]
- 146.Lundkvist M. 2005. Accident risk and environmental assessment: development of an assessment guideline with examination in Northern Sweden. PhD thesis, Uppsala Universitet. Department of Social and Economic Geography; Geografiska Regionstudier Nr 65, 166 pp [Google Scholar]
- 147.Nyberg R, Rapp A. 1998. Extreme erosional events and natural hazards in Scandinavian mountains. Ambio 27, 292–299 [Google Scholar]
- 148.Jonasson C, Nyberg R. 1999. The rainstorm of August 1998 in the Abisko area, northern Sweden: preliminary report on observations of erosion and sediment transport. Geogr. Ann. 81A, 387–390 (doi:10.1111/j.0435-3676.1999.00068.x) [Google Scholar]
- 149.Beylich AA, Gintz D. 2004. Effects of high-magnitude/low-frequency fluvial events generated by intense snowmelt or heavy rainfall in Arctic periglacial environments in northern Swedish Lapland and northern Siberia. Geogr. Ann. 86A, 11–29 (doi:10.1111/j.0435-3676.2004.00210.x) [Google Scholar]
- 150.Jonasson C, Callaghan TV.2010. Climate change and extreme events in the mountains of northern Sweden. In Europés ecological backbone: recognising the true value of our mountains. European Environment Agency Report no. 6. Box 5.1:76, Periglacial Processes 21, 241-25.
- 151.Holmgren B, Tenow O. 1987. Local extreme minima of winter temperature in high latitude mountainous terrain. In Climatological extremes in the mountains. Physical background, geomorphological and ecological consequence (eds Alexandersson H, Holmgren B.), pp. 25–41 Uppsala, Sweden: Uppsala Universitets Naturgeografiska Institution, UNGI rapport no. 65 [Google Scholar]
- 152.Holmgren B, Tjus M. 1996. Summer air temperatures and tree line dynamics. Ecol. Bull. 45, 159–169 [Google Scholar]
- 153.Kallio P, Lehtonen J. 1973. Birch forest damage caused by Oporinia autumnata (Bkh.) and the problem of reforestation. In Fennoscandian tundra ecosystems, Part 2, Ecological studies, vol. 17 (ed. Wielgolaski FE.), pp. 174–180 Berlin, Germany: Springer [Google Scholar]
- 154.Carlsson BÅ, Karlsson PS, Svensson BM. 1999. Alpine and subalpine vegetation. Acta Phytogeogr. Suecica 84, 75–89 [Google Scholar]
- 155.Heliasz M, Johansson T, Lindroth A, Mölder M, Mastepanov M, Friborg T, Callaghan TV, Christensen TR. 2011. Quantification of C uptake in subarctic birch forest after setback by an extreme insect outbreak. Geophys. Res. Lett. 38, L01704 (doi:10.1029/2010GL044733) [Google Scholar]
- 156.Tenow O, Nilssen AC, Holmgren B, Elverum F. 1999. An insect (Argyresthia retinella, Lep., Yponomeutidae) outbreak in northern birch forests, released by climatic changes? J. Appl. Ecol. 36, 111–122 (doi:10.1046/j.1365-2664.1999.00385.x) [Google Scholar]
- 157.Jepsen JU, Hagen SB, Ims RA, Yoccoz NG. 2008. Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. J. Anim. Ecol. 77, 257–264 (doi:10.1111/j.1365-2656.2007.01339.x) [DOI] [PubMed] [Google Scholar]
- 158.Neuvonen S, Bylund H, Tømmervik H. 2005. Forest defoliation risks in birch forest by insects under different climate and land use scenarios in northern Europe. In Plant ecology, herbivory, and human impact in Nordic mountain birch forests (ed. Wielgolaski FE.), pp. 125–138 New York, NY: Springer [Google Scholar]
- 159.Nilssen A, Tenow O. 1990. Diapause, embryo growth and supercooling capacity of Epirrita autumnata eggs from northern Fennoscandia. Entomol. Exp. Appl. 57, 39–55 (doi:10.1111/j.1570-7458.1990.tb01414.x) [Google Scholar]
- 160.Sælthun NR, Barkved L. 2003. Climate change scenarios for the SCANNET region. NIVA Report SNO 4663–2003
- 161.Buck N, Callaghan TV. 1999. Impacts of increased UV-B radiation on the autumn moth caterpillar Epirrita autumnata. Ecol. Bull. 47, pp. 68–76 [Google Scholar]
- 162.Niemelä P. 1980. Dependence of Oporinia autumnata (Lep., Geometridae) outbreaks on summer temperature. Reports from the Kevo Subarctic Research Station 16, 27–30 [Google Scholar]
- 163.Virtanen T, Neuvonen S. 1999. Performance of moth larvae on birch in relation to altitude, climate, host quality and parasitoids. Oecologia 120, 92–101 (doi:10.1007/s004420050837) [DOI] [PubMed] [Google Scholar]
- 164.Wolf A, Kozlov MV, Callaghan TV. 2008. Impact of non-outbreak insect damage on vegetation in northern Europe will be greater than expected during a changing climate. Clim. Change 87, 91–106 (doi:10.1007/s10584-007-9340-6) [Google Scholar]
- 165.Olofsson J, Moen J, Oksanen L. 2002. Effects of herbivory on competition intensity in two Arctic-alpine tundra communities with different productivity. Oikos 96, 265–272 (doi:10.1034/j.1600-0706.2002.960208.x) [Google Scholar]
- 166.Cederlund G, Markgren G. 1987. The development of the Swedish moose population, 1970–1983. Swedish Wildl. Res. (Suppl.) 1, 55–62 [Google Scholar]
- 167.Jeffries MO, Richter-Menge JA, Overland JE. (eds). 2012. Arctic report card 2012. See http://www.arctic.noaa.gov/reportcard [Google Scholar]
- 168.Meltofte H, Christensen TR, Elberling B, Forchhammer MC, Rasch M. 2008. Introduction. In High-Arctic ecosystem dynamics in a changing climate. Advances in Ecological Research, vol. 40 (eds Meltofte H, Christensen TR, Elberling B, Forchhammer MC, Rasch M.), pp. 1–12 New York: Elsevier [Google Scholar]
- 169.INTERACT, International Network for Terrestrial Research and Monitoring in the Arctic See http://www.eu-interact.org.
- 170.Andrews C, Dick J, Jonasson C, Callaghan TV. 2011. Assessment of biological and environmental phenology at a landscape level from 30 years of fixed date repeat photography in Northern Sweden. Ambio 40, 600–609 (doi:10.1007/s13280-011-0167-z) [DOI] [PMC free article] [PubMed] [Google Scholar]