Abstract
This study aimed to determine whether plasma testosterone is associated with the severity of coronary atherosclerosis in a group of 803 men who underwent elective coronary angiography. Testosterone levels were measured in 803 male patients who were categorized into three groups according to testosterone level tertiles. All patients underwent elective coronary angiography, and the severity of coronary artery disease (CAD) was determined by the Gensini score. Moreover, patients were classified into two groups according to Gensini scores (score ≤26 and score >26) using the median values as cutoff points. The plasma testosterone levels were measured by an ELISA kit. The level of testosterone was negatively associated with the Gensini score (r=−0.188; P=0.000). A multiple linear regression analysis revealed that testosterone was an independent risk factor for the Gensini score (β=−0.110; P=0.002) after adjusting for confounding covariates. In a multivariate logistic regression model, the severity of CAD was shown to be significantly lower in the third tertile (highest) of testosterone compared to the first tertile (lowest) of testosterone (odds ratio (OR)=0.465; 95% confidence interval (CI): 0.327–0.662; P=0.000). In this study, patients with lower testosterone levels had higher Gensini scores in a group of 803 men who underwent elective coronary angiography. Additional studies are needed to clarify the direction of causality and possible underlying mechanisms.
Keywords: coronary artery disease (CAD), Gensini score, testosterone
Introduction
Cardiovascular disease remains the leading cause of death in most developed or developing counties,1 and men are at higher risk for coronary artery disease (CAD) when compared to women,2 despite the advanced prevention of known risk factors. Harman et al.3 reported that low serum total testosterone is common in an ageing male population and has a prevalence of 30% in men over the age of 60 years. Phillips et al.4 first reported an inverse relationship between free testosterone levels and the degree of CAD in a series of patients undergoing coronary angiography. However, epidemiological studies have historically found no association between physiologically elevated androgen levels and atherosclerosis.5,6 Yeap et al.7 reported that lower testosterone levels predicted cardiovascular events in older men and are associated with higher cardiovascular and overall mortality. Akishita et al.8 indicated that a low testosterone level was an independent risk factor for cardiovascular disease events in middle-aged Japanese men with coronary risk factors. In addition, a similar relationship has been shown to exist between decreased testosterone concentrations and the extent and severity of coronary atheromas in men undergoing coronary angiography.9 These evidences indicate that testosterone may be involved in the pathogenesis of CAD and may provide a protective role in the development of cardiovascular disease in men. The serum testosterone level has been shown to be decreased with advancing age in men,10 and testosterone supplementation might reduce morbidity, increase longevity and preserve health in ageing men.11
Prior studies had lower reliability and smaller quantities compared to our present study that determine the relationship between plasma testosterone and the severity of coronary atherosclerosis.
Materials and methods
Study subjects
The patients were admitted to the First Affiliated Hospital of Nanjing Medical University, Nanjing (China) between 4 March 2003 and 12 May 2006. A total of 803 consecutive patients, 28–83 years of age (mean 62 years) who were undergoing elective coronary angiography due to CAD were enrolled. Diabetes was diagnosed using the known features of diabetes, including a plasma glucose level of 11.1 mmol l−1, a fasting plasma glucose concentration of 7.0 mmol l−1 after at least 8 h of fasting or a 2-h plasma glucose level of 11.1 mmol l−1 during an oral glucose tolerance test.12 The patients were on pharmacological treatment prior to the coronary angiography.
The exclusion criteria were as follows: inflammatory disease (white blood cell count >10.0×109 l−1; neutrophil percentage >70%); precatheterisation heart failure (Killip class ≥2 after acute myocardial infarction); severe chronic heart failure (New York Heart Association functional class ≥III); renal or thyroid dysfunction (serum creatinine ≥177 µmol l−1 and thyroid-stimulating hormone ≤0.3 mIU l−1 or ≥5 mIU l−1, respectively), end-stage hepatic, familial hypercholesterolaemia and immunological disease.
Written informed consent was obtained from all participants before angiography, and the project was approved by the Ethics Committees of the First Affiliated Hospital of Nanjing Medical University.
Laboratory measurements
Blood samples were collected immediately prior to coronary angiography between 8:00 a.m. and 9:00 a.m. after a 12-h fast. The total cholesterol (TCH, mmol l−1), triglycerides (TG, mmol l−1), fasting blood glucose (mmol l−1), high-density lipoprotein cholesterol (HDL-C, mmol l−1), low-density lipoprotein cholesterol (LDL-C, mmol l−1), creatine kinase (U l−1) and creatinine (µmol l−1) levels were determined by enzymatic procedures on an automated autoanalyser (AU 2700 Olympus; First Chemical Ltd, Tokyo, Japan) at the central clinical laboratory of the First Affiliated Hospital of Nanjing Medical University.
The red blood cell (RBC) count (109 l−1) and haemoglobin level (g l−1) were determined using an automated blood analyser (Bayer Diagnostics ADVIA120; Bayer, Leverkusen, Germany). The total testosterone level (ng ml−1) was measured by enzyme immunoassay in human plasma (11-TESHU-E01-PLAS; ALPCO, Salem, NH, USA). The reference range for testosterone was 0–20 ng ml−1.
Evaluation
Baseline data, including age, smoking status and drinking status, were recorded after hospital admission. Smoking habits were categorized as never smoking and smoking (including formerly smoking or currently smoking). Current and ex-smokers reported the number of cigarettes smoked per day, and those who reported smoking at least one cigarette per day during the preceding year were classified as current smokers. Height and weight were measured in the clinic with participants wearing light clothing and no shoes. The body mass index (BMI) was calculated as the weight (kg) divided by the square of the height (m2).
Gensini coronary score
The severity of coronary stenosis in patients was estimated by the Gensini coronary score.13 The Gensini score is based on the number of stenotic coronary artery segments, including the degree of luminal narrowing and the localisation of the stenosis.14 The Gensini system scores the narrowing of the coronary artery lumen as follows: 1%–25% narrowing=1; 26%–50% narrowing=2; 51%–75% narrowing=4; 76%–90% narrowing=8; 91%–99% narrowing=16; and total occlusion=32. The score is then multiplied by a factor that incorporates the importance of the lesion position in the coronary arterial tree as follows: ×5 for the left main coronary artery; ×2.5 for the proximal left anterior descending or left circumflex coronary artery; ×1.5 for the mid-segment of the left anterior descending; ×1 for the distal left anterior descending, right coronary artery or mid-distal left circumflex; and ×0.5 for any other arteries.13
Statistical analysis
The data were analysed with the Statistical Package for the Social Sciences (version 16.0; SPSS Inc., Chicago, IL, USA). Statistical significance was defined as a P value <0.05. All data were tested using the Kolmogorov–Smirnov test and presented as the mean±standard deviation or median (quartile ranges). The Kruskal–Wallis test or analysis of variance–Sheffe's F test was used for comparisons among groups. Categorical variables were compared by χ2 analysis. Based on the tertile of the testosterone level, patients were classified into three groups as follows: 0.002–3.391 (n=267); 3.392–4.382 (n=269); and 4.383–19.002 (n=267). In addition, patients were classified into two groups according to the Gensini score (score ≤26 and score >26) using the median value as a cutoff point.
The Spearman correlation coefficient (for non-normal distributions) was used to assess the relationship between plasma testosterone concentrations and Gensini score or other risk factors of CAD. To determine the odds ratios (ORs) of the Gensini score by testosterone level tertile, a logistic regression analysis was used, with tertile 1 serving as the reference. We assessed independent predictors of the Gensini score with a stepwise multiple linear regression analysis. All P values were two-tailed.
Results
Baseline, clinical and biochemical characteristics by tertile of the testosterone level in men
Baseline, clinical and biochemical characteristics of the three groups according to the testosterone level (the tertile values that were used as cutoff points) are displayed in Table 1. Age, BMI, creatine kinase, TG, creatinine, glucose, the Gensini score, smoking and diabetes mellitus differed among the three groups (all P<0.05) of patients who underwent elective coronary angiography. However, the TCH, HDL-C, LDL-C, haemoglobin levels and RBC counts were similar among the three groups (P>0.05).
Table 1. Baseline, clinical and biochemical characteristics based on testosterone tertiles in men.
| Variable | Testosterone tertile (ng ml−1) | |||
|---|---|---|---|---|
| 0.002–3.391 (n=267) | 3.392–4.382 (n=269) | 4.383–19.002 (n=267) | P | |
| Age (year) | 66.0 (57.0–72.0) | 64.0 (55.0–71.0) | 60.0 (51.0–69.0) | 0.000 |
| BMI (kg m−2) | 25.5 (22.5–28.5) | 24.8 (21.9–27.7) | 24.6 (21.7–27.5) | 0.001 |
| Gensini score | 40.5 (9.8–84.0) | 20.0 (0.0–51.3) | 16.0 (0.75–48.0) | 0.000 |
| Cholesterol (mmol l−1) | 3.98 (3.45–4.63) | 4.06 (3.37–4.57) | 3.94 (3.31–4.41) | 0.092 |
| Creatine kinase (mmol l−1) | 82.0 (55.8–151.3) | 75.0 (52.8–118.5) | 75.0 (52.8–97.0) | 0.001 |
| Triglycerides (mmol l−1) | 1.37 (1.05–1.89) | 1.34 (0.93–2.01) | 1.38 (0.98–2.00) | 0.022 |
| HDL-C (mmol l−1) | 0.93 (0.78–1.07) | 0.96 (0.85–1.14) | 0.95 (0.82–1.11) | 0.124 |
| Creatinine (µmol l−1) | 81.8 (69.0–97.0) | 79.0 (69.0–91.0) | 75.3 (65.0–89.0) | 0.007 |
| LDL-C (mmol l−1) | 2.42 (1.98–2.99) | 2.30 (1.88–2.86) | 2.32 (1.84–2.71) | 0.062 |
| Glucose (mmol l−1) | 5.20 (4.51–6.32) | 4.73 (4.34–5.36) | 4.67 (4.21–5.09) | 0.000 |
| Red blood cells (109 l−1) | 4.46 (4.02–4.82) | 4.50 (4.15–4.84) | 4.56 (4.11–4.90) | 0.354 |
| Haemoglobin (g l−1) | 138.0 (126.5–148.0) | 139.5 (128.8–147.0) | 139.5 (129.7–150.0) | 0.163 |
| Smoking (yes/no) | 180/87 | 157/112 | 155/112 | 0.041 |
| Diabetes mellitus (yes/no) | 61/206 | 49/220 | 24/243 | 0.000 |
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Spearman correlations between the testosterone level and Gensini score and the risk of CAD
The corrections for the testosterone level and Gensini score and several cardiovascular disease risk factors are shown in Table 2. There was a negative association between the testosterone level and the Gensini score (r=−0.188; P=0.000) based on the study population. The testosterone levels were also correlated with age (r =−0.120; P=0.000), BMI (r=−0.088; P=0.013), TCH (r=−0.089; P=0.013), creatine kinase (r=−0.124; P=0.001), TG (r=−0.089; P=0.013), creatinine (r=−0.104; P=0.004), LDL-C (r=−0.089; P=0.013), glucose (r=−0.195; P=0.000) and haemoglobin levels (r=0.070; P=0.047). No associations existed between the testosterone and HDL-C levels (r=0.055; P=0.123) or the RBC count (r=0.050; P=0.158) in men who underwent elective coronary angiography.
Table 2. Spearman correlations between the testosterone level, Gensini score and CAD risk.
| Variable | Testosterone (ng ml−1) | |
|---|---|---|
| Correlation coefficient | P | |
| Age (year) | −0.120 | 0.001 |
| Gensini score | −0.188 | 0.000 |
| BMI (kg m−2) | −0.088 | 0.013 |
| Cholesterol (mmol l−1) | −0.089 | 0.013 |
| Creatine kinase (mmol l−1) | −0.124 | 0.001 |
| Triglycerides (mmol l−1) | −0.089 | 0.013 |
| HDL-C (mmol l−1) | 0.055 | 0.123 |
| Creatinine (µmol l−1) | −0.104 | 0.004 |
| LDL-C (mmol l−1) | −0.089 | 0.013 |
| Glucose (mmol l−1) | −0.195 | 0.000 |
| Red blood cells (109 l−1) | 0.050 | 0.158 |
| Haemoglobin (g l−1) | 0.070 | 0.047 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
A multiple linear regression analysis with the Gensini score as the dependent variable
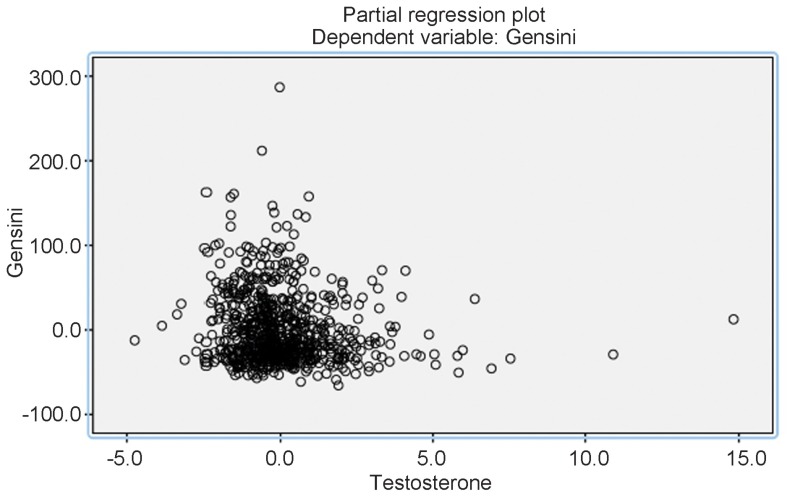
The Gensini score was used as the dependent variable, and the independent variables were age, BMI, RBC count, testosterone, creatine kinase, haemoglobin, glucose, LDL-C, HDL-C, creatinine, TCH, triglyceride levels and smoking. We showed that the testosterone, creatine kinase, haemoglobin, glucose, LDL-C and creatinine levels and age and smoking were independent predictors for the Gensini score (Table 3) in a stepwise regression. Figure 1 presents the partial regression and more clearly shows the relationship between the Gensini score and the testosterone level.
Table 3. The linear regression analysis with the Gensini score as the dependent variable in men.
| Variable | Standardized coefficients (β) | 95%CI | P |
|---|---|---|---|
| Age (year) | 0.105 | 0.129–0.717 | 0.005 |
| Haemoglobin (g l−1) | −0.186 | (−0.678)–(−0.302) | 0.000 |
| Creatine kinase (mmol l−1) | 0.111 | 0.003–0.011 | 0.002 |
| Glucose (mmol l−1) | 0.108 | 0.680–3.041 | 0.002 |
| LDL-C (mmol l−1) | 0.075 | 0.380–8.304 | 0.032 |
| Creatinine (µmol l−1) | 0.096 | 0.053–0.324 | 0.006 |
| Testosterone (ng ml−1) | −0.110 | (−4.785)–(−1.133) | 0.002 |
| Smoking | 0.085 | 1.579–13.623 | 0.013 |
Abbreviations: CI, confidence interval; LDL-C, low-density lipoprotein cholesterol.
Figure 1.
Multiple linear regression analysis with testosterone level as independent predictors for gensini score.
Determinants of the Gensini score in a multivariate logistic regression analysis
The testosterone levels were analysed as tertiles, with tertile 1 serving as the reference, based on the study population. In a multivariate logistic regression model (Table 4) adjusted for smoking, the severity of CAD, as defined by the Gensini score, was shown to be significantly lower in the third testosterone tertile (highest) compared to the first testosterone tertile (lowest) (OR=0.465; 95% confidence interval (CI): 0.327–0.662; P=0.000).
Table 4. The determinants of the Gensini score in a multivariate logistic regression analysis in men.
| Variable | Gensini score | P | OR (95%CI) | |
|---|---|---|---|---|
| ≤26 (n=403) frequency | >26 (n=400) frequency | |||
| Testosterone 1 (lowest) | 109 | 158 | 0.000 | |
| Testosterone 2 | 138 | 131 | 0.021 | 0.660 (0.465–0.938) |
| Testosterone 3 (highest) | 156 | 111 | 0.000 | 0.465 (0.327–0.662) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Discussion
In this study, we demonstrated that the testosterone level was negatively associated with the Gensini score and was an independent predictor for the Gensini score. Thus, testosterone could affect the development of atherosclerotic coronary arteries. Our findings confirm a previous report of an association between the Gensini score and testosterone,14 which involved 87 middle-aged male patients.
Similar results were confirmed thereafter by English et al.,5 who observed lower levels of testosterone in men with CAD when compared to normal coronary angiography. Phillips et al.4 first reported an inverse association between free testosterone levels and the severity of CAD in a series of subjects undergoing coronary angiography. Subsequently, Yeap et al.7 and Akishita et al.8 suggested that endogenous testosterone may have a protective effect against cardiovascular disease in men;16 specifically, testosterone could benefit endothelial function,16 elicit vasodilation and increase blood flow in men17 and in animals.18 An increased frequency of thromboembolic events has also been reported in androgen-deprived men.19,20 These findings provide evidence for a role of endogenous testosterone in the severity of cardiovascular disease. However, Fallah et al.21 reported that lower total testosterone has a preventive effect on CAD, whereas higher values increase the risk of CAD.
Dyslipidaemia also contributes to the pathogenesis of atherosclefrosis. Recent epidemiological and observational studies have generally shown that low endogenous testosterone concentrations are independently associated with dyslipidaemia and an atherogenic lipid profile in men.22 Positive associations have been shown between testosterone levels and HDL-C;23 this is inconsistent with our research in which no such association existed. These discrepancies might be attributed to the sample size and differences in the population characteristics. Further studies should be conducted to explain the underlying mechanism. The observation that lower testosterone levels were also correlated with elevated TCH, TG, LDL-C and fasting glucose levels24 is in agreement with previous reports.23,25,26 Moreover, an inverse association between testosterone and creatine kinase or creatinine levels (r=−0.124; P=0.001 vs. r=−0.104; P=0.004) was shown in the current study.
The Massachusetts Male Aging study showed that decreased testosterone concentrations are associated with an increased risk of developing metabolic syndrome27 in men with a BMI <25 kg m−2. Jockenhovel et al.28 reported that obesity may also act as an inhibitor of the hypothalamic–pituitary–gonadal axis, possibly via agents such as leptin. In our study, we also found that testosterone was negatively correlated with BMI in men (r=−0.088; P=0.013), indicating that the testosterone concentration correlates with adiposity. However, there is no evidence to suggest the direction of possible causality.
We also found that increased testosterone levels were associated with elevated haemoglobin levels in males (r=0.07; P=0.047). This is in agreement with a previous study,29 but no correlation was shown with the RBC count (r=0.050; P=0.158).
The precise mechanism underlying our findings and its clinical relevance require future elucidation. Oestrogen will be an added measurement in a follow-up study. In addition, there are a number of risk factors for CAD; however, we only analysed the most common risk factors in the current study.
In conclusion, men with lower testosterone levels had higher Gensini scores in a group of 803 men who underwent elective coronary angiography. Additional studies are needed to clarify the direction of causality and possible underlying mechanisms.
Author contributions
EZJ conceived and designed the research; LL and CYG collected data and performed the research; TBZ, LSW and KJC contributed new analytical tools and reagents; WZM and ZJY analysed the data; and LL performed the statistical analysis and drafted the manuscript.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 30400173 and No. 30971257).
All authors declare that there are no competing financial interests.
References
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99:1165–72. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Phillips GB, Castelli WP, Abbot RD, McNamara PM. Association of hyperestrogenemia and coronary heart disease in men in the Framingham cohort. Am J Med. 1983;74:863–9. doi: 10.1016/0002-9343(83)91078-1. [DOI] [PubMed] [Google Scholar]
- English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, et al. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000;21:890–4. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–9. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- Yeap BB. Androgens and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2010;17:269–76. doi: 10.1097/MED.0b013e3283383031. [DOI] [PubMed] [Google Scholar]
- Akishita M, Hashimoto M, Ohike Y, Qgawa S, Iijima K, et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210:232–6. doi: 10.1016/j.atherosclerosis.2009.10.037. [DOI] [PubMed] [Google Scholar]
- Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb. 1994;14:701–6. doi: 10.1161/01.atv.14.5.701. [DOI] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–9. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–76. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes—2008. Diabetes Care. 2008;31 Suppl. 1:S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- Hu X, Jiang H, Bai Q, Zhou X, Xu C, et al. Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clin Chim Acta. 2009;406:139–42. doi: 10.1016/j.cca.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Gensini GG. A more meaningful scoring system for determinating the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- Hu X, Rui L, Zhu T, Xia H, Yang X, et al. Low testosterone level in middle-aged male patients with coronary artery disease. Eur J Intern Med . 2011;22:e133–6. doi: 10.1016/j.ejim.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30:1029–34. doi: 10.1291/hypres.30.1029. [DOI] [PubMed] [Google Scholar]
- Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–6. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, et al. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. . Circulation. 1996;94:2614–9. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- Ehdaie B, Atoria CL, Gupta A, Feifer A, Lowrance WT, et al. Androgen deprivation and thromboembolic events in men with prostate cancer. Cancer. 2012;118:3397–406. doi: 10.1002/cncr.26623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L, Basaria S. Adverse effects of androgen deprivation therapy in men with prostate cancer: a focus on metabolic and cardiovascular complications. Asian J Androl. 2012;14:222–5. doi: 10.1038/aja.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah N, Mohammad K, Nourijelyani K, Eshraghian MR, Seyyedsalehi SA, et al. Nonlinear association between serum testosterone levels and coronary artery disease in Iranian men. Eur J Epidemiol. 2009;24:297–306. doi: 10.1007/s10654-009-9336-9. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Mykkanen L, Valdez RA, Katz MS. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J Clin Endocrinol Metab. 1993;77:1610–5. doi: 10.1210/jcem.77.6.8263149. [DOI] [PubMed] [Google Scholar]
- van Pottelbergh I, Braeckman L, de Bacquer D, de Backer G, Kaufman JM. Differential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged men. Atherosclerosis. 2003;166:95–102. doi: 10.1016/s0021-9150(02)00308-8. [DOI] [PubMed] [Google Scholar]
- Lerchbaum E, Pilz S, Boehm BO, Grammer TB, Obermayer- Pietsch B, et al. Combination of low free testosterone and low vitamin D predicts mortality in older men referred for coronary angiography. Clin Endocrinol (Oxf) 2012;77:475–83. doi: 10.1111/j.1365-2265.2012.04371.x. [DOI] [PubMed] [Google Scholar]
- Morris PD, Channer KS.Testosterone and cardiovascular disease in men Asian J Androl2012; 14428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chock B, Lin TC, Li CS, Swislocki A. Plasma testosterone is associated with Framingham risk score. Aging Male. 2012;15:134–9. doi: 10.3109/13685538.2011.654369. [DOI] [PubMed] [Google Scholar]
- Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:840–50. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- Jockenhovel F, Blum WF, Vogel E, Englaro P, Muller-Wieland D, et al. Testosterone substitution normalizes elevated serum leptin levels in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2510–3. doi: 10.1210/jcem.82.8.4174. [DOI] [PubMed] [Google Scholar]
- Gonzales GF, Villena A. Low pulse oxygen saturation in post-menopausal women at high altitude is related to a high serum testosterone/estradiol ratio. Int J Gynaecol Obstet. 2000;71:147–54. doi: 10.1016/s0020-7292(00)00270-8. [DOI] [PubMed] [Google Scholar]