Abstract
Overwhelming oxidative stress and compromised tubular cell antioxidant response have been incriminated for cisplatin (Cis) -induced acute kidney injury (AKI). We hypothesized that Cis-induced KI was the outcome of the deactivated redox-sensitive stress response program (RSSRP). Wild (WT) and heterozygous p66ShcA+/− mice in groups of six were administered either normal saline (WT) or Cis (12.5 mg/kg, intraperitoneal, Cis/WT). Renal biomarkers were collected and kidneys were harvested for renal histology. Cis/WT showed elevated blood urea nitrogen levels and enhanced tubular cell apoptosis, necrosis, and dilated tubules filled with casts when compared to Cis/p66+/−. Cis/p66+/− developed only a clinically occult ARF (normal blood urea levels and only microscopic alterations). Immunoblots from the lysates of renal tissues of Cis/WT displayed enhanced expression of phospho-p66ShcA, and phospho-Foxo3A but attenuated expression of MnSOD and catalase; conversely, p66 deficit prevented these alterations in Cis milieu. In in vitro studies, Cis treated mouse proximal tubular cells (MPTCs) displayed enhanced phosphorylation of p66ShcA and no increase in tubular cell expression of MnSOD. In addition, renal tissues of Cis/WT and Cis-treated MPTCs displayed enhanced phosphorylation of p53 and Bax expression. However, MPTC partially silenced for p66ShcA displayed partial inhibition of Cis-induced tubular cell apoptosis as well as necrosis. These findings indicated that Cis-induced AKI was the outcome of the deactivated RSSRP (attenuated anti-oxidant response) and activation of pro-apoptotic (p53-induced Bax expression) pathway.
Both reactive oxygen species (ROS) and adenosine triphosphate (ATP) depletion have been implicated in cisplatin (Cis)-induced cellular injury (1). Cis is known to cause mitochondrial dysfunction in kidney epithelial cells (19, 20). Cis reduces cellular ATP levels by targeting enzymatic complexes that comprise mitochondrial electron transport chain (19). At high concentrations, Cis promoted tubular cell necrosis through severe ATP depletion (13); whereas, at low concentrations, Cis enhanced tubular cell apoptosis through the release of mitochondrial cytochrome C (19, 20).
Cis has also been demonstrated to enhance tubular cell accumulation of reactive oxygen species (ROS) because of poor antioxidant response in Cis milieu (7, 21). On that account, antioxidants provided protection against Cis-induced tubular cell injury both in vivo and in vitro studies (13, 21). In addition, free radical scavengers attenuated Cis-induced tubular cell apoptosis and renal failure (18). However, the involved mechanism for Cis-induced poor antioxidant response is far from clear. In the present study, we have attempted to determine the involved mechanism in tubular cells for poor antioxidant response in Cis milieu.
Phenotype of the redox-sensitive cells is dependent on their ability to generate free radical scavengers such as MnSOD and catalase to metabolize ROS. This redox -sensitive stress response program (RSRP) acts as a survival strategy for the stressed cells (17). Nonetheless, stressed cell phenotype (cell survival vs. death) would also predict the history in terms of adequacy of stress response. Altered cellular phenotype in Cis milieu has been attributed to inadequate redox-sensitive stress response. However, the exact mechanism of this inadequate redox-sensitive stress response has not been reported so far. We hypothesized that Cis deactivated redox-sensitive stress response program in tubular cells by activating p66ShcA pathway.
The mammalian adaptor protein ShcA has three isoforms, p46, p52, and p66. p66ShcA regulates reactive ROS metabolism and apoptosis through its downstream signaling to FOXO pathway (17). p66ShcA deficient mice and p66ShcA deficient cells displayed reduced levels of intracellular ROS and exhibited resistant to apoptosis (4, 14–167). In that context, a portion of p66ShcA works as a redox enzyme in mitochondria and generates ROS and associated apoptosis (3, 4). Therefore, mitochondrial ROS production is critical for the p66ShcA-induced cellular injury. Since mitochondrial ROS generation plays a critical role in Cis-induced tubular cell injury, we hypothesized that p66 could be contributing to Cis-induced tubular cell injury. In that scenario, we further hypothesized that even a partial p66 deficit status may provide protection against Cis-induced acute renal failure.
Clark et al., (5) in in vitro studies demonstrated that ERK served as the kinase that phosphorylated the pro-apoptotic p66shc protein in Cis-treated renal proximal tubular cells and further demonstrated that over expression of p66ShcA exacerbated, whereas, lack of p66ShcA expression attenuated Cis-induced tubular cell injury. However, these investigators did not explore the role of the redox-sensitive stress response program. In the present study, we have demonstrated that the activation of tubular cell p66ShcA pathway was associated with Foxo3A-mediated deactivation of the redox-sensitive stress response program in Cis-induced acute renal failure model. In addition, a partial deficit of p66 could rescue mice from Cis-induced acute renal failure.
Materials and Methods
Mice
We have used age and sex matched FVB/N (control) and FVBN/p66ShcA+/− mice. Breeding pairs of FVBN were obtained from Jackson Laboratories (Bar Harbor, ME). Breeding pairs to develop FVBN/p66ShcA+/−, SV129p66ShcA+/− mice were obtained from The Mutant Mouse Regional Resource Center U42 RR014817 (MMRC), North Carolina Research Laboratory. Mice were housed in groups of 4 in a laminar-flow facility (Small Animal Facility, Feinstein Institute for Medical research, North Shore LIJ Health System, Manhasset, NY). We are maintaining colonies of these mice in our animal facility. The Ethics Review Committee for Animal Experimentation of Long Island Jewish Medical Center approved the experimental protocol.
Proximal tubular cells
Mouse proximal tubular epithelial cells (MPTC) were a gift from Dr. Poornima Upadhya (Long Island Jewish Medical Center, New Hyde Park, NY). Mouse tubular cells were characterized by their expression for cytokeratin-18,-19 and E-cadherin.
Experimental studies
Control (FVBN) mice or heterozygous (HT) p66 (FVBN/p66ShcA+/−) mice in groups of six were administered either normal saline or cisplatin (12.5 mg/Kg, intraperitoneal, single dose). After 3 days, mice were sacrificed, blood was collected by cardiac puncture and kidneys were isolated for renal histology and molecular markers
Blood urea nitrogen levels
Blood urea nitrogen was measured in control and experimental animals by the standard technique.
Apoptosis studies
Morphologic evaluation of MPTC apoptosis was performed by staining cells with H- 33342 (Molecular Probes, Portland, OR) and propidium iodide (PI, Sigma, St. Louis, MO). Double staining by these two agents provides the percentage of live, apoptotic and necrotic cells under control and experimental conditions. To determine the effect of Cis, MPTCs were grown to subconfluence in 24-well plates. Subsequently, cells were washed twice with phosphate buffered saline (PBS) and incubated in media (including 1% FCS) containing either vehicle or Cis for 24 hours; three sets of experiments were carried out. At the end of the incubation period, cells were stained with H-33342 and PI. The percentage of live, apoptotic and necrosed (primary, intact nuclei and secondary, fragmented nuclei) cells was recorded in eight random fields by two observers unaware of the experimental conditions.
Renal tissues preparation and Disease scoring
Both kidneys were harvested from each mouse. One of the harvested kidneys was used for protein isolation, while a portion of the other kidney was used to prepare a cryo-block. The remaining part of the second kidney was fixed in 10% formalin and embedded in paraffin. Three-micrometer sections were prepared and stained with hematoxylin-eosin and periodic-acid Schiff. Renal injury was scored for four traits related to tubules (necrosis, apoptosis, tubular dilatation, and cast formation). A modified semi quantitative scale was used (9, 23) as follows:
0= No damage
1+ = tubular cell necrosis
2+ = mild tubular dilatation with tubular cell necrosis
3+ = moderate tubular dilatation with tubular cell necrosis
4+ = severe tubular dilatation with cast formation.
Silencing of p66ShcA
MPTECs were transfected with p66Shc (SHC-1 Silencer SelectR Pre- designed siRNA, Cat # 4390771, Ambion, Austin, TX), with Siport Neofax transfection reagent and left in optiMEM media for 48 hrs. Control and trransfected cells were used under control and experimental conditions.
Immunodetetection by Western blot
Renal tissue lysates of control and experimental animals were extracted in RIPA buffer containing 50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1mM EDTA, 1% NP-40, 0.25% Deoxycholate, 0.1% SDS, 1X protease inhibitor cocktail I (Calbiochem, EMD Biosciences, Gibbstan, NJ), 1mM PMSF, and 0.2mM sodium orthovanadate. Protein concentration was measured with the Bradford Protein Assay (Pierce, Rockford, IL). Protein lysates (20 μg) were separated on a 15 % polyacrylamide gels (PAGE, Bio-Rad, Hercules, CA) and transferred onto a nitrocellulose membrane using Bio-Rad miniblot apparatus. Nitrocellulose membranes were then subjected to immunostaining with primary antibodies against p66(ShcA) (recognizes all ShcA isoforms, Cell Signaling, Danvers, MA), mouse monoclonal anti-phospho-ShcA-Ser-36 (EMD Biosciences), anti-phospho-Foxo3a rabbit polyclonal antibody (Cell Signaling), anti-SOD rabbit polyclonal antibody (Calbiochem), anti-catalase antibody (Cell Signaling) anti-phospho-p53 and p53 antibodies (Cell Signaling), anti Bax antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and anti Bcl2 antibody (Santa Cruz) and subsequently treated with horseradish peroxidase labeled appropriated secondary antibodies. The blots were developed using a chemiluminescence detection kit (PIERCE, Rockford, IL) and exposed to X-ray film (Eastman Kodak Co., Rochester, NY). Equal protein loading was confirmed by stripping the blots and immunoblotting for actin using a polyclonal α-Actin antibody (Santa Cruz Technology, Santa Cruz, CA).
Reverse Transcription PCR Analysis
Renal tissues from WT and P66+/− mice were used to quantify mRNA expression of p66ShcA. RNA was extracted using TRIZOL (Invitrogen corp.). For cDNA synthesis, 2 μg of the total RNA was preincubated with 2 nmol of random hexamer (Invitrogen Corp) at 65°C for 5 min. Subsequently, 8 μl of the reverse-transcription (RT) reaction mixture containing Cloned AMV RT, 0.5 mmol each of the mixed nucleotides, 10 mmol dithiothreitol, and 1000 U/mL Rnasin (Invitrogen Corp) was incubated at 42°C for 50 min. For a negative control, a reaction mixture without RNA or reverse transcription (RT) was used. Samples were subsequently incubated at 85°C for 5 min to inactivate the RT. Quantitative PCR was carried out in an ABI Prism 7900HT sequence detection system using the primer sequences as shown below:
| P66 | F | CCTGCCATATCCTGGAGTGT |
| R | GGTTCCTTCCCTGGAAAGTC |
SYBR green was used as the detector and ROX as a stabilizing dye. Results (means ± S.D.) represent number of samples as described in the legend. The data were analyzed using the Comparative CT method (ΔΔCT method). Differences in CT are used to quantify relative amount of PCR target contained within each well. The data were expressed as relative mRNA expression in reference to control, normalized to quantify of RNA input by performing measurements on an endogenous reference gene, GAPDH.
Statistical analysis
For comparison of mean values between two groups, the unpaired t test was used. To compare values between multiple groups, analysis of variance (ANOVA) was used to calculate a P-value. Statistical significance was defined as P<0.05. Results are presented and mean ± SD
Results
p66+/− mice display attenuated tubular cell injury and clinically occult AKI after Cis administration
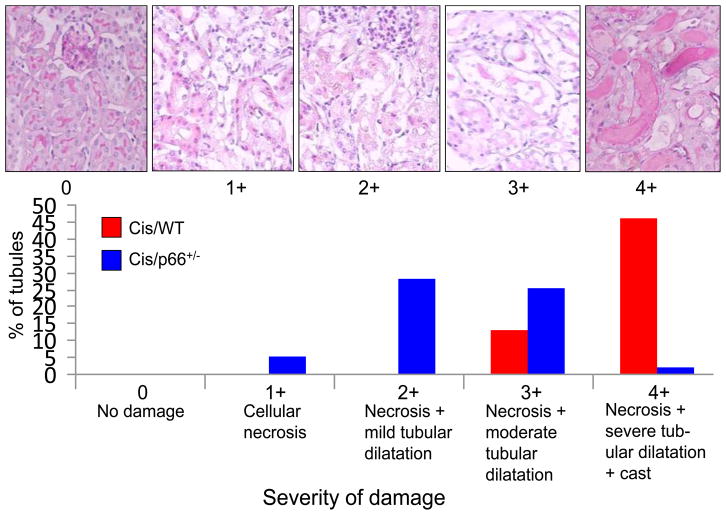
Cis/WT mice displayed massive tubular cell necrosis and dilatation of tubules. Cis/p66+/− mice displayed only mild to moderate amount of tubular cell necrosis and dilatation. Percentages of tubules displaying variable severity of injury are shown in Fig. 1. Approximately 50% tubules displayed 4+ injury in Cis/WT; whereas, only 1.5% of tubules in Cis/p66+/− displayed 4+ injury. Representative renal cortical sections displaying 0 to 4+ tubular cell injuries are shown in Fig. 1. Representative microphotographs of WT, p66+/−, Cis/WT., and Cis/p66+/− are shown in Fig. 2A. Cumulative data on severity of tubular cell injury is shown in Fig. 2B. Cis/WT mice displayed greater (P<0.01) injury score when compared to Cis/P66+/− mice.
Fig. 1. Cis/p66+/− mice display attenuated tubular cell injury.
A. Representative microphotographs showing different grading of tubular cell injury.
B. Cis/WT and Cis/WT mice (n=6) were graded for their tubular cell injury. Percentages of tubules displaying variable severity of injury are shown displayed massive tubular cell necrosis and dilatation of tubules. Approximately 50% tubules displayed 4+ injury in Cis/WT; whereas, only 1.5% of tubules in Cis/p66+/− displayed 4+ injury.
Fig. 2. Cis/p66+/− mice display decreased injury score and only occult AKI.
A. Representative microphotographs of WT, P66+/−, Cis/WT., and Cis/p66+/− mice are shown.
B. Cumulative data (n=6) on injury score is shown
*P<0.01 compared to Cis/WT.
C. Mean BUN levels in WT, P66+/−, Cis/WT., and Cis/P66+/− mice are shown. *P<0.01 compared to other variables.
D. mRNA expression for p66 as assayed by real time PCR studies from control mice and p66+/− mice (n=4). *P<0.01 compared to WT.
Cis/WT mice also showed elevated (P<0.01) BUN levels when compared to WT and Cis/p66+/− (Fig. 2C). Cis/p66+/− mice did not show any elevation of BUN. Normal BUN levels in Cis/p66+/− indicated that they had only clinically occult AKI. To confirm whether p66 transcription is down regulated in renal tissues of p66+/− mice, total RNA was extracted from control mice and p66+/− mice (n=4). mRNA expression for p66 was assayed by real time PCR studies. p66+/− displayed an attenuated mRNA expression for p66 (Fig. 2D).
p66ShcA deficiency partially rescues from Cis-induced tubular cell apoptosis
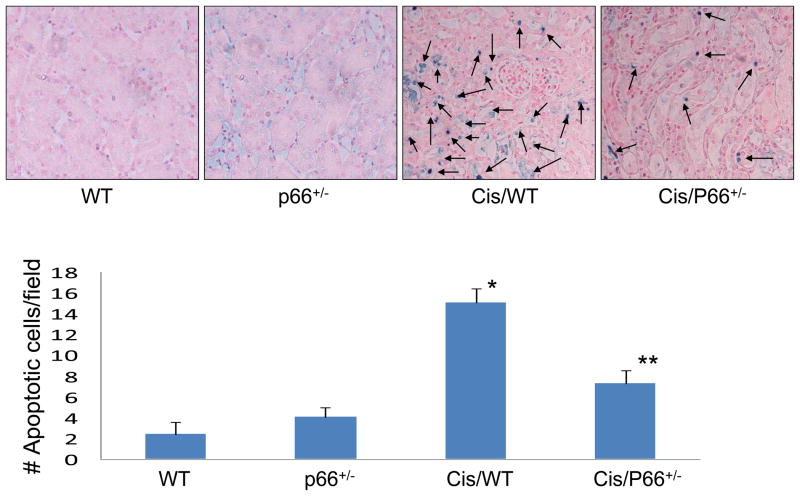
Renal cortical sections of WT, p66+/−, Cis/WT, and Cis/p66+/− mice were labeled for TUNEL +ve cells. Renal cortical sections of Cis/WT mice showed increased (P<0.001) number of TUNEL positive cells when compared to WT and p66+/− mice. Cis/p66+/− mice displayed lower (P<0.01) number of TUNEL +ve cells when compared to Cis/WT mice.
Cis enhances renal tissue p66ShcA and deactivates redox sensitive stress response program
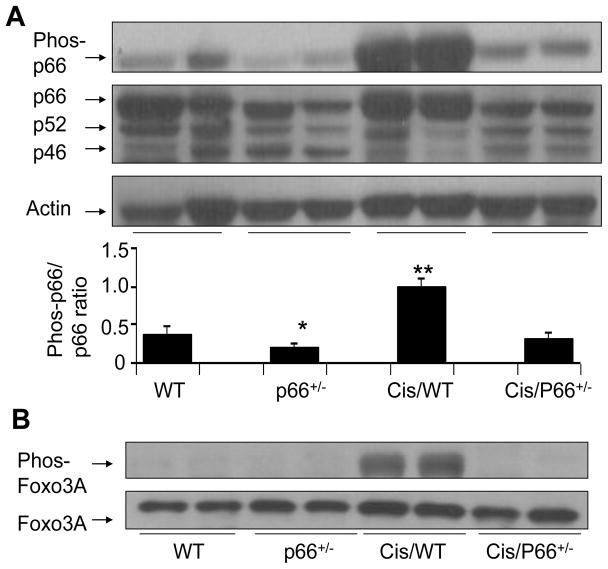
Immunoblots of WT, Cis/WT, p66+/− and Cis/p66+/− were probed for phospho-p66ShcA (n=4). The same blots were stripped and reprobed for total p66 as well as for actin. Representative gels of two different mice are shown in Fig. 4A. p66+/− displayed a diminished expression of p66ShcA when compared to WT mice. Cis/WT mice displayed enhanced phosphorylation of p66ShcA when compared to WT and Cis/p66+/−. Cumulative data of four different mice experiments are shown in the form of bar graphs (Fig. 4A).
Fig. 4. Cis enhances renal tissue p66ShcA and deactivates redox sensitive stress response program.
A. Immunoblots of WT, Cis/WT, P66+/− and Cis/P66+/− were probed for phospho-p66ShcA (n=4). The same nitrocellulose blots were stripped and reprobed for total p66 as well as for actin. Representative gels of the lysates two different animals are shown. Cumulative data of four different mice are shown in the form of bar graphs. *P<0.05 compared to WT; **P<0.01 compared to Cis/p66+/−
B. Renal tissue lysates of WT, Cis/WT, p66+/− and Cis/p66+/− were electrophoresed and then probed for phospho-Foxo3A (n=4). The same Western blots were stripped and reprobed for total Foxo3A.
Cells response to oxidative stress by generating antioxidants such as SOD and catalase (15–17). Nuclear Foxo3A is a transcriptor of SOD and catalase (15–17).However, once Foxo3A is phosphorylated it is translocated to cytosol and gets degraded. We asked whether Cis-induced p66ShcA phosphorylation would be able to phosphorylate Foxo3A and thus make it an inactive moiety transcriptionally.
To determine whether Cis-induced phosphorylation of p66ShcA was also associated with down stream signaling in the form of phosphorylation of Foxo3A, protein lysates from of WT, Cis/WT, p66+/− and Cis/p66+/− were probed for phospho-Foxo3A (n=4). The same blots were stripped and reprobed for total Foxo3A.
Representative gels of renal tissues of two different mice are shown in Fig. 4B. Cis stimulated phosphorylaion of Foxo3A; however, this effect of Cis was partially attenuated by partial deficit of p66.
P66 deficiency restores the renal tissue antioxidant generation
Immunoblots of renal tissues of WT, Cis/WT, p66+/− and Cis/p66+/− were probed for MnSOD and catalase (n=4). The same blots were stripped and reprobed for actin. Representative gels of two different mice are shown in Fig. 5A. Renal tissues of Cis/WT mice displayed attenuated MnSOD expression despite ongoing oxidative stress (Fig. 5A). On the other hand, p66 deficit restored the production of MnSOD in Cis-treated mice. Cumulative data of four mice are shown in the form of a bar diagram.
Fig. 5. p66 deficiency restores the renal tissue antioxidant generation.
A. Protein blots of renal tissues of WT, Cis/WT, p66+/− and Cis/p66+/− were probed for MnSOD and catalase (n=4). The same blots were stripped and reprobed for actin. Representative gels from two different mice are shown. Cumulative data of four mice are shown in the form of a bar diagram. *P<0.05 compared with Cis/WT.
B. Renal tissue lysates of WT, Cis/WT, p66+/− and Cis/p66+/− were electrophoresed and then probed for catalase and reprobed for actin (n=4). Representative gels of two different mice are shown. Renal tissues Cis/WT display attenuated expression of catalase; whereas, renal tissues of Cis/p66+/− display enhanced expression of catalase.
Cis also attenuated renal tissue production of catalase (Fig. 5B); whereas, p66 deficit restored the renal tissue production of catalase in Cis-treated mice. These findings indicated that lack of p66 had a potential to restore Cis-induced deactivated redox-sensitive stress response program.
Cis enhances activation of p53 pathway
Cis has been reported to produce tubular cell apoptosis through the activation of p53 pathway (10). To confirm the activation of p53 pathway, immunoblots obtained from the lysates of renal tissues of WT, Cis/WT, p66+/− and Cis/p66+/− were probed for phospho-p53 (n=4). The Western blots were stripped and reprobed for total p53. Representative gels from two different mice are shown in Fig. 6A. Renal tissues of Cis/WT mice displayed enhanced phosphorylation of p53; on the other hand p66 deficit attenuated this effect of Cis. Cumulative data of four different mice are shown in bar graphs. Since the activation of p53 pathway is associated with enhanced expression of Bax, immunoblots from the lysates of renal tissues of WT, Cis/WT, p66+/− and Cis/p66+/− were probed for Bax (n=4). The same nitrocellulose blots were sripped and reprobed for actin. Representative gels of two different mice are shown in Fig. 6B. Renal tissues of Cis/WT displayed enhanced expression of Bax, however p66 deficit attenuated Cis-induced renal tissue Bax expression.
Fig. 6. Cis enhances activation of p53 pathway.
A. Renal tissue lysates of WT, Cis/WT, p66+/− and Cis/p66+/− were electrophoresed and probed for phospho-p53 (n=4). The same blots were stripped and reprobed for total p53. Representative gels of two different mice are shown. Cumulative data of four different mice are shown in bar graphs. *P<0.001 compared with other variables
B. Immunoblots of renal tissues of WT, Cis/WT, p66+/− and Cis/p66+/− probed for the expression of Bax and actin. Renal tissues of Cis/WT displayed enhanced expression of Bax.
In vitro studies
Cis deactivates redox-sensitive stress response pathway in mouse tubular cells
To determine the direct effect of Cis on mouse tubular cell redox-sensitive stress response program, mouse tubular cells (MPTC) were incubated in media containing either only buffer or different concentrations of Cis (25 or 50 μM) for 12 or 20 hours. Subsequently, transferred protein blots were probed for phospho-p53, phospho-p66ShcA, or MnSOD. After stripping, the same blots were reprobed for actin. Representative gels are displayed in Figs 7A, 7B, and 7C. Cis enhanced tubular cell phosphorylation of p53 and p66ShcA at 12 hours as well as at 20 hours. However, Cis did not enhance tubular cell expression of MnSOD at any concentration at 12 or 20 hours of incubation.
Fig. 7. Cis deactivates redox-sensitive stress response pathway in mouse tubular cells.
MPTCs were incubated in media containing either buffer or variable concentrations of Cis (25 or 50 μM) for 12 or 20 hours. Subsequently, protein blots were probed for phospho-p53, phospho-p66ShcA, or MnSOD. The same blots were reprobed for actin.
A. Representative gels displaying MPTC expression of phospho-p53 and actin under control and Cis-treated conditions.
B. Representative gels displaying MPTC expression of phospho-p66 and actin under control and Cis-treated conditions.
C. Representative gels displaying MPTC expression of MnSOD and actin under control and Cis-treated conditions.
Cis enhances tubular cell expression of Bax but down regulates expression of Bcl2
Cis has been reported to induce tubular cell apoptosis by Bax expression (1, 20). To determine effect of Cis on tubular cell Bax and Bcl2 expression, MPTCs were incubated in media containing either buffer or Cis (25 μM or 50 μM) for 12 hours. Cellular lysates were electrophoresed and probed for Bax and Bcl2. The same blots were reprobed for actin. Representative gels are shown in Fig. 8. Cis enhanced Bax expression by MPTCs. However, Cis down regulated MPTC expression of Bcl2. These findings indicated that Cis not only promotes tubular cell expression of pro-apoptotic protein (Bax) but also down regulates pro-survival protein (Bcl2).
Fig. 8. Cis enhances tubular cell expression of Bax but down regulates expression of Bcl2.

MPTCs were incubated in media containing either buffer or Cis (25 μM or 50 μM) for 12 hours. Subsequently, cellular lysates were immunoelectrophoresed and probed for Bax and Bcl2. The same western blots were reprobed for actin. Representative gels are shown.
p66 is critical for Cis-induced tubular cell apoptosis
To determine the effect of deficit of p66 on Cis-induced tubular cell injury, MPTCs were transfected with siRNA-p66 or scrambled (SCR) siRNA. Immunoblots of control MPTCs and MPTCs transfected with either scrambled siRNA(SCR/MPTC) or p66-siRNA(p66-siRNA/MPTCs) are shown in Fig. 9A. Actin content of stripped and reprobed blots are also displayed. Densitometric data in the form of p66ShcA/Actin ratio are shown in Fig. 9b. Control, SCR-siRNA /MPTC and siRNA-p66/MPTC were incubated in medium containing either buffer or Cis (25 μM) for 24 hours. Subsequently cells were assayed for apoptosis, primary and secondary necrosis. Silencing of p66ShcA inhibited Cis-induced tubular cell apoptosis as well as secondary necrosis (Fig. 9C).
Fig. 9. p66 is critical for Cis-induced tubular cell apoptosis.
A. MPTCs were transfected with either scrambled (SCR) siRNA or p66-siRNA. The upper lane displayed representative immunoblots of control MPTCs, SCR/MPTCs and p66-siRNA/MPTCs. The lower lane shows stripped and reprobed blots for actin.
B. Densitometric data is displayed in the form of bar graphs/
C. Control MPTCs. SCR/MPTCs and p66-siRNA/MPTCs were incubated in medium containing either buffer or Cis (25 μM) for 24 hours (n=3). Subsequently cells were assayed for apoptosis, primary and secondary necrosis. *P<0.01 compared to control and siRNA/p66/Cis; **P<0.05 compared to control; ***P<0.001 compared to control, siRNA/p66/Cis
Discussion
In the present study, Cis/WT mice developed ARF by manifesting elevation levels of blood urea. However, Cis/p66+/− mice did not show any elevation of blood urea, and thus developed only clinically occult ARF. Renal histology revealed extensive tubular cell necrosis and apoptosis in Cis/WT mice. Tubules in Cis/WT mice also displayed marked dilatation and formation of proteinaceous casts. Protein expression from renal tissue of Cis/WT exhibited deactivation of the redox-sensitive stress response program in the form of enhanced expression of phospho-p66ShcA and phospho-Foxo3A and diminished expression of MnSOD and catalase. On the other hand, renal tissue from Cis/p66+/− mice displayed preservation of redox-sensitive stress response program in the form of attenuated phosphorylation of p66ShcA and Foxo3a and restoration of the expression of MnSOD and catalase. Renal tissues from Cis/WT mice also displayed activation of p53 pathway in the form of enhanced phosphorylation of phospho-p53 and increased expression of Bax protein. In in vitro studies, Cis also enhanced phosphorylaion of p53 and p66ShcA in MPTCs. As expected, Cis stimulated MPTC expression of Bax and inhibited expression of Bcl2. Interestingly; MPTCs lacking p66 were resistant to pro-apoptotic/necrotic effect of Cis. These findings indicated that Cis-induced ARF was the outcome of the combined effects- of deactivation of tubular cell redox-sensitive stress response program and activation of the p53 pathway.
Renal tubular cells carry only limited capacity of anaerobic glycolysis; nonetheless, they have enormous ability of aerobic respiration (18). On that account, Cis-induced interruption of aerobic respiration may promote necrosis even in those cells, which were programmed to undergo apoptosis. Moreover, apoptosis is a highly regulated process requiring high amount of energy for its completion, cells with severe ATP depletion will not be able to complete the apoptotic process (12). On that account, Cis-induced severity of tubular cell injury was reported to be concentration dependent- necrosis at high micromolar concentrations and apoptosis at lower concentrations.
There has been discrepancy in the outcome of in vivo studies and in vitro studies in terms of Cis-induced tubular cell phenotype (2, 6. 11, 12). In in vivo studies, Cis- induced tubular cell necrosis was a predominant finding and apoptosis was evident only in a limited number of tubular cells (12). In other studies, Cis-induced tubular cell phenotype depended on its concentration. Thus, it appears that if the higher doses of Cis were used (as for chemotherapy), outcome would be predominantly tubular cell necrosis. Interestingly, in vitro studies, lack of p66 did not prevent primary necrosis but only prevented secondary necrosis, whereas, mice with p66ShcA deficit demonstrated a decrease in both necrosis and apoptosis of tubular cells. Based on our in vitro findings, we speculate that p66 deficit might have prevented secondary necrosis rather than primary necrosis in vivo too. In that contest, occurrence of severity of Cis-induced apoptosis and secondary necrosis determined the AKI phenotype- overt vs. occult AKI.
p53 has been demonstrated to be upstream to p66ShcA signaling (22). p53-null mice displayed attenuated p66ShcA expression and diminished ROS generation; interestingly, down regulation of p66ShcA in this model was not associated with enhanced longevity; on the other hand, these mice died prematurely because of enhanced carcinogenesis (22). Contrary to p53-null mice, p66ShcA knockout mice not only displayed attenuated ROS generation but also lived longer (15, 16). It has been suggested that p53 only induced ROS generation signaling through p66ShcA (22); whereas, its downstream signaling pertaining to tumor suppression was not mediated through p66ShcA pathway. In the present study, Cis also induced activation of the p53 as well as p66ShcA However, p66 deficit was associated with down regulation of phospho-p53 in Cis/p66+/− mice. We propose that p66 deficit enhances generation of antioxidants which prevent accumulation of ROS. Diminished ROS accumulation may lead to a reduction of DNA damage and thus leading to an attenuated activation of p53 pathway (Fig. 10). Our proposed hypothesis is supported by the reported findings of other investigators- free radicals and antioxidants provided protection against Cis-induced tubular cell injury (13, 21).
Fig. 10.
Proposed schematic pathway for Cis-induced tubular cell apoptosis.
We conclude that Cis-induced ARF is the outcome of the deactivation of the tubular cell redox-sensitive stress response; the latter can be restored by tubular cell p66 deficit.
Fig. 3. P66 deficiency partially rescues from Cis-induced apoptosis.
Renal cortical sections of WT, P66+/−, Cis/WT, and Cis/P66+/− mice were labeled for TUNEL +ve cells. Representative Renal cortical sections of WT, P66+/−, Cis/WT., and Cis/P66+/− mice displaying TUNEL +ve cells (blue stained cells).
*P<0.001 compared to WT and P66+/− mice. **P<0.01 compared to Cis/WT mice.
Highlights.
Kidney tubular cells display compromised antioxidant response in cisplatinum induced acute kidney injury (AKI).
The mechanism of poor antioxidant response of cisplatinum-induced AKI is not clear
Cisplatinum-induced poor anti-oxidant response was due to deactivated redox-sensitive stress response program (RSSRP) in tubular cells.
P66ShcA deficit status could restore RSSRP in tubular cells in cisplatinum milieu both in vitro and in vivo.
Acknowledgments
This work was supported by grants RO1DK084910 and RO1 DK083931 (PCS) from National Institutes of Health, Bethesda, MD. We are thankful to the Mutant Mouse Regional Resource Center U42 RR014817 for providing breeding pairs of p66+/− mice. Part of this work was presented at the 43rd Annual Meeting of the American Society of Nephrology in November, 2011 in Philadelphia, PA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 2.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53:394–401. doi: 10.1046/j.1523-1755.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 3.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Luscher TF, Cosentino F. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci USA. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chintapalli J, Yang S, Opawumi D, Goyal SR, Shamsuddin N, Malhotra A, Reiss K, Meggs LG. Inhibition of wild-type p66ShcA in mesangial cells prevents glycooxidant-dependent FOXO3a regulation and promotes the survival phenotype. Am J Physiol Renal Physiol. 2007;292:F523–530. doi: 10.1152/ajprenal.00215.2006. [DOI] [PubMed] [Google Scholar]
- 5.Clark JS, Faisal A, Baliga R, Nagamine Y, Arany I. Cisplatin induces apoptosis through the ERK-p66shc pathway in renal proximal tubule cells. Cancer Lett. 2010;297:165–70. doi: 10.1016/j.canlet.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Fuertesa MA, Castillab J, Alonsoa C, Perez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 2003;10:257–266. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- 7.Husain K, Morris C, Whitworth C, Trammell GL, Rybak LP, Somani SM. Protection by ebselen against cisplatin-induced nephrotoxicity: antioxidant system. Mol Cell Biochem. 1998;178:127–33. doi: 10.1023/a:1006889427520. [DOI] [PubMed] [Google Scholar]
- 8.Inagi R, Kumagai T, Fujita T, Nangaku M. The role of glyoxalase system in renal hypoxia. Adv Exp Med Biol. 2010;662:49–55. doi: 10.1007/978-1-4419-1241-1_6. [DOI] [PubMed] [Google Scholar]
- 9.Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005;67:458–66. doi: 10.1111/j.1523-1755.2005.67102.x. [DOI] [PubMed] [Google Scholar]
- 10.Kang KP, Park SK, Kim DH, Sung MJ, Jung YJ, Lee AS, Lee JE, Ramkumar KM, Lee S, Park MH, Roh SG, Kim W. Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol Dial Transplant. 2011;26:814–22. doi: 10.1093/ndt/gfq528. [DOI] [PubMed] [Google Scholar]
- 11.Kaushal GP, Kaushal V, Hong X, Shah SV. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001;60:1726–1736. doi: 10.1046/j.1523-1755.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 12.Lieberthal W, Nigam SK, Molitoris BA, Weinberg JM, Venkatachalam MA, Zager RA, Nath KA, Goligorsky MS. Acute renal failure. II. Experimental models of acute renal failure: imperfect but indispensable. Am J Physiol Renal Physiol. 2000;278:F1–F12. doi: 10.1152/ajprenal.2000.278.1.F1. [DOI] [PubMed] [Google Scholar]
- 13.Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol Renal Fluid Electrolyte Physiol. 270:F700–F708. 1996. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- 14.Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes. 2006;55:1642–1650. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- 15.Migllaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66Shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;42:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 16.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa M, Nagatomi H, Chang BJ, Sato E, Inoue M. Targeting superoxide dismutase to renal proximal tubule cells inhibits mitochondrial injury and renal dysfunction inuduced by cisplatin. Arch Biochem Biophys. 2001;38:78–84. doi: 10.1006/abbi.2000.2237. [DOI] [PubMed] [Google Scholar]
- 19.Nowak G. Protein kinase C- and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem. 2002;277:43377–43388. doi: 10.1074/jbc.M206373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol. 2002;13:858–865. doi: 10.1681/ASN.V134858. [DOI] [PubMed] [Google Scholar]
- 21.Sadzuka Y, Shoji T, Takino Y. Mechanism of the increase in lipid peroxide induced by cisplatin in the kidneys of rats. Toxicol Lett. 1992;62:293–300. doi: 10.1016/0378-4274(92)90033-g. [DOI] [PubMed] [Google Scholar]
- 22.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura IM, Raker VA, Maccarana M, Petronilli V, Minucci S, Bernardi P, Lanfrancone L, Pelicci PG. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–8. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 23.Weidemann A, Bernhardt WM, Klanke B, Daniel C, Buchholz B, Câmpean V, Amann K, Warnecke C, Wiesener MS, Eckardt KU, Willam C. HIF activation protects from acute kidney injury. J Am Soc Nephrol. 2008;19:486–94. doi: 10.1681/ASN.2007040419. [DOI] [PMC free article] [PubMed] [Google Scholar]