Abstract
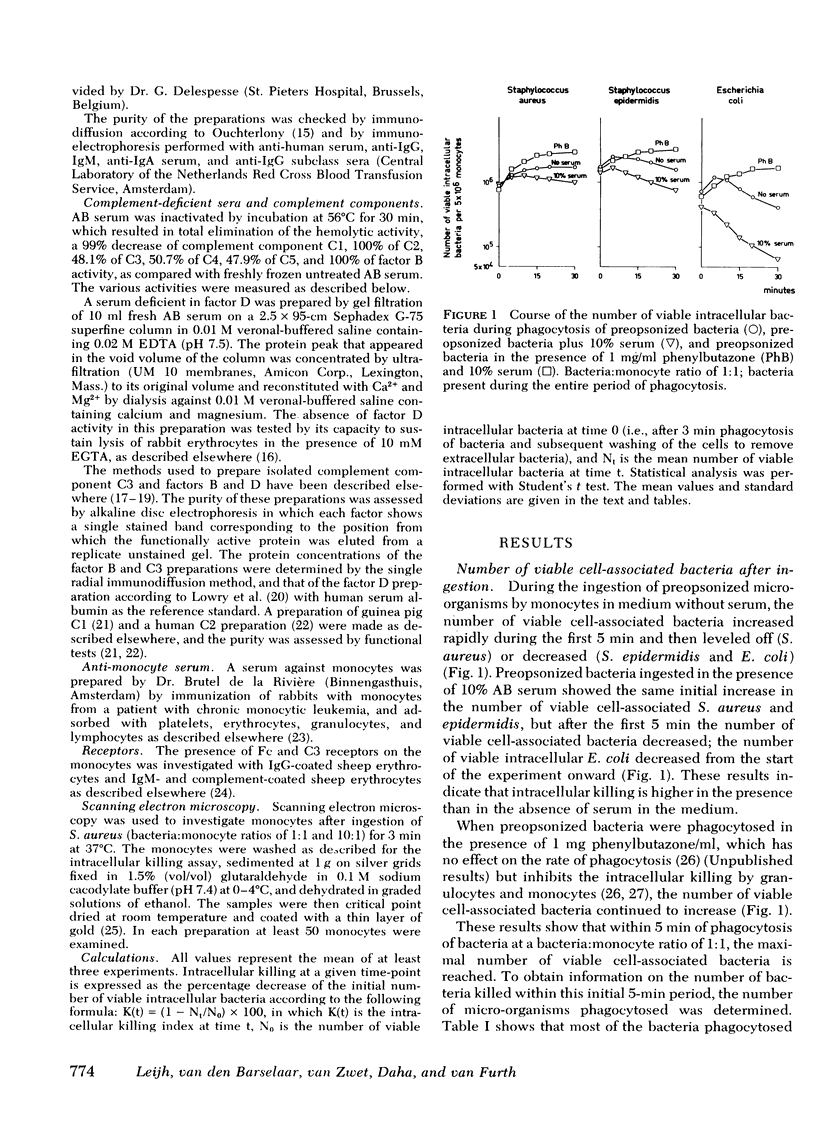
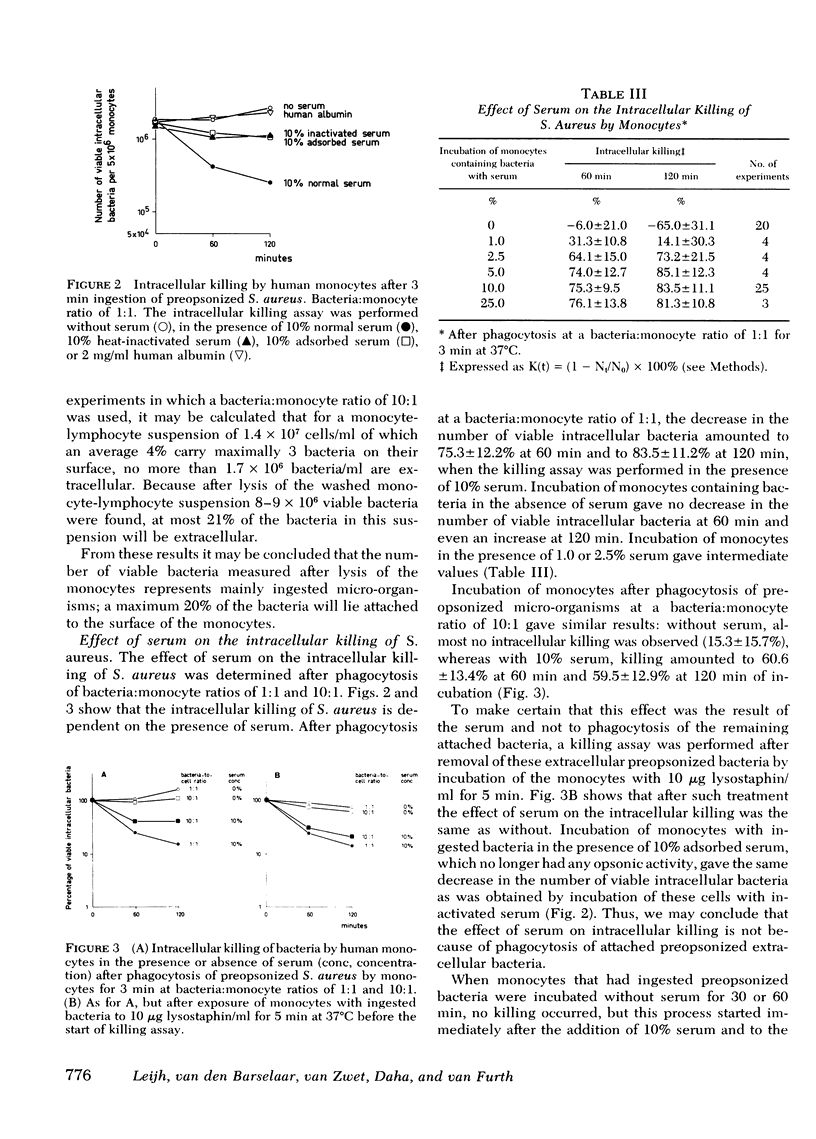
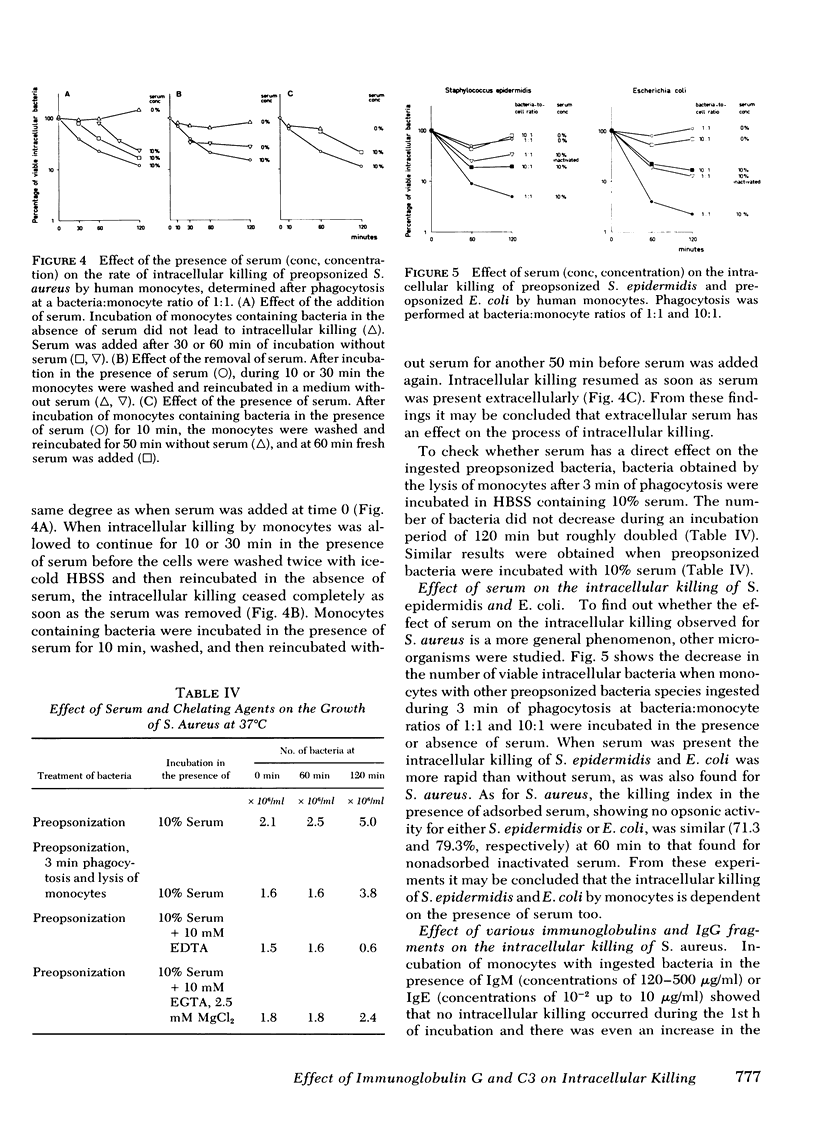
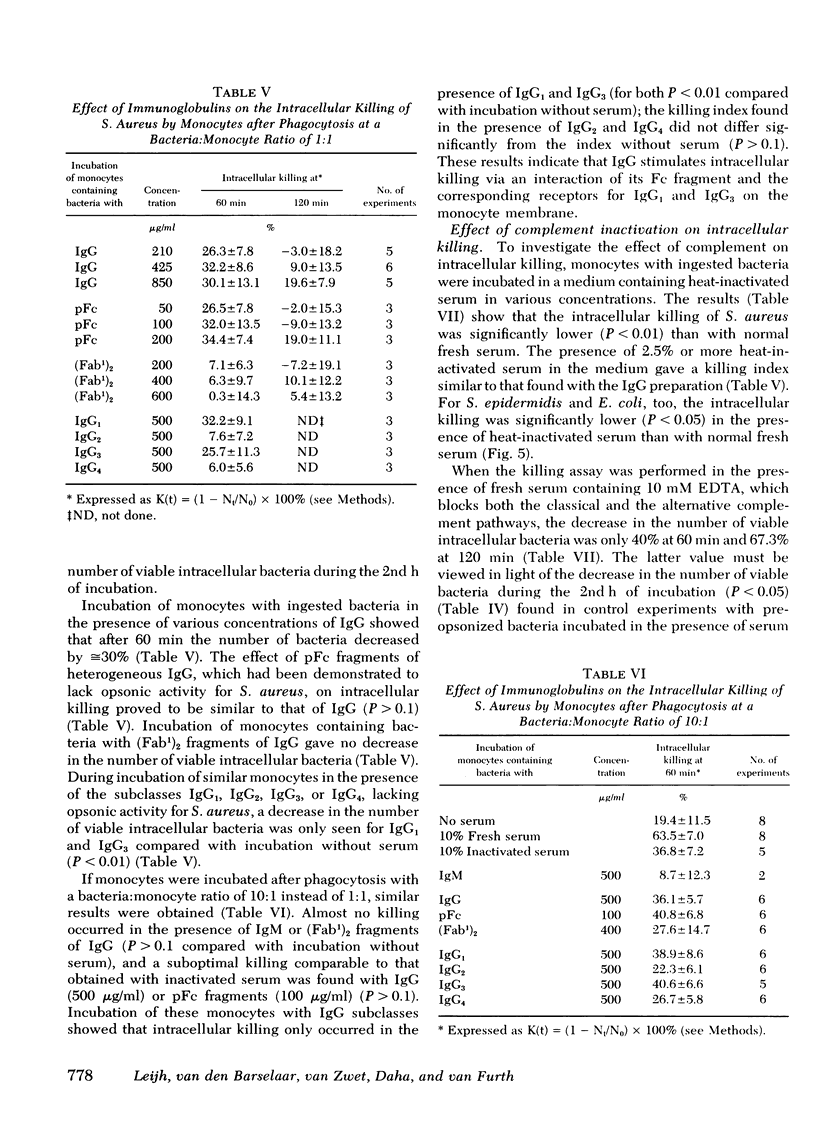
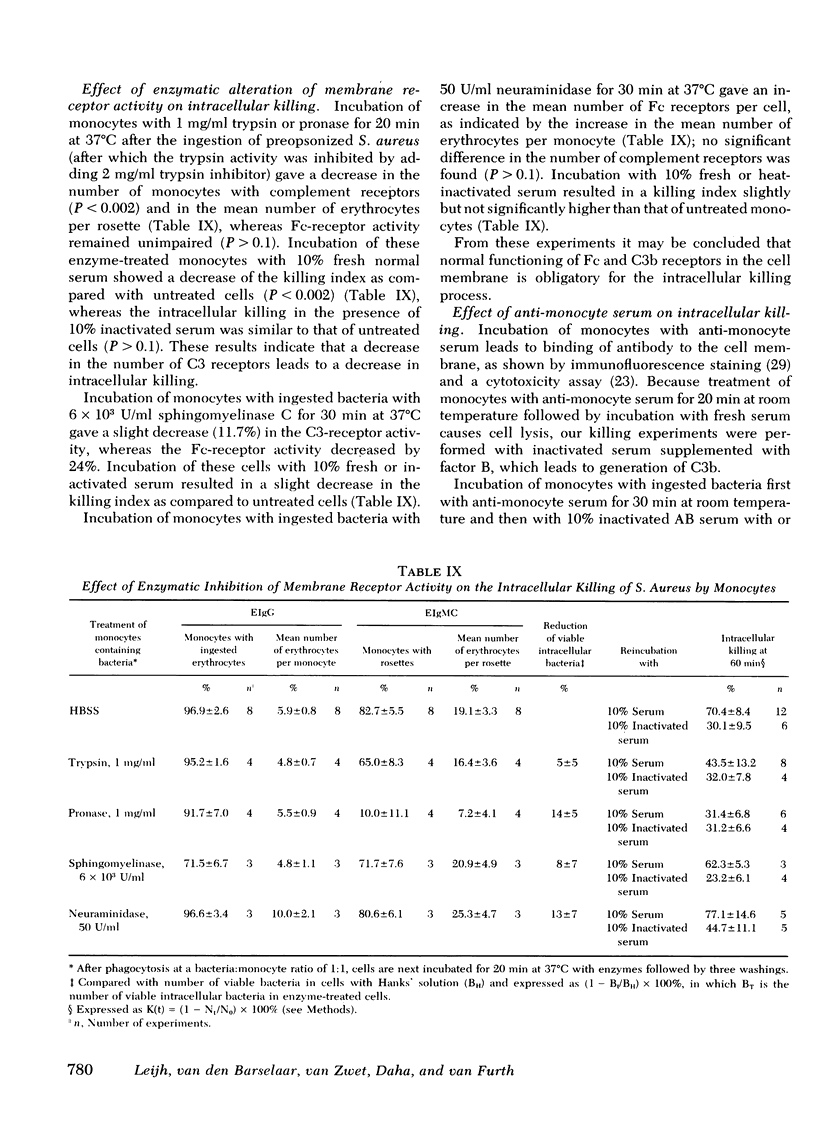
The role of serum factors in the intracellular killing of bacteria by monocytes was studied on the basis of an assay independent of phagocytosis. After 3 min of phagocytosis of preopsonized bacteria and removal of noningested bacteria, the monocytes containing bacteria are reincubated for various periods and the number of unkilled bacteria is determined by a microbiological method after lysis of the cells. Evidence that this assay measures the killing of ingested bacteria was provided by scanning electron microscopy, lysostaphin treatment, and the effect on the rate of intracellular killing of inactivated serum lacking specific opsonic activity. Intracellular killing of Staphylococcus aureaus, S. epidermidis, and Escherichia coli by human monocytes does not occur or is low in the absence of serum, and maximal killing is only reached when fresh serum is present; intermediate values are obtained in the presence of heat-inactivated serum. These findings indicate that complement stimulates intracellular killing. Isolated heterogeneous immunoglobulin (Ig)G, pFc fragments of heterogeneous IgG, and both IgG1 and IgG3 stimulate intracellular killing of S. aureaus by monocytes to the same degree as heat-inactivated serum. Sphingomyelinase, which decreases the number of Fc receptors, and neuraminidase, which increases these receptors, respectively, decreased and increased the intracellular killing, whereas anti-monocyte serum completely abolished the stimulation of intracellular killing by inactivated serum. These results prove that interaction of the Fc receptor with the Fc part of IgG is required for the intracellular killing. Inhibition of the activation of complement components via the alternative pathway gave a considerable reduction in the intracellular killing of S. aureaus; impairment of the activation via the classical pathway had no effect. The addition of complement components to heat-inactivated serum showed that intracellular killing is maximal only when C3b is generated. Reduction of the number of C3b receptors in the membrane by trypsin or pronase decreased intracellular killing in the presence of fresh serum; anti-monocyte serum completely abolished the stimulation of intracellular killing by fresh serum. These results lead to the conclusion that intracellular killing is also dependent on the interaction between C3b and its receptor in the membrane.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brutel de la Rivière A., Verhoef-Karssen P. R., Bosma A., Kr vd Borne A. E., Engelfriet P. Specific antisera against human blood cells applicable in the indirect immunofluorescence technique. Scand J Immunol. 1976;5(9):1065–1074. doi: 10.1111/j.1365-3083.1976.tb03058.x. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie K. E., Solberg C. O., Larsen B., Grov A., Tonder O. Influence of IgG, F(ab')2 and IgM on the phagocytic and bactericidal activities of human neutrophil granulocytes. Acta Pathol Microbiol Scand C. 1976 Apr;84(2):119–123. doi: 10.1111/j.1699-0463.1976.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975 Oct 1;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Radin A., Frosch M. Independent effects of IgG and complement upon human polymorphonuclear leukocyte function. J Immunol. 1976 Oct;117(4):1282–1287. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Silverstein S. C. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med. 1976 Sep 1;144(3):788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Austen K. F. Alternate complement pathway: factors involved in cobra venom factor (CoVF) activation of the third component of complement (C3). J Immunol. 1973 Jan;110(1):128–138. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F., de Bruyn A. M., Radema H. Recognition of human IgM subgroups by quantitative measurement. Clin Exp Immunol. 1973 Sep;15(1):103–111. [PMC free article] [PubMed] [Google Scholar]
- LI I. W., MUDD S., KAPRAL F. A. DISSOCIATION OF PHAGOCYTOSIS AND INTRACELLULAR KILLING OF STAPHYLOCOCCUS AUREUS BY HUMAN BLOOD LEUKOCYTES. J Immunol. 1963 May;90:804–809. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Furth R. Kinetics of phagocytosis and intracellular killing of Candida albicans by human granulocytes and monocytes. Infect Immun. 1977 Aug;17(2):313–318. doi: 10.1128/iai.17.2.313-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages II. Dissociation of the inhibitory effects of 2-deoxyglucose on phagocytosis and ATP generation. J Exp Med. 1976 Dec 1;144(6):1484–1493. doi: 10.1084/jem.144.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J Exp Med. 1976 Dec 1;144(6):1465–1483. doi: 10.1084/jem.144.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C. Phagocytosis in rat Kupffer cells in vitro. Exp Cell Res. 1976 May;99(2):319–327. doi: 10.1016/0014-4827(76)90589-9. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Rabinovitch M. Phagocytosis: the engulfment stage. Semin Hematol. 1968 Apr;5(2):134–155. [PubMed] [Google Scholar]
- Reif A. E. Batch preparation of rabbit gammaG globulin with deae-cellulose. Immunochemistry. 1969 Sep;6(5):723–731. doi: 10.1016/0019-2791(67)90136-x. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J Immunol. 1969 Mar;102(3):533–543. [PubMed] [Google Scholar]
- Schorlemmer H. U., Allison A. C. Effects of activated complement components on enzyme secretion by macrophages. Immunology. 1976 Nov;31(5):781–788. [PMC free article] [PubMed] [Google Scholar]
- Solberg C. O., Christie K. E., Larsen B., Tonder O. Influence of antibodies and thermolabile serum factors on the bactericidal activity of human neutrophil granulocytes. Acta Pathol Microbiol Scand C. 1976 Apr;84(2):112–118. doi: 10.1111/j.1699-0463.1976.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Solberg C. O., Hellum K. B. Influence of serum on the bactericidal activity of neutrophil granulocytes. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Oct;81(5):621–626. doi: 10.1111/j.1699-0463.1973.tb02252.x. [DOI] [PubMed] [Google Scholar]
- Solberg C. O. Influence of therapeutic concentrations of phenylbutazone on granulocyte function. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):100–102. doi: 10.1111/j.1699-0463.1975.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Tan J. S., Watanakunakorn C., Phair J. P. A modified assay of neutrophil function: use of lysostaphin to differentiate defective phagocytosis from impaired intracellular killing. J Lab Clin Med. 1971 Aug;78(2):316–322. [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Wilkinson P. C. Action of sphingomyelinase C and other lipid-specific agents as inhibitors of Fc binding and locomotion in human leucocytes. Immunology. 1977 Sep;33(3):407–412. [PMC free article] [PubMed] [Google Scholar]
- van Rood J. J., van Leeuwen A., Ploem J. S. Simultaneous detection of two cell populations by two-colour fluorescence and application to the recognition of B-cell determinants. Nature. 1976 Aug 26;262(5571):795–797. doi: 10.1038/262795a0. [DOI] [PubMed] [Google Scholar]



