Abstract
Purpose.
To evaluate the expression of ATP-sensitive potassium (KATP) channel subunits and study the effect of KATP channel openers diazoxide and nicorandil on intraocular pressure (IOP) in an in vivo mouse model.
Methods.
Expression of KATP channel subunits in normal C57BL/6 mouse eyes was studied by immunohistochemistry and confocal microscopy. Wild-type C57BL/6 mice were treated with KATP channel openers diazoxide (n = 10) and nicorandil (n = 10) for 14 days. Similar treatments with diazoxide were performed on Kir6.2(−/−) mice (n = 10). IOP was recorded with a handheld tonometer 1 hour, 4 hours, and 23 hours following daily treatment. Posttreatment histology was examined by light and transmission electron microscopy.
Results.
The KATP channel subunits SUR2B, Kir6.1, and Kir6.2 were identified in all tissues within mouse eyes. Treatment with diazoxide in wild-type mice decreased IOP by 21.5 ± 3.2% with an absolute IOP reduction of 3.9 ± 0.6 mm Hg (P = 0.002). Nicorandil also decreased IOP (18.9 ± 1.8%) with an absolute IOP reduction of 3.4 ± 0.4 mm Hg (P = 0.002). Treatment with diazoxide in Kir6.2(−/−) mice had no effect on IOP. No morphological abnormalities were observed in diazoxide- or nicorandil-treated eyes.
Conclusions.
KATP channel openers diazoxide and nicorandil are effective regulators of IOP in mouse eyes. Kir6.2 appears to be a major KATP channel subunit through which IOP is lowered following treatment with diazoxide.
Keywords: intraocular pressure, anterior segment, potassium channel
KATP channel openers diazoxide and nicorandil significantly lowered IOP in wild type mouse eyes. KATP channel subunits SUR2B, Kir6.1 and Kir6.2 were identified in ocular mouse tissues. Studies with Kir6.2(–/–) mice confirmed Kir6.2 as a key subunit involved in diazoxide-mediated IOP reduction.
Introduction
Intraocular pressure (IOP) is the most prevalent and only modifiable risk factor for the treatment of glaucoma,1 the leading cause of irreversible blindness.2,3 Current treatment modalities for glaucoma are aimed at lowering IOP of affected eyes with surgery, drugs, or both. Although surgical procedures lower IOP, their efficacy is variable and generally require additional procedures to maintain IOP reduction. Pharmaceutical agents used to treat glaucoma are also not effective in all cases and many of the commonly used drugs have unwanted side effects.4,5 Most importantly, current treatment modalities for glaucoma only delay the disease progression and do not cure it. Therefore, new agents need to be identified for improved management of glaucoma.6
ATP-sensitive potassium (KATP) channels connect the bioenergetic state of the cells to its membrane potential.7 Opening and closing of KATP channels are known to affect contractility; cell adhesion; gap and tight junction regulation; metabolic protection against ischemia and hypoxia; cell well-being; and cellular adaptation to stress (shear, stretch, pressure, oxidation).8–23 Additionally, KATP channels have been identified as key molecules involved in retinal neuroprotection.24–27 Pharmacologic openers of KATP channels such as diazoxide and nicorandil are used clinically to treat hypertension, ischemic heart disease, and hyperinsulinemia,12,28–31 while KATP channel closers have important applications in the treatment of diabetes mellitus.32
KATP channels are octomeric proteins formed of four inwardly rectifying potassium (Kir) channel subunits along with four auxiliary sulfonylurea (SUR) receptor subunits. The opening and closing of KATP channels are orchestrated by inhibition through the Kir or activation through the SUR subunits.33 Due to multiple subtypes of SUR (SUR1, 2A, and 2B) and Kir (Kir6.1 and 6.2), there is considerable heterogeneity in KATP channel composition. For example, in the heart, KATP channels within atrial myocytes consist of SUR1 and Kir6.2 while in ventricular myocytes, SUR1 is replaced by SUR2A.34 In vascular smooth muscles, both SUR2B/Kir6.1 and SUR2B/Kir6.2 subunit combinations form functional KATP channels.35,36
Our laboratory has identified functional KATP channels in the outflow pathway of normal human eyes.37 Activation of the channels by addition of KATP channel openers diazoxide or nicorandil significantly lowered pressure in ex vivo cultures of human anterior segments and in anesthetized brown Norway rats. Given the fact that functional KATP channels are present in the eye and are involved in IOP regulation, we examined the subunit composition and evaluated the effect of KATP channel openers on IOP in a conscious, clinically relevant murine model.
Methods
Animal Husbandry
The use of animals and all treatment protocols were approved by the Mayo Clinic Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All wild-type C57BL/6 mice (retired breeders, age > 8 months) were purchased from Charles River Laboratories (Wilmington, MA). Kir6.2(−/−) mice were gifts from Andre Terzic (Mayo Clinic, Rochester, MN). Mice were maintained in the Mayo animal care facility under a 12-hour light and dark cycle and received standard rodent chow and water ad libitum. Upon arrival, mice were housed for 5 to 10 days prestudy to acclimatize the animals to the housing conditions.
Immunohistochemistry
Enucleated eyes from untreated wild type C57BL/6 mice were used to study KATP channel subunit composition. Eyes were fixed in 10% neutral buffered formalin (Fisher Scientific Inc., Kalamazoo, MI) for a minimum of 48 hours. Dissected tissues were dehydrated in an ascending series of ethanol concentrations (70%, 80%, 95%, and 100%), cleared with xylene and embedded in paraffin. Paraffin sections of 5 μm were mounted on glass slides (Superfrost/Plus; Fisher Scientific Inc.) and deparaffinized in xylene and rehydrated in a descending series of ethanol (100%, 95%, 80%, and 70%) followed by incubation in phosphate buffered saline. Antigen retrieval was performed by immersing the slides in 1 mM EDTA (pH 8.0) at 95°C for 30 minutes. Sections were blocked in 3% bovine serum albumin and 0.1% Tween 20 (Sigma-Aldrich, St. Louis, MO), and probed with antibodies against SUR1, SUR2B, Kir6.1, and Kir6.2 (Table 1). After being washed in PBS, sections were incubated with appropriate conjugated secondary antibodies (Table 1). Negative controls were incubated with only secondary antibodies. All sections were mounted with mounting media containing DAPI (Vectashield; Vector Laboratories, Burlingame, CA) and examined on a confocal laser microscope (Zeiss 510; Carl Zeiss Inc., Thornwood, NY). Fluorescent images were captured using the same digital settings for control and stained sections.
>Table 1.
Primary and Secondary Antibodies Utilized in Study
|
Primary Antibodies |
Source |
Catalog Number |
Secondary Antibodies |
Source |
Catalog Number |
| Rabbit polyclonal anti-SUR1 | Abcam (Cambridge, MA) | ab32844 | Goat anti-rabbit IgG-AF 488 | Invitrogen (Grand Island, NY) | A11008 |
| Goat polyclonal anti-SUR2B | Santa Cruz Biotechnology (Santa Cruz, CA) | sc32462 | Rabbit anti-goat IgG-AF 568 | Invitrogen (Grand Island, NY) | A11079 |
| Goat polyclonal anti-SUR2A | Santa Cruz Biotechnology (Santa Cruz, CA) | sc5793 | Rabbit anti-goat IgG-AF 568 | Invitrogen (Grand Island, NY) | A11079 |
| Rabbit polyclonal anti-Kir6.1 | Abcam (Cambridge, MA) | ab80972 | Goat anti-rabbit IgG-AF 488 | Invitrogen (Grand Island, NY) | A11008 |
| Rabbit polyclonal anti-Kir6.2 | Novus Biologicals (Littleton, CO) | NBP1-00900 | Goat anti-rabbit IgG-AF 488 | Invitrogen (Grand Island, NY) | A11008 |
For immunostaining with SUR2A, eyes were embedded in optimal cutting temperature (OCT) compound (Sakura Fintek USA Inc., Torrance, CA) and cryosectioned. Slides containing frozen sections were immersed in methanol for 20 minutes at −20°C, blocked in 3% BSA, and stained with SUR2A antibody (Santa Cruz Biotechnology, Dallas, TX) followed by AlexaFluor 568 conjugated secondary antibody (Table 1). Sections were mounted with media containing DAPI (Vector Laboratories) and examined by confocal laser microscopy as described above.
IOP Measurements and Treatment
After acclimatizing the mice in the housing facility, IOP was measured in conscious mice with a handheld rebound tonometer (Icare TonoLab; Colonial Medical Supply, Franconia, NH) once daily for a minimum of 3 days prior to the study to familiarize the animals to handling and IOP measurements. For IOP measurements, the tonometer was held so that the probe hit the cornea perpendicularly, as per the manufacturer's instructions. The tonometer records six readings from the same eye, discards the highest and lowest values, and shows the average of the remaining four values as one IOP reading. Three independent measurements were averaged to obtain the IOP value at a given time point for each eye.
Pretreatment.
IOP was measured in both mouse eyes three times daily for a period of 7 days. The IOP values for the three independent timepoints were averaged and recorded as the daily IOP.
Diazoxide Treatment.
Diazoxide was prepared by diluting a 100 mM stock solution in 10% polyethoxylated castor oil (Cremophor EL; Sigma-Aldrich) in PBS. Use of polyethoxylated castor oil helped maintain diazoxide in solution and served as a cornea permeabilization agent. In C57BL/6 wild-type (n = 10) and Kir6.2(−/−) (n = 10) mice, a 5-μL drop of 5 mM diazoxide was topically administered to one eye of each mouse while the fellow control eye received vehicle (DMSO and 10% polyethoxylated castor oil in the same proportion as the treated eye). IOP was measured daily at 1 hour, 4 hours, and 23 hours following treatment. Average of the three IOP measurements was recorded as the daily IOP. Treatment with diazoxide and vehicle was continued daily for 14 consecutive days.
Nicorandil Treatment.
In C57BL/6 mice (n = 10), one eye of each mouse was treated with nicorandil daily while the fellow eye received vehicle. Like diazoxide, nicorandil was prepared from a 100 mM stock solution (in DMSO), diluted in 10% polyethoxylated castor oil to a final concentration of 5 mM. Nicorandil and vehicle treatments were provided daily for 14 consecutive days.
Posttreatment.
Following the last day of treatment, several wild-type (n = 4 for diazoxide and n = 3 for nicorandil treatment) and Kir6.2(−/−) mice (n = 4) were killed by CO2 asphyxiation. Both control and treated eyes were excised and placed in either 10% neutral buffered formalin or 4% paraformaldehyde in 0.1 M phosphate buffer. With the remaining wild-type (n = 6 for diazoxide and n = 7 for nicorandil treatment) and Kir6.2(−/−) mice (n = 6), treatment was stopped and IOP was measured three times daily for 7 consecutive days.
Histology
For eyes fixed in 10% neutral buffered formalin, whole eyes were dehydrated in a series of ascending ethanol concentrations, cleared in xylene, embedded in paraffin, sectioned at 5 μm, and mounted on glass slides (Fisher Scientific Inc.). For staining, sections were deparaffinized with xylene, rehydrated in descending series of ethanol, and rinsed in running distilled water. Sections were stained with hematoxylin (Electron Microscopy Sciences, Hatfield, PA); washed in running tap water, stained with eosin (Richard Allan Scientific, Kalamazoo, MI); and dehydrated in a series of ascending ethanol concentrations. Stained sections were placed in xylene and mounted under coverslips using a commercial micromount medium (Surgipath; Surgipath Medical Industries, Richmond, IL).
For transmission electron microscopy, whole eyes from diazoxide-treated wild-type mice (n = 2), diazoxide-treated Kir6.2(−/−) mice (n = 2), and nicorandil-treated mice (n = 1), fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, were postfixed in osmium tetroxide, dehydrated in ascending ethanol concentrations, immersed in propylene oxide, and embedded in epoxy resin. Tissue blocks were sectioned at 100 nm, placed on copper grids, and stained with uranyl acetate and lead nitrate. Representative micrographs were taken at ×5000 magnification using a transmission electron microscope (JEOL 1400; JEOL USA Inc., Peabody, MA).
Statistics
All values are expressed as mean ± standard deviation. Analysis by Shapiro-Wilk test revealed several nonparametric data sets. Therefore, differences in IOP between treated and control eyes were compared using Wilcoxon sign rank test. Unpaired datasets were compared by Wilcoxon/Kruskal-Wallis tests (rank sums). P values were compensated for multiple comparisons using Bonferroni correction and results were considered significant when P ≤ 0.002. All statistical calculations were performed using statistical software (JMP; SAS, Cary, NC).
Results
KATP Channel Subunits in the Mouse Eye
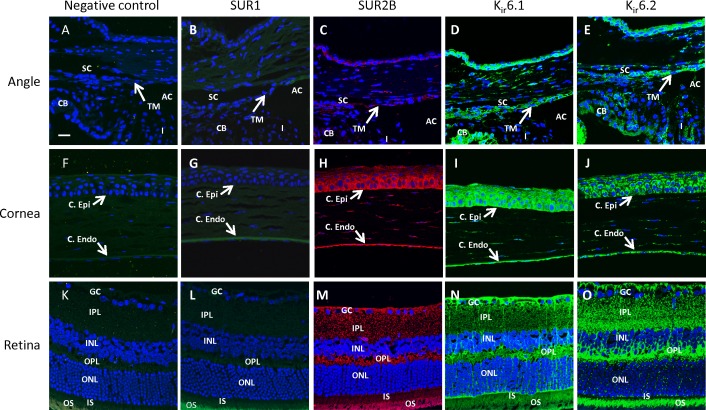
To evaluate which KATP channel subunits are present in the mouse eye, we performed immunohistochemistry on whole eye sections from untreated normal mice. Paraffin sections were used for staining SUR1, SUR2B, Kir6.1, and Kir6.2 (Fig. 1) while SUR2A was stained on frozen tissue sections (Fig. 2). No specific staining was observed for SUR1 (Figs. 1B, 1G, 1L) and SUR2A (Figs. 2B, 2D, 2F) in the various eye tissues that were studied. Only SUR2B of the sulfonylurea family and both Kir6.1 and Kir6.2 subunits of the Kir6 family were identified in the mouse eye (Figs. 1C–E, 1H–J, 1M–O). Both inner and outer wall Schlemm's canal cells and trabecular meshwork cells (but not the trabecular beams) showed the presence of SUR2B, Kir6.1, and Kir6.2 (Figs. 1C–E). These subunits were also found to be present in the cells of the ciliary body and iris (Figs. 1C–E). SUR2B, Kir6.1, and Kir6.2 were present in the corneal epithelium and the endothelium (Figs. 1H–J) as well as in most of the retinal cell layers (Figs. 1M–O). Although the inner and outer photoreceptor segments appeared to stain positive for all the SUR and Kir subunits, autofluorescence of the retinal pigments precluded us from confirming presence of the subunits in these layers since similar fluorescence was observed in the negative controls (Figs. 1K). In frozen tissue sections stained for SUR2A, some nonspecific background autofluorescence was observed in the anterior angle and the retinal inner and outer plexiform layers (Figs. 2B, 2F).
Figure 1.
Expression profile of KATP channel subunits SUR1, SUR2B, Kir6.1, and Kir6.2. SUR1 was not present in mouse eye tissues (B, G, L). SUR2B, Kir6.1, and Kir6.2 were present in the trabecular meshwork (including inner and outer walls of Schlemm's canal) (C–E); corneal epithelium, endothelium, and parts of the stroma (H–J); and in cell layers of the retina (M–O). Fluorescent intensity for the images was set based on the negative controls, which were incubated with secondary antibodies only (A, F, K). C. Epi, corneal epithelium; C. Endo, corneal endothelium; SC, Schlemm's canal; TM, trabecular meshwork; CB, ciliary body; I, iris; AC, anterior chamber; GC, ganglion cells; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segments; OS, outer segments. Red fluorescence: SUR2B. Green fluorescence: SUR1, Kir6.1, and Kir6.2. Blue fluorescence: DAPI. Scale bar: 20 μm for all micrographs.
Figure 2.
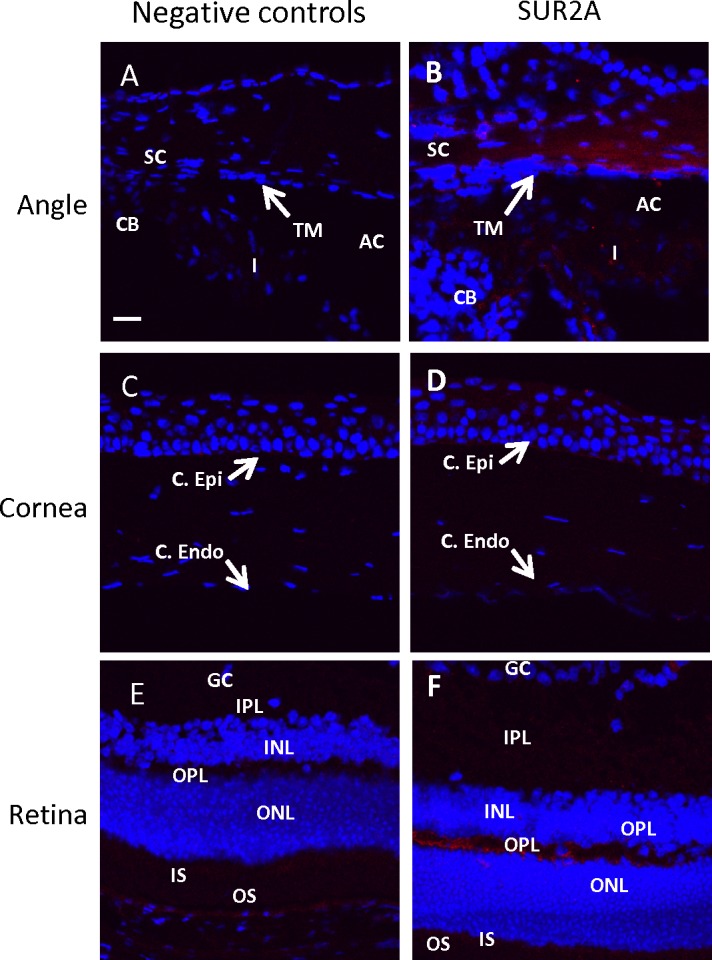
Expression profile of KATP channel subunit SUR2A. SUR2A was not present in the anterior angle (B), cornea (D), or retina (F). Some background autofluorescence was observed in the angle and the retinal IPL and OPL. The negative controls were incubated with secondary antibody only and used to set the fluorescent intensity. Red fluorescence: SUR2A. Blue fluorescence: DAPI. Scale bar: 20 μm for all micrographs.
In Vivo Effect of Diazoxide Treatment on IOP
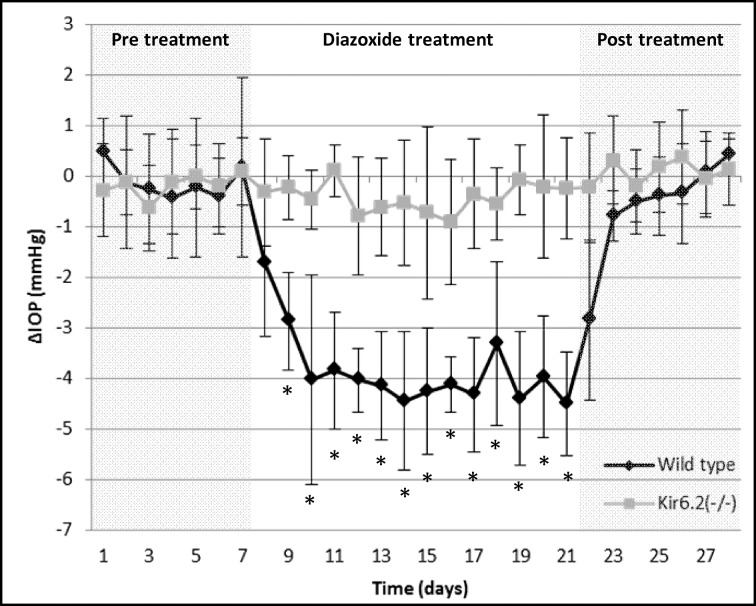
Administration of diazoxide (5 mM) reduced IOP significantly within 48 hours of treatment in C57BL/6 wild-type mice (Fig. 3, black line). Comparison of the treated and control eyes at each timepoint (1 hour, 4 hours, and 23 hours) showed significantly lower IOP (P = 0.002, n = 10), suggesting that a single daily dose of diazoxide lowered IOP over a 24-hour period (Table 2). The daily absolute IOP reduction (average of 1 hour, 4 hours, and 23-hour timepoints) was 3.9 ± 0.6 mm Hg (n = 10 mice, P = 0.002) over the 14 days of treatment with a daily reduction range of 1.7 to 4.5 mm Hg (Fig. 3). This translates to a 21.5 ± 3.2% change in IOP compared with baseline with a daily IOP reduction range of 9.4 to 24.6%. Following treatment cessation, IOP of the treated eye returned to baseline levels within 48 hours (P > 0.002 for IOP comparisons between treated and control eyes).
Figure 3.
Effect of diazoxide on IOP in C57BL/6 wild-type and Kir6.2(−/−) mice. IOP was lowered within 48 hours of diazoxide treatment and maintained an average reduction of 3.9 ± 0.6 mm Hg during the 14-day treatment period (black line). In contrast, Kir6.2(−/−) mice showed no change in IOP on any of the treatment days following diazoxide treatment (gray line). *P = 0.002.
Table 2.
Effect of KATP Channel Opener Treatment on IOP
|
Diazoxide Treated* |
Nicorandil Treated* |
|||||
|
Wild Type C57BL/6 (n
= 10) |
Kir6.2(–/–) (n
= 10) |
Wild Type C57BL/6 (n
= 10) |
||||
|
ΔIOP ± SD, mm Hg |
% Change Compared With Control ± SD |
ΔIOP ± SD, mm Hg |
% Change Compared to Control ± SD |
ΔIOP ± SD, mm Hg |
% Change Compared With Control ± SD |
|
| 1 h | −3.9 ± 0.7† | −21.3 ± 3.2 | −0.6 ± 0.8 | −3.6 ± 4.2 | −3.3 ± 0.4† | −18.5 ± 1.9 |
| 4 h | −3.9 ± 0.9† | −21.7 ± 4.6 | −0.2 ± 0.7 | −1.2 ± 3.7 | −3.4 ± 0.4† | −18.8 ± 2.0 |
| 23 h | −3.8 ± 0.9† | −21.6 ± 4.9 | −0.3 ± 0.6 | −1.7 ± 3.4 | −3.5 ± 0.6† | −19.7 ± 3.1 |
| Average | −3.9 ± 0.6† | −21.5 ± 3.2 | −0.4 ± 0.7 | −2.2 ± 3.5 | −3.4 ± 0.4† | −18.9 ± 1.8 |
SD, standard deviation.
* Numbers represent average of 14-day treatment period.
† P = 0.002.
To confirm specificity and determine subunit importance, we evaluated the effect of diazoxide on IOP in C57BL/6 mice lacking the Kir6.2 subunit. No statistical difference was observed in baseline IOP between the right (15.8 ± 0.6 vs. 17.2 ± 1.1 mm Hg, P = 0.006; α = 0.005 after multiple comparison correction) and left (15.7 ± 0.7 vs. 17.2 ± 1.2, P = 0.009) eyes of wild-type and Kir6.2(−/−) mice (n = 10 wild-type and n = 10 Kir6.2(−/−) mice). However, unlike the wild-type mice, DZ did not lower IOP in the Kir6.2(−/−)– treated eyes at 1 hour, 4 hours, and 23 hours following treatment (Table 2, P > 0.002, n = 10). The average daily IOP difference between treated and control eyes was 0.4 ± 0.7 mm Hg (Fig. 3, gray line, P = 0.03). In these animals, IOP remained stable and did not show any variation from baseline during treatment with diazoxide. Similar levels of Kir6.1 subunits were identified in Kir6.2(−/−) (Supplementary Figs. S1A–F) and wild-type animals, suggesting the knockout of Kir6.2 did not influence expression of Kir6.1.
Histologic evaluation of ocular tissues within the outflow pathway following diazoxide treatment was performed using hematoxylin and eosin (H&E) staining (Figs. 4A–D) and by transmission electron microscopy (Figs. 4E–H). In wild-type and Kir6.2(−/−) mice, there were no structural changes in the anatomy of the outflow pathways. Both the trabecular meshwork and the inner and outer wall of Schlemm's canal appeared normal with similar numbers of viable cells. Overall, treatment with diazoxide or vehicle had no observable changes to cell and tissue histology within the outflow pathway (Figs. 4A–H).
Figure 4.
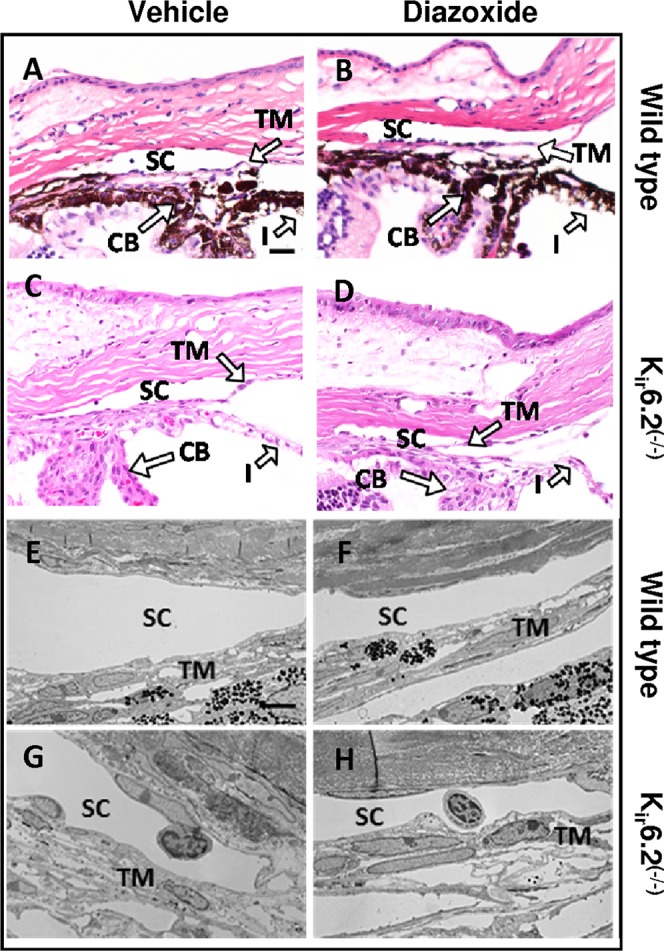
Effect of diazoxide treatment on ocular histology of C57BL/6 wild-type and Kir6.2(−/−) mice. Histologic examination of treated and control eyes in both wild type (A, B, E, F) and Kir6.2(−/−) mice (C, D, G, H) did not show any observable change in cell and tissue appearance within the conventional outflow pathway following diazoxide treatment as evident from H&E staining (A–D) and transmission electron microscopy (E–H). Scale bar: 50 μm for H&E and 5 μm for transmission electron microscopy.
Effect of Nicorandil Treatment on IOP
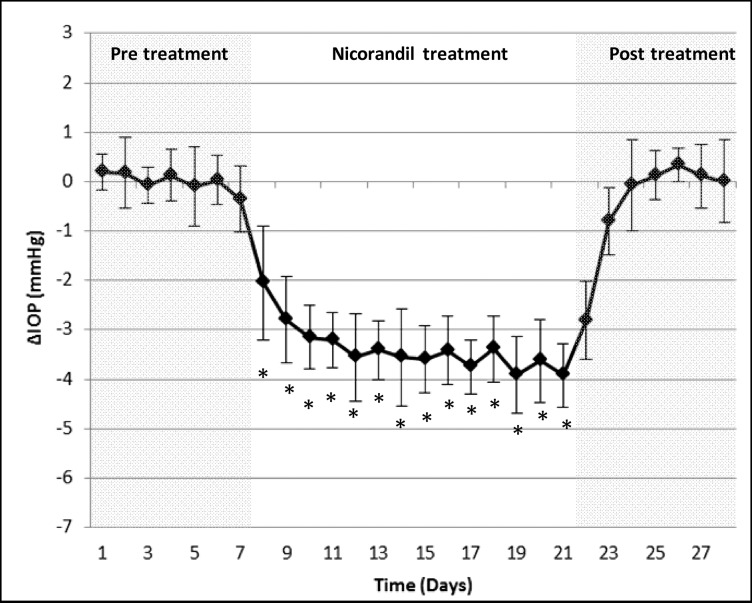
In previous studies on human ex vivo eye cultures, we found that in addition to diazoxide, nicorandil also decreased IOP and increased outflow facility.37 To confirm this in vivo, we used the same experimental design as mentioned above for diazoxide. Similar to what we found with diazoxide, nicorandil-treated C57BL/6 wild-type mice eyes (n = 10) showed a significant reduction in IOP at 1 hour, 4 hours, and 23 hours compared with control (Table 2). Daily IOP values decreased by 3.4 ± 0.4 mm Hg (P = 0.002; Fig. 5) during the 14-day treatment with a daily reduction range of 2.0 to 3.9 mm Hg. This constituted an 18.9 ± 1.8% change between nicorandil-treated and control eyes with a daily reduction range of 12.0 to 21.2%. On completion of the 14 days of treatment, IOP returned to baseline within 48 hours (P > 0.002 for IOP comparisons between control and treated eyes on all posttreatment days). Similar to diazoxide treatment, histologic examination of the eye following nicorandil treatment did not show any adverse side effects to the tissues and cells of the outflow pathways (Figs. 6A–D).
Figure 5.
Effect of nicorandil treatment on IOP of wild-type C57BL/6 mice. Treatment with nicorandil caused a decrease in IOP reaching near maximal reduction levels within 48 hours. Reduction in IOP was maintained throughout the 14-day treatment period with an average reduction of 3.4 ± 0.4 mm Hg compared with control eyes. Following cessation of treatment, IOP returned to baseline within 72 hours. *P = 0.002.
Figure 6.
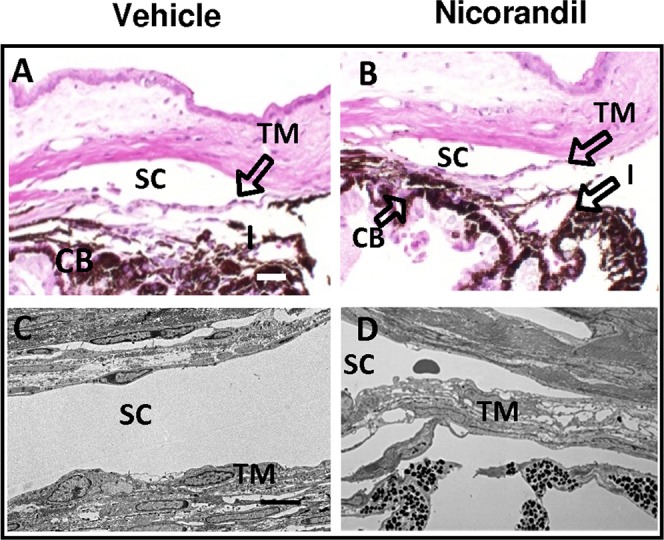
Histology of nicorandil-treated C57BL/6 mice eyes. Ocular morphology of the tissues and cells of the conventional outflow pathway appeared similar between treated and control eyes by either H&E (A, B) or transmission electron microscopy (C, D). Scale bar: 50 μm for H&E and 5 μm for transmission electron microscopy.
Discussion
Current pharmaceutical treatments for glaucoma are often inadequate in patients due to ineffectiveness of the drugs to lower IOP, side effects, or inability of the drugs to address visual field loss.4–6,38 Development of drugs that lower IOP by targeting underlying physiological processes will be helpful in minimizing vision loss. In this study, we characterized the presence of KATP channel subunits in the mouse eye and showed that activation of these channels by KATP channel openers diazoxide and nicorandil are effective at reducing IOP. Additionally, we identified the Kir6.2 subunit as a necessary component for IOP modulation through KATP channels. Results from the current study, along with our previous findings that diazoxide and nicorandil increase outflow facility in ex vivo human anterior segments, indicate that KATP channel openers may be a new class of ocular hypotensive agents.
KATP channels contain four identical SUR subunits (SUR1, SUR2A, or SUR2B) and four identical Kir subunits (Kir6.1 or Kir6.2), making multiple KATP channel subtypes based on subunit composition possible (e.g., SUR1/Kir6.1, SUR2A/Kir6.2, SUR2B/Kir6.1 etc). The heterogeneity of KATP channels imparts considerable differences to their pharmacologic properties. Nevertheless, most KATP channel openers are specific for KATP channels with a particular combination of SUR and Kir subunits. Diazoxide has minimal to no effect on KATP channels composed of SUR2A/Kir6.2 subunits. However, both diazoxide and nicorandil activate SUR2B/Kir6.1 or SUR2B/Kir6.2 containing KATP channels.36,39 SUR2A and SUR2B are alternative splice variants arising from the SUR2 gene with each having a unique 42 amino acid C-terminal end. The difference in amino acid content at the end of SUR2B has been identified as the site required for diazoxide activation.40 In mice, we found KATP channel subunits SUR2B, Kir6.1, and Kir6.2 in the anterior segment, specifically in the trabecular meshwork, Schlemm's canal, iris, ciliary body, and the corneal epithelium and endothelium. Similar to our findings in humans,37 SUR1 and SUR2A were not detected by immunohistochemistry. We cannot completely rule out the possibility that the antibodies used for SUR1 and SUR2A were unable to detect these relevant subunits due to very low abundance.
Because Kir6.2(−/−) and wild-type mice had similar baseline IOP's, Kir6.2 does not appear essential for normal IOP modulation. However, because diazoxide and nicorandil both lowered IOP in wild-type mice, failure of diazoxide to lower pressure in Kir6.2(−/−) mice suggests that the Kir6.2 subunit containing KATP channels are involved in KATP channel–mediated pressure reduction, since unlike the wild-type mice, diazoxide treatment had no effect on IOP in these animals. This indicates that SUR2B/Kir6.2 is the primary KATP channel involved in lowering IOP following treatment with KATP channel openers. This does not preclude the importance of Kir6.1 subunit containing channels since these channels may influence other cellular processes such as maintaining endothelial cell function, protection against cellular atrophy, or overall cellular protection during high pressures. These functions have already been attributed to activation of SUR2B/Kir6.1 containing KATP channels in the heart.41 Alternatively, one cannot rule out the possibility of combination channels. While heterogenic KATP channels have not been identified in vivo, in vitro studies have suggested that single channels made up of Kir6.1 and Kir6.2 are possible.42,43 We did find normal levels of Kir6.1 in Kir6.2(−/−) mice (Supplementary Figs. S1A–F) and therefore functional compensation for the loss of Kir6.2 by the Kir6.1 subunit seems unlikely. Identification of the exact subunit structure making up KATP channels involved in IOP regulation will be important for future drug development.
The Kir6.2(−/−) mice used in this study have been extensively characterized44 and have been used as a viable mouse model to study cardioprotection, glucose metabolism and insulin secretion.11,12,23,44,45 In general, Kir6.2(−/−) mice are susceptible to stress and cardiac overload and have defective glucose-dependent insulin secretion along with increased numbers of α-cells and pancreatic polypeptide positive cells in the pancreatic islets.23,46 These mice have been backcrossed to mice from the C57BL/6 background for multiple generations and hence age-matched C57BL/6 mice were used as wild-type controls for studies involving these knockouts.23,45,47 Overall, under normal conditions, the Kir6.2(−/−) mice appear normal and are fertile with no significant differences in body weight, behavior, or appearance when compared to the wild type.44
Because of the lack of an ocular hypertension model to study drugs that affect the outflow pathways, we were limited to analyzing the effect of KATP channel openers on normotensive animals. In normal mice, all commonly used antiglaucoma agents have been shown to lower IOP; therefore, treating normotensive mice to study efficacies of ocular hypotensive agents is an established practice.48,49 For example, the prostaglandin analog latanoprost has been shown to lower absolute IOP by 3.5 mm Hg in normotensive mice with change in IOP between treated and control eyes of 18%.48,49 In our study, both diazoxide and nicorandil decreased IOP by approximately 20% with absolute IOP change of 3.9 ± 0.6 and 3.4 ± 0.4 mm Hg, respectively, suggesting KATP channel openers have a similar IOP reduction to current antihypertensive agents used to treat glaucoma. Furthermore, both diazoxide and nicorandil were found to significantly lower IOP at individual time points (1 hour, 4 hours, and 23 hours) suggesting that a single daily dose of diazoxide or nicorandil has immediate and lasting effects on IOP.
Previous studies from our laboratory have shown that diazoxide and nicorandil lowered IOP in ex vivo human anterior segment cultures through the conventional outflow pathway.37 Functional similarities exist between human and mouse eyes and drugs affecting the conventional pathway in human eyes have been shown to retain their mode of action in mouse eyes.50 In light of this, there is a strong possibility that diazoxide and nicorandil, which work through the conventional outflow pathway in human eyes, are also affecting the conventional pathway in mice. However, the presence of KATP channel subunits throughout the human and mouse eye suggests that KATP channel openers may also alter unconventional flow and possibly aqueous inflow. Studies assessing aqueous humor dynamics in these mice following KATP channel opener treatment will be necessary to elucidate the specific inflow and outflow pathways involved in KATP channel modulated IOP reduction.
It should be noted that in primary open-angle glaucoma (POAG) patients, an acceptable reduction in IOP with a therapy of frontline drugs is around 20%.51 Combination of multiple medications is often required to achieve a target IOP in POAG patients.52 We have shown that diazoxide, when used in combination with latanoprost free acid, shows an additive effect in reducing pressure in human anterior segment cultures (Roy Chowdhury U, et al. IOVS 2011;52:ARVO E-Abstract 4642), indicating that KATP channel openers may be future candidates for combination therapy for POAG. Of further interest is the fact that KATP channel openers have been shown to provide retinal neuroprotection in rat models.24–26 Given the fact that none of the existing glaucoma medications protect the retina from degeneration, KATP channel openers may be the first drug of its class that shows both hypotensive and neuroprotective properties.
In summary, our study indicates a specific ocular hypotensive effect of KATP channel openers diazoxide and nicorandil in an in vivo murine model. Our data suggest that diazoxide and nicorandil work through the Kir6.2 subunit. Considering that diazoxide and nicorandil act only on SUR2B containing KATP channels,36,39 it is reasonable to conclude that KATP channels composed of Kir6.2/SUR2B are the primary subunit combination involved in KATP-channel–mediated IOP regulation. Although further work is needed to elucidate the exact cellular pathways linking KATP channels to IOP reduction, there is a strong potential for these openers to become future therapeutic agents for the treatment of ocular hypertensive diseases like POAG.
Supplementary Material
Acknowledgments
Supported in part by National Institutes of Health Research Grant EY 21727; Mayo Foundation; Research to Prevent Blindness (MPF is a recipient of a Lew R. Wasserman Merit Award and the Department of Ophthalmology at Mayo Clinic is the recipient of an unrestricted grant).
Disclosure: U.R. Chowdhury, None; B.H. Holman, None; M.P. Fautsch, None
Footnotes
Copyright 2013 Mayo Foundation
References
- 1. Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120: 1268–1279 [DOI] [PubMed] [Google Scholar]
- 2. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008; 53 (suppl 1): S93–S105 [DOI] [PubMed] [Google Scholar]
- 5. Talluto DM, Wyse TB, Krupin T. Topical carbonic anhydrase inhibitors. Curr Opin Ophthalmol. 1997; 8: 2–6 [DOI] [PubMed] [Google Scholar]
- 6. Chader GJ. Key needs and opportunities for treating glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 2456–2460 [DOI] [PubMed] [Google Scholar]
- 7. Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998; 60: 667–687 [DOI] [PubMed] [Google Scholar]
- 8. Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002; 29: 312–316 [DOI] [PubMed] [Google Scholar]
- 9. Gao S, Long CL, Wang RH, Wang H. K(ATP) activation prevents progression of cardiac hypertrophy to failure induced by pressure overload via protecting endothelial function. Cardiovasc Res. 2009; 83: 444–456 [DOI] [PubMed] [Google Scholar]
- 10. Jons T, Wittschieber D, Beyer A, et al. K+-ATP-channel-related protein complexes: potential transducers in the regulation of epithelial tight junction permeability. J Cell Sci. 2006; 119: 3087–3097 [DOI] [PubMed] [Google Scholar]
- 11. Kane GC, Behfar A, Dyer RB, et al. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006; 15: 2285–2297 [DOI] [PubMed] [Google Scholar]
- 12. Kane GC, Behfar A, Yamada S, et al. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004; 53 (suppl 3): S169–S175 [DOI] [PubMed] [Google Scholar]
- 13. Kane GC, Lam CF, O'Cochlain F, et al. Gene knockout of the KCNJ8-encoded Kir6.1 K(ATP) channel imparts fatal susceptibility to endotoxemia. FASEB J. 2006; 20: 2271–2280 [DOI] [PubMed] [Google Scholar]
- 14. Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005; 38: 937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawamura T, Kadosaki M, Nara N, Wei J, Endo S, Inada K. Nicorandil attenuates NF-kappaB activation, adhesion molecule expression, and cytokine production in patients with coronary artery bypass surgery. Shock. 2005; 24: 103–108 [DOI] [PubMed] [Google Scholar]
- 16. Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991; 261: H1675–H1686 [DOI] [PubMed] [Google Scholar]
- 17. Ozcan C, Terzic A, Bienengraeber M. Effective pharmacotherapy against oxidative injury: alternative utility of an ATP-sensitive potassium channel opener. J Cardiovasc Pharmacol. 2007; 50: 411–418 [DOI] [PubMed] [Google Scholar]
- 18. Rocheleau JV, Remedi MS, Granada B, et al. Critical role of gap junction coupled KATP channel activity for regulated insulin secretion. PLoS Biol. 2006; 4: e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003; 81: 133–176 [DOI] [PubMed] [Google Scholar]
- 20. Selivanov VA, Alekseev AE, Hodgson DM, Dzeja PP, Terzic A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol Cell Biochem. 2004; 256-257: 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989; 245: 177–180 [DOI] [PubMed] [Google Scholar]
- 22. Zingman LV, Hodgson DM, Alekseev AE, Terzic A. Stress without distress: homeostatic role for K(ATP) channels. Mol Psychiatry. 2003; 8: 253–254 [DOI] [PubMed] [Google Scholar]
- 23. Zingman LV, Hodgson DM, Bast PH, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002; 99: 13278–13283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atlasz T, Babai N, Reglodi D, et al. Diazoxide is protective in the rat retina against ischemic injury induced by bilateral carotid occlusion and glutamate-induced degeneration. Neurotox Res. 2007; 12: 105–111 [DOI] [PubMed] [Google Scholar]
- 25. Roth S, Dreixler JC, Shaikh AR, Lee KH, Bindokas V. Mitochondrial potassium ATP channels and retinal ischemic preconditioning. Invest Ophthalmol Vis Sci. 2006; 47: 2114–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roth S, Li B, Rosenbaum PS, et al. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci. 1998; 39: 777–785 [PubMed] [Google Scholar]
- 27. Roth S, Shaikh AR, Hennelly MM, Li Q, Bindokas V, Graham CE. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci. 2003; 44: 5383–5395 [DOI] [PubMed] [Google Scholar]
- 28. de Lonlay P, Cuer M, Vuillaumier-Barrot S, et al. Hyperinsulinemic hypoglycemia as a presenting sign in phosphomannose isomerase deficiency: A new manifestation of carbohydrate-deficient glycoprotein syndrome treatable with mannose. J Pediatr. 1999; 135: 379–383 [DOI] [PubMed] [Google Scholar]
- 29. Jahangir A, Terzic A. K(ATP) channel therapeutics at the bedside. J Mol Cell Cardiol. 2005; 39: 99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McNair A, Andreasen F, Nielsen PE. Antihypertensive effect of diazoxide given intravenously in small repeated doses. Eur J Clin Pharmacol. 1983; 24: 151–156 [DOI] [PubMed] [Google Scholar]
- 31. Tomai F, Crea F, Gaspardone A, et al. Ischemic preconditioning during coronary angioplasty is prevented by glibenclamide, a selective ATP-sensitive K+ channel blocker. Circulation. 1994; 90: 700–705 [DOI] [PubMed] [Google Scholar]
- 32. O'Meara NM, Shapiro ET, Van Cauter E, Polonsky KS. Effect of glyburide on beta cell responsiveness to glucose in non-insulin-dependent diabetes mellitus. Am J Med. 1990; 89: 11S–16S discussion 51S–53S [DOI] [PubMed] [Google Scholar]
- 33. Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010; 90: 291–366 [DOI] [PubMed] [Google Scholar]
- 34. Flagg TP, Kurata HT, Masia R, et al. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ Res. 2008; 103: 1458–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cui Y, Tran S, Tinker A, Clapp LH. The molecular composition of K(ATP) channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol. 2002; 26: 135–143 [DOI] [PubMed] [Google Scholar]
- 36. Lawson K. Potassium channel openers as potential therapeutic weapons in ion channel disease. Kidney Int. 2000; 57: 838–845 [DOI] [PubMed] [Google Scholar]
- 37. Chowdhury UR, Bahler CK, Hann CR, et al. ATP-sensitive potassium (KATP) channel activation decreases intraocular pressure in the anterior chamber of the eye. Invest Ophthalmol Vis Sci. 2011; 52: 6435–6442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boland MV, Ervin AM, Friedman D, et al. Treatment for Glaucoma: Comparative Effectiveness. Rockville: Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 39. Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Phys. 1998; 274: C25–C37 [DOI] [PubMed] [Google Scholar]
- 40. Isomoto S, Kondo C, Yamada M, et al. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996; 271: 24321–24324 [DOI] [PubMed] [Google Scholar]
- 41. Tang Y, Long CL, Wang RH, Cui W, Wang H. Activation of SUR2B/Kir6.1 subtype of adenosine triphosphate-sensitive potassium channel improves pressure overload-induced cardiac remodeling via protecting endothelial function. J Cardiovasc Pharmacol. 2010; 56: 345–353 [DOI] [PubMed] [Google Scholar]
- 42. Cui Y, Giblin JP, Clapp LH, Tinker A. A mechanism for ATP-sensitive potassium channel diversity: Functional coassembly of two pore-forming subunits. Proc Natl Acad Sci U S A. 2001; 98: 729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pountney DJ, Sun ZQ, Porter LM, et al. Is the molecular composition of K(ATP) channels more complex than originally thought? J Mol Cell Cardiol. 2001; 33: 1541–1546 [DOI] [PubMed] [Google Scholar]
- 44. Miki T, Nagashima K, Tashiro F, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998; 95: 10402–10406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park YB, Choi YJ, Park SY, et al. ATP-sensitive potassium channel-deficient mice show hyperphagia but are resistant to obesity. Diabetes Metab J. 2011; 35: 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seino S, Iwanaga T, Nagashima K, Miki T. Diverse roles of K(ATP) channels learned from Kir6.2 genetically engineered mice. Diabetes. 2000; 49: 311–318 [DOI] [PubMed] [Google Scholar]
- 47. Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci. 2005; 8: 1742–1751 [DOI] [PubMed] [Google Scholar]
- 48. Akaishi T, Odani-Kawabata N, Ishida N, Nakamura M. Ocular hypotensive effects of anti-glaucoma agents in mice. J Ocul Pharmacol Ther. 2009; 25: 401–408 [DOI] [PubMed] [Google Scholar]
- 49. Ota T, Murata H, Sugimoto E, Aihara M, Araie M. Prostaglandin analogues and mouse intraocular pressure: effects of tafluprost, latanoprost, travoprost, and unoprostone, considering 24-hour variation. Invest Ophthalmol Vis Sci. 2005; 46: 2006–2011 [DOI] [PubMed] [Google Scholar]
- 50. Boussommier-Calleja A, Bertrand J, Woodward DF, Ethier CR, Stamer WD, Overby DR. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest Ophthalmol Vis Sci. 2012; 53: 5838–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perry CM, McGavin JK, Culy CR, Ibbotson T. Latanoprost: an update of its use in glaucoma and ocular hypertension. Drugs Aging. 2003; 20: 597–630 [DOI] [PubMed] [Google Scholar]
- 52. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701–713 discussion 729–730 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.