Abstract
Epididymal tumour incidence is at most 0.03% of all male cancers. It is an enigma why the human epididymis does not often succumb to cancer, when it expresses markers of stem and cancer cells, and constitutively expresses oncogenes, pro-proliferative and pro-angiogenic factors that allow tumour cells to escape immunosurveillance in cancer-prone tissues. The privileged position of the human epididymis in evading tumourigenicity is reflected in transgenic mouse models in which induction of tumours in other organs is not accompanied by epididymal neoplasia. The epididymis appears to: (i) prevent tumour initiation (it probably lacks stem cells and has strong anti-oxidative mechanisms, active tumour suppressors and inactive oncogene products); (ii) foster tumour monitoring and destruction (by strong immuno-surveillance and -eradication, and cellular senescence); (iii) avert proliferation and angiogenesis (with persistent tight junctions, the presence of anti-angiogenic factors and misplaced pro-angiogenic factors), which together (iv) promote dormancy and restrict dividing cells to hyperplasia. Epididymal cells may be rendered non-responsive to oncogenic stimuli by the constitutive expression of factors generally inducible in tumours, and resistant to the normal epididymal environment, which mimics that of a tumour niche promoting tumour growth. The threshold for tumour initiation may thus be higher in the epididymis than in other organs. Several anti-tumour mechanisms are those that maintain spermatozoa quiescent and immunologically silent, so the low incidence of cancer in the epididymis may be a consequence of its role in sperm maturation and storage. Understanding these mechanisms may throw light on cancer prevention and therapy in general.
Keywords: anti-oxidation, cell proliferation, metabolic reprogramming, tumour dormancy, tumour suppression
Introduction
Cancer is not common, despite the constant genetic insults on cells, because of the effectiveness of endogenous tumour suppression.1 Although cancers occur in the testis of young men, and in the prostate of old men, malignant neoplasms of the epididymis are rare.2, 3, 4 There has been surprisingly little interest in this intriguing observation. The epididymis lies over the testis and receives the non-functional spermatozoa produced by the testis (Figure 1). The epithelia in its different regions (Figure 2) provide luminal environments that foster sperm maturation and permit quiescent sperm storage.5 As knowledge about the organ's evasion of tumours could be useful for the design of cancer therapies in general, this review explores the current understanding of tumour initiation and suppression, the general hallmarks of cancer and their relevance to the epididymis. Epidemiological data were extracted from cancer registries in the public domain and calculated from cases reported in the China Hospital Knowledge Database (CHKD) under China National Knowledge Infrastructure (http://www.chkd.cnki.net) from 1979 to 2010.
Figure 1.
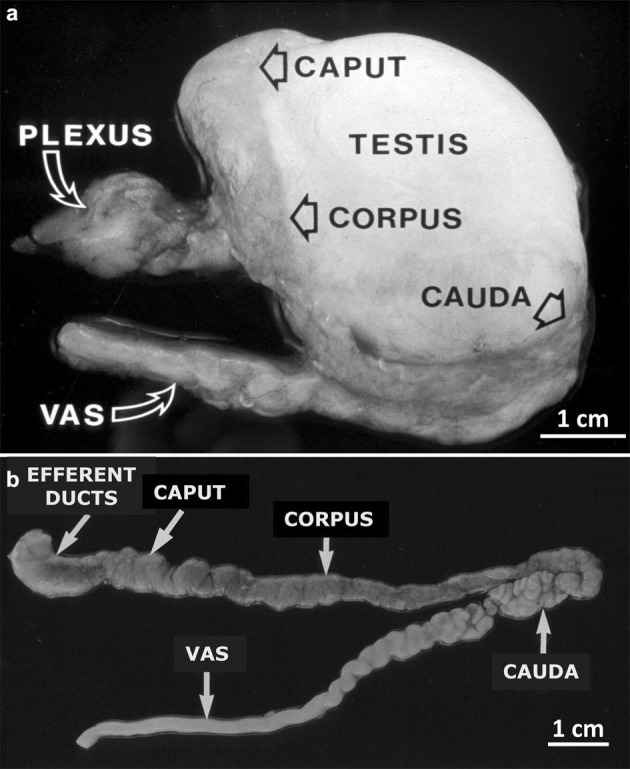
Macroscopic appearance of the human epididymis. (a) Photograph of a human epididymis attached to the testis from a 74-year-old man with prostatic cancer. The head of the epididymis (comprising efferent ducts and caput epididymidis (CAPUT)) is partially hidden behind the testis, the entire corpus epididymidis (CORPUS) is visible, and the cauda epididymidis (CAUDA) is partially hidden behind the testis but is continuous with the vas deferens (VAS). PLEXUS indicates the site of entry and exit of blood and lymphatic vessels. Scale bar=1 cm. (b) The same organ dissected from the testis showing the general regions of efferent ducts, caput, corpus and cauda epididymidis and vas deferens. Unlike that of other species, the human epididymis does not conform to the usual mammalian model of a thin capsule revealing the outline of the underlying convoluted tubules in clear-cut caput, corpus and cauda regions. The efferent ducts within the proximal head regions are often dark. Scale bar=1 cm.
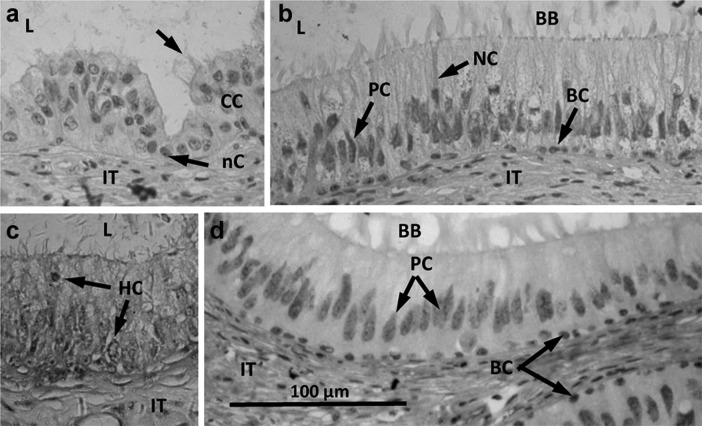
Figure 2.
Photomicrographs of the human post-testicular duct system revealing various cell types present in the epithelium. (a) Efferent ducts. Arrows indicate an nC and the cilia of a CC. (b) Caput epididymidis. Arrows indicate the cytoplasm of an NC and nuclei of PCs and BCs. (c) Caput epididymidis. Arrows indicate HCs among principal cells. (d) Corpus epididymidis. Arrows indicate nuclei of PCs and BCs. Scale bar in (D) 100 µm, applicable to all micrographs. BB, brush border; BC, basal cell; CC, ciliated cell; HC, halo cell; IT, interstitium; L, lumen; nC, non-ciliated cell; NC, narrow cell; PC, principal cell.
In most cancer registry reports, the epididymis is not entered as a separate tumour site. In some, it is under ‘male genital other than testis, prostate and penis' (Table 1) or not at all. Rare as they are, statistics show epididymal cancer incidence rates up to 0.03% of all male cancers, in sharp contrast to almost 20% in Western countries for the most common, prostate cancer. The tumour incidence in the epididymis is about 50 times as rare as that in the testis, whereas the kidney, with the same embryonic origin as the epididymis, is about 100 times more likely to develop tumours (Table 1). Such data are not available in China, but screening of the CHKD for men from 1979 to 2010 revealed 328, 18 387 and 54 550 reports of cases of tumours in the epididymis, testis and kidney, respectively. The relative numbers of these published cases for these three organs resemble the relative incidences above.
Table 1. Statistics on tumour incidence from three cancer registriesa, (i) as a percentage of total male cancers and (ii) as number of cases per 100 000 of the male population.
| ICD code | Primary site of tumour | Dhaka (i) | Ireland (i) | New York (ii) |
|---|---|---|---|---|
| C60.9 | Penis | 0.28 | 0.16 | 1.0 |
| C61.9 | Prostate | 1.64 | 19.07 | 166.9 |
| C62.9 | Testis | 1.63 | 1.19 | 5.6 |
| C63.0 | Epididymis | 0.03 | ||
| C63.1 | Spermatic cord | 0.04 | ||
| C63.2 | Scrotum | 0.06 | ||
| C63.7 | Seminal vesicle | 0.01 | ||
| C63 | Other male genitalb | 0.02 | 0.2 | |
| C64.9 | Kidney | 0.99 | 1.87 | 21.7 |
| C66.9 | Ureter | 0.07 | 0.06 | 0.7 |
| C67.9 | Bladder | 1.97 | 2.34 | 42.5 |
| C68.0 | Urethra | 0.05 | ||
| Comparison with epididymal tumours (n-fold)c | ||||
| Testis/epididymis | 59 | 75 | 28 | |
| Prostate/epididymis | 55 | 954 | 835 | |
| Kidney/epididymis | 36 | 117 | 109 | |
Abbreviation: ICD, International Classification of Diseases.
Sources: Dhaka: World Health Organization cancer registry report for National Institute of Cancer Research and Hospital in Dhaka, Bangladesh in 2005–2007; Ireland: Ireland national cancer registry report, yearly averaged rate in 2005–2009; New York: Cancer registry report for whole of New York State in 2004–2008.
Male genital organs (including epididymis) other than penis, prostate and testis.
The entry for C63 was used for calculation when epididymis was not a separate entry.
Bold entries highlight values used in comparison of epididymis with other organs.
The CHKD reports that epididymal tumours can occur unilaterally or bilaterally, at any age after puberty (13–83 years) with mean ages in the thirties and forties, and 82% of cases are not malignant. Of the 328 cases, 55% belonged to the ‘adenomatoid' type (occurring in the epithelial compartment), 15% were smooth muscle tumours and 5% were in blood or lymph vessels. In 73% of cases, a primary tumour was stated in the diagnosis, only two stated metastasis. Both the invasion of primary epididymal tumours into other organs, and metastases of tumours in other organs spreading to the epididymis have been reported.3 In a Western survey of 257 benign epididymal tumours, 73% were adenomatoid, whereas of 84 malignant cases, 51% were primary or metastatic carcinomas (epithelial) and 44% were sarcomas (stromal4). The most studied human epididymal cancer is cystadenoma, a benign cystic tumour of the gland.
Cells-of-origin of epididymal tumours
Epididymal stem cells
Tumour cells often originate from normal tissue stem cells.6 OCT4, SOX2, NANOG, KLF4 and NOTCH1 are factors that maintain pluripotency in embryonic stem cells. Whereas human embryonic and adult stem cells, and cancer stem cells express OCT4, differentiated normal cells in adults have lost this expression.7 Both principal and basal cells of the human adult epididymal epithelium express OCT4 and SOX2 in the nuclei and NANOG in the cytoplasm.8 KLF4 is highly expressed by the whole epithelium of the epididymis of the mouse.9 Notch1 mRNA is in all epididymal regions, without cellular identity;10 Notch1 is only detectable by in situ hybridisation in hyperplastic epididymal epithelial cells of constitutively active Notch1-transgenic mice.11 These embryonic stem cell factors may have other functions, such as cellular differentiation, in the adult epididymis.
Markers of adult stem cells found in other organs are also present in the epididymis (Table 2), but as they are expressed by most epithelial cells, no particular type is a likely candidate, for which only a small positive population is anticipated (<2% in the kidney12 and <5% in the prostate13). However, the formation in human epididymal cell culture of epithelial spheres,8 a characteristic of ‘cancer stem cells' or ‘tumour initiating cells',14 suggests that these cells do exist.
Table 2. Molecular markers of normal adult stem cells in various organs, and their expression in the epididymis without evidence that they are stem cell markers.
| Braina | Liver | Intestine | Kidney | Prostate | Epididymal transcriptb | Epididymal proteinc | |
|---|---|---|---|---|---|---|---|
| Mouse/human | Human | Mouse/human | Human | Mouse/human | Human | Mouse/bull/human | |
| Abcg2 | + | + (Ref. 20) | Mouse caput epithelial microvilli, caput and corpus interstitial endothelial cells165 | ||||
| Ascl 2 | + | + (Ref. 20) | |||||
| Bmi-1 | + | + | |||||
| CD9 | + | + (Ref. 20) | Bull corpus and cauda epithelium166 | ||||
| CD24 | + | + (Refs. 20 and 38) | |||||
| CD29 | + | Human cultured cells8 | |||||
| CD44 | + | + (Refs. 20 and 38) | Human cultured cells; mouse basal cells8 | ||||
| CD49b | + | ||||||
| CD49f | + | ||||||
| CD73 | + | ||||||
| CD81 | + | + (Refs. 20 and 38) | |||||
| CD90 | + | ||||||
| CD95 | + | ||||||
| CD117 | + | ||||||
| CD133 | + | + | + | + | Human cultured cells (j); mouse principal cells microvilli except initial segment167 | ||
| CD144 | + | ||||||
| CD146 | + | + | |||||
| CD184 | + | ||||||
| CK6a | + | ||||||
| DCAMKL-1 | + | + (Ref. 20) | |||||
| EpCAM | + | ||||||
| Lgr5 | + | + (Ref.20) | |||||
| Musashi-1 | + | ||||||
| mTERT | + | ||||||
| Nestin | + | + (Ref. 38) | |||||
| OLFM4 | + | + (Ref. 20) | |||||
| PTEN | + | + (Ref. 20) | Mouse caput epithelium apical surface, especially initial segment stereocilia21 | ||||
| Sca-1 | + | ||||||
| sFRP5 | + | + (Ref. 20) | |||||
| SOX4 | + | + (Ref. 20) | |||||
| SOX9 | + | + (Ref. 20) | |||||
| WIP1 | + | ||||||
| Reference | 6 | 161 | 162 | 12 | Murine,163, 164 | 20, 38 | 8, 21, 165–167 |
| human13 |
Epididymal cancer progenitor cells
In the absence of epithelial stem cells, cells giving rise to tumours can only be mutated progenitor cells located in the epithelium or stroma. Epithelial cells are more likely than stromal cells to be the cancer cells-of-origin, since 55% of all epididymal tumours are adenomatoid, of which 88% are adenomas and 12% cystadenomas (CHKD). Both epithelial and peritubular progenitor cells can be the cancer cell-of-origin in mouse models of prostatic cancer, whereas peritubular cells have been implicated in the human.6 In the Pten+Vhl double gene-knockout mouse model of epididymal cystadenoma, an increase in epithelial basal cells suggested to Frew et al.15 that the neoplasm was generated by basal cell proliferation without differentiation.
Regulation of cell proliferation
Growth suppressors
Tumour progression is sustained by cell proliferation following the loss of normal cell controls, which could reflect responses to excessive proliferation signals or evasion of growth suppressors. Retinoblastoma protein 1 (RB1) is a nuclear transcriptional suppressor that interacts with many regulatory proteins, depending on its phosphorylation state.16 Dysfunction of RB1 is associated with many human cancers.17
Rb1 RNA is expressed in the mouse,18 rat19 and human epididymis.20 The expression of the tumour suppressor genes Pten and Rb1 counter-balances the activity of pro-proliferative signal proteins in the proximal epididymis of mice.21 Xu et al.21 have suggested that the rarity of epididymal cancer could be attributable to growth suppressor genes, including DUSP6 (dual specificity phosphatase 6) and related proteins. DUSPs inactivate phosphorylated proteins in the mitogen-activated protein kinase signal pathway and terminate proliferative mechanisms, and DUSP6 exerts anti-tumour effects on lung cancer cell lines.22 DUSP6 mRNA is found in the human epididymis,20 but its comparative expression in other cancer-prone organs is not known.
Cell proliferation rate
High proliferation rates are likely to be associated with high chances of unregulated cell division. In the rat epididymis and prostate, such rates estimated from 3H-thymidine labelling of DNA, decrease rapidly after birth to similar rates for the two organs in the adult, slightly higher than those of liver and skeletal muscle.23 In the epididymal epithelium, proliferation rates decline during early adulthood from around 2% to stabilize at around 0.5% of labelled cells.24, 25 Similarly low proliferation rates (1.3%) have been confirmed in the adult rat prostate.26 It seems unlikely that proliferation rates alone can account for the far higher tumour incidence in the prostate than that of the epididymis.
Growth promoters
Wingless/int (Wnt) signalling pathways are important for normal cell proliferation, differentiation and maintenance of cellular homeostasis. Disruption of these, especially the canonical β-catenin-dependent pathway, has profound effects on human cancer development.27 The binding of Wnt to the frizzled receptors (Fzd) releases β-catenin from a destructive complex; the stabilized β-catenin then enters the nucleus and activates pro-proliferative target genes.28 Humans and mice share the same gene components of Wnt and Fzd (http://www.stanford.edu/group/nusselab/cgi-bin/wnt/) and almost all Wnt signalling pathway component genes are transcribed in the adult mouse epididymis (http://www.mrg.genetics.washington.edu/index.cgi); many are in the adult human epididymis.20
β-catenin is expressed in the rat epididymis at the junctional complexes between epithelial cells,29 in association with E-cadherin, which is also expressed in the human epididymis.30 Rat epididymal β-catenin released from E-cadherin following castration accumulates in the cytoplasm, not the nucleus, and there is no subsequent hyperplasia.29 A high expression of the non-canonical pathway Wnt4, which can inhibit the canonical Wnt pathway through competition for common components,31 has been reported in the epididymis.32 The regulation and role of Wnt pathways in epididymal tumourigenesis remain unexplored.
Endogenous protection against mutagenic microenvironments
Tumourigenic transformation requires a stressful mutagenic microenvironment causing abnormal DNA transcription and uncontrolled cell division, which can be brought on by oxidative attack on DNA itself or by the activation of oncogenes.
Antioxidant protection of epididymal cell DNA
The production of reactive oxygen species (ROS) is normally limited by the low oxygen tension in epididymal tissue33 and epididymal lumen.34 This may stem from the epithelial principal cells' expressing high activities of indoleamine-2,3-dioxgenase (IDO) and tryptophan-2,3-dioxgenase (TDO), both of which can incorporate molecular oxygen into their substrates,35 and from the high number of luminal spermatozoa, which consume oxygen even when concentrated and immotile.36 Complementing this non-oxidative environment are anti-oxidant enzymes and components removing superoxide radicals and hydrogen peroxide,37 some of which are reported in the human epididymal proteome.38, 39 These anti-oxidant strategies make excessive oxidative damage to epididymal cell DNA unlikely.
Oncogene expression in the normal epididymis
Naturally occurring cancers and cancerous growths induced in vitro are associated with the expression of oncogenes, which are often mutated versions of normal proteins not normally expressed in adult tissues. When present in non-metastatic tissue, the non-mutated forms (proto-oncogenes) may have other cellular roles. For example, c-ros in many developing mouse organs declines in expression as differentiation proceeds and stops in the adult, but is upregulated in many tumours.40 Activation of the mutated oncogenes or overexpression of proto-oncogenes in tissues can give rise to tumours within them (see section on ‘Dormancy of early tumour cells').
Many proto-oncogenes are expressed in the normal adult epididymis. In contrast to the common postnatal downregulation of c-ros, it is upregulated in the proximal caput epididymidis around puberty, when the initial segment differentiates, and sustained in adulthood.41 c-Ros deletion leads to male-specific infertility because of epididymal maldevelopment.41 c-ROS has been identified in the human epididymis.42 In adult mice, epididymal expression of c-Mos, A-raf, B-Raf, c-Raf, L-Myc, N-Myc, B-Myc, C-Myc, c-Ret and Met have been identified.43, 44
Explanations for epididymal oncogene expression not being accompanied by typical oncogenic activity include: (i) the counter-balancing of antiproliferative gene expression (see above)—the expression of antiproliferative B-Myc protein exceeds that of pro-proliferative c-Myc;45 (ii) the triggering, by high expression of some oncogenes (e.g., Ras family and C-Myc), of fail-safe mechanisms that induce senescence or apoptosis (see section on ‘Intrinsic barriers to tumour formation'), instead of inducing proliferation;46 (iii) the provision of protection against tumours by endogenous oncogenes. The expression of K-Ras, B-Raf and Myc, expressed in the adult human epididymis,20 enhances the basal expression of the transcription factor Nrf2 (nuclear factor E2-related factor 2), which controls intracellular levels of ROS through an inducible antioxidant programme.47 Nfe2l2 (Nrf2) mRNA is highly expressed throughout the rat19 and mouse epididymis18 and also found in that of man.20 Adult Nrf2−/− male mice suffer reduced antioxidants and raised lipid peroxidation in the epididymis, accompanied by reduced epididymal sperm motility.48 Whether the constitutively expressed oncogenes in the epididymis contribute to its especially strong anti-oxidation defence mechanisms (see section on ‘Antioxidant protection of epididymal cell DNA') warrants investigation; and (iv) non-oncogenic functions of oncogene proteins in the epididymis. The regulation of A-Raf and B-Myc by androgens, and of B-Myc and c-Ros (in the initial segment) but not A-Raf (in the caput) by testicular fluid,49, 50 mirrors that of cancer-unrelated epididymal proteins. Similarly, ‘cancer markers' such as metastasis-associated protein 151 and human epididymal protein 4,52, 53 which are normally expressed, may serve other functions.
Cell–cell junctions and cancer
Tight junctions
Tight junctions are the most distinctive cell junctions in epithelia54 and constitute an inherent barrier to aberrant cell proliferation by mediating the meiotic block upon cell contact,55 maintaining adhesion with adjacent cells and generating a barrier that restricts the paracellular passage of fluid and separates luminal and basolateral compartments. Tight junctional disruption allows luminal growth factors to interact with their basolateral receptors, which encourages mitogenesis.55 Expression of the main junction-associated proteins (occludins, claudins) is altered in cancerous tissue.56 Of more than 100 tight junction proteins, some are themselves tumour suppressors,57 whereas others are hubs for signal transduction leading to cell proliferation and tumourigenesis.58
Tight junctions in the epididymis are the physical component of the blood–epididymis barrier59 and the major tight-junction proteins have been localized in the human epididymis.60 When Sertoli cell tight junctions undergo seasonal regression, epididymal tight junctions remain intact in the mink61, 62 and are even reinforced in bats.63 In a related situation in men (non-obstructive azoospermia), caput epididymidal junctions appear normal, with no change in expression and location of most claudins and tight junction proteins.64 Stable and persistent tight junctions may be one component of epididymal resistance to cancer.
Other junctions
Epithelial cell adhesion is also mediated by adherens junctions and desmosomes, both of which contain cadherins,65 whereas gap junctions, formed from connexins, provide intercellular communication. Cadherin and catenin expression is altered in cancers66 and there is evidence for an inhibitory role of connexins in the progression of primary tumours, despite suggestions of their enhancing tumour cell adhesion and migration in cancer invasion and metastasis.67 Adherens junctions, desmosomes and gap junctions are found in the epididymis.61, 62, 68 Of the connexins considered primary tumour suppressors, Cx26, Cx3269 and Cx4370 have been localized in the gap junctions of the rat epididymis.
Intrinsic barriers to tumour formation
Apoptosis
Tumour suppression mechanisms include the prevention of cellular proliferation by apoptosis, which can be activated by intrinsic and extrinsic factors. Apoptosis can be induced by the oncogenes C-Myc, E1A and E2F, and regulated by the p53 (tumour suppressor protein 53) pathway.71 Epididymal apoptosis is normally non-detectable by TUNEL and DNA ladder analysis,72 and is rare in the epithelium.73 Such low apoptosis may reflect the presence of anti-apoptotic B-cell lymphoma-2 (Bcl-2) and absence of pro-apoptotic Fas.74 The normal mouse epididymis expresses p53 and the apoptosis effector caspase 3,75 yet apoptosis in this organ following cryptepididymis and castration is independent of p53 and Fas/FasL,75, 76 as is rat epididymal apoptosis after efferent duct ligation.73
Senescence
Another mechanism of intrinsic tumour suppression is age-independent ‘senescence', a signal transduction programme leading to irreversible arrest of cell proliferation, accompanied by distinct changes in cellular phenotype.77 Senescence can be induced by oxidative, bacterial, genotoxic and oncogenic stresses; for example, continuous overexpression of Ras leads to irreversible cell arrest.77, 78 In mouse prostatic tumours induced by Pten inactivation, and in human prostatic tumours,79 oncogene-induced senescence is detected before neoplasia develops, but not afterwards when this barrier is overcome.
The most common and consistent molecular marker of oncogene-induced senescence in mouse and man is the enzyme ‘senescence-associated β-galactosidase' (SABG), with cytoplasmic activity detectable at pH 6, contrasting with the lysosomal optimum of pH 4.77, 80 Among the factors regulating this senescence programme, p53 is the best established.71 In mice, many prostatic cells become positive for p53 and SABG during cancer development following Pten inactivation.79
β-galactosidase (β-Gal) with neutral pH activity is constitutively expressed in the caput and corpus epididymidal epithelium of young adult rats and mice.81 It is located in the supranuclear region of principal cells in the initial segment, but not in adjacent caput regions,82 and in the corpus.42 It is not known if this β-Gal is identical to SABG in oncogene-induced senescence. The SABG-encoding gene GLB179 mRNA is found in the human epididymal transcriptome.20 A rat epididymis-specific β-galactosidase-like protein (GLB1L4), absent from the initial segment, has immunoreactivity in the remaining principal cells of the caput,83 but has no enzymatic activity.
The dual role of p53 in apoptosis and senescence makes p53-null mice excellent models for the study of cancers resulting in various tissues.84 Epididymides from such mice exhibit cribriform hyperplasia, which is characterized by intraepithelial protein-filled vacuoles, infrequent apoptosis and rare mitotic figures.84 These changes are not considered neoplastic, but a variant of normal histology that can be seen in aging mice85 and occasionally in the human epididymis.86 Such abnormal intraepithelial vacuoles can be induced in the epithelium of prepubertal, but not adult, mice following deprivation of upstream regulatory luminal factors by ductal ligation.87 It may reflect the presence of an occult cancer.1 The lack of relationship of epididymal p53 with apoptosis (see section on ‘Apoptosis'), the possible role of p53 in senescence, and the constitutive activity of β-Gal in the epididymis support the hypothesis that senescence, induced by the constitutively expressed oncogenes (see section on ‘Oncogene expression in the normal epididymis'), constitutes a persistent barrier to cancer in the epididymis.
Endogenous barriers to tumour formation: immunosurveillance
Upon failure of intrinsic protective mechanisms, when cellular transformation to tumourigenesis occurs, a second line of defence, ‘immunoediting', is triggered. Most cancer-forming cells are immunogenic and routinely eliminated in immunocompetent hosts by this process.88, 89
Eradication of early tumour cells
The molecular signals from early tumour cells that trigger their eradication remain to be identified, but such cell removal involves innate and adaptive immunocompetent cells,89 including macrophages, natural killer (NK) cells and NK T lymphocytes (NKT), dendritic cells, as well as CD4+ and CD8+ T lymphocytes. Leukocytes within the normal epididymal epithelium are considered to block the development of immunity against luminal spermatozoa. In human epididymides from organ donors, no B lymphocytes are found but T cells are numerous, making up around 13% of epithelial cells.90 Of these, the cytotoxic T cells are predominant in the epithelium, suggesting selective recruitment from the interstitium where the much lower cytotoxic/regulatory T-cell ratio reflects that of blood.
In epididymides from young and old men, intraepithelial macrophages are reported, some in direct contact with lymphocytes.91 When young adult rats age, the numbers of CD4+ T cells, CD8+ T cells and macrophages in the epithelium change, leading to the proximal regions having more intraepithelial immunocompetent cells than the distal.92 Although quantitative data are lacking for men, the same may be true, as the cauda epididymidis is the site with the highest tumour incidence: of the 218 cases of epididymal cancer in the CHKD where the site of occurrence was given, 30% were in the caput, 8% in the corpus and 60% in the cauda, with <3% occurring throughout the whole epididymis.
A chemokine secreted by activated T cells to induce movement of immune cells from the bloodstream, RANTES (Ccl5), is expressed at the RNA and protein levels in all cells of the normal mouse epididymis and efferent ducts.93 Its function here is unclear and the authors suggested a reproductive rather than immune role in view of its presence on luminal spermatozoa.
Dendritic cells are antigen-presenting cells mediating communication between the innate and adaptive immune cells. Cells with dendritic phenotype forming a dense network have been described at the base of the mouse epididymal epithelium, sending cytoplasmic extensions between epithelial cells towards the lumen.94 NK and NKT cells, innate immune cells participating in the elimination phase of tumour immunoediting,89 have not been studied in the epididymis, other than in its associated adipose tissue. In contrast to ∼2% of all lymphocytes in blood or lymph nodes being NK or NKT cells, these cells account for ∼12% in inguinal fat and exceed 30% in the epididymal fat pad.95 Although their presence may explain the low cancer rates in adipose tissue, there is no prominent epididymal fat pad in the human, as there is in laboratory rodents, and no attention has been paid to any adipose tissues adhering tightly over the caput and corpus.
In many organs, the innate immune system operates in non-immune epithelial cells. These recognize bacterial, fungal and viral components through membrane-bound and intracellular Toll-like receptors (TLRs), retinoic acid-inducible-gene-1-like receptors and Nod-like receptors, and respond by killing the pathogens or secreting cytokines to attract destructive immune cells. TLR are constitutively present within the rat epididymal epithelium,96 whereas Nod2 is upregulated in the mouse epididymis upon TLR stimulation by lipopolysaccharide.97 The rodent epididymis responds to lipopolysaccharide challenge by upregulation of tumour necrosis factor97 and secretion of interleukins.98 Lipopolysaccharide-binding protein is present within the apex of principal cells of the human epididymis.99
Dormancy of early tumour cells
When the elimination of early tumour cells is incomplete, the surviving cells go through a ‘dormancy' phase when the adaptive immunity of the tissue suppresses tumour outgrowth. This is achieved by CD4+ and CD8+ T cells, interleukin-12, interferon-γ and other factors.89 This equilibrium phase can last as long as the host lives, or the dormant tumour can remain undetected until it escapes suppression upon acquiring the ability to circumvent immune recognition and destruction (see section on ‘Escape of early tumour cells from immunological surveillance').
Evidence that transformed cells experience dormancy in the epididymis is provided by reports of the resistance of the epididymis to tumourigenesis, when other organs succumb, in response to the imposition of tumourigenic challenges, whether they be oncogene activation, downregulation of regulators or overexpression of growth factors: (i) in vivo activation of c-erbB-2 (the human homologue of c-neu) leads to preneoplastic tumours in the kidney and lung, yet only hyperplasia and hypertrophy in the epididymis;100 (ii) activation of c-neu itself,101 and of Notch-related int-3,102 also lead only to epididymal hyperplasia in animals that develop mammary adenocarcinoma; (iii) activation of H-ras produces only epididymal hyperplasia in animals that develop hepatocarcinoma and polycystic kidney disease;103 (iv) constitutive expression of stabilized β-catenin leads to hyperplasia in both epididymis and prostate, but only to transdifferentiation in the latter;104 (v) silencing the p53 gene induces only hyperplasia in the epididymis of mice that develop other tissue cancers;84 (vi) targeting the simian virus 40 large T antigen to the caput epididymidis results in the GPX5Tag-1 line to full tumours in the prostate but only non-malignant hyperplasia and dysplasia of the epididymis;105 (vii) overexpressing transforming growth factor alpha (TGF-α) leads to hyperplasia of p63-positive basal cells in the prostate but not in the epididymis;106 and (viii) overexpressing vascular endothelial growth factor (VEGF) leads to epididymal hyperplasia without malignancy.107
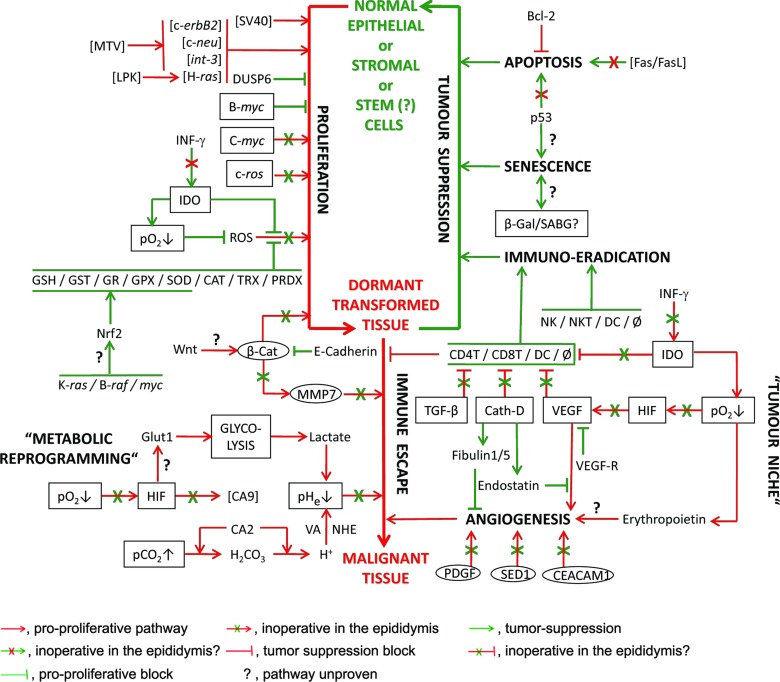
All these experimental models indicate that while the epididymis may succumb to unregulated proliferation, the resulting hyperplastic tissue is prevented from further development into tumours, which could represent a condition of dormancy (Figure 3).
Figure 3.
Representative factors found in the epididymis that are involved in pro-cancer and tumour suppression mechanisms in cancer-prone tissues. Tumours follow transformation of a susceptible cell type to a potentially cancerous form (via proliferation) that can either be suppressed (immune suppression) or remain dormant until conditions allow progression into malignant tissues (by immune escape). Pro-cancer forces are shown in red; anti-cancer in green. Boxed factors are constitutively expressed in the epididymis, but only upregulated in cancer cells upon induction by pro-tumour factors. Circled factors are in the epididymal epithelium but not localized where they are in cancerous tissues. Square brackets enclose factors that have not been proven to be in the epididymis or non-endogenous factors that have been used to drive tumourigenesis. Factors not in parentheses are present in the epididymis as RNA or protein (see text for details). The major causes of tumourigenicity, ROS and oncogene expression, are either heavily suppressed or inoperative, respectively. There is no evidence that the boxed factors elicit the responses that occur in cancer-prone tissues. Even when induction of tumourigenesis is attempted (e.g., by introduction of SV40 or driving exogenous oncogenes by MTV or LPK), no progression beyond epididymal hyperplasia occurs, suggesting that immunosurveillance maintains the tissue in a dormant state. Bcl-2, B-cell lymphoma-2; β-CAT, beta-catenin; β-Gal, beta-galactosidase; CA2,9, carbonic anhydrase 2,9; CAT, catalase; Cath-D, cathepsin D; CD4T/8T, CD4-/8-positive T-lymphocytes; CEACAM1, carcino-embryonic antigen-related cell adhesion molecule; DC, dendritic cells; DUSP6, dual-specificity phosphatise 6; Fas, Fas receptor CD95; FasL, Fas ligand CD95L; Glut1, glucose transporter 1; GPX, glutathione peroxidases; GR, glutathione reductase; GSH, glutathione; GST, glutathione S-transferase; HIF, hypoxia-induced factor; IDO, indoleamine 2,3-dioxygenase; INF-γ, interferon gamma; LPK, L-type pyruvate kinase gene promoter; MMP7, matrix metalloproteinase 7 (matrilysin); MTV, mouse mammary tumour virus long terminal repeat; NHE, sodium-hydrogen exchanger; Nrf2, nuclear factor E2-related factor 2; NK, natural killer cells; NKT, natural killer T-lymphocytes; p53, tumour suppressor protein 53; pCO2↑, high tissue carbon dioxide; PDGF, platelet-derived growth factor; pHe↓, high extra-cellular acidity; pO2↓, low tissue oxygen tension; PRDX, peroxiredoxins; ROS, reactive oxygen species; SABG, senescence-associated beta-galactosidase; SED1, secreted protein 1 with two EGF repeats and discoidin domains; SOD, superoxide dismutase; SV40, Simian virus 40 large T antigen; TGF-β, transforming growth factor beta; TRX, thioredoxin peroxidases; VA, vacuolar ATPase; VEGF, vascular endothelial growth factor; VEGF-R, VEFG receptor; Wnt, Wingless/int pro-tumour protein; φ, macrophage.
Escape of early tumour cells from immunological surveillance
To circumvent immunosurveillance and rejection, tumour cells downregulate expression of classical tumour antigens (HLA class I) and upregulate non-classical (HLA-G) antigens, which prevent stimulation of cytotoxic CD8+ T cells and NK cells. They also induce changes in their microenvironment (e.g., upregulating IDO, VEGF, TGF-β, cathepsin D) that render it hostile to the cells responsible for the immunosurveillance.108 In tumour niches, VEGFs inhibit immune dendritic cells and recruit myeloid-derived suppressor cells that block T-cell function, whereas IDO has a dual action, both removing the amino-acid tryptophan essential for the survival of immune cells, and converting it to products that are toxic to these cells. TGF-β inhibits the proliferation of T cells and NK cells, and suppresses their cytotoxic function against tumour cells.109 Independent of its proteolytic activity, cathepsin D attenuates the effect of anti-tumour cytokines secreted by dendritic cells and is highly expressed in prostate and breast cancer cells.110
The epididymis expresses many of the above factors, employed by tumour cells to evade immunological attack, but a pro-tumour role for them in the epididymis is unlikely. VEGF is constitutively expressed in the normal adult epididymal epithelium of rodents and men (see section on ‘Atypical responses to hypoxia in the epididymis'). TGF-β expression has been reported in the epithelium (TGF-β3) and interstitium (TGF-β1) of the rat epididymis;111 in the marmoset, TGF-β1 is located in apical cells and its receptor in the principal cells.112
In contrast to its interferon-γ-induced expression in most tumourigenic organs, IDO is constitutively expressed in the epididymis113 with an activity far exceeding that of many other organs.114 IDO1 is located in principal cells,114 as well as in basal and apical cells, but not in the interstitium. Enzyme expression is highest in the caput and corpus, but absent from the cauda epididymidis. There is an increased expression of inflammatory markers in the caput region of IDO1-null mice,115 attesting to a role for this enzyme in regulating local immune status. IDO-like proteins (INDOL, IDO2) have been found in man and mouse, with highest activity in the epididymis after the kidney.116
Although TGF-α, VEGF, IDO and cathepsin D, which act against anti-tumour immune cells in cancers, are expressed by the epididymis, immune cells persist there (see section on ‘Eradication of early tumour cells'). This may reflect the absence of necessary coeffectors for attacking anti-tumour immune cells in the healthy organ. Alternatively, the constitutive and persistent, rather than inducible and transient, expression of these factors in the epididymis may lead to the unresponsiveness of the targeted immune cells, via exhaustion or clonal anergy. The retention of active immune cells, keeping check on any neoplastic epididymal cells, would prevent progression to full tumours, consistent with the observations cited in the section ‘Dormancy of early tumour cells'.
Angiogenesis and its endogenous inhibitors in tumourigenesis
Pro-angiogenic factors
Angiogenesis is considered essential for tumour progression and metastasis; the process may be an early tumourigenic event. In healthy adult organs, there is a balance between anti- and pro-angiogenic factors to maintain vasculature stability. The activation of the vasculature in pre-malignant lesions, mainly by overexpression of VEGF by hyperplastic or neoplastic epithelial cells, is characterized by endothelial cell proliferation and sprouting of vessels from pre-existing vessels, resulting in increased microvessel density.117
In the normal human epididymis, VEGF is expressed in basal and principal cells, and in peritubular myoid cells.118 Since neovascularisation is rarely observed, a paracrine role for epididymal VEGF in regulating vessel permeability is possible. In adult rats, VEGF is localized in the principal cells of the whole epididymis.119 Interestingly, in addition to the main VEGF isoforms, the mRNA of the splice variant VEGF144 is detected, which is rarely expressed in adult organs except when they are cancerous.120 In mice, overexpression of VEGF leads to epididymal hyperplasia associated with increased capillary density, fenestrations and permeability.107 Isoforms of another pro-angiogenic factor, platelet-derived growth factor (PDGF) and its receptor121 are present in the epididymis of adult animals, not in the stroma, but in the epithelium,122 a site inconsistent with a vascular role.
The adhesion molecule CEACAM1 has pro-angiogenic properties in endothelial cells123 and can promote cell migration.124 It is expressed in normal human epididymal tissue at the luminal surface of the epithelium.125 The luminal rather than stromal protein location casts doubt on a pro-angiogenic function. Similarly, SED1 in rodents and its homologue Del1 in man, are associated with tumour neovascularisation,126 but both are located in the epididymal epithelium, not stroma.127
By digesting the stromal matrix, matrix metalloproteinases (MMPs) have been considered angiogenic, although they also act as tumour suppressors.128 MMP7 (matrilysin) is a target gene of the Wnt/β-catenin pathway129 (see section on ‘Growth promoters') and is expressed in the efferent ducts and initial segment of mice.130 This unusual presence in epithelial cells suggests that the protein is involved in tissue development rather than tumourigenesis;131 indeed, transgenic mice overexpressing MMP7 in the epididymis exhibit the protein in a structurally normal organ.132 Erythropoietin promotes tumour growth by stimulating angiogenesis.133 It is present in the epididymal interstitium, but whether there is an angiogenic role is uncertain.134
As well as mediating peritubular contractility and sperm passage through the epididymal lumen, endothelins and their receptors (ER) are associated with cancer.135 ER-A is involved in cell proliferation, migration, invasion and angiogenesis, and ER-B in countering tumour progression by promoting apoptosis and binding ET-1.136 Endothelin-1 and these receptors are present in the human epididymal epithelium and peritubular muscle, respectively.137
Anti-angiogenic factors
Many endogenous inhibitors of angiogenesis are fragments of extracellular matrix molecules making up the vascular basement membrane. They are released upon proteolysis of the extracellular matrix by enzymes such as MMPs, cathepsins and elastases,138 and one such proteolytic product is endostatin, the C-terminal fragment of collagen 18. In the human epididymis, collagen 18 is synthesized by the epithelial cells and secreted to form the basement membrane. Endostatin is highly expressed in the epithelial cells, but only weakly expressed in the interstitium of the normal organ, and is absent from human epididymal adenomatoid tissue, except in blood vessels within and around the tumour.139
Cathepsin D degrades endothelial basement membrane into fibulin-1 fragments and fibulin-5, which inhibit angiogenesis and suppress tumour growth.140 In mice, the epididymis has the highest expression of fibulin-1D and fibulin-2, possibly attributable to their interaction with interstitial sex hormone-binding globulin.141 The transcript of fibulin-5, which antagonizes VEGF signalling and inhibits endothelial sprouting, is expressed in the entire epididymis of rats and mice, especially in the corpus region (http://www.mrg.genetics.washington.edu/). Cathepsin D, fibulin-1 and -5 are reported in the human epididymal transcriptome.20
Non-matrix-derived angiogenesis inhibitors, interferons and certain interleukins,138 are present in the rat epididymis,98 human epididymal cysts and spermatocoele fluid.142 Vasostatin, a potent inhibitor of neovascularisation in tumour growth, acting by preventing endothelial cell attachment to laminin, is the N-terminal domain of human calreticulin.138, 143 Although calreticulin is highly expressed in rodent (http://www.mrg.genetics.washington.edu/) and the human epididymis,38 its fragment, vasostatin, is unreported.
Non-endothelial cell VEGF receptor (VEGFR) is considered an inhibitor of angiogenesis by binding to its ligand, thereby preventing VEGF signalling. Whereas VEGF is localized in the principal cells of the whole epididymis, VEGFR1 (FLT-1) is found in the caput and cauda in the rat.119 Human FLT-1 has high expression in the efferent ducts and colocalizes with VEGF in epithelial cells and in luminal macrophages, but in the epididymis proper it is only confined to the interstitial lymph vessels, and non-detectable in the epithelium where VEGF is positive.118 Interstitial FLT-1 is well situated to antagonize any VEGF secreted there. VEGFR2 (KDR) is localized in efferent duct epithelial cells and in basal cells of the epididymal tubule in addition to endothelial cells.
Angiogenesis in epididymal tumours
A rare quantitative study of the angiogenic status of epididymal adenomatoid tissues revealed blood vessel densities similar to, or less than, those of normal tissues125 and almost the whole tumour tissue was negative for the pro-angiogenic factor CEACAM1, in contrast to the upregulation of endothelial expression reported in cancers of other organs such as the prostate. Whether poor angiogenesis is a common feature of epididymal tumours awaits more evidence; nevertheless, it may provide one explanation of the rarity of epididymal tumours.
The metabolic characteristics of cancer cells
A hallmark of cancer cells is their switch to enhanced glycolysis under aerobic conditions, which generates energy and intermediate metabolites needed for tumour growth and proliferation.144 This is effected through the signalling of oncogenes, including AKT kinase that upregulates hypoxia-induced factors (HIF-1α and HIF-1β), which in turn upregulate glycolysis. Under normal oxygen tension, HIF-1α is degraded after polyubiquitination by the VBC complex; in hypoxia, the VBC complex dissociates, allowing intact HIF-1α to interact with the constitutively present HIF-1β/ArNT and become an active dimer. Unless hypoxia sets in, the VBC complex keeps HIF inactive and suppresses both angiogenesis145 and the upregulation of glycolysis, which would favour tumour cell metabolism.88
Active HIFs enable normal cells to adapt to hypoxic conditions, by binding to sites in the promoter regions of genes involved in energy metabolism, angiogenesis, stress and O2 delivery. In cancers, HIF upregulates the glucose transporter GLUT-1 which provides glucose to the tissue, carbonic anhydrase 9 which produces carbonic acid from respiratory carbon dioxide146 and VEGF which stimulates angiogenesis and thereby improves blood flow.147, 148 The combination of low oxygen tension, increased aerobic glycolysis and the production of extracellular carbonic and lactic acids causes a reduction in extracellular pH, which selects and retains cancer cells in the tumour niche.149 Proton export by the tumour cells raises their intracellular pH, which is an effective proliferative trigger.150
Atypical responses to hypoxia in the epididymis
VHL, a protein component of the VBC complex, is encoded by a cancer suppressor gene, since its mutation in patients with von Hippel–Lindau (VHL) disease is responsible for a certain group of tumours found at various sites including the epididymis.4 The multifocal epithelial tumourlets in the epididymis associated with VHL disease are confined to the efferent ducts,146, 148 which comprise much of the head of the human epididymis151 (Figure 1b). Thus, even with a non-functional VHL gene, only the efferent ducts develop non-malignant tumourlets, and the epididymis proper is even more resistant to tumourigenesis. Upregulation of HIF1, HIF2, VEGF, GLUT1 and CAIX (CA9) occurs in human efferent duct cystadenomatous tissue.146, 148
Whereas VHL expression in the mouse epididymis is marginal, elongin B (Tceb2, the second component of the VBC complex) expression is distinctively positive (http://www.mrg.genetics.washington.edu/), and cullin 2, the third component of the VBC complex, is absent. The presence of a VBC complex in the epididymis is thus doubtful; its absence would permit constitutive expression of intact HIF-1α and render the epididymis unresponsive to changes in local oxygen tension. Indeed, the transcription of HIF-1α in normoxic rats does occur in all regions of the epididymis, to an extent similar to that in the liver and kidney,152 with less in the caput than the cauda.153 In contrast to the ischemic testis, where HIF protein increases over that of the normoxic, neither caput nor cauda HIF content changes in experimental ischemia in vivo in rats153 or mice.154 The pro-angiogenic erythropoietin is, however, upregulated by acute hypoxia in the mouse epididymis.132
Constitutively-expressed HIF protein in the epididymis should enhance glycolysis under normal situations, rather than in hypoxia as occurs in other tissues. The epididymis does glycolyse aerobically and the rate does not increase under anaerobic conditions—it secretes lactate under both conditions.155 A target gene of HIF, Glut1 (Slc2a1), is especially expressed in the rodent cauda epididymidis (http://www.mrg.genetics.washington.edu/), and Glut1 mRNA is found in the human epididymis.20 Another target of HIF, CA9, is not present in the rat epididymis.156
Epididymal fluid has a higher osmolality, lower ionic strength, lower Na+/K+ ratio than blood plasma,157 a low pH,158, 159 low pO2,34 and high pCO2.159 Many of these characteristics mirror those of the tumourigenic niche.149 Whereas such a niche promotes tumours in neoplastic cells in other organs, epididymal cells normally survive in this environment.
Summary
The privileged position of the human epididymis in evading tumourigenicity, confirmed in transgenic mouse models, appears to reflect a continuum of prevention, suppression and lowered sensitivity to otherwise tumourigenic insults. These may combine to create: (i) a low chance of cells becoming cancerous (no stem cells, strong anti-oxidative mechanisms, active tumour suppressors, and inactive oncogene products) with (ii) immune eradication and senescence of any affected cells (strong immunosurveillance that restricts hyperplastic cells to dormancy), and (iii) blockade of mechanisms favouring metaplasia (persistent tight junctions, presence of anti-angiogenic factors, misplaced pro-angiogenic factors and inoperative immune escape) (Figure 3). In response to the constitutive expression of normally inducible pro-proliferative factors, and an environment mimicking that of the tumour niche, epididymal cells may have evolved to be less responsive than others to tumourigenic stimuli, thereby raising the threshold for tumour initiation above that of other organs. As an anti-oxidative and immune-suppressive environment is also what maintains high numbers of spermatozoa quiescent and immunologically silent, the low incidence of cancer in the epididymis may be a direct consequence of its role in sperm maturation and storage. Such hypotheses are open to experimentation, but they may not explain the similarly low incidence of tumours in the seminal vesicles160 (Table 1), which also deserves study. Further investigation into the regulation of the epididymal anti-tumourigenic activity may well offer insights that can be used in the prevention or treatment of cancers in general.
Author contributions
CHY initiated and designed the study, collected and analysed data, planned and wrote the review. KW searched and analysed the Chinese databases and wrote parts of the review. TGC initiated and designed the study, planned and wrote the review.
Acknowledgments
We thank Ning Li (Central Laboratory, YuHuangDing hospital, Shandong, China) for assistance with preparing the micrographs, Yan Wang (School of Pharmacy, Yantai University, Shandong, China) for help with the Chinese cancer database screening, Professor Jianyuan Li (Shandong Stem Cell Engineering and Technology Research Centre, YuHuangDing hospital, Shandong, China) for his encouragement and support for this work, and anonymous referees for helpful suggestions.
The authors have no competing financial interests.
References
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–9. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MA, Young RH, Scully RE. Adenocarcinoma of the epididymis: a report of four cases and review of the literature. Am J Surg Pathol. 1997;21:1474–80. doi: 10.1097/00000478-199712000-00010. [DOI] [PubMed] [Google Scholar]
- Ganem JP, Jhaveri FM, Marroum MC. Primary adenocarcinoma of the epididymis: case report and review of the literature. Urology. 1998;52:904–8. doi: 10.1016/s0090-4295(98)00275-1. [DOI] [PubMed] [Google Scholar]
- Odrzywolski KJ, Mukhopadhyay S. Papillary cystadenoma of the epididymis. Arch Pathol Lab Med. 2010;134:630–3. doi: 10.5858/134.4.630. [DOI] [PubMed] [Google Scholar]
- Robaire B, Hinton BT.eds). The Epididymis. From Molecules to Clinical Practice A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens New York; Kluwer Academic/Plenum Publishers; 2002 [Google Scholar]
- Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–22. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Trosko JE. From adult stem cells to cancer stem cells: Oct-4 gene, cell–cell communication, and hormones during tumor promotion. Ann NY Acad Sci. 2006;1089:36–58. doi: 10.1196/annals.1386.018. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Nielsen JE, Kalisz M, Dalgaard MD, Audouze K, et al. OCT4 and downstream factors are expressed in human somatic urogenital epithelia and in culture of epididymal spheres. Mol Hum Reprod. 2010;16:835–45. doi: 10.1093/molehr/gaq008. [DOI] [PubMed] [Google Scholar]
- Godmann M, Kosan C, Behr R. Kruppel-like factor 4 is widely expressed in the mouse male and female reproductive tract and responds as an immediate early gene to activation of the protein kinase A in TM4 Sertoli cells. Reproduction. 2010;139:771–82. doi: 10.1530/REP-09-0531. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yoshinaga A, Ohno R, Ishii N, Kamata S, et al. Expression of the p63 and Notch signaling systems in rat testes during postnatal development: comparison with their expression levels in the epididymis and vas deferens. J Androl. 2004;25:692–8. doi: 10.1002/j.1939-4640.2004.tb02843.x. [DOI] [PubMed] [Google Scholar]
- Lupien M, Dievart A, Morales CR, Hermo L, Calvo E, et al. Expression of constitutively active Notch1 in male genital tracts results in ectopic growth and blockage of efferent ducts, epididymal hyperplasia and sterility. Dev Biol. 2006;300:497–511. doi: 10.1016/j.ydbio.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Romagnani P. Toward the identification of a ‘renopoietic system'. Stem Cells. 2009;27:2247–53. doi: 10.1002/stem.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Moll R, Hesse U, Prasad AR, Gandolfi JA, et al. Identification of a stem cell candidate in the normal human prostate gland. Eur J Cell Biol. 2005;84:341–54. doi: 10.1016/j.ejcb.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhagon MA, Hurt EM, Sotelo-Silveira JR, Zhang X, Farrar WL. Genomic profiling of tumor initiating prostatospheres. BMC Genomics. 2010;11:324–40. doi: 10.1186/1471-2164-11-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew IJ, Minola A, Georgiev S, Hitz M, Moch H, et al. Combined VHLH and PTEN mutation causes genital tract cystadenoma and squamous metaplasia. Mol Cell Biol. 2008;28:4536–48. doi: 10.1128/MCB.02132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnam M, Goodrich DW. RB1, development, and cancer. Curr Top Dev Biol. 2011;94:129–69. doi: 10.1016/B978-0-12-380916-2.00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen ES, Wang JY. Targeting the RB-pathway in cancer therapy. Clin Cancer Res. 2010;16:1094–9. doi: 10.1158/1078-0432.CCR-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, et al. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–13. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- Jelinsky SA, Turner TT, Bang HJ, Finger JN, Solarz MK, et al. The rat epididymal transcriptome: comparison of segmental gene expression in the rat and mouse epididymides. Biol Reprod. 2007;76:561–70. doi: 10.1095/biolreprod.106.057323. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Liu Q, Li YM, Hall SH, French FS, et al. Genome-wide profiling of segmental-regulated transcriptomes in human epididymis using oligo microarray. Mol Cell Endocrinol. 2006;250:169–77. doi: 10.1016/j.mce.2005.12.041. http://www.humanet.scbit.org/spatial.jsp [DOI] [PubMed] [Google Scholar]
- Xu B, Yang L, Lye RJ, Hinton BT. p-MAPK1/3 and DUSP6 regulate epididymal cell proliferation and survival in a region-specific manner in mice. Biol Reprod. 2010;83:807–17. doi: 10.1095/biolreprod.110.085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kobayashi S, Borczuk AC, Leidner RS, Laframboise T, et al. Dual specificity phosphatase 6 (DUSP6) is an ETS-regulated negative feedback mediator of oncogenic ERK signaling in lung cancer cells. Carcinogenesis. 2010;31:577–86. doi: 10.1093/carcin/bgq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujarit S, Jones RC. [3H]thymidine uptake by the epididymis, seminal vesicles and prostate gland during postnatal development of the rat. Reprod Fertil Dev. 1991;3:313–9. doi: 10.1071/rd9910313. [DOI] [PubMed] [Google Scholar]
- Sun EL, Flickinger CJ. Proliferative activity in the rat epididymis during postnatal development. Anat Rec. 1982;203:273–84. doi: 10.1002/ar.1092030209. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Flannery J. Mitotic activity in the epithelium of the epididymis in young and old adult rats. Biol Reprod. 1970;3:283–92. doi: 10.1093/biolreprod/3.3.283. [DOI] [PubMed] [Google Scholar]
- Berges RR, Furuya Y, Remington L, English HF, Jacks T, et al. Cell proliferation, DNA repair, and p53 function are not required for programmed death of prostatic glandular cells induced by androgen ablation. Proc Natl Acad Sci USA. 1993;90:8910–4. doi: 10.1073/pnas.90.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero OM, Dawson DW, Moon RT, Chien AJ. A re-evaluation of the ‘oncogenic' nature of Wnt/beta-catenin signaling in melanoma and other cancers. Curr Oncol Rep. 2010;12:314–8. doi: 10.1007/s11912-010-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBellefeuille S, Hermo L, Gregory M, Dufresne J, Cyr DG. Catenins in the rat epididymis: their expression and regulation in adulthood and during postnatal development. Endocrinology. 2003;144:5040–9. doi: 10.1210/en.2002-0139. [DOI] [PubMed] [Google Scholar]
- Marín-Briggiler CI, Veiga MF, Matos ML, Echeverría MF, Furlong LI, et al. Expression of epithelial cadherin in the human male reproductive tract and gametes and evidence of its participation in fertilization. Mol Hum Reprod. 2008;14:561–71. doi: 10.1093/molehr/gan053. [DOI] [PubMed] [Google Scholar]
- Kharaishvili G, Simkova D, Makharoblidze E, Trtkova K, Kolar Z, et al . Wnt signaling in prostate development and carcinogenesis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:11–8. doi: 10.5507/bp.2011.016. [DOI] [PubMed] [Google Scholar]
- Deshpande SN, Vijayakumar G, Rao AJ. Oestrogenic regulation and differential expression of WNT4 in the bonnet monkey and rodent epididymis. Reprod Biomed Online. 2009;18:555–61. doi: 10.1016/s1472-6483(10)60134-4. [DOI] [PubMed] [Google Scholar]
- Cross BA, Silver IA. Neurovascular control of oxygen tension in the testis and epididymis. J Reprod Fertil. 1962;3:377–95. doi: 10.1530/jrf.0.0030377. [DOI] [PubMed] [Google Scholar]
- Free MJ, Schluntz GA, Jaffe RA. Respiratory gas tensions in tissues and fluids of the male rat reproductive tract. Biol Reprod. 1976;14:481–8. doi: 10.1095/biolreprod14.4.481. [DOI] [PubMed] [Google Scholar]
- Werner ER, Werner-Felmayer G. Substrate and cofactor requirements of indoleamine 2,3-dioxygenase in interferon-gamma-treated cells: utilization of oxygen rather than superoxide. Curr Drug Metab. 2007;8:201–3. doi: 10.2174/138920007780362482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch RN, Armstrong VL, Clulow J, Jones RC. Relationship between motility and oxygen consumption of sperm from the cauda epididymides of the rat. Reprod Fertil Dev. 1999;11:87–94. doi: 10.1071/rd99039. [DOI] [PubMed] [Google Scholar]
- Noblanc A, Kocer A, Chabory E, Vernet P, Saez F, et al. Glutathione peroxidases at work on epididymal spermatozoa: an example of the dual effect of reactive oxygen species on Mammalian male fertilizing ability. J Androl. 2011;32:641–50. doi: 10.2164/jandrol.110.012823. [DOI] [PubMed] [Google Scholar]
- Li JY, Liu FJ, Wang HY, Liu X, Liu J, et al. Systematic mapping and functional analysis of a family of human epididymal secretory sperm-located proteins. Mol Cell Proteom. 2010;9:2517–28. doi: 10.1074/mcp.M110.001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu F, Liu X, Liu J, Zhu P, et al. Mapping of the human testicular proteome and its relationship with that of the epididymis and spermatozoa. Mol Cell Proteom. 2011;10:M110.004630. doi: 10.1074/mcp.M110.004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta. 2009;1795:37–52. doi: 10.1016/j.bbcan.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Sonnenberg-Riethmacher E, Walter B, Riethmacher D, Godecke S, Birchmeier C. The c-ros tyrosine kinase receptor controls regionalization and differentiation of epithelial cells in the epididymis. Genes Dev. 1996;10:1184–93. doi: 10.1101/gad.10.10.1184. [DOI] [PubMed] [Google Scholar]
- Legare C, Sullivan R. Expression and localization of c-ros oncogene along the human excurrent duct. Mol Hum Reprod. 2004;10:697–703. doi: 10.1093/molehr/gah087. [DOI] [PubMed] [Google Scholar]
- Winer MA, Wadewitz AG, Wolgemuth DJ. Members of the raf gene family exhibit segment-specific patterns of expression in mouse epididymis. Mol Reprod Dev. 1993;35:16–23. doi: 10.1002/mrd.1080350104. [DOI] [PubMed] [Google Scholar]
- Hsia N, Cornwall GA. DNA microarray analysis of region-specific gene expression in the mouse epididymis. Biol Reprod. 2004;70:448–57. doi: 10.1095/biolreprod.103.021493. [DOI] [PubMed] [Google Scholar]
- Gregory MA, Xiao Q, Cornwall GA, Lutterbach B, Hann SR. B-Myc is preferentially expressed in hormonally-controlled tissues and inhibits cellular proliferation. Oncogene. 2000;19:4886–95. doi: 10.1038/sj.onc.1203851. [DOI] [PubMed] [Google Scholar]
- Berger AH, Knudson AG, Pandolfi PP. A continuum model for tumour suppression. Nature. 2011;476:163–9. doi: 10.1038/nature10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortes MM, et al. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic Biol Med. 2010;49:1368–79. doi: 10.1016/j.freeradbiomed.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer MA, Wolgemuth DJ. The segment-specific pattern of A-raf expression in the mouse epididymis is regulated by testicular factors. Endocrinology. 1995;136:2561–72. doi: 10.1210/endo.136.6.7750478. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Collis R, Xiao Q, Hsia N, Hann SR. B-Myc, a proximal caput epididymal protein, is dependent on androgens and testicular factors for expression. Biol Reprod. 2001;64:1600–7. doi: 10.1095/biolreprod64.6.1600. [DOI] [PubMed] [Google Scholar]
- Ma L, Li W, Zhu HP, Li Z, Sun ZJ, et al. Localization and androgen regulation of metastasis-associated protein 1 in mouse epididymis. PLoS One. 2010;5:e15439. doi: 10.1371/journal.pone.0015439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff C. Molecular characterization of epididymal proteins. Reproduction. 1998;3:86–95. doi: 10.1530/ror.0.0030086. [DOI] [PubMed] [Google Scholar]
- Galgano MT, Hampton GM, Frierson HF. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19:847–53. doi: 10.1038/modpathol.3800612. [DOI] [PubMed] [Google Scholar]
- Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010;2:a002907. doi: 10.1101/cshperspect.a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K, Troy TC. Junctions gone bad: claudins and loss of the barrier in cancer. Biochim Biophys Acta. 2011;1816:73–9. doi: 10.1016/j.bbcan.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Ouban A, Ahmed AA. Claudins in human cancer: a review. Histol Histopathol. 2010;25:83–90. doi: 10.14670/HH-25.83. [DOI] [PubMed] [Google Scholar]
- Feigin ME, Muthuswamy SK. Polarity proteins regulate mammalian cell–cell junctions and cancer pathogenesis. Curr Opin Cell Biol. 2009;21:694–700. doi: 10.1016/j.ceb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Tight junctions in the testis: new perspectives. Philos Trans R Soc Lond B Biol Sci. 2010;365:1621–35. doi: 10.1098/rstb.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital P, Hinton BT, Dufour JM. The blood–testis and blood–epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–8. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé E, Dufresne J, Chan PT, Hermo L, Cyr DG. Assessing the role of claudins in maintaining the integrity of epididymal tight junctions using novel human epididymal cell lines. Biol Reprod. 2010;82:1119–28. doi: 10.1095/biolreprod.109.083196. [DOI] [PubMed] [Google Scholar]
- Pelletier RM. Blood barriers of the epididymis and vas deferens act asynchronously with the blood barrier of the testis in the mink (Mustela vison) Microsc Res Techniq. 1994;27:333–49. doi: 10.1002/jemt.1070270408. [DOI] [PubMed] [Google Scholar]
- Pelletier RM. Freeze-fracture study of cell junctions in the epididymis and vas deferens of a seasonal breeder; the mink (Mustela vison) Microsc Res Techniq. 1995;30:37–53. doi: 10.1002/jemt.1070300104. [DOI] [PubMed] [Google Scholar]
- Crichton EG, Suzuki F, Krutzsch PH, Hammerstedt RH. Unique features of the cauda epididymidal epithelium of hibernating bats may promote sperm longevity. Anat Rec. 1993;237:475–81. doi: 10.1002/ar.1092370406. [DOI] [PubMed] [Google Scholar]
- Dubé E, Hermo L, Chan PT, Cyr DG. Alterations in gene expression in the caput epididymides of nonobstructive azoospermic men. Biol Reprod. 2008;78:342–51. doi: 10.1095/biolreprod.107.062760. [DOI] [PubMed] [Google Scholar]
- Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–17. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drivalos A, Papatsoris AG, Chrisofos M, Efstathiou E, Dimopoulos MA. The role of the cell adhesion molecules (integrins/cadherins) in prostate cancer. Int Braz J Urol. 2011;37:302–6. doi: 10.1590/s1677-55382011000300002. [DOI] [PubMed] [Google Scholar]
- Naus CC, Laird DW. Implications and challenges of connexin connections to cancer. Nat Rev Cancer. 2010;10:435–41. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- Pointis G, Fiorini C, Defamie N, Segretain D. Gap junctional communication in the male reproductive system. Biochim Biophys Acta. 2005;1719:102–16. doi: 10.1016/j.bbamem.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Dufresne J, Finnson KW, Gregory M, Cyr DG. Expression of multiple connexins in the rat epididymis indicates a complex regulation of gap junctional communication. Am J Physiol Cell Physiol. 2003;284:C33–43. doi: 10.1152/ajpcell.00111.2002. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Hermo L, Laird DW. Immunocytochemical localization and regulation of connexin43 in the adult rat epididymis. Endocrinology . 1996;137:1474–84. doi: 10.1210/endo.137.4.8625926. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Fan X, Robaire B. Orchidectomy induces a wave of apoptotic cell death in the epididymis. Endocrinology. 1998;139:2128–36. doi: 10.1210/endo.139.4.5888. [DOI] [PubMed] [Google Scholar]
- Turner TT, Riley TA. p53 independent, region-specific epithelial apoptosis is induced in the rat epididymis by deprivation of luminal factors. Mol Reprod Dev. 1999;53:188–97. doi: 10.1002/(SICI)1098-2795(199906)53:2<188::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Matsuzawa A, Iguchi T. Down regulation of Bcl-2 is the first step on Fas-mediated apoptosis of male reproductive tract. Oncogene. 1996;13:31–7. [PubMed] [Google Scholar]
- Jara M, Esponda P, Carballada R. Abdominal temperature induces region-specific p53-independent apoptosis in the cauda epididymidis of the mouse. Biol Reprod. 2002;67:1189–96. doi: 10.1095/biolreprod67.4.1189. [DOI] [PubMed] [Google Scholar]
- Sugihara A, Yamada N, Tsujimura T, Iwasaki T, Yamashita K, et al. Castration induces apoptosis in the male accessory sex organs of Fas-deficient lpr and Fas ligand-deficient gld mutant mice. In Vivo. 2001;15:385–90. [PubMed] [Google Scholar]
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–7. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Murga M, Martinez-Pastor B, Ortega-Molina A, Soria R, et al. Limited role of murine ATM in oncogene-induced senescence and p53-dependent tumor suppression. PLoS One. 2009;4:e5475. doi: 10.1371/journal.pone.0005475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–79. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TT, Bomgardner D, Jacobs JP, Nguyen QA. Association of segmentation of the epididymal interstitium with segmented tubule function in rats and mice. Reproduction. 2003;125:871–8. doi: 10.1530/rep.0.1250871. [DOI] [PubMed] [Google Scholar]
- Avram CE, Cooper TG. Development of the caput epididymidis studied by expressed proteins (a glutamate transporter, a lipocalin and beta-galactosidase) in the c-ros knockout and wild-type mice with prepubertally ligated efferent ducts. Cell Tissue Res. 2004;317:23–34. doi: 10.1007/s00441-004-0892-8. [DOI] [PubMed] [Google Scholar]
- Zhen W, Li P, He B, Guo J, Zhang YL. The novel epididymis-specific beta-galactosidase-like gene Glb1l4 is essential in epididymal development and sperm maturation in rats. Biol Reprod. 2009;80:696–706. doi: 10.1095/biolreprod.108.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Perle KM, Blomme EA, Sagartz JE, Capen CC. Epididymal cribriform hyperplasia with nuclear atypia in p53 homozygous knockout mice on a mixed 129/Sv-FVB/N background. Comp Med. 2002;52:568–71. [PubMed] [Google Scholar]
- Haines DC, Chattopadhyay S, Ward JM. Pathology of aging B6:129 mice. Toxicol Pathol. 2001;29:653–61. doi: 10.1080/019262301753385988. [DOI] [PubMed] [Google Scholar]
- Nistal M, Iniguez L, Paniagua R. Pitted pattern in the human epididymis. J Reprod Fertil. 1990;89:655–61. doi: 10.1530/jrf.0.0890655. [DOI] [PubMed] [Google Scholar]
- Abe K, Takano H, Ito T. Interruption of the luminal flow in the epididymal duct of the corpus epididymidis in the mouse, with special reference to differentiation of the epididymal epithelium. Arch Histol Jpn. 1984;47:137–47. doi: 10.1679/aohc.47.137. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- El-Demiry MI, Hargreave TB, Busuttil A, James K, Ritchie AW, et al. Lymphocyte sub-populations in the male genital tract. Br J Urol. 1985;57:769–74. doi: 10.1111/j.1464-410x.1985.tb07051.x. [DOI] [PubMed] [Google Scholar]
- Wang YF, Holstein AF. Intraepithelial lymphocytes and macrophages in the human epididymis. Cell Tissue Res. 1983;233:517–21. doi: 10.1007/BF00212221. [DOI] [PubMed] [Google Scholar]
- Serre V, Robaire B. Distribution of immune cells in the epididymis of the aging Brown Norway rat is segment-specific and related to the luminal content. Biol Reprod. 1999;61:705–14. doi: 10.1095/biolreprod61.3.705. [DOI] [PubMed] [Google Scholar]
- Li Z, Sun ZJ, Liao CG, Ma L, Ma BF, et al. Regulated upon activation normal T-cell expressed and secreted originating from the epididymis differentially associates with viable and defective spermatozoa. Fertil Steril. 2010;93:2661–7. doi: 10.1016/j.fertnstert.2010.01.053. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, et al. A dense network of dendritic cells populates the murine epididymis. Reproduction. 2011;141:653–63. doi: 10.1530/REP-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, et al. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579:3487–92. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Immunophysiology and pathology of inflammation of the testis and epididymis. J Androl. 2011;32:625–40. doi: 10.2164/jandrol.111.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlbauer M, Cheely AW, Yenugu S, Jobin C. Regulation and functional impact of lipopolysaccharide induced Nod2 gene expression in the murine epididymal epithelial cell line PC1. Immunology. 2008;124:256–64. doi: 10.1111/j.1365-2567.2007.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YT, Guo JH, Wu ZL, Xiong Y, Zhou WL. Innate immune responses of epididymal epithelial cells to Staphylococcus aureus infection. Immunol Lett. 2008;119:84–90. doi: 10.1016/j.imlet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Malm J, Nordahl EA, Bjartell A, Sørensen OE, Frohm B, et al. Lipopolysaccharide -binding protein is produced in the epididymis and associated with spermatozoa and prostasomes. J Reprod Immunol. 2005;66:33–43. doi: 10.1016/j.jri.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Stöcklin E, Botteri F, Groner B. An activated allele of the c-erbB-2 oncogene impairs kidney and lung function and causes early death of transgenic mice. J Cell Biol. 1993;122:199–208. doi: 10.1083/jcb.122.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Activated neu induces rapid tumor progression. J Biol Chem. 1996;271:7673–8. doi: 10.1074/jbc.271.13.7673. [DOI] [PubMed] [Google Scholar]
- Jhappan C, Gallahan D, Stahle C, Chu E, Smith GH, et al. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 1992;6:345–55. doi: 10.1101/gad.6.3.345. [DOI] [PubMed] [Google Scholar]
- Gilbert E, Morel A, Tulliez M, Maunoury R, Terzi F, et al. In vivo effects of activated H-ras oncogene expressed in the liver and in urogenital tissues. Int J Cancer. 1997;73:749–56. doi: 10.1002/(sici)1097-0215(19971127)73:5<749::aid-ijc23>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, et al. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22:3875–87. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- Sipilä P, Cooper TG, Yeung CH, Mustonen M, Penttinen J, et al. Epididymal dysfunction initiated by the expression of simian virus 40 T-antigen leads to angulated sperm flagella and infertility in transgenic mice. Mol Endocrinol. 2002;16:2603–17. doi: 10.1210/me.2002-0100. [DOI] [PubMed] [Google Scholar]
- Yoshio Y, Ishii K, Arase S, Hori Y, Nishikawa K, et al. Effect of transforming growth factor alpha over-expression on urogenital organ development in mouse. Differentiation. 2010;80:82–8. doi: 10.1016/j.diff.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Korpelainen EI, Karkkainen MJ, Tenhunen A, Lakso M, Rauvala H, et al. Over-expression of VEGF in testis and epididymis causes infertility in transgenic mice: evidence for non-endothelial targets for VEGF. J Cell Biol. 1998;143:1705–12. doi: 10.1083/jcb.143.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Katunuma N. Involvement of cathepsins in the invasion, metastasis and proliferation of cancer cells. J Med Invest. 2005;52:1–9. doi: 10.2152/jmi.52.1. [DOI] [PubMed] [Google Scholar]
- Tomsig JL, Turner TT. Growth factors and the epididymis. J Androl. 2006;27:348–57. doi: 10.2164/jandrol.05182. [DOI] [PubMed] [Google Scholar]
- Bomgardner D, Wehrenberg U, Rune GM. TGF-β could be involved in paracrine actions in the epididymis of the marmoset monkey (Callithrix jacchus) J Androl. 1999;20:375–83. [PubMed] [Google Scholar]
- Hansen AM, Ball HJ, Mitchell AJ, Miu J, Takikawa O, et al. Increased expression of indoleamine 2,3-dioxygenase in murine malaria infection is predominantly localised to the vascular endothelium. Int J Parasitol. 2004;34:1309–19. doi: 10.1016/j.ijpara.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Dai X, Zhu BT. Indoleamine 2,3-dioxygenase tissue distribution and cellular localization in mice: implications for its biological functions. J Histochem Cytochem. 2010;58:17–28. doi: 10.1369/jhc.2009.953604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jrad-Lamine A, Henry-Berger J, Gourbeyre P, Damon-Soubeyrand C, Lenoir A, et al. Deficient tryptophan catabolism along the kynurenine pathway reveals that the epididymis is in a unique tolerogenic state. J Biol Chem. 2011;286:8030–42. doi: 10.1074/jbc.M110.172114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–13. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Raica M, Cimpean AM, Ribatti D. Angiogenesis in pre-malignant conditions. Eur J Cancer. 2009;45:1924–34. doi: 10.1016/j.ejca.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Ergün S, Luttmer W, Fiedler W, Holstein AF. Functional expression and localization of vascular endothelial growth factor and its receptors in the human epididymis. Biol Reprod. 1998;58:160–8. doi: 10.1095/biolreprod58.1.160. [DOI] [PubMed] [Google Scholar]
- Ai QY, Tian H, Zhang J, Ma L, Miao NZ, et al. Expressions of VEGF and Flt-1 in the testis, epididymis and epididymal sperm of adolescent rats. Zhonghua Nan Ke Xue. 2008;14:871–5. Chinese. [PubMed] [Google Scholar]
- Lun SW.Vascular endothelial growth factors (VEGF) and VEGF receptor expression and localization in the rat epididymisMPhil thesis, Chinese University of Hong Kong, China, 2003
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basciani S, Mariani S, Arizzi M, Brama M, Ricci A, et al. Expression of platelet-derived growth factor (PDGF) in the epididymis and analysis of the epididymal development in PDGF-A, PDGF-B, and PDGF receptor beta deficient mice. Biol Reprod. 2004;70:168–77. doi: 10.1095/biolreprod.103.019232. [DOI] [PubMed] [Google Scholar]
- Ergün S, Kilik N, Ziegeler G, Hansen A, Nollau P, et al. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell. 2000;5:311–20. doi: 10.1016/s1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- Moh MC, Shen S. The roles of cell adhesion molecules in tumour suppression and cell migration. A new paradox. Cell Adh Migr. 2009;3:334–6. doi: 10.4161/cam.3.4.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic N, Tilki D, Ergün B, Seitz M, Stief CG, et al. Epithelial versus endothelial CEACAM1 expression and angiogenesis in epididymal adenomatoid tumor. Anticancer Res. 2010;30:2651–7. [PubMed] [Google Scholar]
- Raymond A, Ensslin MA, Shur BD. SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem. 2009;106:957–66. doi: 10.1002/jcb.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AS, Shur BD. A novel role for SED1 (MFG-E8) in maintaining the integrity of the epididymal epithelium. J Cell Sci. 2009;122:849–58. doi: 10.1242/jcs.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decock J, Thirkettle S, Wagstaff L, Edwards DR. Matrix metalloproteinases: protective roles in cancer. J Cell Mol Med. 2011;15:1254–65. doi: 10.1111/j.1582-4934.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–91. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Heppner KJ, Rudolph LA, Matrisian LM. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell. 1995;6:851–69. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28:123–36. doi: 10.1016/1357-2725(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Rudolph-Owen LA, Cannon P, Matrisian LM. Over-expression of the matrix metalloproteinase matrilysin results in premature mammary gland differentiation and male infertility. Mol Biol Cell. 1998;9:421–35. doi: 10.1091/mbc.9.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis. 2003;24:1021–9. doi: 10.1093/carcin/bgg060. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Yanase H, Iwanaga T, Sasaki R, Nagao M. Epididymis is a novel site of erythropoietin production in mouse reproductive organs. Biochem Biophys Res Commun. 2002;296:145–51. doi: 10.1016/s0006-291x(02)00832-x. [DOI] [PubMed] [Google Scholar]
- Wang R, Dashwood RH. Endothelins and their receptors in cancer: identification of therapeutic targets. Pharmacol Res. 2011;63:519–24. doi: 10.1016/j.phrs.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato A, Loizidou M, Pflug BR, Curwen J, Growcott J. Role of the endothelin axis and its antagonists in the treatment of cancer. Br J Pharmacol. 2011;163:220–33. doi: 10.1111/j.1476-5381.2011.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri A, Fantoni G, Granchi S, Vannelli GB, Barni T, et al. Endothelin-1 is synthesized and biologically active in human epididymis via a paracrine mode of action. Steroids. 1998;63:294–8. doi: 10.1016/s0039-128x(98)00036-1. [DOI] [PubMed] [Google Scholar]
- Ribatti D. Endogenous inhibitors of angiogenesis: a historical review. Leuk Res. 2009;33:638–44. doi: 10.1016/j.leukres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Tilki D, Kilic N, Herbst H, Reich O, Seitz M, et al. High level of endostatin in epididymal epithelium: protection against primary malignancies in this organ. Histochem Cell Biol. 2008;130:527–35. doi: 10.1007/s00418-008-0440-9. [DOI] [PubMed] [Google Scholar]
- Xie L, Palmsten K, MacDonald B, Kieran MW, Potenta S, et al. Basement membrane derived fibulin-1 and fibulin-5 function as angiogenesis inhibitors and suppress tumor growth. Exp Biol Med. 2008;233:155–62. doi: 10.3181/0706-RM-167. [DOI] [PubMed] [Google Scholar]
- Ng KM, Catalano MG, Pinos T, Selva DM, Avvakumov GV, et al. Evidence that fibulin family members contribute to the steroid-dependent extravascular sequestration of sex hormone-binding globulin. J Biol Chem. 2006;281:15853–61. doi: 10.1074/jbc.M512370200. [DOI] [PubMed] [Google Scholar]
- Kocak I, Dundar M, Yenisey C, Serter M, Gunaydin G. Pro-inflammatory cytokine response of the fluid contents of spermatoceles and epididymal cysts. Andrologia. 2002;34:112–5. doi: 10.1046/j.0303-4569.2001.00486.x. [DOI] [PubMed] [Google Scholar]
- Bee YS, Sheu SJ, Ma YL, Lin HC, Weng WT, et al. Topical application of recombinant calreticulin peptide, vasostatin 48, alleviates laser-induced choroidal neo-vascularization in rats. Mol Vis. 2010;16:756–67. [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–4. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Giaccia AJ. The role of hypoxia-induced factors in tumorigenesis. Cell Death Diff. 2008;15:678–85. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläsker S, Tran MG, Shively SB, Ikejiri B, Lonser RR, et al. Epididymal cystadenomas and epithelial tumourlets: effects of VHL deficiency on the human epididymis. J Pathol. 2006;210:32–41. doi: 10.1002/path.2029. [DOI] [PubMed] [Google Scholar]
- Leung SY, Chan AS, Wong MP, Yuen ST, Fan YW, et al. Expression of vascular endothelial growth factor in von Hippel–Lindau syndrome-associated papillary cystadenoma of the epididymis. Hum Pathol. 1998;29:1322–4. doi: 10.1016/s0046-8177(98)90265-9. [DOI] [PubMed] [Google Scholar]
- Mehta GU, Shively SB, Duong H, Tran MGB, Moncrief TJ, et al. Progression of epididymal maldevelopment into Hamartoma-like neoplasia in VHL disease. Neoplasia. 2008;210:1146–53. doi: 10.1593/neo.08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49 Suppl 2:24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- Gillies RJ, Martinez-Zaguilan R, Martinez GM, Serrano R, Perona R. Tumorigenic 3T3 cells maintain an alkaline intracellular pH under physiological conditions. Proc Natl Acad Sci USA. 1990;87:7414–8. doi: 10.1073/pnas.87.19.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CH, Cooper TG, Bergmann M, Schulze H. Organization of tubules in the human caput epididymidis and the ultrastructure of their epithelia. Am J Anat. 1991;191:261–79. doi: 10.1002/aja.1001910306. [DOI] [PubMed] [Google Scholar]
- Powell JD, Elshtein R, Forest DJ, Palladino MA. Stimulation of hypoxia-inducible factor-1alpha (HIF-1alpha) protein in the adult rat testis following ischemic injury occurs without an increase in HIF-1alpha messenger RNA expression. Biol Reprod. 2002;67:995–1002. doi: 10.1095/biolreprod.101.002576. [DOI] [PubMed] [Google Scholar]
- Palladino MA, Powell JD, Korah N, Hermo L. Expression and localization of hypoxia-inducible factor-1 subunits in the adult rat epididymis. Biol Reprod. 2004;70:1121–30. doi: 10.1095/biolreprod.103.023085. [DOI] [PubMed] [Google Scholar]
- Marti HH, Katschinski DM, Wagner KF, Schaffer L, Stier B, et al. Isoform-specific expression of hypoxia-inducible factor-1alpha during the late stages of mouse spermiogenesis. Mol Endocrinol. 2002;16:234–43. doi: 10.1210/mend.16.2.0786. [DOI] [PubMed] [Google Scholar]
- Brooks DE. Androgenic regulation of metabolic pathways in the rat epididymis. Biol Reprod. 1978;18:629–38. doi: 10.1095/biolreprod18.4.629. [DOI] [PubMed] [Google Scholar]
- Hermo L, Chong DL, Moffatt P, Sly WS, Waheed A, et al. Region- and cell-specific differences in the distribution of carbonic anhydrases II, III, XII, and XIV in the adult rat epididymis. J Histochem Cytochem. 2005;53:699–713. doi: 10.1369/jhc.4A6575.2005. [DOI] [PubMed] [Google Scholar]
- Cooper TG.Epididymis. Encyclopedia of ReproductionIn: Neill JD, Knobil E. San Diego; Academic Press; 1999. Vol. 2, pp1–17. [Google Scholar]
- Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971;213:557–70. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caflisch CR, DuBose TD. Direct evaluation of acidification by rat testis and epididymis: role of carbonic anhydrase. Am J Physiol. 1990;258:E143–50. doi: 10.1152/ajpendo.1990.258.1.E143. [DOI] [PubMed] [Google Scholar]
- Xu LW, Wu HY, Yu YL, Zhang ZG, Li GH. Large phyllodes tumour of the seminal vesicle: case report and literature review. J Int Med Res . 2010;38:1861–7. doi: 10.1177/147323001003800534. [DOI] [PubMed] [Google Scholar]
- Tarnok A, Ulrich H, Bocsi J. Phenotypes of stem cells from diverse origin. Cytometry A. 2010;77:6–10. doi: 10.1002/cyto.a.20844. [DOI] [PubMed] [Google Scholar]
- Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–70. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–8. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- Tsujimura A, Fujita K, Komori K, Takao T, Miyagawa Y, et al. Prostatic stem cell marker identified by cDNA microarray in mouse. J Urol. 2007;178:686–91. doi: 10.1016/j.juro.2007.03.092. [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol. 2007;72:967–75. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- Kaewmala K, Uddin MJ, Cinar MU, Grosse-Brinkhaus C, Jonas E, et al. Association study and expression analysis of CD9 as candidate gene for boar sperm quality and fertility traits. Anim Reprod Sci. 2011;125:170–9. doi: 10.1016/j.anireprosci.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Fargeas CA, Joester A, Missol-Kolka E, Hellwig A, Huttner WB, et al. Identification of novel Prominin-1/CD133 splice variants with alternative C-termini and their expression in epididymis and testis. J Cell Sci. 2004;117:4301–11. doi: 10.1242/jcs.01315. [DOI] [PubMed] [Google Scholar]
- Li JY, Wang HY, Liu J, Liu Q, Zhang JS, et al. Transcriptome analysis of a cDNA library from adult human epididymis. DNA Res. 2008;15:115–22. doi: 10.1093/dnares/dsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]