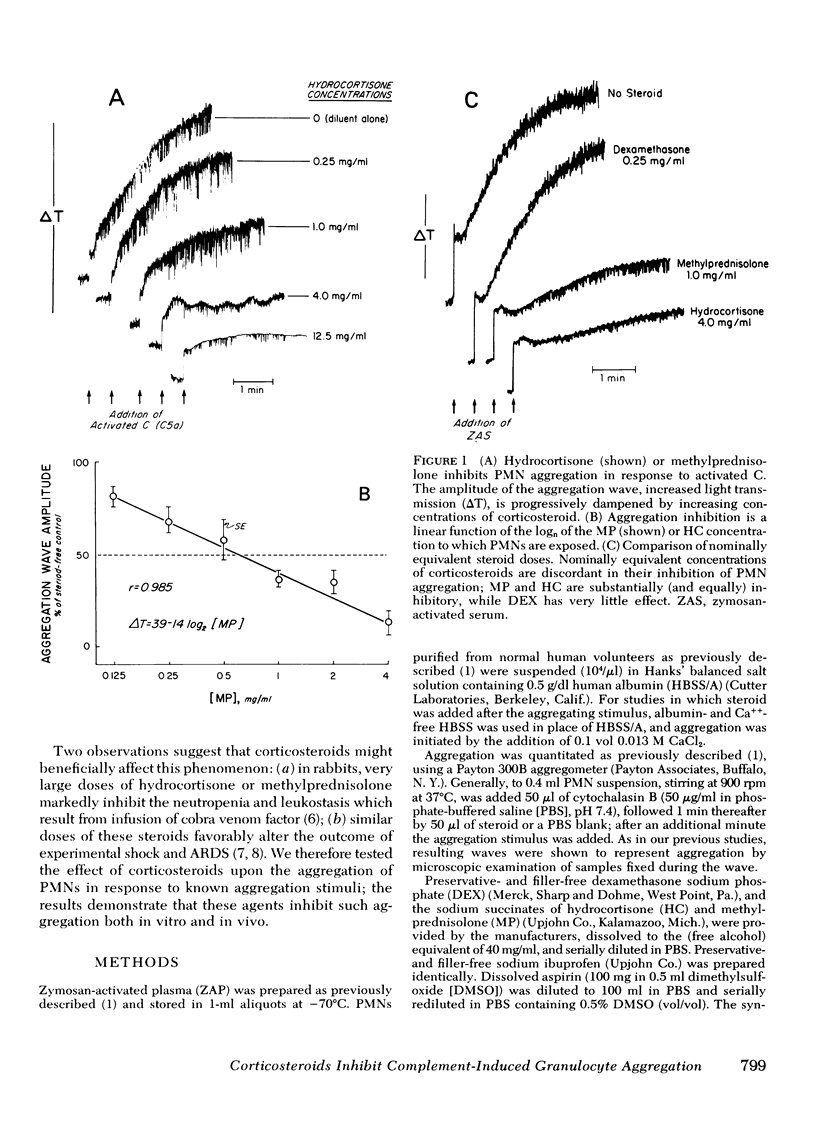
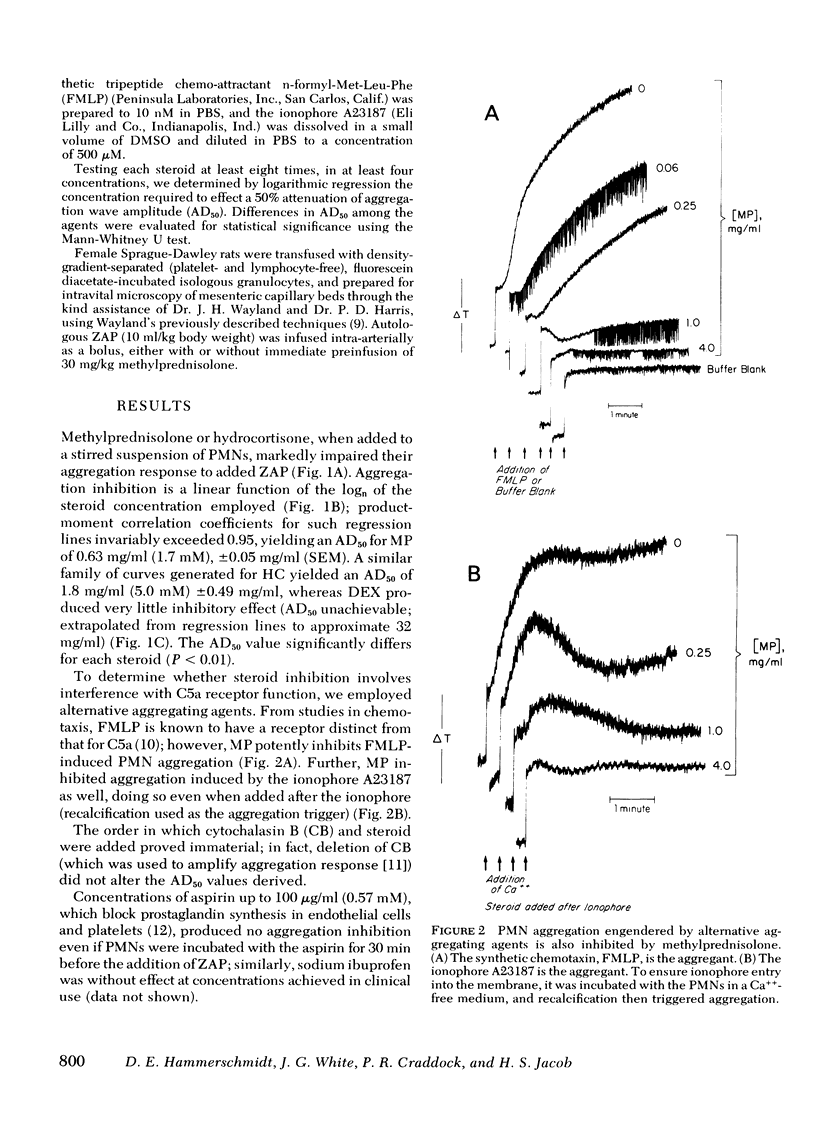
Abstract
Granulocyte (PMN) aggregation and embolization may underlie complement (C)-mediated organ dysfunction in such syndromes as hemodialysis neutropenia and Purtscher's ischem;c retinopathy. Because of clinical and pathologic parallels, we have further suggested a role for this phenomenon in the genesis of the adult respiratory distress syndrome (ARDS). Because corticosteroids are commonly used in immune diseases, and have particularly been claimed efficacious in shock and ARDS, we tested the capability of methylprednisolone (MP), hydrocortisone (HC), and dexamethasone (DEX) to inhibit PMN aggregation. Aggregation engendered in vitro by zymosan-activated plasma (ZAP) was inhibited by MP and HC at concentrations approximating plasma levels achieved with the large bolus (30 mg/kg i.v) therapy advocated in shock states; DEX was almost without effect. Using intravital fluorescence microscopy, we observed PMN aggregation and embolization in the mesenteric vessels of rats given intra-arterial infusions of ZAP; this was also prevented by pretreatment with 30 mg/kg MP. Steroid inhibition of aggregation seemed not to involve disruption of receptor function, because aggregation induced by alternative agents, n-formyl-Met-Leu-Phe and the ionophore A23187, was also inhibited by MP. Moreover, corticosteroid inhibition of PMN prostaglandin synthesis is also an unlikely explanation for our results, since aspirin and ibuprofen failed to block aggregation and arachidonic acid neither effected aggregation itself nor ameliorated the steroid effect. Our studies provide a plausible rationale for the empiric observation that high-dose corticosteroids may benefit patients with syndromes associated with microvascular leukostasis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Dillender M. J., Majerus P. W. Cultured human skin fibroblasts and arterial cells produce a labile platelet-inhibitory prostaglandin. Biochem Biophys Res Commun. 1977 Sep 9;78(1):294–301. doi: 10.1016/0006-291x(77)91253-0. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., White J. G., Jacob H. S. Potentiation of complement (C5a)-induced granulocyte aggregation by cytochalasin B. J Lab Clin Med. 1978 Mar;91(3):490–499. [PubMed] [Google Scholar]
- Goldstein I. M., Malmsten C. L., Kindahl H., Kaplan H. B., Rådmark O., Samuelsson B., Weissmann G. Thromboxane generation by human peripheral blood polymorphonuclear leukocytes. J Exp Med. 1978 Sep 1;148(3):787–792. doi: 10.1084/jem.148.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Craddock P. R., McCullough F., Kronenberg R. S., Dalmasso A. P., Jacob H. S. Complement activation and pulmonary leukotasis during nylon fiber filtration leukapheresis. Blood. 1978 Apr;51(4):721–730. [PubMed] [Google Scholar]
- Hong S. L., Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc Natl Acad Sci U S A. 1976 May;73(5):1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Craddock P. R., Jacob H. S. Mechanism of anti-complementary activity of corticosteroids in vivo: possible relevance in endotoxin shock. Proc Soc Exp Biol Med. 1977 Feb;154(2):206–209. doi: 10.3181/00379727-154-39638. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandritter W., Mittermayer C., Riede U. N., Freudenberg N., Grimm H. Shock lung syndrome (a general review). Pathol Res Pract. 1978 May;162(1):7–23. doi: 10.1016/s0344-0338(78)80128-9. [DOI] [PubMed] [Google Scholar]
- Wayland H. Laser stimulation of fluorochromes in intravital microscopy using a mirror objective. Bibl Anat. 1973;11:19–24. [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. W. Treatment or prevention of pulmonary cellular damage with pharmacologic doses of corticosteroid. Surg Gynecol Obstet. 1972 Apr;134(4):675–681. [PubMed] [Google Scholar]