Abstract
Despite regional variations in the prevalence of coronary artery disease (CAD), men are consistently more at risk of developing and dying from CAD than women, and the gender-specific effects of sex hormones are implicated in this inequality. This ‘Perspectives' article reviews the current evidence regarding the cardiovascular effects of testosterone in men including an examination of the age-related decline in testosterone, the relationship between testosterone levels and coronary disease, coronary risk factors and mortality. We also review the vaso-active effects of testosterone, and discuss how these have been used in men with heart failure and angina. We discuss the ‘cause' versus ‘effect' controversy, regarding low testosterone levels in men with coronary heart disease, as well as concerns over the use of testosterone replacement therapy in middle aged and elderly men. The article concludes with a discussion regarding the future direction for work in this interesting area, including the relative merits of screening for, and treating hypogonadism with testosterone replacement therapy in men with heart disease.
Keywords: atherosclerosis, chronic heart failure, ischaemic heart disease, replacement therapy, testosterone
Introduction
Irrespective of regional variations in the prevalence of coronary heart disease, the burden of coronary disease in men is approximately three times that of women.1 Moreover, men develop coronary disease approximately 10 years ahead of women. Multiple logistic regression analyses have shown that these differences are not explained by simple differences in coronary risk factor profiles.2 The relationship between male gender and the prevalence of coronary heart disease suggests a role for sex hormones in the aetiology of cardiovascular disease. Historically, much attention has been paid to the cardioprotective effects of female sex hormones in women. In women, physiological levels of oestrogen appear protective against atherosclerosis, whereas conditions associated with oestrogen deficiency such as early menopause or bilateral oopherectomy are associated with an increased burden of coronary disease.3, 4, 5 In animal models, oestrogen deficiency (for example, in the aromatase knockout mouse) has been shown to adversely affect lipid handling and encourage features of the metabolic syndrome.6 Conversely, conditions associated with higher female androgen levels, such as in polycystic ovarian syndrome, are associated with an excess of coronary artery disease.7 Despite promising early reports, hormone replacement strategies in postmenopausal women have demonstrated an increased risk of breast and endometrial malignancy, and thrombo-embolic disease with a resulting increased mortality.8, 9
Studies of men who abuse anabolic steroids have clearly demonstrated higher risk of myocardial infarction and sudden cardiac death.10, 11, 12 In men, exogenous oestrogen therapy has also been trialled for secondary prevention of coronary disease, following acute myocardial infarction.13 This trial was terminated early due to a twofold increase in re-infarction and a significant increase in mortality. Similar results were found more recently in men receiving oestrogens for treatment of prostatic carcinoma.14, 15 The effects of sex hormones are, therefore, gender-specific and their roles are more sophisticated than first perceived. The combination of: (i) the male preponderance of coronary disease; (ii) the cardioprotective effects of oestrogens in pre-menopausal women; and (iii) the increased cardiovascular death in men abusing anabolic steroids, have led to the view that testosterone is deleterious to the male heart. Contrary to this view, current evidence suggests that normal and physiological levels of testosterone are not deleterious to the male heart and are, in fact, beneficial. It is hypotestosteronaemia which is associated with adverse coronary risk profiles and with coronary morbidity and mortality in men. Moreover, androgen replacement therapy has positive effects on coronary risk factor profile and acts as a vasodilator demonstrating potential, because it is an anti-ischaemic agent.
Physiology and decline with age
Testosterone is a steroid hormone synthesized, predominantly, by the testicular Leydig cells under the control of the gonadotrophins, chiefly, luteinizing hormone. Testosterone secretion demonstrates both diurnal and circannual secretion, peaking in the early morning and in the autumn. Once synthesized, it circulates bound to serum proteins with approximately 68% tightly bound to sex hormone binding globulin and 30% bound more loosely to albumin. Only about 2% circulates freely and it is this free portion along with the albumin bound portion that make up the biologically available (bioavailable) testosterone. Testosterone is metabolized by 5-α reductase to dihydrotestosterone or by aromatase, in adipose tissue, to oestrogens. Men with increased abdominal fat, therefore, metabolize more testosterone to oestrogen, resulting in gynaecomastia and a reduction in secondary sexual characteristics.
Multiple cross-sectional studies have demonstrated a fall in androgen levels with advancing age.16, 17, 18, 19, 20, 21, 22, 23, 24, 25 However, unlike women, men do not experience the well characterized, sudden and rapid decline in sex hormone levels and cessation of reproductive capability as they age. In men, the decline in sex hormone levels is much more variable appearing to spare some men, fall unknowingly in some, and result in frank and symptomatic hypogonadism in others. Contrasting with the female menopause, the male ‘andropause' often results in rather non-specific symptoms, including reduced libido, fatigue, weakness, depression, dry skin and poor concentration, symptoms which are often regarded simply as a natural part of the aging process. Clinical signs can include fine-wrinkled dry skin, low hairline, gynaecomastia and muscle wasting. Due to the non-specific nature of the symptoms, hypogonadism often remains undiagnosed and thus untreated in many cases. In others, it is diagnosed but remains untreated due to a perceived concern regarding adverse iatrogenic effects on the prostate and heart. Harman et al.25 investigated the nature and potential aetiological factors involved in the change in sex hormone levels with age in the Baltimore Longitudinal Study of Aging. They found that in 890 generally healthy men, both total and free testosterone decreased at a constant rate from the third to ninth decade. Total testosterone fell at a rate of 0.11 nmol l−1 year−1, but the fall in free testosterone was more impressive and due, at least in part, to the significant rise of sex hormone binding globulin with age. These observations were independent of obesity, comorbid illnesses, medication, smoking and alcohol consumption.
Testosterone and coronary risk factors
It was previously believed that the higher prevalence of coronary disease in men may be explained by differences in risk factor profiles between genders. It is widely regarded that men display behaviours which are considered, cardiologically, more risky with increased levels of smoking and with diets richer in saturated fats.2 However, multiple logistic regression analysis has shown that differences in behavioural profiles do not account for the excess burden of coronary disease in men.2, 26 The development and progression of coronary atherosclerosis is heavily influenced by the interaction of multiple risk factors. The lipid profile in men is naturally more pro-atherogenic than in women, a difference that has, in the past, been attributed to higher circulating testosterone levels. However, it is low, not normal, nor high, testosterone levels that have been found to be associated with adverse lipid profiles. Testosterone levels are found to correlate positively with the cardio-protective high-density lipoprotein (HDL) cholesterol and negatively with atherogenic low-density lipoprotein (LDL) cholesterol and triglycerides.27, 28, 29, 30, 31 Some studies have demonstrated that testosterone causes a fall in the cardio-protective HDL cholesterol.32 However, any observed decline in HDL cholesterol is generally smaller and less pronounced than the positive effects on the other lipid fractions. Hypotestosteronaemia is associated with raised pro-inflammatory cytokines (tumour-necrosis factor-αand interleukin 6) and reduced anti-inflammatory cytokines (interleukin 10)33 which are, in turn associated with pro-atherosclerotic and inflammatory states. Additionally, low testosterone is associated with raised fibrinogen and hypercoagulable states,34, 35, 36 theoretically, promoting atherosclerosis and atherosclerotic plaque instability and thus acute coronary syndromes. The metabolic syndrome is a well-recognized risk factor for atherosclerosis and coronary morbidity and mortality. All of the components of the syndrome—hypertension, dyslipidaemia, insulin resistance, type II diabetes and hyperglycaemia—are independently associated with hypotestosteronaemia and frank hypogonadism. Moreover, there is a negative correlation between the number of components of the metabolic syndrome and the absolute serum testosterone level with a 10-fold increase in the relative risk of frank hypogonadism, if all four of the components of the metabolic syndrome are present.37 In an analysis of data from the Massachusetts Male Aging Study, it was found that, over a 15-year period, in nonobese men, low testosterone at baseline led to a two- to fourfold increased risk of developing metabolic syndrome. The authors concluded that low testosterone may act as an early warning sign for the development of the metabolic syndrome, and provide an opportunity for early (primary) intervention.38
Testosterone levels in men with coronary heart disease
Contrary to the notion that higher testosterone levels account for the higher burden of coronary disease in men than women, there is an increasing body of literature indicating that men with coronary artery disease (CAD) have significantly lower testosterone levels than men without CAD. Cross-sectional studies comparing men with and without CAD have repeatedly demonstrated significantly lower levels of both total and bioavailable testosterone in men with CAD than in controls with normal coronary arteries.39 However, there is heterogeneity in the consistency of these findings, especially in earlier reports.40 Explanations for the apparent inconsistency of earlier reports include variability in study design, in the assays applied, in the measures of testosterone quoted and in the definitions of ‘hypogonadism' and ‘cardiovascular disease' used. Some authors performed retrospective analyses from frozen samples as the original intention was not to study testosterone at all, and these studies took no account of the instability in testosterone level when frozen samples are stored over many years. Some studies measured total testosterone level, others used free or bioavailable testosterone or used calculated free-androgen index. There are different opinions and criteria regarding what ‘hypogonadism' actually is in terms of assay, cut-off and whether the criteria should include associated symptoms. More modern studies have been more consistent in design and definition, especially when the primary hypothesis was related to the question of the link between testosterone blood level and CAD. Despite some heterogeneity, the majority of studies that investigated androgen levels in men with coronary disease, showed that testosterone levels were significantly lower in men with coronary disease than in matched controls.
Do levels of total and free testosterone truly reflect androgenisation? Testosterone circulates partly bound to albumin (weakly) and partly bound to sex hormone binding globulin (strongly) and only a small fraction is free. The albumin-bound and free portions are biologically available to the tissues. It could be argued that bioavailable testosterone quantification would provide a more accurate measurement. It is possible to measure bioavailable testosterone by the method of Tremblay and Dube,41 but the assay is labour-intensive and time-consuming and, as such, is mainly restricted to the research laboratory. It is believed by some that this fraction more accurately reflects the true serum androgen level. Bioavailable testosterone assays have been utilized in several studies of men with coronary artery disease and more consistently demonstrate decline with age. One study of over 900 men found that both total and bioavailable testosterone were significantly lower in men with coronary artery disease than in those without.42 The magnitude of the difference in testosterone levels between men with coronary artery disease and those without is clinically significant. The same study demonstrated a prevalence of hypogonadism of 24% in men with coronary artery disease, by strict criteria, which is approximately three times higher than the expected background rate.
One question which remains unanswered is whether low testosterone levels accelerate the development of CAD or whether they are simply a consequence of chronic illness?
Cause or consequence?
Coronary artery disease is a chronic illness and patients with CAD often have other associated chronic illnesses such as hypertension, diabetes and hypercholesterolaemia. Maybe this is the cause of the lower testosterone levels? Regression analysis has demonstrated that even when the effects of such comorbid conditions are controlled for the relationship between CAD and lower testosterone levels remains.2, 26 Furthermore, if hypogonadism was a consequence of CAD, it might be expected that patients with more severe CAD might have lower testosterone levels than those with milder disease. This hypothesis has not been proven. The prevalence of hypogonadism in men with asymptomatic coronary plaque is similar to the prevalence in men with symptomatic CAD and both groups have lower levels of testosterone than men with normal coronary arteries, supporting a causative role more than a symptomatic consequence (Morris PD, 2001. unpublished data).
Studies in male animals have shown accelerated atherosclerosis after castration—an effect that is abrogated by androgen replacement therapy.43, 44
Risk factors for coronary disease such as diabetes are also associated with lower testosterone levels and testosterone supplementation in these men improves their risk factor profile with improvements in glycaemic control, adiposity and lipid profiles.45 Individuals affected by hypogonadal hypogonadism such as men with Klinefelter's syndrome are known to have increased levels of insulin resistance, dyslipidaemia and central obesity46—all the constituents of the metabolic syndrome, which carries a strong assocation with coronary disease and morbidity. Aside from coronary disease, Klinefelter's patients have been shown to have higher rates of congenital heart disease, mitral valve prolapse, reduced left ventricular function and more procoagualable states.47, 48, 49 Furthermore, accelerated coronary artery disease has been demonstrated in patients treated with testosterone-suppressive therapy. In a study of over seventy thousand men (73 196) treated with androgen suppressive therapy for prostate cancer, there was a 44% increase in the risk of developing diabetes and 16% increase in the risk of cardiovascular death or myocardial infarction, effects which were evident as early as 1–4 months.13 Similar conclusions were drawn in a study of men treated by orchidectomy, where, over a 10 year period, there was a twofold increase of cardiovascular mortality.50 Androgen suppressive therapy has also been linked with increased central blood pressure, insulin resistance, and hyperglycaemia.51, 52, 53, 54 However, one must be careful to consider the difference in androgen levels between the moderate hypotestosteronaemia associated with aging and the more extreme low testosterone levels associated with androgen suppressive therapy used in prostate cancer treatment.
A recently published meta-analysis of 19 prospective studies40 investigated some of the previously found heterogeneity in the results and design of studies in this area. Although the analysis failed to confirm that low testosterone increases the risk of cardiovascular disease in middle aged men, it did find a significant inverse association between testosterone and coronary disease in men older than 70 years.
Whether low testosterone is a ‘cause or consequence' of coronary disease remains unknown. There appears to be evidence supporting both sides of this controversy. It is of course possible that it plays a causative role and is also a consequence of illness and frailty. A great deal of research work will be needed to carefully untangle all the possible mechanisms underlying this relationship. Further work will undoubtedly expand our knowledge regarding the underlying mechanisms and relationships between low testosterone and coronary disease and this will be of great interest. If low testosterone is found to be a significant aetiological factor in the development of acceleration of coronary disease then, given the high prevalence of hypogonadism in this population, it would be worth considering screening for testosterone levels in men with coronary disease.
Vaso-active properties of testosterone
Although some people have suggested that the reported positive effects of androgens in cardiovascular disease may simply reflect non-specific effects on skeletal muscle function and mood, it has been demonstrated that testosterone does have direct vaso-active effects. It is known that testosterone levels inversely correlate with penile artery smooth muscle compliance with men with lower testosterone levels more likely to suffer erectile dysfunction.55 In animal models, testosterone causes vasodilatation of isolated coronary, femoral and pulmonary arteries in a dose-dependent fashion.56, 57, 58, 59 Interestingly, these effects are not mediated by the endothelium (unlike oestrogen) nor via the nuclear androgen receptor. In these models, testosterone appeared to act directly on the vascular smooth muscle, having an antagonistic action on calcium channels similar in effect to that of the anti-anginal drug nifedipine.60 In vitro studies of isolated male arteries have demonstrated similar vasodilatory actions. In vivo studies have also demonstrated a vasodilatory action for testosterone. One study showed that acute intracoronary administration of testosterone, at physiological concentrations, induces coronary artery dilatation and increases coronary blood flow in men with established coronary artery disease.61 Other studies of acute intravenous testosterone therapy have demonstrated increased cardiac output mediated by a reduction in the systemic vascular resistance and increased ischaemic threshold in men with CAD.62, 63 Clinical trials have demonstrated that chronic and physiological dose testosterone supplementation significantly improves anginal symptoms and the time to electrocardiographic ischaemia on exercise treadmill testing,64, 65, 66, 67 an effect which is proposed to be mediated by testosterone's vasodilatory action.
Testosterone levels and mortality
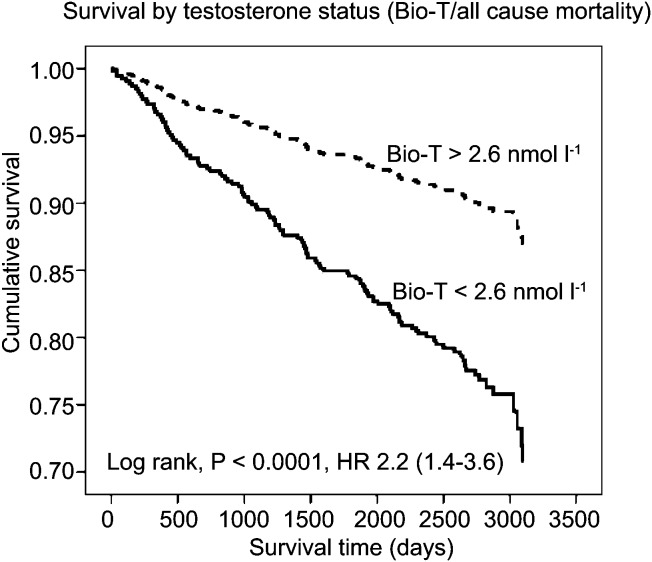
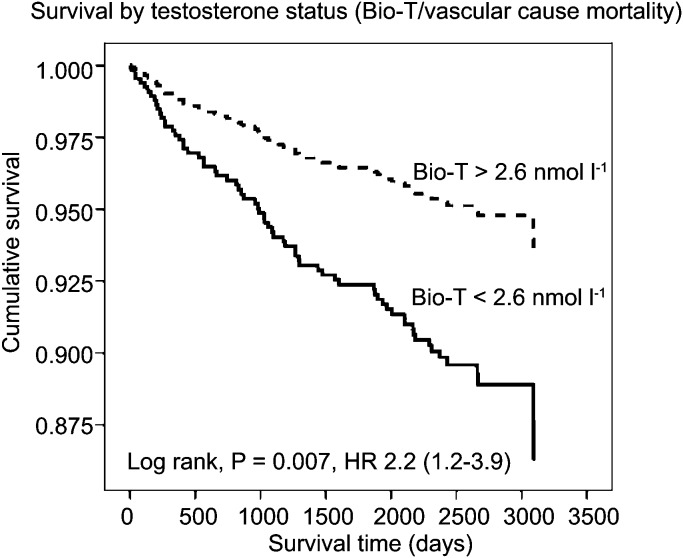
The aforementioned decline in testosterone in some men has previously been regarded by some simply as part of the natural physiology of ageing. However, five recent studies have demonstrated that lower baseline testosterone levels are a significant predictive marker for mortality even after controlling for the effects of comorbid conditions. In 2004, Shores et al.68 reported that hypotestosteronaemia was a marker for mortality in a group of 44 geriatric inpatients within a 6-month period. In a following study, the same group performed a computerized analysis of the Veteran's Affair's clinical database.69 They looked at 850 men over a 4- to 8-year period controlling for comorbid conditions which would affect mortality, e.g. concurrent cancer. They found that men with low testosterone levels had an 88% (20.1% vs. 34.9%, P<0.001) relative increase in all-cause mortality risk when compared with those with normal testosterone levels at baseline. In 2007, the InCHIANTI study demonstrated that an age-associated fall in bioavailable testosterone was associated with increased risk of death.70 In a 6-year follow-up study of 410 men aged over 65 years, they found that this effect was made more pronounced and more statistically significant when low testosterone was associated with similar decline in insulin-like growth factor and dehydroepiandrosterone sulphate. In contrast to men with all three hormones above the lowest quartiles, men with one, two or three hormones in the lowest quartiles were increasingly at more risk of death. In 2008, Laughlin et al.71 studied an older group (mean age of 71 years) of 794 men over a period of up to 20 years. They found a significant fall in bioavailable testosterone but not total testosterone with age. The risk of death was greater for men in the lowest baseline quartile of both total and bioavailable testosterone compared with those in the highest quartile. After adjusting for age, adiposity and lifestyle choices, the risk of death was 44% greater between the lowest and highest quartiles for total testosterone (hazard ratio (HR): 1.44; 95% confidence interval (CI): 1.12–1.84) and 50% higher between the lowest and highest quartiles for bioavailable testosterone (HR: 1.50; 95% CI: 1.15–1.96). In the largest study to date investigating the effects of endogenous testosterone levels and mortality, the European Prospective Investigation into Cancer Norfolk study72 prospectively investigated all-cause and cardiovascular mortality in 11 606 healthy men between the ages 40 and 79 years at baseline. Over a 6- to 10-year follow-up period, they observed a statistically significant association between baseline serum testosterone level and all-cause (HR: 0.75; 95% CI: 0.55–1.00), cardiovascular (HR: 0.62; 95% CI: 0.45–0.84) and cancer related (HR: 0.59; 95% CI: 0.42–0.85) deaths (P<0.001) for each association after controlling for comorbid conditions and behaviours. They found that every one standard deviation increase in baseline testosterone (∼6 nmol l−1) was associated with a ∼14% risk reduction in mortality over the study period. Even more relevant to the current article is the recently published study by Malkin et al.42 In this study, 930 men with angiographically proven CAD were prospectively followed up over a 7-year period. They observed a baseline prevalence of hypogonadism in this group (by a strict criteria) to be 24%. In this androgen deficient group, the mortality was 21% versus only 12% in the eugonadal group (P=0.002). Low bioavailable testosterone but not total testosterone significantly influenced the all-cause and cardiovascular mortality after the multivariate analysis (Figures 1 and 2), suggesting that this is the more sensitive assay in detecting pathological deficiency and risk compared with other assays. Low testosterone therefore, appears to be a marker for mortality. However, a similar ‘cause or consequence' argument arises. Does low testosterone have a causative role in promoting worsening cardiovascular health or does it simply mark out a population of less healthy men, or both?
Figure 1.
A survival curve of all-cause mortality based on baseline Bio-T. The solid line represents patients with baseline Bio-T less than 2.6 nmol l−1, the broken line represents patients with Bio-T greater than 2.6 nmol l−1. Reproduced from Malkin et al. (2010).42 Bio-T, bioavailable testosterone; HR, hazard ratio.
Figure 2.
A survival curve of vascular mortality based on baseline Bio-T. The solid line represents patients with baseline Bio-T less than 2.6 nmol l−1, the broken line represents patients with Bio-T greater than 2.6 nmol l−1. Reproduced from Malkin et al. (2010).42 Bio-T, bioavailable testosterone; HR, hazard ratio.
Testosterone and heart failure
Coronary heart disease is the biggest underlying cause of heart failure in the Western world. However, the specific relationship between testosterone and heart failure has not been studied to the same degree as that of testosterone and coronary disease. Heart failure is characterized by a catabolic state with activation of inflammatory cytokines, vasodilator incapacity and maladaptive neurohormonal activation. As described above, testosterone exerts an effect which opposes all of these adaptations. Serum testosterone levels have been shown to correlate positively with cardiac output in men with heart failure and in one study acute, intravenous administration of testosterone acutely increased cardiac output.63 The effects of chronic testosterone supplementation have also been studied. In a small randomized placebo controlled clinical trial, Pugh et al.73 demonstrated improvements in exercise capacity and in symptom scores after 12 weeks of testosterone therapy in men with heart failure. Similar results were found in larger placebo-controlled randomized controlled trials with improvements in exercise capacity, symptom scores, VO2 max, maximal strength, insulin resistance and a reduction in electrocardiographic Q-T dispersion.74, 75, 76 Although these early studies are positive, more evaluation is needed to elucidate the mechanisms of action of testosterone in heart failure and on the long-term effects of supplementation.
Testosterone therapy in cardiovascular disease
Evidence regarding the cardiovascular effects of testosterone therapy can be broadly divided into two groups: the effects on cardiovascular risk factors, such as lipid profiles, blood pressure, etc., which exert an indirect effect on coronary artery disease and the direct clinical effects of testosterone therapy on the heart itself.
Testosterone supplementation in men with type II diabetes has been shown to reduce total and LDL cholesterol and lipoprotein a, even in men already established on statin therapy.77, 78 In studies of elderly and hypogonadal men, testosterone therapy has been associated with improved lipid profiles with reductions in total and LDL cholesterol.31, 79, 80, 81 Hypotestosteronaemia is associated with hypertension and arterial stiffening. There have been several trials of testosterone replacement therapy in eugonadal, hypogonadal and obese men which have observed impressive reductions in both systolic and diastolic blood pressure over periods as short as 6 months and for as long as 10 years.82, 83, 84 Similar beneficial effects of chronic testosterone therapy were demonstrated in reducing body mass index in a study of testosterone therapy over a 12-month period.67 Testosterone therapy induces increased insulin sensitivity and improved glycaemic control in type II diabetic men.77 Studies of testosterone therapy have also observed beneficial modifications in pro- and anti-inflammatory cytokine profiles.33
Rosano et al.39 investigated the acute effects of intravenous testosterone therapy in a group of men about to perform exercise, treadmill testing. When compared to baseline and a placebo, time to ischaemia was significantly prolonged after intravenous testosterone. English et al.65 demonstrated similar effects, but in the context of chronic testosterone therapy. In men with angiographically-proven coronary disease, 12 weeks of trans-dermal testosterone therapy significantly increased time to ischaemia at exercise testing. The anti-ischaemic effect was greatest in those men with the lowest baseline testosterone levels. Malkin et al.66 advanced this hypothesis by performing a similar, blinded, placebo-controlled and crossover study of testosterone therapy in men with angina, but only recruited men with significant hypogonadism. At treadmill testing, the increase in time to ischaemia was even greater (74 s). In addition, there were significant improvements in symptom scores and beneficial changes in lipid profiles and reductions in the proinflammatory cytokine tumour-necrosis factor-α. In a further study by the same group, the anti-ischaemic effects of testosterone therapy were demonstrated up to the end of the study period at 1 year.67 Table 1 illustrates the studies investigating the effects of testosterone supplementation in men with coronary disease.
Table 1. Review of all published studies of testosterone in men with angina (Channer et al., in press).
| Author | Drug dosage | Primary study outcome | n | Result |
|---|---|---|---|---|
| Hamm85 | Variable | 7 | ↓ Frequent angina | |
| Walker86 | Variable | 9 | ↑ Exercise tolerance | |
| Sigler87 | Low | 16 | ↑ Exercise duration | |
| Lesser88 | Low | 92 | Improvement in 85 | |
| Jaffe89 | Physiological | Exercise test | 50 | ↓ Segment depression depression |
| Wu and Weng90 | Variable | Holter | 62 | ↓ Ischaemia on Holter |
| Rosano et al.39 | High | Exercise test | 14 | ↑ Time to ischaemia |
| Webb et al.62 | High | Exercise test | 14 | ↑ Time to ischaemia |
| English et al.65 | Physiological | Exercise test | 46 | ↑ Time to ischaemia |
| Thompson et al.91 | High and physiological | Exercise test Single photon emission computed tomography scan | 32 | Neutral |
| Malkin et al.66 | Physiological | Exercise test | 10 | ↑ Time to ischaemia |
| Mathur et al.67 | Physiological | Exercise test | 13 | ↑ Time to ischaemia |
In a recent review paper by Saad et al.,92 the beneficial effects of testosterone therapy on cardiovascular risk factors including on body composition, lipids profile, glycaemic control and blood pressure were described. They found that the beneficial effects of testosterone therapy started to become apparent after 3 months of therapy onwards. However, continuing benefit was observed with therapy up to 9 months in the case of improvements in blood pressure, 12 months in the case of improved glycamemic control and up to 2 years in the case of improved lipid profiles.
Despite historical concerns over testosterone therapy in aging males, there is now a large and rapidly increasing body of evidence suggesting that testosterone replenishment in men with cardiovascular disease is safe and effective. However, the effect of testosterone replacement therapy on mortality and patient outcome will need to be subject to large, prospective and randomized controlled trials. This, surely, will be the next big step in this interesting area. With millions of men affected by CAD worldwide, and a prevalence of hypogonadism estimated at approximately one-quarter in this population, the rewards for successfully replacing testosterone in affected males are potentially very large indeed.
Concerns over testosterone therapy in men
Historically, there have been two main concerns regarding testosterone therapy in middle aged and older men. The first was the concern that it might promote coronary heart disease and acute coronary syndromes. The second was that testosterone supplementation might promote prostate cancer. Hopefully, the current article has dealt with the former concern and has brought reassurance regarding physiological levels of testosterone and the male heart. In one recent interventional study of frail hypogonadal men, supraphysiological dosages of testosterone replacement therapy was used in an attempt to improve muscle strength.93 The study showed that pharmacological doses of testosterone significantly increased muscle strength, but the trial was stopped early because of an excess of cardiovascular side effects. The authors reported that 23 patients taking testosterone had cardiovascular complications compared with five in the placebo group and on this basis stopped the trial. Critical review of this paper shows that in fact there were only six hard end points in the treatment group compared with one in the placebo group. About half the group had a history of cardiovascular disease and the rest had significant cardiovascular risk factors. Our view is that this study showed that men with hypogonadism should be treated only with physiological doses of testosterone for true replacement therapy. The literature showed that testosterone replacement should be managed in the same way as thyroid hormone replacement. Replacement dosages should aim to maintain normal physiological levels. If the Basaria trial had been done in hypothyroid patients with high cardiovascular risk and replacement had aimed at supra-physiological levels, the same (or worse) results would have been seen.
The second concern is likely to be fuelled by the knowledge that prostate cancer can be successfully treated by androgen suppressive therapy. However, over the last decade, epidemiological and clinical investigations have failed to demonstrate any association between underlying testosterone levels and the risk of developing prostate cancer. Similarly, no studies have demonstrated that lower than normal testosterone levels are protective against developing prostate cancer.94, 95, 96, 97 In the study by English et al.,65 where men with proven angina were given trans-dermal testosterone, prostate-specific antigen levels were monitored over the study period and did not change significantly. Furthermore, testosterone levels decline with age as the prevalence of prostate carcinoma rises—could it be normal and physiological levels of testosterone that are in fact protective against the development of prostate cancer? In men with prostate cancer, testosterone therapy is clearly contra-indicated and lower levels of testosterone are beneficial. However, to our knowledge, there is no evidence supporting a causative role of testosterone supplementation on the levels in the physiological normal range, with the risk of developing prostate cancer. In fact, in view of the data concerning low testosterone and increased mortality, it has even been suggested that testosterone suppressive therapy could be withheld from elderly men with T1 to T2 localized prostate cancer due to reduced survival.98 Any future trials looking at the effects of chronic testosterone therapy in patients with coronary disease should monitor the effects on prostate-specific antigen and look for any deleterious effects on the prostate gland.
Discussion
In elderly and middle aged men with coronary disease, about one-quarter will have testosterone deficiency and symptomatic hypogonadism.42 In men with coronary disease, it remains unclear what role testosterone deficiency might play in the aetiology and progression of atherosclerosis or whether hypotestosteronaemia simply reflects chronic illness and frailty. However, it has now been demonstrated in several large longitudinal cohort studies of men with and without coronary disease that low baseline testosterone is a significant risk marker of increased all-cause and cardiovascular mortality. More importantly, once hypogonadism is diagnosed, replacement therapy has a beneficial anti-ischaemic effect, along with beneficial effects on lipids, glucose metabolism, inflammatory cytokine profiles, lean body mass, blood clotting profiles and patient well-being. Moreover, there are no data suggesting that testosterone supplementation into the normal physiological range leads to an increased risk of developing prostate cancer. Hormone replacement is one of the central tenets of endocrinology. Replacing testosterone in men is simple, cheap and easy to monitor both clinically and biochemically. Are we failing to treat a large population of men who would benefit from hormone replacement therapy?
The next challenge in this interesting field of work will be to perform large randomized controlled trials of testosterone replacement in men with coronary artery disease.
Another unanswered question regards the possible role for screening men with coronary artery disease for hypogonadism. At least nine out of the 10 ‘Wilson and Junger criteria' for screening99 are satisfied by screening for hypogonadism in men with CAD. Studies estimate the prevalence of symptomatic hypogonadism in men with coronary disease at approximately one-quarter (24%) and that these men have poorer cardiovascular outcomes than those with normal androgen levels. Worldwide, there are millions of men suffering from coronary artery disease. This means that there may be a huge population of men with symptomatic hypogonadism that would benefit from replacement therapy, not only from a symptomatic and endocrine perspective, but also from improved cardiovascular outcomes.
Clearly, further research will be needed in:
bringing some standardisation and consistency to what measurement of androgenisation should be used and what should define hypogonadism and hypotestosteronaemia;
elucidating the long term cardiovascular effects of testosterone therapy in men with coronary disease in large, prospective, randomized, placebo controlled trials;
elucidating any additional benefit in hypogonadal men with coronary disease;
clarifying the safety of testosterone therapy in men with coronary disease;
investigating the role of screening for hypogonadism in men with coronary disease.
The authors declare no competing financial interests.
References
- Wingard DL, Suarez L, Barrett-Conor E. The sex differential in mortality from all causes and ischaemic heart disease. Am J Epidemiol. 1983;117:165–72. doi: 10.1093/oxfordjournals.aje.a113527. [DOI] [PubMed] [Google Scholar]
- Njølstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark Study. Circulation. 1996;93:450–6. doi: 10.1161/01.cir.93.3.450. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: The Framingham Study. Ann Intern Med. 1976;85:447–52. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Barrett-Connor E, Wingard DL. Hysterectomy, oophorectomy, and heart disease risk factors in older women. Am J Public Health. 1997;87:676–80. doi: 10.2105/ajph.87.4.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, et al. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–10. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, et al. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003;144:1474–80. doi: 10.1210/en.2002-221123. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Decarli A, Franceschi S, Gentile A, Negri E, et al. Menstrual and reproductive factors and the risk of myocardial infarction in women under fifty-five years of age. Am J Obstet Gynecol. 1987;157:1108–12. doi: 10.1016/s0002-9378(87)80271-5. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Womens Health Initiative Risks and benefits of estrogen plus progestin in healthy postmenopauseal women. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Bagatell CJ, Bremmer WJ. Androgens in men—uses and abuses. N Engl J Med. 1996;334:707–14. doi: 10.1056/NEJM199603143341107. [DOI] [PubMed] [Google Scholar]
- Dickerman RD, McConathy WJ, Zachariah NY, Schaller F. Cardiovascular complications and anabolic steroids. Eur Heart J. 1996;17:1912–5. doi: 10.1093/oxfordjournals.eurheartj.a014812. [DOI] [PubMed] [Google Scholar]
- Sullivan ML, Martinez CM, Gennis P, Gallagher EJ. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis. 1998;41:1–15. doi: 10.1016/s0033-0620(98)80019-4. [DOI] [PubMed] [Google Scholar]
- Coronary Drug Project Research Group The coronary drug project. Findings leading to discontinuation of the 2.5mg day estrogen group. JAMA. 1973;226:652–7. [PubMed] [Google Scholar]
- Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–5. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Rubens R, Verdonck L. Testosterone secretion and metabolism in male senescence. J Clin Endocrinol Metab. 1972;34:730–5. doi: 10.1210/jcem-34-4-730. [DOI] [PubMed] [Google Scholar]
- Rubens R, Dhont M, Vermeulen A. Further studies on Leydig cell function in old age. J Clin Endocrinol Metab. 1974;39:40–5. doi: 10.1210/jcem-39-1-40. [DOI] [PubMed] [Google Scholar]
- Baker HW, Burger HG, de Kretser DM, Hudson B, O'Connor S, et al. Changes in the pituitary testicular system with age. Clin Endocrinol. 1976;5:349–72. doi: 10.1111/j.1365-2265.1976.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Pirke KM, Doerr P. Age related changes in free plasma testosterone, dihydrotestosterone and oestradiol. Acta Endocrinol (Copenh) 1975;80:171–8. doi: 10.1530/acta.0.0800171. [DOI] [PubMed] [Google Scholar]
- Purifoy FE, Koopmans LH, Mayes DM. Age differences in serum androgen levels in normal adult males. Hum Biol. 1981;53:499–511. [PubMed] [Google Scholar]
- Bremner WJ, Prinz PN. A loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–81. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- Tenover JS, Matsumoto AM, Plymate SR, Bremner WJ. The effects of aging in normal men on bioavailable testosterone and luteinizing hormone secretion: response to clomiphene citrate. J Clin Endocrinol Metab. 1987;65:1118–26. doi: 10.1210/jcem-65-6-1118. [DOI] [PubMed] [Google Scholar]
- Gray A, Berlin JA, McKinlay JB, Longcope C. An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol. 1991;44:671–84. doi: 10.1016/0895-4356(91)90028-8. [DOI] [PubMed] [Google Scholar]
- Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1988;147:750–4. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Raynor M, Mockford C, Boaz A. London; British Heart Foundation; 1998. Coronary heart disease statistics. [Google Scholar]
- Mendoza SG, Osuna A, Zerpa A, Gartside PS, Glueck CJ. Hypertriglyceridemia and hypoalphalipoproteinemia in azoospermic and oligospermic young men: relationships of endogenous testosterone to triglyceride and high density lipoprotein cholesterol metabolism. Metabolism. 1981;30:481–6. doi: 10.1016/0026-0495(81)90184-0. [DOI] [PubMed] [Google Scholar]
- Dai WS, Gutai JP, Kuller LH, Laporte RE, Falvo-Gerard L, et al. Relation between plasma high-density lipoprotein cholesterol and sex hormone concentrations in men. Am J Cardiol. 1984;53:1259–63. doi: 10.1016/0002-9149(84)90075-4. [DOI] [PubMed] [Google Scholar]
- Heller RF, Wheeler MJ, Micallef J, Miller NE, Lewis B. Relationship of high density lipoprotein cholesterol with total and free testosterone and sex hormone binding globulin. Acta Endocrinol (Copenh) 1983;104:253–6. doi: 10.1530/acta.0.1040253. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E.Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus Ann Intern Med199;117807–11. [DOI] [PubMed] [Google Scholar]
- Simon D, Charles MA, Nahoul K, Orssaud G, Kremski J, et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: The Telecom Study. J Clin Endocrinol Metab. 1997;82:682–5. doi: 10.1210/jcem.82.2.3766. [DOI] [PubMed] [Google Scholar]
- Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med. 2001;111:261–9. doi: 10.1016/s0002-9343(01)00833-6. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Glueck HI, Stroop D, Speirs J, Hamer T, et al. Endogenous testosterone, fibrinolysis, and coronary heart disease risk in hyperlipidemic men. J Lab Clin Med. 1993;122:412–20. [PubMed] [Google Scholar]
- Caron P, Bennet A, Camare R, Louvet JP, Boneu B, et al. Plasminogen activator inhibitor in plasma is related to testosterone in men. Metabolism. 1989;38:1010–5. doi: 10.1016/0026-0495(89)90014-0. [DOI] [PubMed] [Google Scholar]
- Bonithon-Kopp C, Scarabin PY, Bara L, Castanier M, Jacqueson A, et al. Relationship between sex hormones and haemostatic factors in healthy middle-aged men. Atherosclerosis. 1988;71:71–6. doi: 10.1016/0021-9150(88)90303-6. [DOI] [PubMed] [Google Scholar]
- Corona G, Mannucci E, Schulman C, Petrone L, Mansani R, et al. Psychobiologic correlates of the metabolic syndrome and associated sexual dysfunction. Eur Urol. 2006;50:595–604; discussion 604. doi: 10.1016/j.eururo.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–50. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- Rosano GM, Leonardo F, Pagnotta P, Pelliccia F, Panina G, et al. Acute anti-ischaemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–70. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- Ruige JB, Mahmoud AM, de Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;7:870–5. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- Tremblay RR, Dube JY. Plasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptives. Contraception. 1974;10:599–605. doi: 10.1016/0010-7824(74)90099-7. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, et al. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010;96:1821–5. doi: 10.1136/hrt.2010.195412. [DOI] [PubMed] [Google Scholar]
- Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res. 1999;84:813–19. doi: 10.1161/01.res.84.7.813. [DOI] [PubMed] [Google Scholar]
- Nettleship JE, Jones TH, Channer KS, Jones RD. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation. 2007;116:2427–34. doi: 10.1161/CIRCULATIONAHA.107.708768. [DOI] [PubMed] [Google Scholar]
- Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study Int J Androl4(6 Pt 1)2011528–40. [DOI] [PubMed]
- Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, et al. The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29:1591–8. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006;91:1254–60. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- Fricke GR, Mattern HJ, Schweikert HU, Schwanitz G. Klinefelter's syndrome and mitral valve prolapse. an echocardiographic study in twenty-two patients. Biomed Pharmacother. 1984;38:88–97. [PubMed] [Google Scholar]
- Andersen NH, Bojesen A, Kristensen K, Birkebaek NH, Fedder J, et al. Left ventricular dysfunction in Klinefelter syndrome is associated to insulin resistance, abdominal adiposity and hypogonadism. Clin Endocrinol. 2008;69:785–91. doi: 10.1111/j.1365-2265.2008.03211.x. [DOI] [PubMed] [Google Scholar]
- Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–24. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–7. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93:2042–9. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- Kintzel PE, Chase SL, Schultz LM, O'Rourke TJ. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy. 2008;28:1511–22. doi: 10.1592/phco.28.12.1511. [DOI] [PubMed] [Google Scholar]
- Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29:534–9. doi: 10.2164/jandrol.108.005454. [DOI] [PubMed] [Google Scholar]
- Aversa A, Isidori AM, de Martino MU, Caprio M, Fabbrini E, et al. Androgens and penile erection: evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. Clin Endocrinol. 2000;53:517–22. doi: 10.1046/j.1365-2265.2000.01118.x. [DOI] [PubMed] [Google Scholar]
- English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res. 2001;33:645–52. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol. 2001;281:H1720–7. doi: 10.1152/ajpheart.2001.281.4.H1720. [DOI] [PubMed] [Google Scholar]
- English KM, Jones RD, Jones TH, Morice AH, Channer KS. Testosterone acts as a coronary vasodilator by a calcium channel antagonist action. J Endocrinol Invest. 2002;25:455–8. doi: 10.1007/BF03344037. [DOI] [PubMed] [Google Scholar]
- Jones RD, Pugh PJ, English KM, Mandour O, Steeds R, et al. Isolated arteries from testicular feminised mice have maintained dilator responses to testosterone but reduced vascular reactivity to acetylcholine. Br J Pharmacol. 2001;135:P129. [Google Scholar]
- Scragg JL, Jones RD, Channer KS, Jones TH, Peers C. Testosterone is a potent inhibitor of L-type Ca2+ channels. Biochem Biophys Res Commun. 2004;318:503–6. doi: 10.1016/j.bbrc.2004.04.054. [DOI] [PubMed] [Google Scholar]
- Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–6. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- Webb CM, Adamson DL, de Zeigler D, Collins P. Effect of acute testosterone on myocardial ischaemia in men with coronary artery disease. Am J Cardiol. 1999;83:437–963. doi: 10.1016/s0002-9149(98)00880-7. [DOI] [PubMed] [Google Scholar]
- Pugh PJ, Jones TH, Channer KS. Acute haemodynamic effects of testosterone in men with chronic heart failure. Eur Heart J. 2003;24:909–15. doi: 10.1016/s0195-668x(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, et al. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res. 2007;19:176–82. doi: 10.1038/sj.ijir.3901504. [DOI] [PubMed] [Google Scholar]
- English KM, Steeds RP, Jones TH, Diver MJ, Channer KS.Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study Circulation2000 17;1021906–11. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Morris PD, Kerry KE, Jones RD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90:871–6. doi: 10.1136/hrt.2003.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, et al. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161:443–9. doi: 10.1530/EJE-09-0092. [DOI] [PubMed] [Google Scholar]
- Shores MM, Moceri VM, Gruenewald DA, Brodkin KI, Matsumoto AM, et al. Low testosterone is associated with decreased function and increased mortality risk: a preliminary study of men in a geriatric rehabilitation unit. J Am Geriatr Soc. 2004;52:2077–81. doi: 10.1111/j.1532-5415.2004.52562.x. [DOI] [PubMed] [Google Scholar]
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–5. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Ling SM, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167:2249–54. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- Pugh PJ, Jones RD, West JN, Jones TH, Channer KS. Testosterone treatment for men with chronic heart failure. Heart. 2004;90:446–7. doi: 10.1136/hrt.2003.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, et al. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006;27:57–64. doi: 10.1093/eurheartj/ehi443. [DOI] [PubMed] [Google Scholar]
- Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–27. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Morris PD, Pugh PJ, English KM, Channer KS. Effect of testosterone therapy on QT dispersion in men with heart failure. Am J Cardiol. 2003;92:1241–3. doi: 10.1016/j.amjcard.2003.07.044. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care. 2011;34:828–37. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgliczynski S, Ossowski M, Slowinska-Srzednicka J, Brzezinska A, Zgliczynski W, et al. Effect of testosterone replacement therapy on lipids and lipoproteins in hypogonadal and elderly men. Atherosclerosis. 1996;121:35–43. doi: 10.1016/0021-9150(95)05673-4. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Shah P, Lakshmy R, Reddy KS. Effect of testosterone replacement on whole body glucose utilisation and other cardiovascular risk factors in males with idiopathic hypogonadotrophic hypogonadism. Horm Metab Res. 1998;30:642–5. doi: 10.1055/s-2007-978950. [DOI] [PubMed] [Google Scholar]
- Uyanik BS, Ari Z, Gümüs B, Yiğitoğlu MR, Arslan T. Beneficial effects of testosterone undecanoate on the lipoprotein profiles in healthy elderly men. A placebo controlled study. Jpn Heart J. 1997;38:73–82. doi: 10.1536/ihj.38.73. [DOI] [PubMed] [Google Scholar]
- Mårin P, Holmäng S, Gustafsson C, Jönsson L, Kvist H, et al. Androgen treatment of abdominally obese men. Obes Res. 1993;1:245–51. doi: 10.1002/j.1550-8528.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92:3844–53. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- Anderson FH, Francis RM, Faulkner K. Androgen supplementation in eugonadal men with osteoporosis-effects of 6 months of treatment on bone mineral density and cardiovascular risk factors. Bone. 1996;18:171–7. doi: 10.1016/8756-3282(95)00441-6. [DOI] [PubMed] [Google Scholar]
- Hamm L. Testosterone propionate in the treatment of angina pectoris. J Clin Endocrinol. 1942;2:325–8. [Google Scholar]
- Walker TC. The use of testosterone priopionate and estrogenic substance in the treatment of essential hypertension, angina and peripheral vascular disease. J Clin Endoc. 1942;2:560–8. [Google Scholar]
- Sigler LH. Treatment of angina pectoris by testosterone propionate. NY J Med. 1943;43:1424–8. [Google Scholar]
- Lesser MA. Testosterone propionate therapy in one hundred cases of angina pectoris. J Clin Endocrinol. 1946;6:549–57. doi: 10.1210/jcem-6-8-549. [DOI] [PubMed] [Google Scholar]
- Jaffe MD. Effect of testosterone cypionate on postexercise ST segment depression. Br Heart J. 1977;39:1217–222. doi: 10.1136/hrt.39.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SZ, Weng XZ. Therapeutic effects of an androgenic preparation on myocardial ischemia and cardiac function in 62 elderly male coronary heart disease patients. Chin Med J. 1993;106:415–8. [PubMed] [Google Scholar]
- Thompson PD, Ahlberg AW, Moyna NM, Duncan B, Ferraro-Borgida M, et al. Effect of intravenous testosterone on myocardial ischaemia in men with coronary artery disease. Am Heart J. 2002;143:249–56. doi: 10.1067/mhj.2002.120144. [DOI] [PubMed] [Google Scholar]
- Saad F, Aversa A, Isidori AM, Zafalon L, Zitzmann M, et al. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011;165:675–85. doi: 10.1530/EJE-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. New Eng J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stattin P, Lumme S, Tenkanen L, Alfthan H, Jellum E, et al. High levels of circulating testosterone are not associated with increased prostate cancer risk: a pooled prospective study. Int J Cancer. 2004;108:418–24. doi: 10.1002/ijc.11572. [DOI] [PubMed] [Google Scholar]
- Travis RC, Key TJ, Allen NE, Appleby PN, Roddam AW, et al. Serum androgens and prostate cancer among 643 cases and 643 controls in the European Prospective Investigation into Cancer and Nutrition Int J Cancer200;1211331–8. [DOI] [PubMed] [Google Scholar]
- Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–83. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish AM, Saad F, Feeley RJ, Guay A. The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl. 2009;30:477–94. doi: 10.2164/jandrol.108.007245. [DOI] [PubMed] [Google Scholar]
- Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–81. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J, Junger G. WHO; 1968. Principles of screening for disease. pp. 26–39. [Google Scholar]