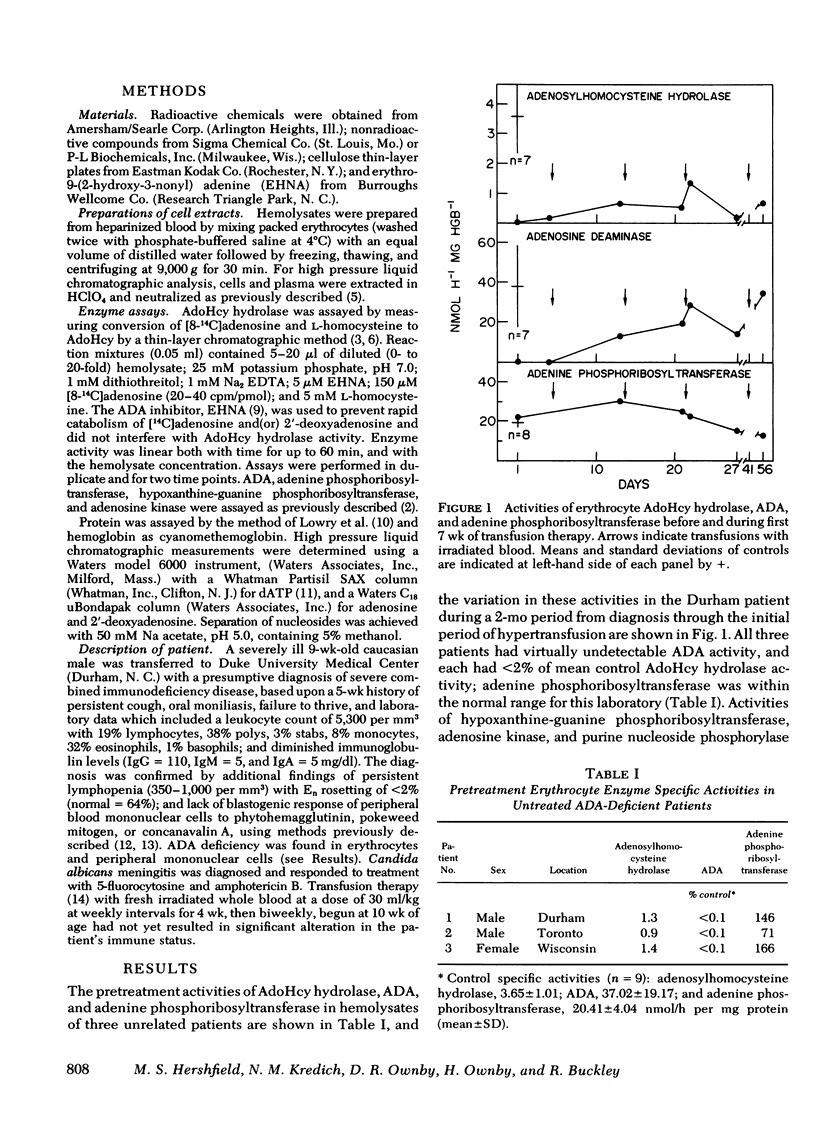
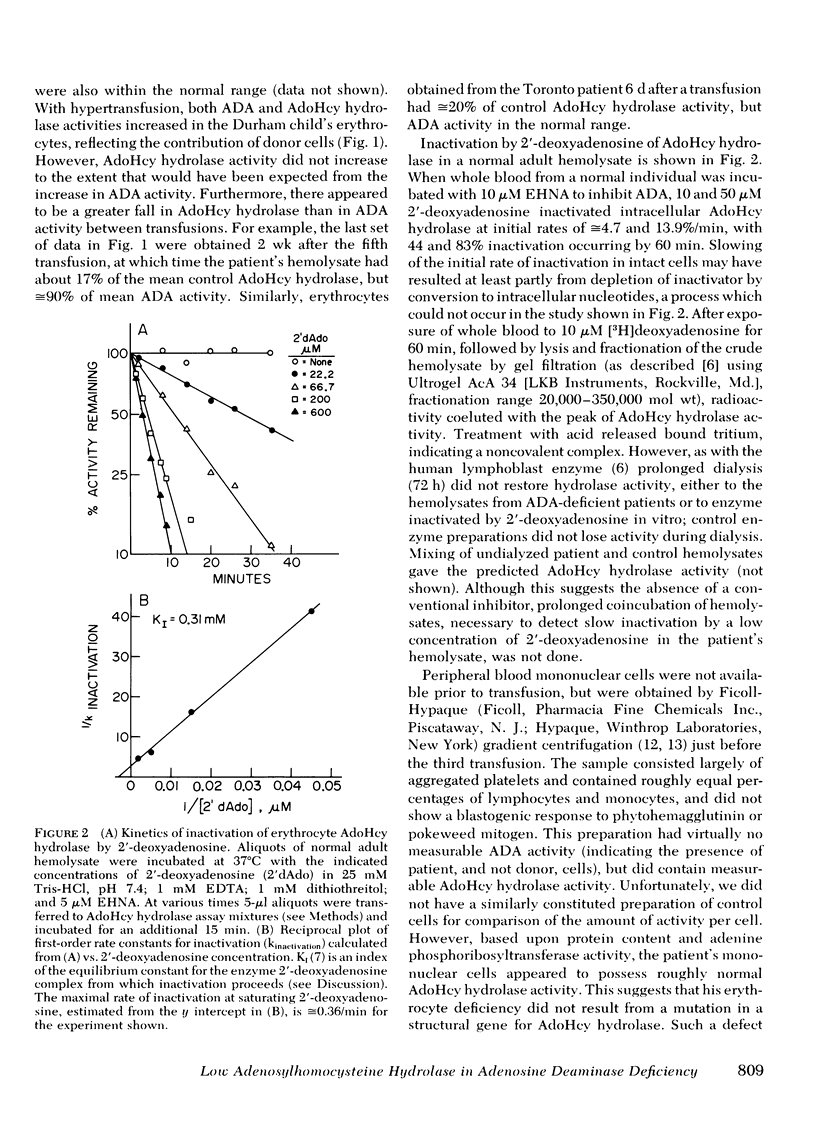
Abstract
The cytotoxic nucleoside 2'-deoxyadenosine is excreted in excessive amounts by individuals with genetic deficiency of adenosine deaminase, and may be in part responsible for the severe combined immune dysfunction from which they suffer. Earlier studies from this laboratory showed that 2'-deoxyadenosine causes the irreversible inactivation of the enzyme S-adenosylhomocysteine hydrolase by an active site-directed, "suicide-like" process. In this communication we have demonstrated similar inactivation of S-adenosylhomocysteine hydrolase in hemolysate and in intact erythrocytes, as well as a striking deficiency of S-adenosylhomocysteine hydrolase activity in the erythrocytes of three adenosine deaminase-deficient patients. In vivo suicide-like inactivation of S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine may contribute to the cytotoxicity of 2'-deoxyadenosine and to the immune dysfunction in adenosine deaminase deficiency.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- DE LA HABA G., CANTONI G. L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959 Mar;234(3):603–608. [PubMed] [Google Scholar]
- Donofrio J., Coleman M. S., Hutton J. J., Daoud A., Lampkin B., Dyminski J. Overproduction of adenine deoxynucleosides and deoxynucletides in adenosine deaminase deficiency with severe combined immunodeficiency disease. J Clin Invest. 1978 Oct;62(4):884–887. doi: 10.1172/JCI109201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S. Apparent suicide inactivation of human lymphoblast S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine and adenine arabinoside. A basis for direct toxic effects of analogs of adenosine. J Biol Chem. 1979 Jan 10;254(1):22–25. [PubMed] [Google Scholar]
- Hershfield M. S., Krodich N. M. S-adenosylhomocysteine hydrolase is an adenosine-binding protein: a target for adenosine toxicity. Science. 1978 Nov 17;202(4369):757–760. doi: 10.1126/science.715439. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Snyder F. F., Seegmiller J. E. Adenine and adenosine are toxic to human lymphoblast mutants defective in purine salvage enzymes. Science. 1977 Sep 23;197(4310):1284–1287. doi: 10.1126/science.197600. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Martin D. V., Jr Role of S-adenosylhomocysteine in adenosinemediated toxicity in cultured mouse T lymphoma cells. Cell. 1977 Dec;12(4):931–938. doi: 10.1016/0092-8674(77)90157-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Polmar S. H., Stern R. C., Schwartz A. L., Wetzler E. M., Chase P. A., Hirschhorn R. Enzyme replacement therapy for adenosine deaminase deficiency and severe combined immunodeficiency. N Engl J Med. 1976 Dec 9;295(24):1337–1343. doi: 10.1056/NEJM197612092952402. [DOI] [PubMed] [Google Scholar]
- Schiff R. I., Buckley R. H., Gilbertsen R. B., Metzgar R. S. Membrane receptors and in vitro responsiveness of lymphocytes in human immunodeficiency. J Immunol. 1974 Jan;112(1):376–386. [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Cohen A., Martin D. W., Jr Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease. Cell. 1978 Jun;14(2):365–375. doi: 10.1016/0092-8674(78)90122-8. [DOI] [PubMed] [Google Scholar]
- Walsh C. Recent developments in suicide substrates and other active site-directed inactivating agents of specific target enzymes. Horiz Biochem Biophys. 1977;3:36–81. [PubMed] [Google Scholar]



