Abstract
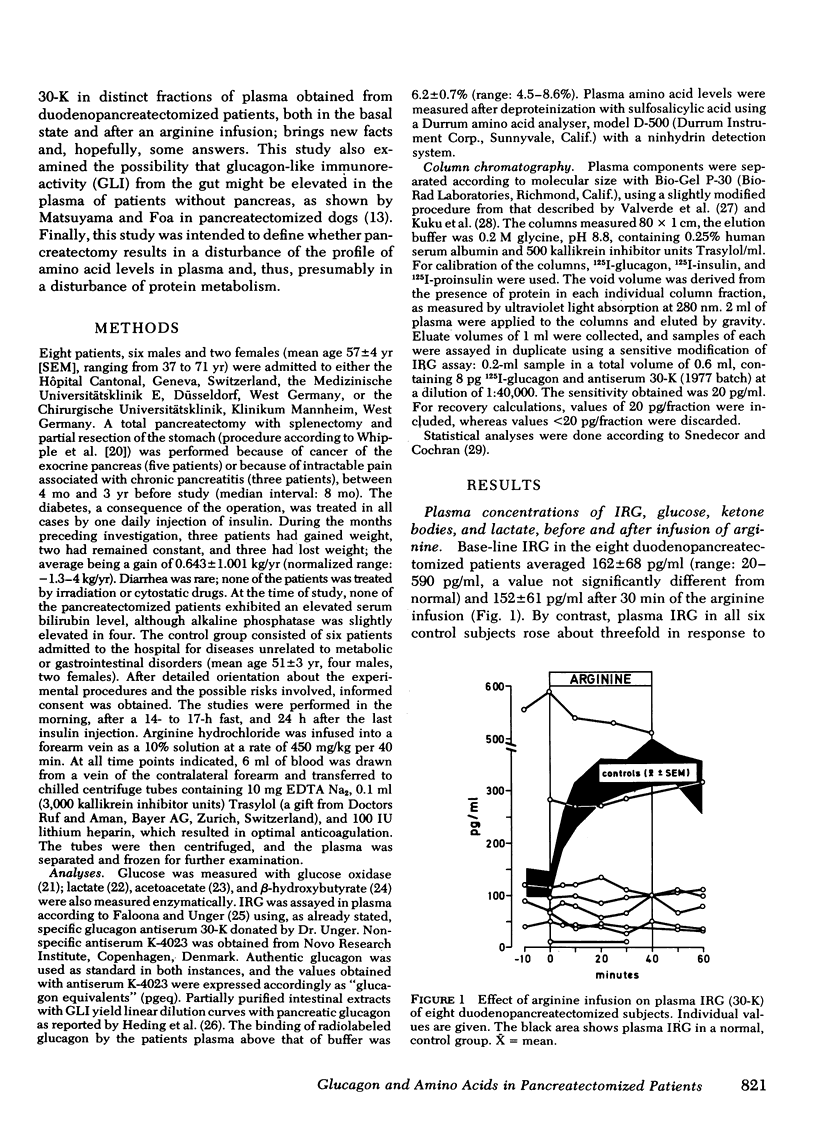
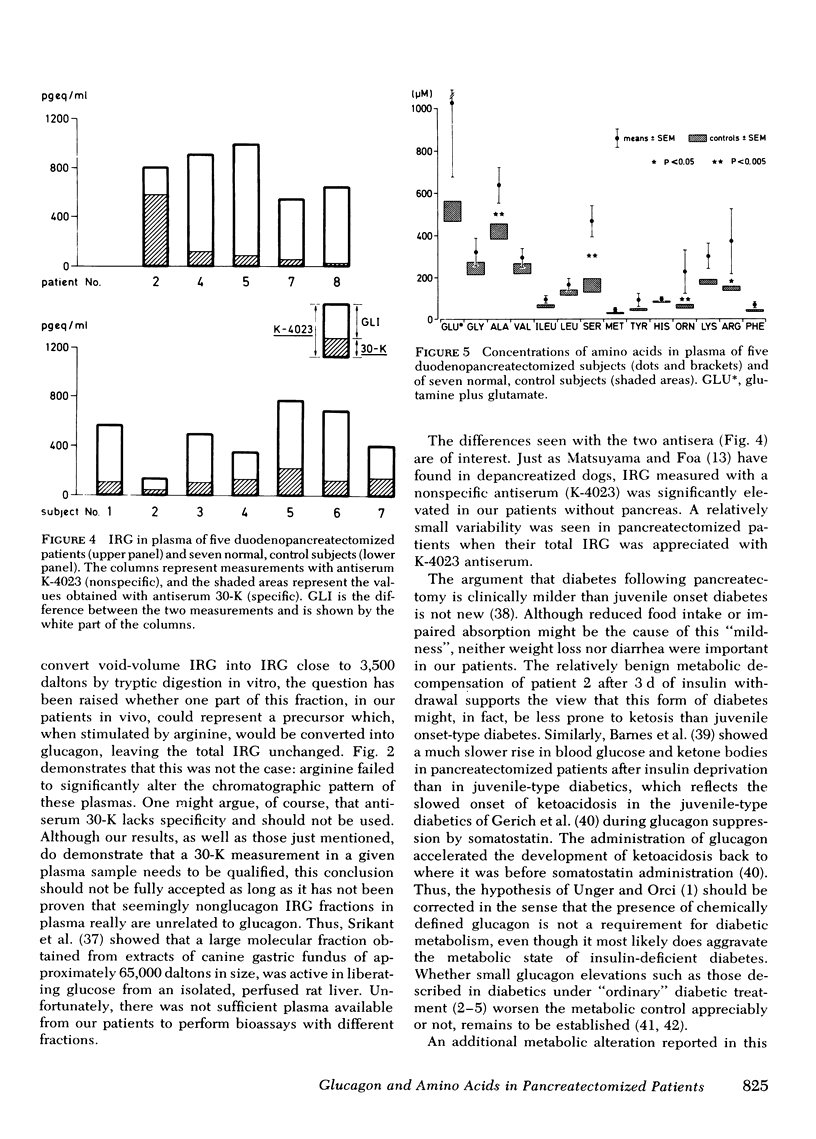
Glucogon immunoreactivity (IRG) was measured in plasma of duodenopancreatectomized subjects with a nonspecific (K-4023) and a specific (30-K) glucagon antiserum. After an overnight fast, plasma IRG (K-4023) was significantly (P < 0.05) higher in the subjects without pancreas, averaging 782±79 (SEM) pgeq/ml, than in the controls (482±80 pgeq/ml). IRG (30-K) of 162±68 pg/ml did not change during an infusion of arginine (450 mg/kg per 40 min). Insulin deprivation during 3 d in one patient did not restore the IRG response to arginine as reported in depancreatized dogs.
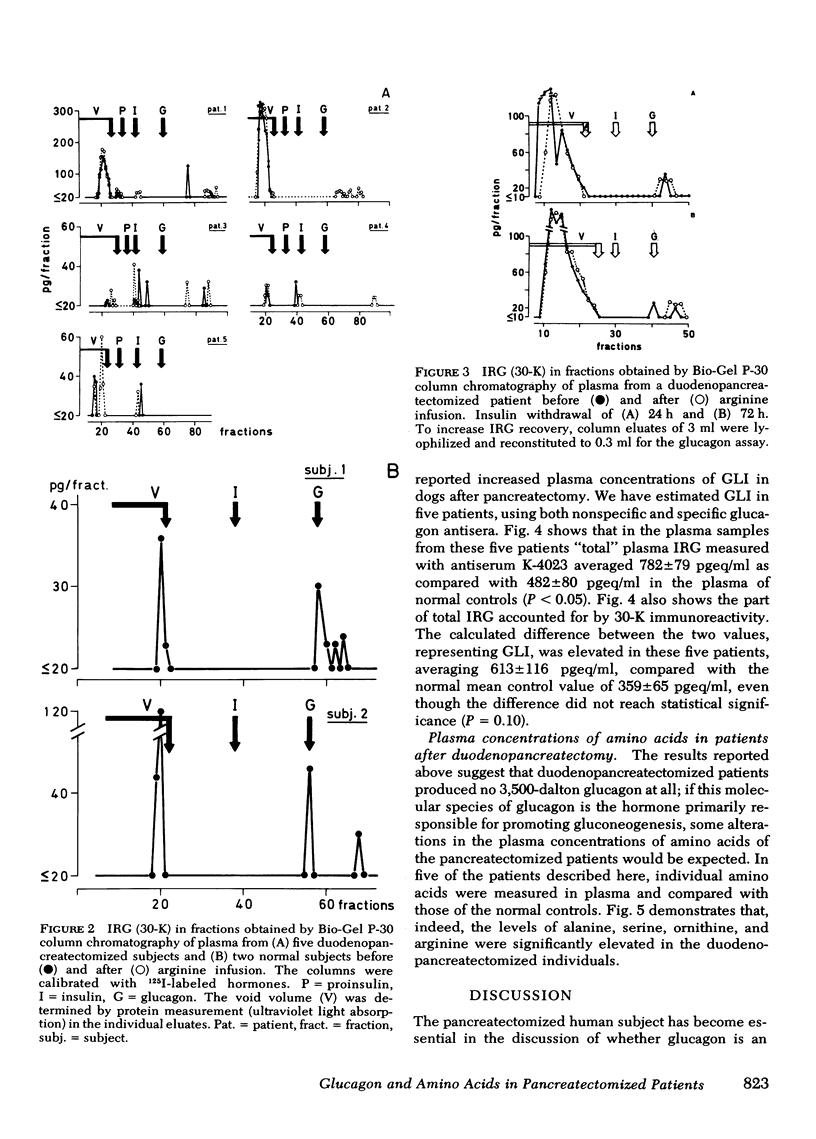
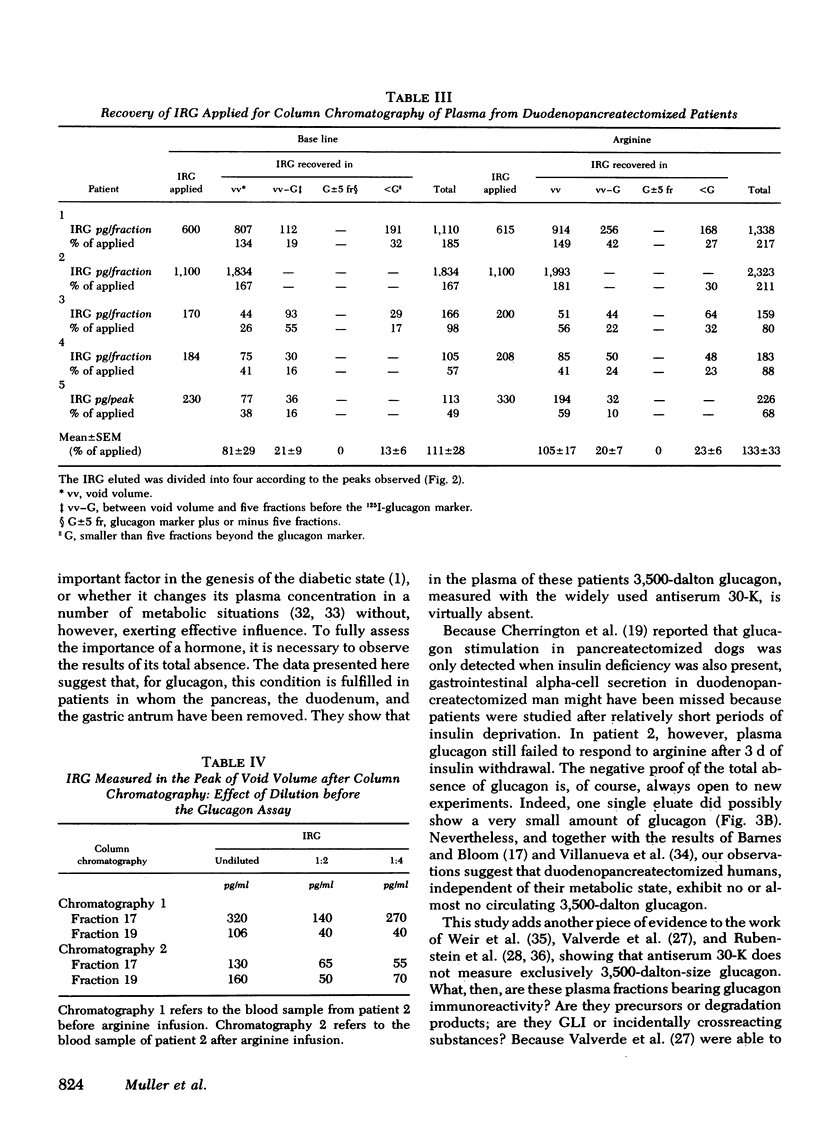
Bio-Gel P-30 column chromatography revealed that virtually all IRG (30-K) measured in whole plasma was of different molecular weight than glucagon, and primarily of a mol wt ≥ 40,000. Intravenous arginine did not significantly alter the chromatographic pattern of these plasmas. Thus, as postulated by others, duodeno-pancreatectomized humans have virtually no circulating 3,500-dalton glucagon. Hence, the presence of 3,500-dalton glucagon in plasma is not a condition for the diabetic state. It might, nevertheless, when present in normal or excessive amounts, worsen the metabolic state of diabetic patients.
Among 14 amino acids measured in plasma of these patients, the concentrations of alanine, serine, ornithine, and arginine were significantly (P < 0.05) elevated to approximately twice that of normal: alanine and serine are both substrates for gluconeogenesis, whereas ornithine and arginine are involved in the formation of urea, the second product of hepatic gluconeogenesis. As the concentrations of branched chain amino acids were not grossly altered, it is hypothesized that this amino acid pattern is a consequence of glucagon deficiency rather than secondary to the diabetic state of these patients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes A. J., Bloom S. R., Goerge K., Alberti G. M., Smythe P., Alford F. P., Chisholm D. J. Ketoacidosis in pancreatectomized man. N Engl J Med. 1977 Jun 2;296(22):1250–1253. doi: 10.1056/NEJM197706022962202. [DOI] [PubMed] [Google Scholar]
- Barnes A. J., Bloom S. R., Mashiter K., Alberti K. G., Smythe P., Turnell D. Persistent metabolic abnormalities in diabetes in the absence of glucagon. Diabetologia. 1977 Jan;13(1):71–75. doi: 10.1007/BF00996330. [DOI] [PubMed] [Google Scholar]
- Barnes A. J., Bloom S. R. Pancreatectomised man: A model for diabetes without glucagon. Lancet. 1976 Jan 31;1(7953):219–221. doi: 10.1016/s0140-6736(76)91339-8. [DOI] [PubMed] [Google Scholar]
- Berger M., Zimmermann-Telschow H., Berchtold P., Drost H., Müller W. A., Gries F. A., Zimmermann H. Blood amine acid levels in patients with insulin excess (functioning insulinoma) and insulin deficiency (diabetic ketosis). Metabolism. 1978 Jul;27(7):793–799. doi: 10.1016/0026-0495(78)90214-7. [DOI] [PubMed] [Google Scholar]
- Blazquez E., Muñoz-Barragan L., Patton G. S., Orci L., Dobbs R. E., Unger R. H. Gastric A-cell function in insulin-deprived depancreatized dogs. Endocrinology. 1976 Nov;99(5):1182–1188. doi: 10.1210/endo-99-5-1182. [DOI] [PubMed] [Google Scholar]
- Buchanan K. D., McCarroll A. M. Abnormalities of glucagon metabolism in untreated diabetes mellitus. Lancet. 1972 Dec 30;2(7792):1394–1395. doi: 10.1016/s0140-6736(72)92964-9. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Kawamori R., Pek S., Vranic M. Arginine infusion in dogs. Model for the roles of insulin and glucagon in regulating glucose turnover and free fatty acid levels. Diabetes. 1974 Oct;23(10):805–815. doi: 10.2337/diab.23.10.805. [DOI] [PubMed] [Google Scholar]
- Chesney C. M., Harper E., Colman R. W. Human platelet collagenase. J Clin Invest. 1974 Jun;53(6):1647–1654. doi: 10.1172/JCI107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J. L., Anderson J. Abnormalities of glucagon metabolism in diabetes mellitus. Clin Endocrinol (Oxf) 1973 Jul;2(3):211–217. doi: 10.1111/j.1365-2265.1973.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Felig P., Marliss E., Ohman J. L., Cahill C. F., Jr Plasma amino acid levels in diabetic ketoacidosis. Diabetes. 1970 Oct;19(10):727–728. doi: 10.2337/diab.19.10.727. [DOI] [PubMed] [Google Scholar]
- Felig P., Pozefsky T., Marliss E., Cahill G. F., Jr Alanine: key role in gluconeogenesis. Science. 1970 Feb 13;167(3920):1003–1004. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Bier D. M., Tsalikian E., Schneider V., Karam J. H., Forsham P. H. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. J Clin Invest. 1976 Apr;57(4):875–884. doi: 10.1172/JCI108364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heding L. G., Frandsen E. K., Jacobsen H. Structure-function relationship: immunologic. Metabolism. 1976 Nov;25(11 Suppl 1):1327–1329. doi: 10.1016/s0026-0495(76)80134-5. [DOI] [PubMed] [Google Scholar]
- Herberg L., Coleman D. L. Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism. 1977 Jan;26(1):59–99. doi: 10.1016/0026-0495(77)90128-7. [DOI] [PubMed] [Google Scholar]
- Jaspan J. B., Rubenstein A. H. Circulating glucagon. Plasma profiles and metabolism in health and disease. Diabetes. 1977 Sep;26(9):887–904. doi: 10.2337/diab.26.9.887. [DOI] [PubMed] [Google Scholar]
- Katsilambros N., Rahman Y. A., Hinz M., Fussgänger R., Schröder K. E., Straub K., Pfeiffer E. F. Action of streptozotocin on insulin and glucagon responses of rat islets. Horm Metab Res. 1970 Sep;2(5):268–270. doi: 10.1055/s-0028-1095056. [DOI] [PubMed] [Google Scholar]
- Kuku S. F., Jaspan J. B., Emmanouel D. S., Zeidler A., Katz A. I., Rubenstein A. H. Heterogeneity of plasma glucagon. Circulating components in normal subjects and patients with chronic renal failure. J Clin Invest. 1976 Sep;58(3):742–750. doi: 10.1172/JCI108521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinson C. N., Bloom S. R., Warin A. P., Salmon P. R., Cox B. A glucagonoma syndrome. Lancet. 1974 Jul 6;2(7871):1–5. doi: 10.1016/s0140-6736(74)91343-9. [DOI] [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Unger R. H., Soeldner J. S., Cahill G. F., Jr Glucagon levels and metabolic effects in fasting man. J Clin Invest. 1970 Dec;49(12):2256–2270. doi: 10.1172/JCI106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiter K., Harding P. E., Chou M., Mashiter G. D., Stout J., Diamond D., Field J. B. Persistent pancreatic glucagon but not insulin response to arginine in pancreatectomized dogs. Endocrinology. 1975 Mar;96(3):678–693. doi: 10.1210/endo-96-3-678. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Foà P. P. Plasma glucose, insulin, pancreatic, and enteroglucagon levels in normal and depancreatized dogs. Proc Soc Exp Biol Med. 1974 Oct;147(1):97–102. doi: 10.3181/00379727-147-38288. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Brennan M. F., Tan M. H., Aoki T. T. Studies of glucagon secretion in pancreatectomized patients. Diabetes. 1974 Jun;23(6):512–516. doi: 10.2337/diab.23.6.512. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Girardier L., Seydoux J., Berger M., Renold A. E., Vranic M. Extrapancreatic glucagon and glucagonlike immunoreactivity in depancreatized dogs. A quantitative assessment of secretion rates and anatomical delineation of sources. J Clin Invest. 1978 Jul;62(1):124–132. doi: 10.1172/JCI109096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971 Sep;50(9):1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhooda A. F., Like A. A., Chappel C. I., Wei C. N., Marliss E. B. The spontaneously diabetic Wistar rat (the "BB" rat). Studies prior to and during development of the overt syndrome. Diabetologia. 1978 Mar;14(3):199–207. doi: 10.1007/BF00429781. [DOI] [PubMed] [Google Scholar]
- Raskin P., Unger R. H. Effects of exogenous hyperglucagonemia in insulin-treated diabetics. Diabetes. 1977 Nov;26(11):1034–1039. doi: 10.2337/diab.26.11.1034. [DOI] [PubMed] [Google Scholar]
- Saunders S. J., Truswell A. S., Barbezat G. O., Wittman W., Hansen J. D. Plasma free aminoacid pattern in protein-calorie malnutrition. Reappraisal of its diagnostic value. Lancet. 1967 Oct 14;2(7520):795–797. doi: 10.1016/s0140-6736(67)92233-7. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Fisher M., Hendler R., Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med. 1976 Feb 26;294(9):455–461. doi: 10.1056/NEJM197602262940901. [DOI] [PubMed] [Google Scholar]
- Srikant C. B., McCorkle K., Unger R. H. Characteristics of tissue IRGs in the dog. Metabolism. 1976 Nov;25(11 Suppl 1):1403–1404. doi: 10.1016/s0026-0495(76)80151-5. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Orci L. Physiology and pathophysiology of glucagon. Physiol Rev. 1976 Oct;56(4):778–826. doi: 10.1152/physrev.1976.56.4.778. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975 Jan 4;1(7897):14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- Valverde I., Villanueva M. L., Lozano I., Marco J. Presence of glucagon immunoreactivity in the globulin fraction of human plasma ("big plasma glucagon"). J Clin Endocrinol Metab. 1974 Dec;39(6):1090–1098. doi: 10.1210/jcem-39-6-1090. [DOI] [PubMed] [Google Scholar]
- Villanueva M. L., Hedo J. A., Marco J. Plasma glucagon immunoreactivity in a totally pancreatectomized patient. Diabetologia. 1976 Dec;12(6):613–616. doi: 10.1007/BF01220639. [DOI] [PubMed] [Google Scholar]
- Vranic M., Pek S., Kawamori R. Increased "glucagon immunoreactivity" in plasma of totally depancreatized dogs. Diabetes. 1974 Nov;23(11):905–912. doi: 10.2337/diab.23.11.905. [DOI] [PubMed] [Google Scholar]
- WHITFIELD A. G., CRANE C. W., FRENCH J. M., BAYLEY T. J. LIFE WITHOUT A PANCREAS. Lancet. 1965 Mar 27;1(7387):675–677. doi: 10.1016/s0140-6736(65)91829-5. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Knowlton S. D., Martin D. B. High molecular weight glucagon-like immunoreactivity in plasma. J Clin Endocrinol Metab. 1975 Feb;40(2):296–302. doi: 10.1210/jcem-40-2-296. [DOI] [PubMed] [Google Scholar]



