Abstract
Rice bran chemical profiles differ across rice varieties and have not yet been analyzed for differential chemopreventive bioactivity. A diverse panel of 7 rice bran varieties was analyzed for growth inhibition of human colorectal cancer (CRC) cells. Inhibition varied from 0–99%, depending on the variety of bran used. Across varieties, total lipid content ranged 5–16%, individual fatty acids had 1.4 to 1.9 fold differences, vitamin E isoforms (α-, γ-, δ- tocotrienols and tocopherols) showed 1.3 to 15.2 fold differences, and differences in γ- oryzanol and total phenolics ranged between 100–275 ng/mg and 57–146 ng GAE/mg, respectively. Spearman correlation analysis was used to identify bioactive compounds implicated in CRC cell growth inhibitory activity. Total phenolics and γ- tocotrienol were positively correlated with reduced CRC cell growth (p < 0.05). Stoichiometric variation in rice bran components and differential effects on CRC viability merit further evaluation elucidate their role in dietary CRC chemoprevention.
Keywords: Chemoprevention, Colorectal Cancer, Bioactive Components, γ-tocotrienol, Phenolics, Rice Bran, Vitamin E
1. INTRODUCTION
Colorectal cancer (CRC) is one of the most common causes of cancer related deaths (Siegel, Naishadham & Jemal, 2012). It has been estimated that dietary changes have the potential to decrease CRC incidence by 60–70% (Donaldson, 2004), and recent evidence supports that consumption of brown rice at least once weekly reduces the risk of CRC polyp formation by 40% (Tantamango, Knutsen, Beeson, Fraser & Sabate, 2011). Rice bran, the outer layer of the brown rice grain, has repeatedly been shown to contain phytochemicals with biological activities associated with preventing CRC (Henderson et al., 2012; Li, Chou & Shih, 2011). In carcinogen induced CRC pre-clinical models, rice bran components have shown anticancer activity in isolation and as a whole food ingredient. Furthermore, the rice bran oil fraction has been shown to significantly reduce tumor burden in rats when compared to other plant oils (Panala, Verghese, Boateng, Field, Shackelford & Walker, 2009).
Rice brans are comprised of specific lipids in distinct ratios, yet little is known about the role for lipid profiles across rice varieties to differentially influence the CRC fighting activity of rice bran although epidemiological evidence supports that dietary fatty acid profiles are associated with decreased CRC incidence (Rao, Hirose, Indranie & Reddy, 2001). In addition to fatty acids, rice bran also comprises polyphenolics, γ-oryzanol, and vitamin E isoforms. These bioactive rice bran components were found to display a range of antioxidant activities that could be directly related to their anticancer activity (Hudson, Dinh, Kokubun, Simmonds & Gescher, 2000). For instance, gamma-oryzanol, which contains sterols and ferulic acids called cycloartanyl ferulate or triterpene alcohol ferulate, has anti-cancer properties and is unique to the rice plant (Srinivasan, Sudheer & Menon, 2007). The distinct ratio of tocotrienols:tocopherols in rice bran has also been widely studied; however, the relative stoichiometric contribution of these vitamin E isoforms to anticancer activity is unknown. Despite the large, diverse and complex nature of rice bran compounds, most studies thus far have focused on single compounds or a specific group of compounds that are structurally related. This focus may be too narrow as emerging evidence supports the role of the complex mixtures of bioactive compounds in whole food for chemoprevention (Ricciardiello, Bazzoli & Fogliano, 2011). Therefore, studies of rice bran and its role in CRC chemoprevention should attempt to examine rice bran as a dietary source of multiple and varied bioactive compounds.
Medicinal plants have been traditionally screened for anticancer activity for nutraceutical development of the most active compounds. While an IC50 (concentration needed for 50% cell growth inhibition) is often used to compare chemotherapeutic efficacy across single compounds, this approach has been minimally applied in the evaluation of the complex mixtures in rice bran. This study identifies and compares the IC50 of rice bran extracts across several rice varieties in order to explore their effects on the inhibition of CRC cell growth. A total of seven rice bran varieties that exhibit wide variation in lipid contents were selected in order to test the hypothesis that specific rice bran components are positively correlated with growth inhibition of CRC cells. The major objectives of this study were to 1) determine rice bran varietal differences in anti-cancer activity, 2) characterize bran phytochemical extracts from genetically diverse rice varieties for stoichiometric differences in bioactive compounds, and 3) correlate rice bran compounds with inhibition of CRC cell growth. This experimental approach provides a foundation for evaluating the relative contribution of individual rice bran components while maintaining the integrity of the rice bran mixture of compounds associated with CRC growth inhibition.
2 MATERIALS AND METHODS
2.1 Rice Varieties
Seven rice varieties were obtained from the USDA-ARS Dale Bumpers National Rice Research Center (Stuttgart, AR) that represented several different sub-populations of Oryza sativa, bran colors and grain characteristics (Table 1). The varieties Wells (PI 612439), Cypress (PI 561734), and Jasmine 85 (PI 595927) are long grains with brown bran that have been commercially grown in the southern USA. Shufeng 121 (Shu 121) (PI 615015)is a long grain, brown bran accession from China. The two red bran varieties include Red Wells which is a result of a single gene mutation of Wells (Brooks, Yan, Jackson & Deren, 2008) and IL 121-1-1, a breeding line (BC3 F4) selected from a backcross using Jefferson (PI 593892), a tropical japonica cultivar, as the recurrent parent and an accession of the wild related species Oryza rufipogon (IRGC-105491) from Malaysia (Thomson et al, 2003). Rice with red bran has been shown to have elevated levels of proanthocyanidins due to the Rc gene on chromosome 7 that regulates proanthocyanidins (Sweeney, Thomson, Pfeil & McCouch, 2006). IAC 600 is a purple bran rice cultivar that was developed and commercialized in Brazil (Bastos, personal communication) and has been demonstrated to synthesize anthocyanins (Min, McClung & Chen, 2011) and possess health beneficial properties (Salgado, de Oliveira, Mansi, Donado-Pestana, Bastos & Marcondes, 2010). The varieties Wells and Red Wells were produced at Beaumont, TX during 2009 and IL 121-1-1was produced at the same location in 2010. All other varieties were produced at Stuttgart, AR during 2011.
Table 1.
Description of rice varieties selected for differences in bran components and bioactive inhibition of CRC viability.
| Rice Variety | Bran Color | Sub Population | Grain Type |
|---|---|---|---|
| Jasmine 85 | Brown | Indica | Long |
| IAC 600 | Purple | Temperate Japonica | Short |
| Red Wells | Red | Tropical Japonica | Long |
| IL 121-1-1 | Red | Tropical Japonica | Long |
| Cypress | Brown | Tropical Japonica | Long |
| Wells | Brown | Tropical Japonica | Long |
| Shu 121 | Brown | Indica | Long |
2.2 Rice Bran Isolation and Extraction for Cell Treatments
Rough rice from each variety was dehulled and milled using a Satake One Pass Mill (Pearler, Model SKD, Australia) and the bran was collected. The bran was heat stabilized using a commercial dryer (STERIS, Mentor, OH) at 110°C for 3 minutes, placed in a vacuum sealed plastic pouch, and stored at −20°C until further use.
The rice bran extraction method used for cell culture studies has been previously described (Ryan, Heuberger, Weir, Barnett, Broeckling & Prenni, 2011). Briefly, heat stabilized rice bran (200g) from each variety was incubated with ice-cold 80% methanol, single-phase aqueous-alcohol solvent for 1 hour to break down proteins and extract soluble small molecules. This rice bran-methanol suspension was centrifuged, after which supernatant was removed and subjected to speed vacuum evaporation. The weight of the remaining dried extract containing methanol-soluble free metabolites was used to determine appropriate dosing of bioactive components in cell culture assays. After weighing, the dried extract was re-suspended into methanol. This 80% methanol rice bran extract is referred to as the rice bran cell treatment extract.
2.3 Cell Culture and Treatment Conditions
HT-29, Caco-2 and SW-480 human colon cancer cell lines were purchased from American Type Culture Collection (Manassas, VA). CRC cell lines Caco-2 and SW480 were cultured in RPMI medium (Mediatech Inc, Manassas, VA), whereas HT-29 was cultured in DMEM (Hyclone laboratories, Logan, UT) media. All media was supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO), 2 mM L-glutamine (Mediatech Inc), 10 mg/mL penicillin, 10,000 IU/mL streptomycin, 25mg/mL amphotericin, 1 mM sodium pyruvate (Mediatech Inc), and 1x MEM nonessential amino acids (Mediatech Inc). All cells were grown to confluence at 37°C and were used for experimentation at similar passage numbers. Rice bran extracts were resuspended in cell culture medium at concentrations of 1, 3, and 5 mg/ml. All treatment doses contained 2.5% methanol from the rice bran extract suspension. Vehicle control treatments were also prepared in culture medium with 2.5% methanol.
2.4 Cell Viability Analysis
Colon cancer cell lines (Caco-2, HT-29, SW-480) were plated to a density of 2.5×105 cells/mL in 96-well flat-bottom plates and allowed to adhere overnight. Culture medium was removed and cell lines were incubated in the presence of rice bran extracts for 24 hours. Treatment medium was removed after 24 h and replaced with a solution consisting of cell culture medium and 1% resazurin sodium salt (AlamarBlue, Invitrogen, Carlsbad, CA). Plates were then incubated in the dark at 37°C for 1 hour. Fluorescence was measured at 530 nm (excitation)/590 nm (emission) (Bio-Tek Synergy HT Multi-Mode Microplate Reader) and viability was expressed as percent fluorescence relative to the vehicle control. Cell experiments were replicated 3 times and conducted in triplicate.
2.5 Lipid Content and Fatty Acid Profile Analysis
Total lipids were extracted using chloroform:methanol (2:1, v/v) via a modified Folch procedure (Dunbar & Bauer, 2002; Folch, Lees & Stanley, 1957) and the triacylglycerol fraction was subfractionated via thin layer chromatography. Fatty acid methyl esters were prepared after recovery of this fraction and fatty acid profiles were determined via capillary gas chromatography and flame ionization detection (GC-FID) as previously reported (Dunbar et al, 2002).
2.6 Rice Bran Extraction and Phytochemical Detection
The concentration of tocopherols, tocotrienols and γ-oryzanol in the whole rice bran and cell treatment extracts were quantified using published methods (Min et al., 2011). Briefly, tocopherols (α-, γ-, and δ-tocopherols), tocotrienols (α-, γ-, and δ-tocotrienols), and γ-oryzanols were determined using HPLC (Waters, Milford, MA). The HPLC was equipped with a Waters 2695Alliance Separation Module, a Waters 2996 Photodiode Array Detector (PDA), a Waters 474 Scanning Fluorescence Detector, and EmpowerTM 2 software for data acquisition. For whole-bran phytochemical quantification, rice bran was extracted with 100% methanol using a bran to solvent ratio of 1 to 33 (w/v) and is referred to as whole-bran extract. The mixture was flushed with nitrogen gas and shaken overnight at 22°C. After centrifugation at 2000 × g for 10 min at 22°C, the supernatant was filtered through a 0.45 µm polyvinylidene fluoride (PVDF) membrane (Waters), injected through a Symmetryshield RP C-18 guard column (3.5 µm, 3.0 × 20 mm; Waters) and separated on a Symmetryshield RP C-18 analytical column (3.5 µm, 3.0 × 150 mm; Waters). The filtrate was eluted with a gradient mobile phase consisting of (A) 100% acetonitrile, (B) 100% methanol, and (C) 1% acetic acid in 50 % methanol at 0.5 mL/min at 25°C controlled by an HPLC column heater (TL-105, Timberline Instruments, Boulder, CO). The tocopherol and tocotrienol homologs were detected by the fluorescence detector at the excitation and emission wavelengths of 298 and 328 nm, respectively, and the γ-oryzanols by PDA at 325 nm. The peak identification for each substance was performed by comparing the retention time with those of standards. The concentration of each tocopherol and tocotrienol homolog and γ − oryzanol fraction was calculated using a standard curve, which was obtained by plotting the peak area against a series of concentrations of each tocopherol and tocotrienol homolog and γ − oryzanol standard and indicated as µg/g rice bran.
2.7 Total Phenolics Assay
Total phenolic concentrations in rice bran cell treatment extracts were determined as previously described, with minor modifications (Heuberger, Lewis, Chen, Brick, Leach & Ryan, 2010). Briefly, 150 µL of Folin-Ciocalteu reagent/water (1:9) was added to 35 µL of rice metabolite extract and incubated at 22°C for 5 minutes. Sodium bicarbonate (115 µL of a 7.5% solution) was then added and samples were incubated at 37°C for 30 minutes. Samples were allowed to cool to 22°C and absorbance was measured at 765 nm (Bio-Tek Synergy HT Multi- Mode Microplate Reader). Metabolite extractions were performed in triplicate. Total phenolics were calculated using a standard curve generated from a series of gallic acid concentrations; values were expressed as ng of gallic acid equivalents (GAE) per mg of rice bran.
2.8 Statistical Analysis
A one-way ANOVA with Bonferroni correction was applied to evaluate the significance of chemical content differences across varieties. A two-way ANOVA with Bonferroni correction was used to determine the significance of differences in cell growth inhibition across varieties and by dose and rice variety for cell culture assays. Differences in rice bran extract effects on CRC cell viability across the cell lines and between rice varieties were determined using a two-way ANOVA and Tukey’s HSD. A Dunnet’s 2-tailed comparison was used to confirm significant differences between treatments (rice varieties) and control (vehicle). Data are presented as mean ± SEM. Correlation between the concentration of each bioactive component in the rice bran cell treatment extracts and percent inhibition of colon cancer cell viability, as well as concentrations of whole rice bran lipids, fatty acids, and bioactive compounds was determined using Spearman’s rank correlation coefficient. The rice bran extract’s potential for inhibition of cell growth was compared by determining an IC50 using liner regression. These tests were performed using GraphPad Prism (v 5.0, GraphPad Software, Inc., La Jolla, CA). Results were considered significant at P < 0.05.
3. RESULTS AND DISCUSSION
3.1. CRC Cell Growth Inhibition is Dependent on Rice Bran Variety and Extract Dose
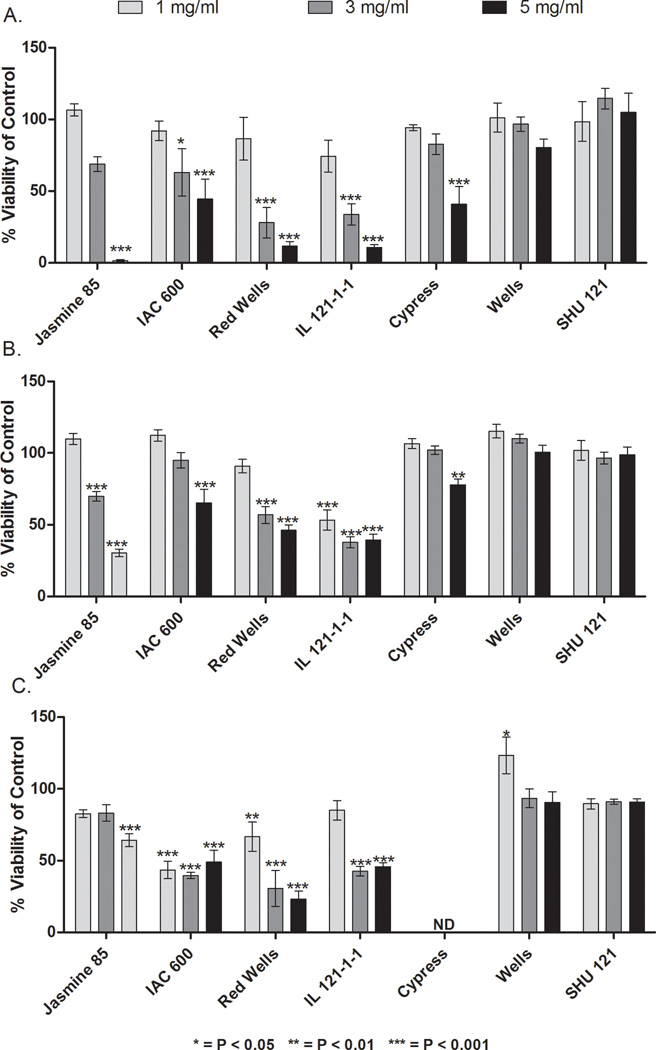
Methanolic rice bran cell treatment extracts showed a range of a 33 fold difference in CRC cell growth inhibition (Figure 1). No significant difference in the growth inhibitory effects of rice bran cell treatment extracts was detected across the three human CRC cell lines tested. HT-29, Caco-2 and SW-480 human colon cancer cell lines were selected for this experiment based on their differences in p53 mutations and varying degrees of differentiation and invasion characteristics (Pai, Nakamura, Moon & Tarnawski, 2003; von Kleist, Chany, Burtin, King & Fogh, 1975). We rationalized that by selecting these three different cell lines, we would be able to assess and establish both selectivity and specificity of our agents as well as differential effects, if any, in different human CRC cells. Figure 1A illustrates Caco2 cells treated with rice bran at a concentration of 5 mg/ml, Jasmine 85 had the greatest inhibitory effect with cell viability at 1.62% ± 0.54%, followed by IL 121-1-1 (10.82% ± 1.92%), Red Wells (11.86% ± 3.30%), Cypress (40.92% ± 12.31%), and IAC 600 (44.39% ± 14.03%). Wells and Shu 121 showed no inhibitory effect on Caco2 cells. In the HT-29 cells treated with the highest rice bran concentration of 5mg/ml, Jasmine 85, IL 121-1-1, and Red Wells were again the most inhibitory rice varieties, with cell viabilities of 30.37% ± 2.56%, 43.24% ± 3.97%, and 46.24% ± 3.71%, respectively (Figure 1B). IAC 600 had less of an inhibitory effect on HT-29 cells compared to Caco2 cells (65.30 ± 9.48%), while Cypress, Shu 121 and Wells did not have an inhibitory effect. In the 5mg/ml-treated SW-480 cells (Figure 1C), the most potent rice bran extract was Red Wells (23.21% ± 5.67%), followed by IAC 600 (49.10% ± 8.05%) and IL 121-1-1 (45.84% ± 2.80%). Wells and Shu 121 did not inhibit SW−480 cell growth. Cypress was not evaluated on the SW−480 cell line because the amount of extract available from this rice variety was limited. Across all cell lines, Jasmine 85, IL 121-1-1, Red Wells, and IAC 600 inhibited CRC cell growth while Wells, Cypress, and Shu 121 exhibited minimal CRC growth inhibition.
FIG 1. Dose-Dependent and Rice Bran Variety Differences in CRC Cell Viability following Treatment with Bran Extracts.
CRC cell viability after 24 hours compared to control. A. Caco-2 cell line, B. HT-29 and C. SW- 480. ND = not determined. Differences from control were considered significant when p < 0.05.
In order to compare the inhibitory potential of each rice bran extract on growth in each CRC cell line, an IC50 was determined for each rice variety (Table 2). These results were then used to rank the relative anticancer activity of each variety of rice bran extract. IC50 values could not be calculated for Shu 121 and Wells due to their low inhibitory activity on the CRC cell lines. Bran extracts from the IAC 600 (1.13–4.1 mg/ml) and Red Wells (1.8–3.6 mg/ml), purple and red bran varieties, had the lowest IC50 range across the cell lines tested. These results demonstrate that the in vitro CRC inhibitory properties of rice bran extracts differ based on rice variety. 216
Table 2.
Rice bran varietal differences for inhibition of CRC viability.
| Rice Variety | IC50* (mg/ml) |
||
|---|---|---|---|
| HT 29 | Caco2 | SW-480 | |
| Jasmine 85 | 2.5 | 3.0 | 5.53 |
| IAC 600 | 3.1 | 4.2 | 1.13 |
| Red Wells | 3.6 | 2.6 | 1.81 |
| IL 121-1-1 | 3.2 | 2.4 | 5.19 |
| Cypress | 5.9 | 3.6 | ND |
| Wells | NA | 11.0 | 13.64 |
| Shu 121 | NA | NA | NA |
IC50 results are based off the milligrams per milliliter of rice bran at which 50% cell death was achieved.
ND: not determined.
NA: An IC50 for these varieties could not be calculated due to their low inhibition of CRC cell growth.
3.2 Total Phenolic Concentration Across Rice Bran Varieties is Correlated with CRC Cell Growth Inhibition
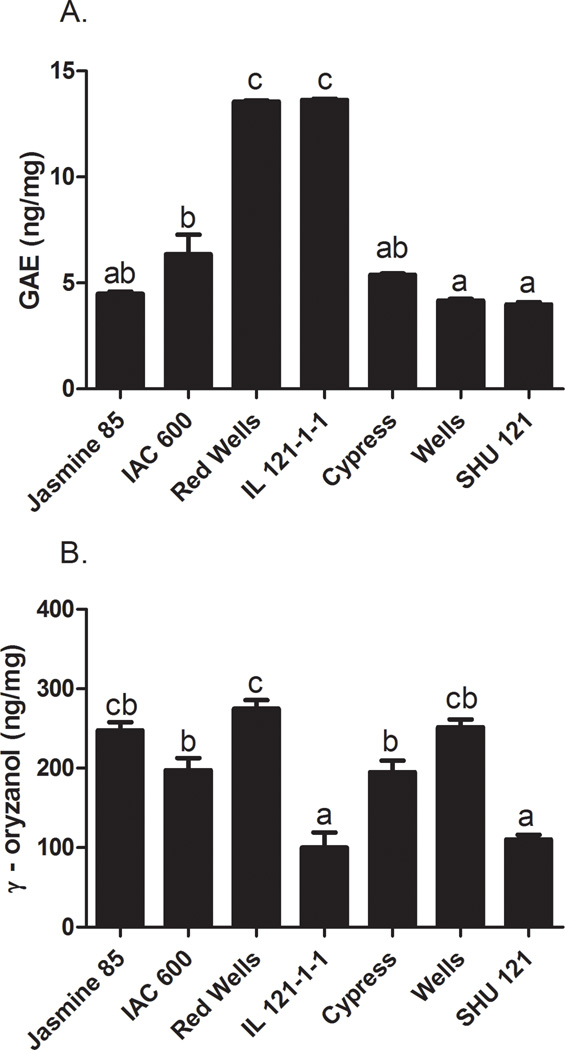
There was a 3.4-fold difference in total soluble phenolic concentrations between the 7 different rice bran cell treatment extracts (Fig. 2A). IL 121-1-1 and Red Wells had the highest phenolic concentrations (13.64 ± 0.05, 13.56 ± 0.06 ng/mg, respectively) followed by IAC 600 (6.35 ± 0.93 ng/mg). Cypress, Jasmine 85 and Shu 121 had the lowest concentrations (5.40 ± 0.06, 4.50 ± 0.10, and 3.99 ± 0.11 ng/mg, respectively).
FIG 2. Gamma-Oryzanol and Total Phenolics Concentrations in Rice Bran Cell Treatment Extracts of Seven Rice Varieties.
A. Total phenolic concentration expressed as ng gallic acid equivalents (GAE)/mg. B. Gamma-oryzanol concentrations (ng/mg). Columns marked by the same letter are not significantly different from each other (p < 0.05).
Total soluble phenolic concentration was strongly correlated with inhibition of cell viability (–0.71 to –0.81, p<0.05, Table 3). This finding supports previous work demonstrating the chemopreventive activity of multiple phenolic compounds in rice bran against CRC cell lines Hudson et al, 2000). Interestingly, IAC 600 demonstrated strong CRC cell inhibition but only contained moderate levels of total soluble phenolics. Jasmine 85 had one of the lowest total soluble phenolic levels and the lowest IC50 in the HT 29 cell line. This observation suggests that while important, phenolic compounds alone may not be responsible for the chemopreventive activity of the extracts, but may contribute to the bioactivity of the complex rice bran phytochemical mixture. This finding highlights the importance of multiple compounds working in concert to prevent chronic disease.
Table 3.
Spearman Correlations between Bioactive Rice Bran Components and CRC Viability.
| Cell Line | Caco2 | HT 29 | SW-480 | Vit E | δT3 | γT3 | αT3 | δT | γT | αT | γ-Oryzanol | Phenolics |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaCo2 | 1.00 | 0.83 | 0.64 | −0.27 | −0.29 | −0.88* | −0.45 | −0.23 | −0.01 | −0.50 | 0.06 | −0.73* |
| HT 29 | 0.83 | 1.00 | 0.72 | −0.33 | −0.38 | −0.19 | −0.38 | −0.26 | −0.08 | −0.42 | 0.13 | −0.81* |
| SW-480 | 0.64 | 0.72 | 1.00 | −0.33 | −0.42 | −0.17 | −0.54* | −0.01 | −0.15 | −0.54* | 0.25 | −0.71* |
Components with a significant correlation (P < 0.05)
Vitamin (Vit) E, sum of all isoforms of tocotrienols (T3) and tocopherols (T). δ, γ, and α, isoform symbols of delta, gamma and alpha, respectively.
Given the emphasis on rice bran oil for conferring bioactivity (Panala et al., 2009) , we decided to evaluate the extracts for bioactive lipophilic compounds. Variation in the amount of such compounds between rice varieties may contribute to the observed differences in anti-cancer activity across the bran extracts.
3.3 γ-Oryzanol Content is Not Correlated with CRC Cell Growth Inhibition
Gamma-oryzanol concentrations ranged from 100 to 274 ng/mg in the rice bran cell treatment extracts for a 2.74 fold difference across rice bran varieties. Red Wells, Wells and Jasmine 85 varieties had the highest concentrations of γ-oryzanol (274 ± 11, 252 ± 10, and 247 ± 11 ng/mg, respectively) followed by IAC 600 and Cypress (197 ± 15 and 195 ±14 ng/mg, respectively, Fig. 2B). Shu 121 and IL 121-1-1 varieties had the lowest levels (110 ± 6 and 100 ± 19 ng/mg, respectively).
Gamma-oryzanol content in the cell treatment extract did not significantly correlate with CRC cell growth inhibition, contrary to expectations from published in vivo data (Henderson et al, 2012; Kim, Kang, Nam & Friedman, 2012). The limited range in γ-oryzanol content in the rice bran cell treatment extract across the 7 rice varieties analyzed may explain the lack of correlation (Table 3). Furthermore, in vivo studies may be better suited to confirm the concentration of γ-oryzanol needed for CRC inhibition.
3.4. Total Vitamin E and Isoforms and CRC Cell Inhibition Correlations
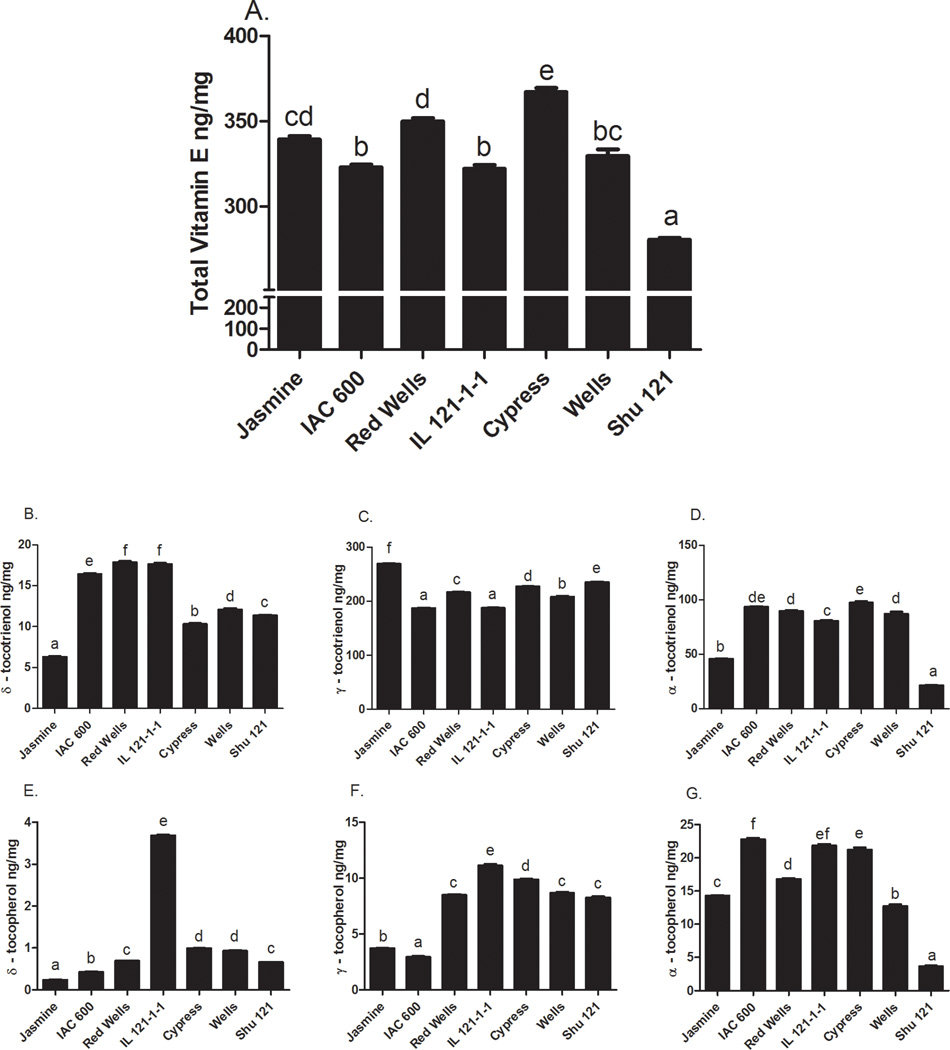
The rice bran cell treatment extracts from the 7 varieties have significantly different total vitamin E levels (Fig. 3A); in addition, there were marked differences across the isoforms,namely α-, γ-, and δ-tocotrienols and tocopherols (Fig 3B–G). Cypress had the highest total vitamin E concentration (367 ± 2.48 ng/mg) followed by Red Wells (350 ± 1 ng/mg) and Jasmine 85 (339 ± 2 ng/mg). Wells had the next highest total vitamin E concentration (330 ± 4 ng/mg), followed by IAC 600 (323 ± 2 ng/mg) and IL 121-1-1 (322± 2 ng/mg). Shu 121 had the lowest total vitamin E concentration (281 ± 1 ng/mg). The most prevalent vitamin E isoform was γ- tocotrienol followed by α-tocotrienol, α-tocopherol, δ-tocotrienol, γ-tocopherol, and δ- tocopherol. Variation in vitamin E profile determinations was consistent with previous reports (Huang & Ng, 2011; Min et al., 2011).
FIG 3. Quantification of Vitamin E Isoforms in Rice Bran Cell Treatment Extracts.
Rice bran extracts from seven varieties were evaluated for the concentrations (ng/mg) of A: Total vitamin E (represents the sum of tocotrienols and tocopherols); B: delta-tocotrienol, C. gamma-tocotrienol; D: alpha-tocotrienol; E: delta-tocopherol; F: gamma-tocopherol; G: alpha-tocopherol. Columns marked by the same letter are not significantly different from each other (p < 0.05).
Table 3 shows that γ-tocotrienol was highly correlated with CRC cell growth inhibition in the Caco-2 cell line (−0.88, p<0.05). In the SW-480 cell line, α-tocotrienol and α-tocopherol were significantly correlated with cell growth inhibition. Across cell lines, there were no consistent associations found between δ- tocotrienol, δ- tocopherol, or γ- tocopherol and CRC cell growth inhibitory activity.
Correlation analysis revealed the importance of total soluble phenolics, γ- tocotrienol, α- tocotrienol and α- tocopherol in the phytochemical extract mixture of rice bran for CRC cell growth inhibition in vitro (Table 3). The relative concentrations of vitamin E isoforms, γ- oryzanol, and total phenolic concentrations in the rice bran treatment extracts were not explained by the observed range in total percent rice bran lipid levels (Table 4). These findings show that the stoichiometry or relative ratios of lipophilic compounds in rice bran extracts do not parallel total lipid contents. Taken together, these data suggest that correlation analysis can reveal lipophilic rice bran compounds for CRC growth inhibition that should be considered individually as total lipid content may not be associated with bioactivity. Furthermore, a wide range in the concentrations of single compounds may be needed to evaluate their role in complex phytochemical mixtures.
Table 4.
Rice bran fatty acid profiles of selected varieties.
| % of total lipid |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice Variety |
*Total lipid (%) |
Myristic Acid |
Palmitic Acid |
Palmitoleic Acid |
Stearic Acid |
Oleic Acid |
Vaccenic Acid |
Linoleic Acid |
a-Linolenic Acid |
Arachidic Acid |
Gadoleic Acid |
Behenic Acid |
Lignoceric Acid |
| Jasmine 85 | 5.02 | 0.31 | 18.95 | 0.22 | 2.22 | 44.23 | 0.98 | 29.15 | 1.20 | 1.08 | 0.61 | 0.41 | 0.64 |
| IAC 600 | 9.80 | 0.36 | 16.63 | 0.22 | 3.06 | 38.89 | 1.44 | 34.82 | 1.09 | 1.27 | 0.55 | 0.48 | 0.81 |
| Red Wells | 9.96 | 0.31 | 13.73 | 0.16 | 2.32 | 43.32 | 0.99 | 35.22 | 1.09 | 1.00 | 0.69 | 0.40 | 0.78 |
| IL 121-1-1 | 12.1 | 0.34 | 14.21 | 0.14 | 2.84 | 43.50 | 0.95 | 33.80 | 0.97 | 1.37 | o.67 | 0.44 | 0.68 |
| Cypress | 12.3 | 0.26 | 13.25 | 0.15 | 1.91 | 44.30 | 0.93 | 35.50 | 1.22 | 0.85 | 0.72 | 0.32 | 0.58 |
| Wells | 12.6 | 0.26 | 14.76 | 0.17 | 1.63 | 42.36 | 1.02 | 36.27 | 1.17 | 0.75 | 0.70 | 0.32 | 0.60 |
| Shu 121 | 16.2 | 0.35 | 19.70 | 0.19 | 1.81 | 40.78 | 1.74 | 31.08 | 1.27 | 1.46 | 0.66 | 0.40 | 0.57 |
Reported as % of rice bran.
3.4.1 Variations in Whole Rice Bran Lipid Soluble Compounds Across Varieties
Given the demonstrated potential of this cell culture model system to screen rice bran varieties for CRC chemopreventive activity, we next evaluated fatty acid profiles and the concentrations of vitamin E isoforms and γ-oryzanol in whole rice bran from the 7 rice varieties, and determined the relationship between these bioactive compounds in the rice bran cell treatment extract versus the whole bran. The 7 rice varieties that were selected for this study showed a wide range of total lipid content (Table 4). The Jasmine 85 (5.02%) and Shu 121 (16.20%) varieties displayed the lowest and one of the highest percent lipid contents, respectively. The Red Wells and IAC 600 varieties have 9.80% and 9.96% lipid contents and the Cypress, Wells, and IL 121-1-1 showed a 12.1–12.6% range in lipid content.
Complete fatty acid profiles of the bran from these 7 rice varieties were evaluated as a percent of total lipids, and specific fatty acids ranged from 1.4 to 1.9 fold differences across varieties (Table 4). Variation in the most abundant fatty acids, namely palmitic acid (13.73–19.72%), oleic acid (38.8–44.3%), and linoleic acid (29.1–36.2%) was detected. Stearic acid (1.63–3.06%), alpha-linolenic acid (0.97–1.27%) and arachidic acid (0.85–1.46%) comprised a smaller percentage of the total fatty acid content, yet demonstrated a range across rice varieties.
Concentrations of vitamin E isoforms and γ-oryzanol in whole bran are shown in Table 5. The highest γ-oryzanol content was found in whole rice bran of Wells. These data from whole bran take into consideration the discrepancies and potential limitations of screening ‘extracts’ in in vitro assays compared to evaluating whole rice bran varietal differences with dietary feeding studies in vivo. The in vitro cell culture assay was an excellent tool to identify compounds that have chemopreventive bioactivity against CRC, and the resultant information could be used to guide rice crop improvement programs for screening concentrations of bioactive bran compounds. The data presented herein and from others has demonstrated that vitamin E isoforms (particularly γ-tocotrienol, α-tocotrienol, and α-tocopherol), γ-oryzanol and fatty acids have the potential to suppress CRC cell growth and to modulate colon tumorigenesis (Kim et al, 2012; Rao et al, 2001). These substances are all present in the crude rice bran oil fraction of whole rice bran. Some of these compounds have limited solubility in 80% methanol that was used for testing bioactivity in vitro, and merit further evaluation of chemopreventive bioactivity in vivo. Thus, tandem evaluation of rice bran extracts and the whole rice bran could further discern rice varietal differences. Accordingly, the results from this study emphasize that screening rice varieties is a reliable method for investigating the relative contributions of specific bran components; in addition, this method enables such investigation without reducing or isolating the specific components from the whole rice bran.
Table 5.
Concentrations* of vitamin E isoforms and γ-oryzanol in whole rice bran.
| Variety | δT3 | γT3 | αT3 | δT | γT | αT | γ-Oryzanol |
|---|---|---|---|---|---|---|---|
| Jasmine 85 | 2.1 ± 0.0 e | 231.6 ± 0.2b | 28.8 ± 3.6b | 0.0 ± 0.0e | 3.3 ± 0.7d | 24.1 ± 3.9d | 3564.7 ± 49.9cd |
| IAC 600 | 6.0 ± 0.2 b | 103.5 ± 5.4f | 98.5 ± 3.8a | 0.3 ± 0.0de | 1.3 ± 0.4d | 56.6 ± 1.6bc | 3633.3 ± 141.4cd |
| Red Wells | 6.7 ± 0.0 a | 154.0 ± 0.6d | 108.3 ± 2.1a | 0.6 ± 0.1cd | 14.0 ± 0.6c | 56.0 ± 0.5bc | 4266.7 ± 0.0b |
| IL 121-1-1 | 6.5 ± 0.0 a | 126.9 ± 1.4e | 109.7 ± 2.8a | 4.3 ± 0.2a | 28.8 ± 0.6b | 96.5 ± 0.8a | 932.8 ± 0.7e |
| Cypress | 3.6 ± 0.0 d | 180.2 ± 2.6c | 117.9 ± 14.8a | 1.0 ± 0.1bc | 23.3 ± 1.0b | 71.2 ± 7.8b | 3864.5 ± 3.0c |
| Wells | 5.5 ± 0.0 c | 184.2 ± 1.8c | 98.7± 6.4a | 1.3 ± 0.1b | 24.4 ± 0.5b | 41.6 ± 3.2c | 5133.3 ± 94.3a |
| Shu 121 | 5.8 ± 0.0 bc | 272.2 ± 3.3a | 13.1± 0.1b | 1.4 ± 0.1b | 41.0 ± 4.1a | 5.0 ± 0.3e | 3533.3 ± 0.0d |
values are mean ± SD (n = 2) of ug/g bran. Similar letters in the same column are not significantly different.
4.0 CONCLUSION
Rice (Oryza sativa) is a staple food and primary source of dietary calories for half of humanity, and has been intensively investigated for chemopreventive components (Henderson et al, 2012). Our study revealed that the relative proportions of rice bran bioactive components that inhibit CRC cell growth differ significantly across rice varieties. Among the compounds we investigated, total phenolic and γ- tocotrienol concentrations were most strongly correlated with CRC cell growth inhibition (Table 3). These findings build upon previous studies that highlight the role of rice bran phytic acid (Norazalina, Norhaizan, Hairuszah & Norashareena, 2010), tricin and flavonoids (Cai et al, 2005) in CRC chemoprevention activity. Dietary chemoprevention studies have shown that the magnitude of anticancer activity is greater with the whole food or whole food extract, when compared to isolated compounds (Ricciardiello et al, 2011; Shukla & George, 2011). Moreover, the safety and toxicity profile of dietary exposures to whole foods versus isolated supplements is rapidly gaining medical research attention from animal and human studies (Fahey, Talalay & Kensler, 2012). Thus, the evaluation of the profile of bioactive rice bran components across diverse rice varieties was an innovative approach for determining anticancer efficacy of complex phytochemical mixtures, and our findings support similar observations with beans (Messina, 1999), wheat (Lv et al, 2012), and berries (Tulipani et al, 2008). A rapidly growing body of scientific information points to the nutritional complexity of rice genetic diversity (Heuberger et al, 2010). Results from this study support variation in rice bran bioactivity with respect to CRC growth inhibition, and demonstrates the importance of reporting specific rice varieties used in pre-clinical and clinical investigations that may advance rice bran as a functional food ingredient.
Highlights.
Rice bran extracts differentially inhibit colorectal cancer (CRC) cell growth.
Different rice varieties have unique lipophilic bioactive compounds.
Total soluble phenolic content was correlated with CRC cell inhibition.
Vitamin E isoforms γ-T3, a-T3, and a-T were correlated with CRC cell inhibition.
Rice bran chemoprevention is due to the complex phytochemical mixture.
ACKNOWLEDGEMENTS
The authors thank Dr. Amy Keller for technical assistance with CRC viability assays, Dr. Ann Hess for providing statistical analysis support, and Cadie Tillotson and Erica Borresen with editorial aspects of manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report. The Shipley Foundation and NIH-NCI R03CA150070 supported the work reported in this manuscript. Lipid studies were supported by the Mark L. Morris Professorship of Clinical Nutrition at Texas A&M University.
REFERENCES
- Brooks SA, Yan WG, Jackson AK, Deren CW. A natural mutation in rc reverts white-rice-pericarp to red and results in a new, dominant, wild-type allele: Rc-g. Theoretical and Applied Genetics. 2008;117(4):575–580. doi: 10.1007/s00122-008-0801-8. [DOI] [PubMed] [Google Scholar]
- Cai H, Al-Fayez M, Tunstall RG, Platton S, Greaves P, Steward WP, Gescher AJ. The rice bran constituent tricin potently inhibits cyclooxygenase enzymes and interferes with intestinal carcinogenesis in apcmin mice. Molecular Cancer Therapeutics. 2005;4(9):1287–1292. doi: 10.1158/1535-7163.MCT-05-0165. [DOI] [PubMed] [Google Scholar]
- Donaldson MS. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutrition Journal. 2004;3:19. doi: 10.1186/1475-2891-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar BL, Bauer JE. Conversion of essential fatty acids by delta 6-desaturase in dog liver microsomes. Journal of Nutrition. 2002;132(6):1701S–1703S. doi: 10.1093/jn/132.6.1701S. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Talalay P, Kensler TW. Notes from the field: "green"chemoprevention as frugal medicine. Cancer Prevention Research. 2012;5(2):179–188. doi: 10.1158/1940-6207.CAPR-11-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Chemical Biology. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Henderson AJ, Ollila CA, Kumar A, Borresen EC, Raina K, Agarwal R, Ryan EP. Chemopreventive properties of dietary rice bran: Current status and future prospects. Advances in Nutrition. 2012;3(5):643–653. doi: 10.3945/an.112.002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuberger AL, Lewis MR, Chen MH, Brick MA, Leach JE, Ryan EP. Metabolomic and functional genomic analyses reveal varietal differences in bioactive compounds of cooked rice. Plos One. 2010;5(9) doi: 10.1371/journal.pone.0012915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, Ng LT. Quantification of tocopherols, tocotrienols, and gamma-oryzanol contents and their distribution in some commercial rice varieties in Taiwan. Journal of Agricultural and Food Chemistry. 2011;59(20):11150–11159. doi: 10.1021/jf202884p. [DOI] [PubMed] [Google Scholar]
- Hudson EA, Dinh PA, Kokubun T, Simmonds MSJ, Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiology, Biomarkers & Prevention. 2000;9(11):1163–1170. [PubMed] [Google Scholar]
- Kim SP, Kang MY, Nam SH, Friedman M. Dietary rice bran component γ-oryzanol inhibits tumor growth in tumor-bearing mice. Molecular Nutrition & Food Research. 2012;56(6):935–944. doi: 10.1002/mnfr.201200057. [DOI] [PubMed] [Google Scholar]
- Li SC, Chou TC, Shih CK. Effects of brown rice, rice bran, and polished rice on colon carcinogenesis in rats. Food Research International. 2011;44(1):209–216. [Google Scholar]
- Lv J, Yu L, Lu Y, Niu Y, Liu L, Costa J, Yu LL. Phytochemical compositions, and antioxidant properties, and antiproliferative activities of wheat flour. Food Chemistry. 2012;135:325–331. doi: 10.1016/j.foodchem.2012.04.141. [DOI] [PubMed] [Google Scholar]
- Messina MJ. Legumes and soybeans: Overview of their nutritional profiles and health effects. The American Journal of Clinical Nutrition. 1999;70(3):439S–450S. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- Min B, McClung AM, Chen MH. Phytochemicals and antioxidant capacities in rice brans of different color. Journal of Food Science. 2011;76(1):C117–126. doi: 10.1111/j.1750-3841.2010.01929.x. [DOI] [PubMed] [Google Scholar]
- Norazalina S, Norhaizan ME, Hairuszah I, Norashareena MS. Anticarcinogenic efficacy of phytic acid extracted from rice bran on azoxymethane-induced colon carcinogenesis in rats. Experimental and Toxicologic Pathology. 2010;62:259–268. doi: 10.1016/j.etp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Pai R, Nakamura T, Moon WS, Tarnawski AS. Prostaglandins promote colon cancer cell invasion; signaling by cross-talk between two distinct growth factor receptors. Federation of American Societies for Experimental Biology. 2003;17(12):1640–1647. doi: 10.1096/fj.02-1011com. [DOI] [PubMed] [Google Scholar]
- Panala V, Verghese J, Boateng J, Field R, Shackelford L, Walker LT. A comparison of rice bran, corn oil and soybean oil against azoxymethane induced colon cancer in a fisher 344 rat model. International Journal of Cancer Research. 2009;5(1):25–35. [Google Scholar]
- Rao CV, Hirose Y, Indranie C, Reddy BS. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Research. 2001;61(5):1927–1933. [PubMed] [Google Scholar]
- Ricciardiello L, Bazzoli F, Fogliano V. Phytochemicals and colorectal cancer prevention -- myth or reality? Nature Reviews. Gastroenterology & Hepatology. 2011;8(10):592–596. doi: 10.1038/nrgastro.2011.149. [DOI] [PubMed] [Google Scholar]
- Ryan EP, Heuberger AL, Weir TL, Barnett B, Broeckling CD, Prenni JE. Rice bran fermented with saccharomyces boulardii generates novel metabolite profiles with bioactivity. Journal of Agriculture and Food Chemistry. 2011;59(5):1862–1870. doi: 10.1021/jf1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado JM, de Oliveira AGC, Mansi DN, Donado-Pestana CM, Bastos CR, Marcondes FK. The role of black rice (Oryza sativa l.) in the control of hypercholesterolemia in rats. Journal of Medicinal Food. 2010;13(6):1355–1362. doi: 10.1089/jmf.2009.0246. [DOI] [PubMed] [Google Scholar]
- Shukla Y, George J. Combinatorial strategies employing nutraceuticals for cancer development. Annals of the New York Academy of Sciences. 2011;1229:162–175. doi: 10.1111/j.1749-6632.2011.06104.x. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: Therapeutic potential through its antioxidant property. Journal of Clinical Biochemistry and Nutrition. 2007;40(2):92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MT, Thomson MJ, Pfeil BE, McCouch S. Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. The Plant Cell. 2006;18(2):283–294. doi: 10.1105/tpc.105.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantamango YM, Knutsen SF, Beeson WL, Fraser G, Sabate J. Foods and food groups associated with the incidence of colorectal polyps: The Adventist health study. Nutrition and Cancer. 2011;63(4):565–572. doi: 10.1080/01635581.2011.551988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson MJ, Tai TH, McClung AM, Lai XH, Hinga ME, Lobos KB, Xu Y, Martinez CP, McCouch SR. Mapping quantitative trait loci for yield, yield components and morphological traits in an advanced backcross population between oryza rufipogon and the oryza sativa cultivar jefferson. Theoretical and Applied Genetics. 2003;107(3):479–493. doi: 10.1007/s00122-003-1270-8. [DOI] [PubMed] [Google Scholar]
- Tulipani S, Mezzetti B, Capocasa F, Bompadre S, Beekwilder J, De Vos CHR, Capanoglu E, Bovy A, Battino M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. Journal of Agriculture and Food Chemistry. 2008;56(3):696–704. doi: 10.1021/jf0719959. [DOI] [PubMed] [Google Scholar]
- von Kleist S, Chany E, Burtin P, King M, Fogh J. Immunohistology of the antigenic pattern of a continuous cell line from a human colon tumor. Journal of the National Cancer Institute. 1975;55(3):555–560. doi: 10.1093/jnci/55.3.555. [DOI] [PubMed] [Google Scholar]